Abstract
Insomnia is a highly prevalent disorder that occurs frequently in its acute form and at a rate of approximately 10% in its chronic form. There is a high prevalence of insomnia in a variety of medical and psychiatric conditions. Cognitive–behavioral therapy (CBT) may be employed for chronic insomnia as well as for insomnia in the context of other conditions such as chronic pain conditions. In such cases, some simple adaptations to standard CBT for insomnia are useful. This article reviews the typical assessment and CBT for adult insomnia, which have substantial empirical support for its efficacy. A case illustrates the core treatment processes and demonstrates that improving sleep in the context of conditions like chronic pain can lead to better management of such conditions.
Keywords: insomnia, treatment, cognitive–behavioral therapy, comorbidity, chronic pain
Chronic insomnia, whether it presents alone or in combination with another medical or psychiatric disorder, tends to persist in the absence of direct intervention. Cognitive–behavioral therapy for insomnia (CBT-I) is one treatment with strong support for its efficacy and growing support for its effectiveness (Leshner, 2005). The individual components of CBT-I, which include psycho-education, behavioral strategies, cognitive therapy, and relaxation training, can be delivered as monotherapies. It is widely accepted, however, that multicomponent CBT-I is the preferred approach. In this article, I review the typical assessment strategies and differential diagnoses for adult insomnia. I then review the core treatment components of CBT-I. A case illustration provides a template for how the treatment process can unfold when applying the core treatments that comprise CBT-I.
Insomnia is a fairly straightforward complaint, but two major dimensions of this disorder make thorough assessments a critical step in formulating and delivering successful, individualized CBT. First, because the diagnostic criteria for insomnia (presented in this issue in the article by Taylor & Roane, 2010, pp. 000–000) are somewhat minimal and insomnia itself is highly prevalent, differential diagnosis is quite important. Primary insomnia accounts for only 10–20% of all insomnia presentations. Most insomnia, then, occurs in the context of medical conditions and psychiatric disorders. When this is the case, the evaluation may point the clinician to consulting with the patient’s primary care provider and/or other professionals to discuss possible contraindications for CBT-I and/or developing joint treatment planning. For instance, some conditions do warrant targeted intervention prior to treating the presenting insomnia. These may include untreated or unstable gastroesophageal reflux disease, cardiovascular disease, bipolar disorder, alcohol or substance dependence, and other sleep disorders such as sleep apnea, restless legs syndrome, or circadian rhythm disorders.
Nonetheless, depending on the condition, insomnia may still be treated in conjunction with such conditions or in some cases (e.g., some chronic pain conditions) may be a front-line treatment. Regardless of the co-occurring condition, clinical experience and increasing research suggest that foregoing insomnia interventions altogether in the belief that insomnia is a symptom that will ameliorate in concert with the “primary” condition ill serves a sizable number of patients. Instead, an assessment of how the co-occurring condition interacts with insomnia in a particular patient aids in modifying CBT-I to best serve the individual presentation.
Second, the pathophysiology of insomnia can be somewhat complex and multifactorial. The major etiologic contributions to insomnia (Perlis, Smith, & Pigeon, 2005b) are believed to include hyperarousal, circadian dysregulation, and sleep homeostasis abnormalities coupled with maladaptive behaviors and dysfunctional thoughts and beliefs about sleep. The particular presentation of insomnia may differ based on the relative contribution of these factors, further informing how CBT-I may be structured.
The assessment of insomnia includes a thorough sleep, medical, and mental health history via a sleep-oriented clinical interview (Schutte-Rodin, Broch, Buysse, Dorsey, & Sateia, 2008). Particular attention is given to factors that contributed to the initiation of insomnia and to various life stressors and factors that maintain insomnia. The daily sleep diary, which is considered the gold standard subjective report of sleep, is an invaluable tool for both assessment and treatment. Patients are asked to complete these on a daily basis for 1–2 weeks. By entering daily values for time to bed and time out of bed along with minutes to fall asleep and minutes awake during the time in bed, weekly averages of several variables can be calculated by the therapist (latency to sleep, wake time, average time in bed, total sleep time, and sleep efficiency [sleep time divided by time in bed]). Such values may also be obtained objectively via wrist-worn actigraphy to either corroborate or replace sleep diary data, though most therapists prefer to use actigraphy in addition to (rather than instead of) diaries. A full overnight sleep study (a polysomnograhic recording), on the other hand, is not indicated in the standard assessment of insomnia. Finally, among the several validated, self-report instruments for the assessment of sleep disturbance, the most widely used when assessing insomnia are the Pittsburgh Sleep Quality Index (Buysee, Reynolds, Monk, Berman, & Kupfer, 1989), which provides a global assessment of sleep, and the more disease-specific Insomnia Severity Index (Bastien, Vallieres, & Morin, 2001). The comprehensive assessment of insomnia sets the stage for successful CBT-I.
