Abstract
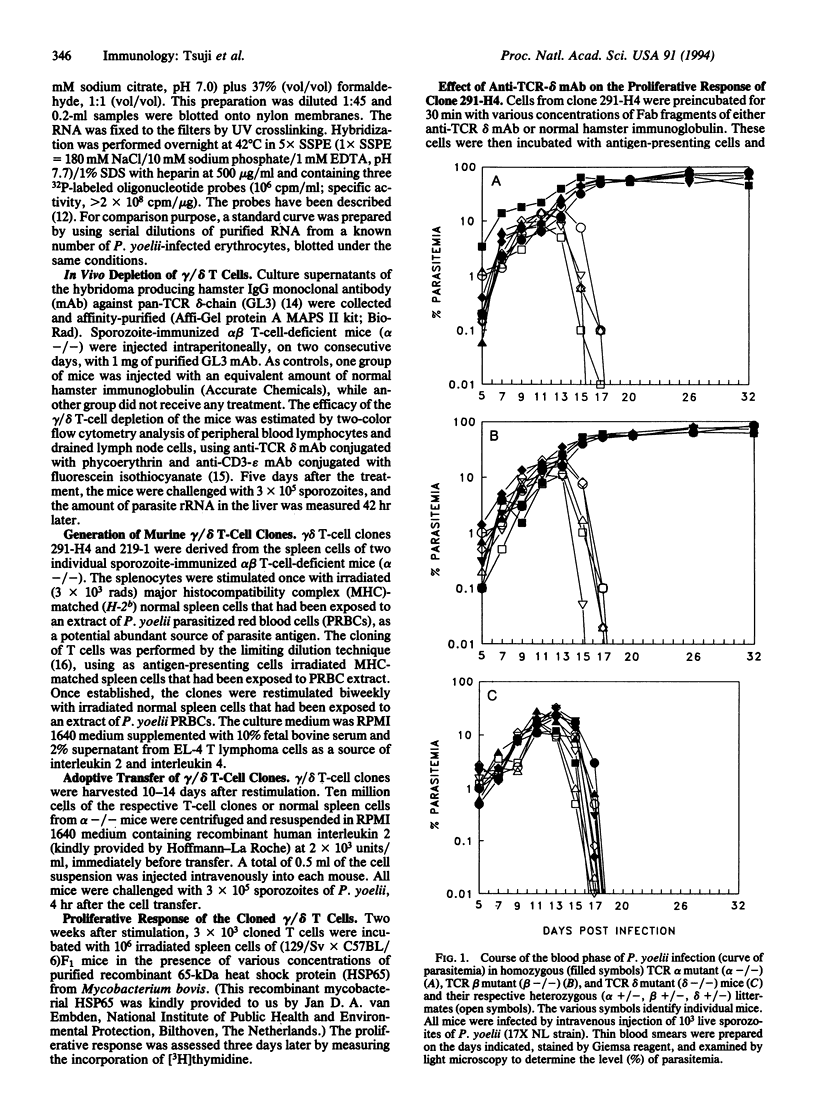
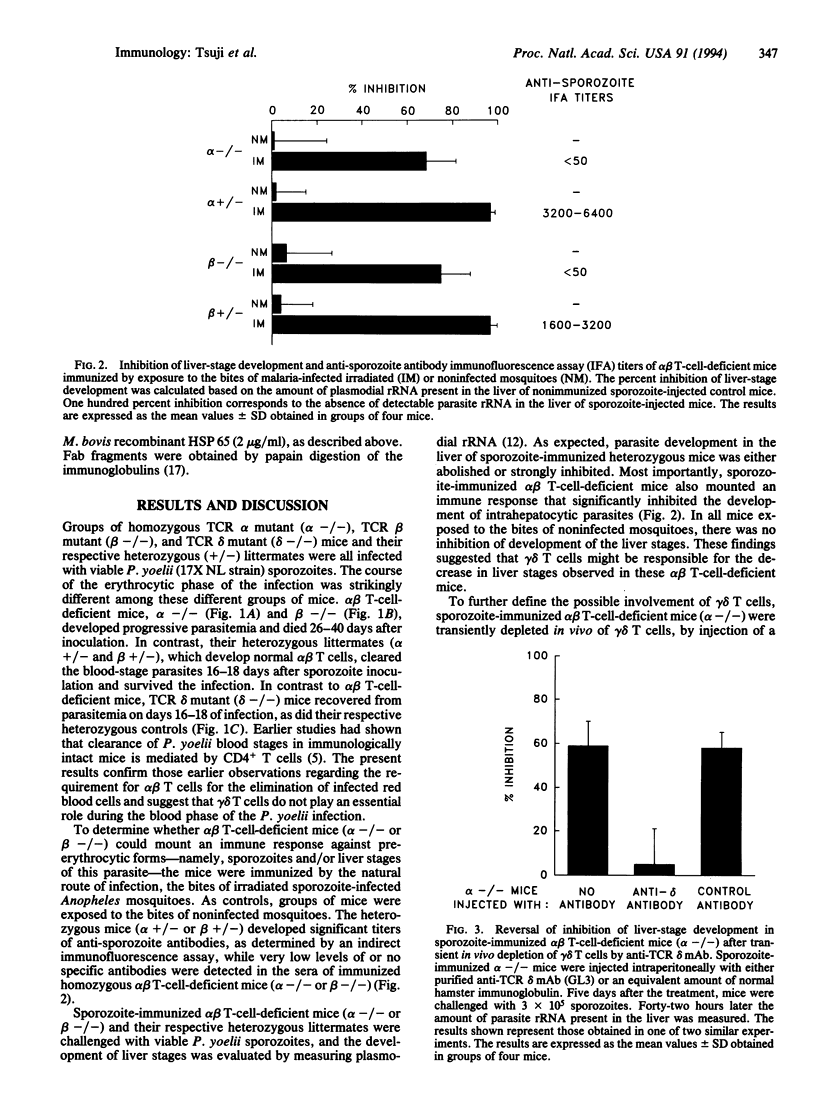
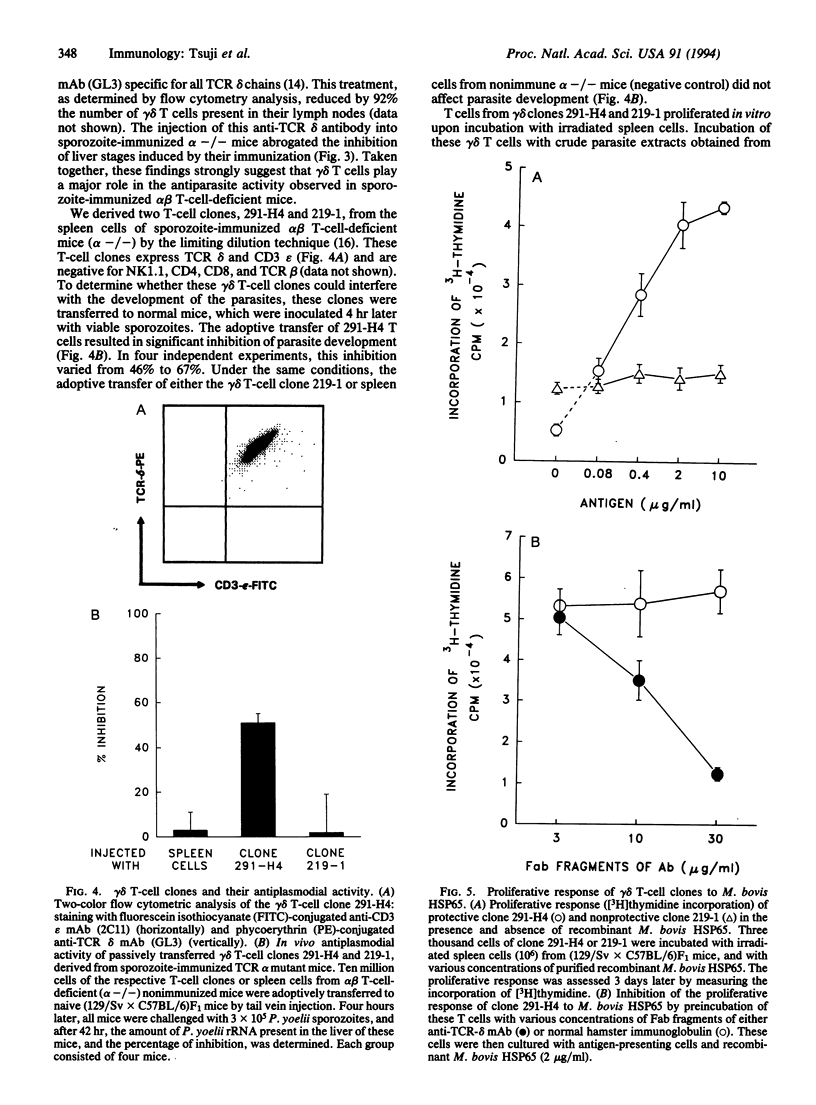
The functional role of gamma delta T cells (expressing the gamma delta heterodimeric T-cell receptor for antigen) in infectious diseases remains largely unknown. We have therefore attempted to define the possible role of these T cells in the immune response against the various developmental stages of malaria parasites. For this purpose, we monitored the immune response and the development of liver and blood stages of Plasmodium yoelii, a rodent malaria parasite, in immunized and nonimmunized alpha beta T-cell-deficient and gamma delta T-cell-deficient mice. Immunization of alpha beta T-cell-deficient mice with irradiated sporozoites induced an immune response that significantly inhibited the development of the parasite's liver stages. This inhibitory immune response was abolished by an antibody-mediated transient in vivo depletion of gamma delta T cells. Two gamma delta T-cell clones were derived from malaria-immunized alpha beta T-cell-deficient mice. The adoptive transfer of one of these gamma delta T-cell clones to normal mice inhibited the development of liver stages, following sporozoite inoculation. These results provide evidence for