Cognitive–Behavioral Therapy for Insomnia
The underpinnings of CBT-I flow from (a) the application of both operant and classical conditioning paradigms in the from of stimulus control instructions (Bootzin & Nicassio, 1978); (b) the focus on sleep-interfering behaviors in the form of sleep hygiene (Hauri, 1982); (c) the recognition of and focus on reducing the hyperarousal features of insomnia (Lichstein, 1988); (d) the improvement of circadian and sleep homeostasis regulation of sleep with sleep scheduling and limited, partial sleep deprivation (Spielman, Caruso, & Glovinsky, 1987); and (e) the adaptation of cognitive therapy to insomnia (Morin & Azrin, 1988). Essentially, the guiding model of insomnia and the development of multicomponent CBT-I are based on targeting factors that interfere with initiating and maintaining sleep. Cognitive–behavioral therapy for insomnia is a combined strategy that addresses the multiple putative causes and perpetuators of insomnia.
An influential conceptual model of insomnia espouses that insomnia occurs acutely in relation to both predisposing and precipitating factors. This stage model (3P) focuses especially on how perpetuating factors, such as maladaptive coping strategies, can maintain insomnia in a way that it becomes both learned and chronic. Today, most models of insomnia start by blending this 3P model of insomnia with the observations that repeated pairings of the sleep environment with wakefulness, frustration, and anxiety promote conditioned arousal (Bootzin & Nicassio, 1978).
The standard delivery of CBT-I is structured to allow for weekly sessions to occur over 6–8 weeks. Much of the efficacy data is based on studies of this length (Morin, Culbert, & Schwartz, 1994; Smith et al., 2002), it allows the patient and clinician to monitor progress together for some weeks, and it provides enough time for the patient’s total sleep time to improve. Nonetheless, briefer versions of CBT-I have been delivered with good efficacy (Edinger & Sampson, 2003). Of course, the number of sessions can be altered to match the patient’s needs and strengths. Indeed, as with other conditions, some patients may be quite capable of self-administering treatment with minimal therapeutic guidance. Cognitive–behavioral therapy for insomnia has also been delivered in both group and individual formats. The order of delivering the treatment can vary, but it is somewhat standard to begin with sleep education and treatment rationale and to follow with stimulus control therapy and sleep restriction therapy. Other components of therapy (sleep hygiene, cognitive therapy, relaxation training) follow, although their individual order can vary.
Sleep education and treatment rationale serve an important purpose in CBT-I as the therapy can be challenging for both patient and practitioner, especially early in the therapeutic process. Demonstrating good knowledge of sleep science and the principles behind the treatment probably enhances treatment compliance and thus desired outcomes. As with most therapies, CBT-I practitioners find it important to both educate and work collaboratively with the client throughout the treatment process. An overview of normal sleep including stages of sleep and how the normal sleeper cycles through these stages is a good start. This can be followed by an overview of how predisposing (e.g., basal hyperarousal, general anxiety), precipitating (e.g., stressful life events), and perpetuating factors (e.g., spending excessive time in bed) contribute to the development and maintenance of insomnia (Spielman et al., 1987). This provides an opportunity for the patient to offer their personal examples for each of these factors.
During the interview and psychoeducation process, the therapist can evaluate patient goals and discuss whether they can be realistically met given their current life circumstances. This can involve some discussion underscoring that even good sleepers have some bad nights and seldom get 8 uninterrupted hours of sleep per night. Realistic goals also include informing patients that there is very strong evidence for the treatment program on which they are about to embark, but that CBT-I can be difficult and importantly, that improvements in sleep consolidation will not lead to feeling better right away.
It is helpful to both therapist and patient to keep graphs of client progress throughout treatment. Especially early in treatment, seeing that “statistical” gains are being made and that sleep is coming under some control provides a reward for early efforts.
Finally, before initiating the first steps of treatment, providing rationale for these steps is equally useful. This involves educating the patient about (a) the difference between sleep ability and sleep opportunity, noting that the mismatch disrupts sleep homeostasis and regulation of sleep; and (b) the importance of a good stimulus environment for sleep.
Stimulus control and sleep restriction address two of the main perpetuating factors of insomnia. The first, remaining in bed even while awake, promotes conditioned arousal through repeated pairings of the sleep environment with wakefulness, frustration, and anxiety. The second, extending sleep opportunity, includes the tendency of patients with insomnia to go to bed earlier and/or to get out of bed later than their preinsomnia sleep schedules. Both are maladaptive strategies intended to increase the possibility of getting more sleep and often adopted under the rationale that staying in bed is at least “restful.” Both strategies, however, are ineffective and may exacerbate sleep problems. When sleep opportunity exceeds sleep ability, the consequence is more frequent and longer awakenings. Staying in bed while awake, meanwhile, strengthens the association between bed/bedroom and wakefulness/hyperarousal. These two behaviors often occur concurrently and buoy one another. Cognitive–behavioral therapy for insomnia almost immediately targets these behaviors as follows.
Stimulus control therapy limits the amount of time patients spend awake in bed or in the bedroom and also begins to develop a more consistent sleep schedule. Typical instructions include (1) use an alarm to keep a fixed wake time every day, regardless of how much sleep you get during the night; (2) do not nap during the day; (3) avoid any behavior in the bed or bedroom other than sleep or sexual activity; (4) lie down to go to sleep only when you are sleepy; (5) leave the bedroom when awake for approximately 15 minutes or when you begin to feel frustrated about not sleeping; and (6) return to bed only when sleepy. The combination of these instructions decreases the bed and bedroom as cues for arousal, reestablishes the bed and bedroom as strong cues for sleep, and promotes a more regular circadian sleep–wake cycle. Patients need to be cautioned not to “clock-watch.” Some therapists make this part of the stimulus control instructions and others may have patient’s put their clock on the floor or turn their clock around even before stimulus control therapy begins.
Sleep restriction therapy limits the amount of time that patients spend in bed to an amount that matches their ability to fill this with mostly sleep. Typical instructions include (a) determine average total sleep time (TST) from 1–2 weeks of daily sleep diaries; (b) establish a fixed wake time; (c) set a “sleep window” equal to the total sleep time plus 30 minutes and place this window by working backwards from the wake time to arrive at the prescribed bed time; (d) keep weekly sleep diaries and adjust the sleep window based on weekly sleep efficiency (TST/sleep window) derived from the prior weeks sleep diaries (if sleep efficiency is ≥90%, increase sleep window by 15 minutes, if efficiency is <90% and ≥85%, keep the sleep window unchanged; if sleep efficiency is <85% decrease sleep window by 15 minutes; and (e) continue weekly adjustments until daytime functioning reaches an adequate or desired level and/or until sleep efficiency >90% cannot be maintained. These instructions include some slight modifications from the original and other modifications can be considered such as not setting sleep window <5 hours even if TST is shorter than this amount or increasing the sleep window by >15 minutes.
Following these instructions increases the drive for sleep and tends to result in quicker onset to sleep with fewer and briefer awakenings and “deeper” sleep. It also can increase fatigue and sleepiness in the first few weeks; cautions to patients about this possibility can be worthwhile. Sleep restriction is contraindicated in patients with untreated hypersomnolence and also in those with histories of bipolar disorder or seizures (both of which can be exacerbated by sleep deprivation/sleep loss).
Sleep hygiene is a catch-all term for a set of instructions geared toward helping the patient maintain good sleep habits, such as keeping an environment and routine conducive to sleep, maintaining a regular bed and wake time, and avoiding tobacco, alcohol, large meals, and vigorous exercise for several hours prior to bed. Sleep hygiene instructions are not helpful when provided as a monotherapy (Lacks & Morin, 1992) and are perhaps best delivered in an interactive and coexperimenter approach where the therapist and patient review each of the sleep hygiene items, discussing how each is related to sleep and whether any apply to the patient. Those items that may be contributing to insomnia become therapeutic targets. Goals (and action steps to achieve them) are set to change each identified sleep hygiene factor.
Cognitive therapy may take one of several forms that differ slightly with respect to emphasis, although all focus on the negative thoughts and/or maladaptive beliefs about sleep, insomnia, and its consequences. As in cognitive therapy applied to other conditions, helping patients to challenge the veracity and usefulness of these unhelpful thoughts and beliefs and then to change or modify them is the goal. This is believed to decrease the anxiety and arousal associated with insomnia both in terms of daytime concerns and nighttime worry and rumination.
Relaxation training has hyperarousal as its obvious target. Any variety of relaxation techniques may be used as part of the CBT-I package. The optimal relaxation method for insomnia may be the technique that is the most acceptable to and/or easiest to learn for the patient.
Psychotherapists differ somewhat in terms of when to prescribe relaxation practice. Certainly, while learning the technique, it may be best to avoid bedtime practice as this may cause additional frustration. Some people find some relaxation strategies refreshing and alerting, which is generally a positive outcome, but not 5 minutes before bedtime. It may also be useful to underscore that the goal of relaxation is not necessarily sleep, but an overall decrement in the basal arousal rate. This requires consistent practice and once achieved it matters not when the practice is undertaken.
Cognitive–behavioral therapy for insomnia can be deployed for a variety of insomnias, and the standard insomnia patient encountered in clinical practice is seldom free of comorbidities. In the following case illustration, we will explore modest adaptations to standard CBT-I for a condition that, due to its high prevalence rate and association with sleep processes (Pigeon, Park, & Sateia, 2004; Smith & Haythornthwaite, 2004), is a frequent complaint in both primary care and mental health settings: chronic pain.
Case Illustration
Presenting Problem and Client Description
Jerry is a 40-year-old married man with two children aged 10 and 7; he currently works as a delivery driver and is a volunteer firefighter. He presented to his primary care physician (PCP) after having to curtail social/recreational activities and resign from the fire station due to increasing levels of disability from low back pain (LBP), fatigue, and difficulty staying asleep. He is not responding well to physical therapy. His current medications include Flexeril® (cyclobenzaprine) 10mg three times daily for low back pain, trazodone 100 mg at bedtime for sleep, and lisinopril 10 mg in the morning for hypertension. He has no prior history of psychological disorders or treatment and is referred for insomnia treatment as he has declined a prescription for an additional sleep medication.
Jerry reports recurrent LBP over the past 10 years that has previously responded to rest and stretching or to physical therapy. His latest pain episode began approximately 6 months ago when lifting a heavy package at work. Notes from the PCP suggest that there is no radiographic evidence indicating need for surgical intervention, but is noteworthy for a lumbar “bulging disk.” The patient reports that he initially “worked through the pain,” but found that the pain did not resolve and has not responded to physical therapy or to nonsteroidal antiinflammatories. He is considering applying for workers compensation, but has heard this is an onerous process and he’d like to “just get better and get on with his life.”
Jerry is approximately 20 lbs overweight and reports that most of those pounds were added in these past 6 months. He reports his blood pressure has “always” been borderline high, but was notably higher at his last physical 3 months ago when he was started on an antihypertensive medication.
His main sleep complaint is repeated awakenings and difficulty returning to sleep, which occurs nearly every night. Jerry approximates his total sleep time as about 5 hours. His bedtime varies from 10 p.m. to 1 a.m., though he typically gets up by alarm at 6 a.m. to get to work during the week and sleeps in until 9 or 10 on weekends. He reports no difficulty falling asleep regardless of his bedtime, but awakens after 3–4 hours for an extended period and then “tosses and turns” and feels as though he only sleeps lightly for brief stretches for the rest of the night. Jerry notes that his wife has complained that his snoring is getting worse. He does get sleepy during breaks at work and has “nodded off” a few times, though not while driving. In past episodes of insomnia he had difficulty falling asleep and staying asleep when under stress, but these always seemed to resolve after a few weeks or with over-the-counter (OTC) sleep aids. The current episode of sleep disturbance began a few weeks after his most recent back injury. The OTC sleep aids no longer provide any relief. Trazodone does help him sleep soundly at the beginning of the night, but he’s not sure that he feels any better or more refreshed in the morning.
Jerry reports problems with concentration and memory, though the main reason he has curtailed activity is due to low energy. He also stopped playing softball, going to the gym, and coaching his children’s’ sports teams due to both fatigue and back pain. His score on the Insomnia Severity Index (Bastien, Vallieres, & Morin, 2001) was 18, which corresponds to a moderate severity of insomnia on this 0–28 scale. He completed a one-week sleep diary that was mailed to him prior to the assessment appointment and the following average values were calculated: sleep latency (10minutes), number of awakenings (8.0) wake after sleep onset (110 minutes), time in bed (500 minutes), total sleep time (380 minutes), and sleep efficiency (380/500 = 76%).
Initial Case Formulation
Jerry meets the diagnostic criteria for insomnia. The additional presentation of daytime sleepiness, increased snoring, and hypertension suggested the possible presence of obstructive sleep apnea. A decision was made to defer insomnia treatment and recommend to the PCP that Jerry be referred for evaluation for obstructive sleep apnea.
Patients presenting with chronic pain of diverse etiologies have a high prevalence of sleep disorders, including insomnia, but also with obstructive sleep apnea (OSA). The latter is believed to occur for two reasons. First, the longer the condition persists, the higher the likelihood that patients become more sedentary, experience weight gain, and develop OSA. Second, patients may be taking some pain medications (e.g., muscle relaxants) that can cause obstructive events or exacerbate existing OSA. Both of these conditions were true for Jerry. In some cases, treatment of OSA may alleviate altogether, but in all cases it is unlikely that insomnia can be well-managed in the presence of untreated OSA.
Representation and Reformulation
Jerry returned one month later after his overnight sleep evaluation at a sleep disorders center. The study was completed off trazodone and off Flexeril® and the sleep report confirmed the Jerry’s self-report of multiple awakenings, extended periods of wakefulness, diminished total sleep time, diminished slow-wave sleep, and delayed rapid eye movement (REM) sleep onset. There was no evidence of excessive motor activity, but there was an apnea–hypopnea index of 19, which represents mild–moderate OSA, with which he was diagnosed the following day. At that time, Jerry agreed to be oriented to, fitted for, and tested with a continuous positive airway pressure device (CPAP) in a subsequent overnight sleep study. On that night, he still had extended wakefulness, but his OSA was controlled and he had fewer awakenings and more normal slow-wave sleep and REM sleep.
Jerry presented after wearing CPAP for 2 weeks, which is controlling his OSA, but which he “hates” wearing. He is motivated to lose weight to see if he can reverse the OSA. He reports that he is now sleeping without trazodone, which he discontinued on his own. He still readily falls asleep, but still wakes up often. If anything, Jerry thought he may actually spend more time awake in the middle of the night as he feels the 3–4 hours of consolidated sleep he does get feels more refreshing. His newly completed one-week sleep diary still reflects an average total sleep time of approximately 6 hours of sleep with 8 hours in bed (75% sleep efficiency). On average, he still feels as fatigued, but he thinks he may be a bit less sleepy during the day. He has not made any other changes to his sleep.
Following sleep education and treatment rationale, Jerry agrees to begin sleep restriction and stimulus control. His sleep window was set for 6.5 hours and anchored to his desired wake time of 6:00 a.m., making his prescribed bedtime 11:30 p.m. He is instructed to keep this schedule even on weekends and is given a blank sleep diary.
Course of Treatment
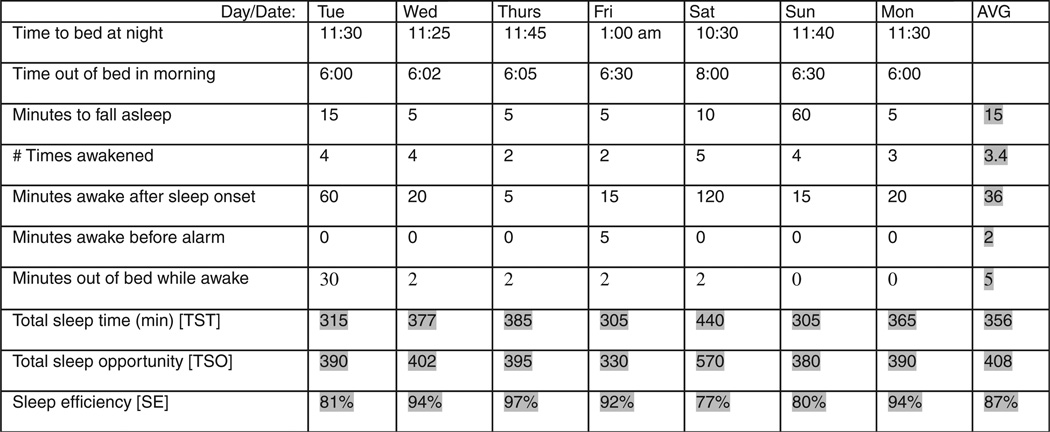
Jerry returned one week later with the sleep diary in Figure 1, about which several observations can be made:
Overall, there was good compliance with sleep restriction’s sleep window with the exception of the weekend. Note, that going to bed later than the prescribed sleep time is “allowable,” but that extending sleep opportunity by going to bed earlier or sleeping in later than prescribed times is to be avoided.
Jerry appears to have applied the stimulus control instruction of getting out of bed while awake on Tuesday night but not on Sunday night.
Sleep latency remains low, but waking during the middle of the night has decreased significantly. Overall total sleep time is still right around 6 hours, and sleep efficiency was close to our 90% target.
The only significant adherence problem occurred on Saturday night when Jerry went to bed 1 hour early and stayed in bed 2 hours later than prescribed and did not get out of bed despite extended periods of wakefulness.
Figure 1.
Patient sleep diary at intake.
After some discussion of these aspects of the diary and his ability to adhere to the plan most nights, the following exchange took place:
Therapist: Why don’t you tell me what happened on Saturday?
Patient: Oh, I was just hammered from staying up late and still almost getting up on time. I always sleep in on Saturdays, so 6:30 was a victory for me. And then by nighttime, I was just literally falling asleep on the couch, so I just went up to bed, which seemed fine but then I woke up around 2 and couldn’t fall back asleep.
Therapist: Hmm…and you didn’t get out of bed like you had earlier in the week when you were awake for more than 15 minutes?
Patient: I thought about it, but I was just so tired and it was the middle of the night, so (sheepishly) I just turned the TV on for awhile and used our headphones to not disturb my wife.
Therapist: Smiling Right…and you couldn’t watch TV down in the living room? Listen, remember how I said this was really hard to do. I understand that getting out of bed at 3 a.m. is not fun.
Patient: Yeah, a little harder than it sounded at the time... and my back was stiff and I thought, even though I’m awake at least I’m resting.
Therapist: Right. But you also know that I’m going to say something like “you can rest somewhere other than the bedroom.” Let’s look at the positive side by looking closely at the sleep diary. You followed the plan and had some decent, not great, but decent, sleep a few nights. The weekend came. Do you see how things went astray?
Patient: I guess so. I went to bed too early and even though I got a good 3–4 hours but then I was in trouble.
Therapist: And next time…?
Patient: Right, next time I just get up because really, I just dozed on and off even after I stayed up with the TV on.
This patient–therapist interaction included a very common issue that can arise with chronic pain patients, which bears some additional discussion. Namely, following stimulus control instructions to get out of bed when awake can be thwarted when patients report “my doctor told me I needed bed rest” or some variation of the rationale used by Jerry (“at least I’m resting.”). Bed may actually provide some comfort that is not available standing or sitting. It may be physically difficult to get out of bed and/or walk down a set of stairs to another room. Patients may be especially whetted to the idea that lying in bed awake is far more restful (and less painful) then getting up or that they are practicing good health behavior for their pain condition. When presented with any such variant, an appropriate response can be some matching variation of “You may rest, you just need to do it somewhere other than the bedroom.”
After the sleep diary is reviewed and any problems implementing stimulus control or sleep restriction instructions are addressed, the focus may move to sleep hygiene. For Jerry, removing the TV from the bedroom is an appropriate target for sleep hygiene (and one that also fits with stimulus control). In addition, particularly for patients with chronic pain conditions, comments like “I can’t get comfortable” or “My bed is terrible” may arise and provide other sleep hygiene targets that address promoting a comfortable sleep environment. For instance, the emphasis on a comfortable mattress and pillows may need to be more of an imperative among low back pain patients who complain of an old soft mattress. Pain patients have probably heard it before or are doing it already, but strategic use of extra pillows can relieve some discomfort (e.g., a pillow between the knees when sleeping on one’s side).
Also included in sleep hygiene is a discussion about how exercise can enhance sleep and that late-night exercise may be overly alerting. Recall that Jerry had weight reduction as a goal to improve the sleep apnea that seemed to be weight-related. Even if this were not the case, a compelling rationale for activity/exercise is easy to come by: Regular exercise can improve sleep, chronic pain, and/or depression. As such, it is a natural fit for enhancing CBT-I delivered to chronic pain patients. For patients who have stretching or modest physical therapy exercises assigned by another clinician for their pain, psychotherapists can suggest that patients do these exercises as part of a winding down bedtime routine. If the patient has a physical or occupational therapist, it is of course good clinical practice to seek their guidance regardless of the level of activity being considered. Jerry had an exercise plan developed by his physical therapist that he was not following regularly, so this was added to the CBT-I treatment goals (and it was added as a line in the sleep diary so that minutes of daily exercise could be recorded) at the end of the session.
The following week, Jerry returned with completed sleep diaries including daily exercise filled in. Sleep latency and wake minutes after sleep onset had fallen further, sleep efficiency had increased to 93%, and total sleep time to a little over 6 hours. The sleep window was, therefore, increased by 15 minutes to 6 hours and 45 minutes (11:15 p.m. to 6:00 a.m.).
The following exchange took place:
Therapist: So, overall you had a slight improvement in total sleep time and you stuck to the plan so well (even on the weekend), that your sleep efficiency jumped up over our 90% target. That’s great. This is still fairly early in the treatment program, but this is exactly the progress that sets the right foundation for continued improvement. Good work.
Patient: Thanks. It makes sense. It’s just a matter of doing it I guess, and I do feel hopeful.
Therapist: You should. There are good signs in here. I see you got almost the full 61/2 sleep on Tuesday and again on Thursday.
Patient: God, I felt awful the next morning. I could barely get out of bed. I remember this happening some time when I was taking the trazodone. It was like I hadn’t moved all night.
Therapist: Wow. If feeling awful is the reward for sleeping well, then we’re in trouble. Maybe…
Patient: I didn’t quite say that. My back and legs hurt something awful, but overall I could tell that I slept better.
Therapist: Really, how?
Patient: Oh I think I felt a little sharper. You know, I almost felt rested.
Therapist: Oh, that’s different then. So, on the one hand, you slept better—in fact perhaps the best you have yet—and this resulted in both some increased soreness and feeling more rested or refreshed. On the whole, this seems maybe two steps forward and one step back.
Patient: Better than the other way around I guess.
Embedded in the above exchange is another common concern of chronic pain patients that might be stated as, “If I sleep too well, I wake up more stiff and sore than usual.” This may seem like an odd complaint: Someone with insomnia is complaining of sleeping well. One response to such observations is the matching observation that this provides evidence of restful sleep (as presumably there were limited amounts of tossing, turning, and position changing). Jerry provided his own reframing of the situation and the therapist needed only reaffirm this. Suggestions that may be helpful for this type of complaint include:
Point out that even when they are sleeping soundly every night, patients will (like good sleepers) wake several times a night. They can take this opportunity to switch sleeping positions (if they don’t already do so).
Morning stretching, if not already part of their routine, may be indicated for patients with this waking complaint of soreness, which may quickly alleviate morning stiffness/soreness and set the stage for a positive day.
Similarly, when patients are out of bed practicing stimulus control, stretching would be a good suggested activity.
In other cases, the psychotherapist can save such statements for their use in cognitive therapy, which was introduced to Jerry in the second half of this session. Eliciting other automatic thoughts and emotions that occurred on that morning may also be instructive and lead to identification (and challenges) of other cognitive distortions. Typically, the “C” in CBT-I focus mainly on thoughts and beliefs related to sleep. As with other facets of the therapy it may prove useful to begin with a didactic exercise, one that uses an exercise in how an event can elicit automatic thoughts and emotions that are counterproductive to sleep. After this exercise, one fruitful place to find such thoughts (and how to address them) is demonstrated in the following:
Therapist: So, let’s say you’re lying in bed awake at 2 a.m. and sleep is not coming. What is going through your mind?
Patient: Well, I would think how frustrating this is and how I’d better get to sleep soon or else.
Therapist: Or else what?
Patient: Or else, I’ll have a rough day tomorrow.
Therapist: OK, give me the worst case scenario.
Patient: I don’t know. I’ll have an accident.
Therapist: OK. Good. Thanks for playing along. Now, I know this may not happen a lot, but let’s use this as an example gets up and starts writing on the white board. So the event is “I’m awake” and the thought is “if I don’t fall asleep soon, I’ll get in an accident tomorrow” and the emotion is what, anxiety?
Patient: Yeah, feeling worried.
Therapist: And on a scale of 0–100% how worried are you?
Patient: Oh, pretty worried, I can really work myself up. Maybe 90%.
Therapist: Alright, and when you’re this worried, how certain are you that you will have an accident.
Patient: laughs Well, in the moment, pretty certain.
Therapist: 100% certain? 75%?
Patient: Oh, 90% or so.
Therapist: OK. And how many accidents have you had at work?
Patient: I see where you’re going, but none. But I have had some close calls.
Therapist: OK. How many? Let’s say in the last 6 months since your sleep has been particularly bad.
Patient: Well, one that I can think of.
Therapist: OK, so now let’s do some math. Let’s say you’ve worked 25 weeks and say 4 nights per week you’ve had poor sleep. That’s 100 nights with 90% certainly, so by that logic you should have had about 90 close calls. But you’ve only had one.
Patient: I get it.
Yet, another common thought that similar patients have is “My pain wakes me up and then I can’t get back to sleep.” This may be very true. In general, increased pain does “lighten” sleep, produce miniarousals, and even some full awakenings. So, this thought can be partly supported. Typically, however, this is either when pain levels are high and not controlled well or when there are bursts of increased pain, such as a muscle spasm. Pain at a moderate level does not necessarily cause full awakenings. Therefore, it is useful to educate the patient about the number of awakenings that are “normal.” In addition, although a subtle point, it is not necessarily pain that jerks someone awake from sleep; instead, pain typically is associated with less-sound sleep (more stage 1 and stage 2 sleep) from which it is easier to be aroused by a number of stimuli.
Why go to the trouble to address these thoughts? Often, one of the unstated beliefs here can be some version of “since my pain is not going to improve, my sleep can’t improve.” Such a belief leads to resignation and noncompliance. Clinically, we want to do two things: normalize the awakenings to some extent and focus work on what happens after the awakenings. Specifically, attention to what the patient’s mind and body are doing during those awakenings is useful in determining their contribution to extended awakenings. In such cases, standard cognitive therapy and stimulus control can be applied.
In this or a subsequent session, relaxation training may be introduced. It is not uncommon for chronic pain patients to report that progressive muscle relaxation (PMR) causes muscle spasms or soreness. Typically this can be avoided by delivering several cautions during introduction to PMR (and on any tapes that you may make with PMR instructions) in which you inform patients of this possibility and instruct them not to tighten muscles as much as they can, but to just tighten them a small amount so that they can feel a little more muscle tension than they do when the muscle is relaxed. Alternatively, PMR may be avoided altogether and a different relaxation technique chosen.
Finally, sleep diaries are maintained throughout treatment; discussion and charting of progress continues; and sessions are used to troubleshoot and fine-tune approaches until the patient can continue on their own.
Outcome and Prognosis
Jerry completed a total six sessions of CBT-I with positive results. His sleep window expanded to 7 hours and 30 minutes (10:30 p.m. to 6:00 a.m.). His score on the Insomnia Severity Index decreased to 9 (mild insomnia) from 18. His final sleep diary average values (vs. intake values) were sleep latency (8 minutes vs. 10 minutes), number of awakenings (3.4 vs. 8.0), wake after sleep onset (22 minutes vs. 110 minutes), time in bed (450 minutes vs. 500 minutes), total sleep time (420 minutes vs. 380 minutes), and sleep efficiency (93% vs. 76%). In addition, both daytime sleepiness and fatigue were reduced. Jerry had continued to exercise almost daily, had lost 5 lbs and was still wearing a CPAP. His pain level was only slightly decreased, but he felt he had more energy and he did return to some of his social and family activities. Importantly, Jerry believed that he had regained control over his sleep and that this was giving him the energy to manage his chronic low back pain better.
Overall, Jerry was a motivated patient who was provided with information, skills, and strategies to pursue self-management of his health. It remains to be seen whether he can shed the additional pounds that he hopes may improve his sleep apnea. Jerry’s consistent daily practice and adherence to treatment recommendations on multiple fronts will likely be the largest determinant of meeting his overall health goals.
Clinical Practices and Summary
Cognitive–behavioral therapy for insomnia can be applied when insomnia presents wholly on its own. In common comorbid conditions like chronic pain, CBT-I can be equally effective when considerations of the chronic pain patient are addressed. There is now enough research evidence reinforced by practice successes in patients with co-occurring chronic pain and insomnia to recommend CBT-I for most of such patients. Indeed, although improvements in sleep may not improve pain markedly, they may certainly set the stage for the optimal management of pain (and similar conditions) to occur.
Acknowledgments
The author thanks the following sources of support: U.S. National Institutes of Health Grant # NR010408 and the Veterans Administration Center of Excellence at Canandaigua, Canandaigua, NY and the Center for Integrated Healthcare, Syracuse, NY.
Selected References and Recommended Readings
- Bastien C, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Bootzin RR, Nicassio P. Behavioral treatments for insomnia. In: Hersen M, Eisler RH, Miller PM, editors. Progress in behavior modification. New York: Academic Press; 1978. pp. 1–47. [Google Scholar]
- Buysee DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Carney CE. Overcoming insomnia: A cognitive-behavioral therapy approach therapist guide. New York: Oxford University Press; 2008. [Google Scholar]
- Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26:177–182. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- Hauri P. Sleep disorders: Current concepts (2nd ed.) Kalamazoo, MI: Upjohn Company; 1982. [Google Scholar]
- Lacks P, Morin CM. Recent advances in the assessment and treatment of insomnia. Journal of Consulting & Clinical Psychology. 1992;60:586–594. doi: 10.1037//0022-006x.60.4.586. [DOI] [PubMed] [Google Scholar]
- Leshner A. NIH State-of-the-Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults. In: Lichstein KL, editor. Clinical relaxation strategies. New York: Wiley; 2005. [Google Scholar]
- Morin CM, Azrin NH. Behavioral and cognitive treatments of geriatric insomnia. Journal of Consulting & Clinical Psychology. 1988;56:748–753. doi: 10.1037//0022-006x.56.5.748. [DOI] [PubMed] [Google Scholar]
- Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: A meta-analysis of treatment efficacy. American Journal of Psychiatry. 1994;151:1172–1180. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- Morin CM, Espie CA. Insomnia: A clinical guide to assessment and treatment. New York: Kluwer Academic/Plenum Press; 2003. [Google Scholar]
- Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive behavioral treatment of insomnia. New York: Springer; 2005a. [Google Scholar]
- Perlis ML, Smith MT, Pigeon WR. Etiology and pathophysiology of insomnia. In: Kryger M, Roth T, Dement WC, editors. Principle and practice of sleep medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005b. pp. 714–725. [Google Scholar]
- Pigeon W. Sleep manual. Hauppauge, NY: Barron’s; 2010. [Google Scholar]
- Pigeon W, Park R, Sateia M. Sleep and pain. In: Lader M, Cardinali D, Pandi-Perumal SR, editors. Sleep and sleep disorders: A neuropsychological approach. Georgetown, TX: Landes Bioscience; 2004. [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. Journal of Clinical Sleep Medicine. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Medicine Reviews. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Smith MT, Perlis ML, Park A, Smith MS, Pennington J, Giles DE, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. American Journal of Psychiatry. 2002;159:5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- Spielman A, Caruso L, Glovinsky P. A behavioral perspective on insomnia treatment. Psychiatric Clinics of North America. 1987;10:541–553. [PubMed] [Google Scholar]
- Taylor DJ, Roane BM. Treatment of insomnia in adults and children: A practice-friendly review of research. Journal of Clinical Psychology: In Session. 2010;66 doi: 10.1002/jclp.20733. 000–000. [DOI] [PubMed] [Google Scholar]