gamma delta T-cell-mediated protective immunity against parasites, in the absence of alpha beta T cells. As for the blood phase of the infection, both normal mice and gamma delta T-cell-deficient mice cleared the blood stages of the nonlethal strain of P. yoelii, while alpha beta T-cell-deficient mice failed to control the parasitemia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton S. M., van der Zee R., Goodacre J. A. Inflammation activates self hsp60-specific T cells. Eur J Immunol. 1993 Jan;23(1):33–38. doi: 10.1002/eji.1830230107. [DOI] [PubMed] [Google Scholar]
- Arreaza G., Corredor V., Zavala F. Plasmodium yoelii: quantification of the exoerythrocytic stages based on the use of ribosomal RNA probes. Exp Parasitol. 1991 Jan;72(1):103–105. doi: 10.1016/0014-4894(91)90127-i. [DOI] [PubMed] [Google Scholar]
- Behr C., Dubois P. Preferential expansion of V gamma 9 V delta 2 T cells following stimulation of peripheral blood lymphocytes with extracts of Plasmodium falciparum. Int Immunol. 1992 Mar;4(3):361–366. doi: 10.1093/intimm/4.3.361. [DOI] [PubMed] [Google Scholar]
- Born W., Hall L., Dallas A., Boymel J., Shinnick T., Young D., Brennan P., O'Brien R. Recognition of a peptide antigen by heat shock--reactive gamma delta T lymphocytes. Science. 1990 Jul 6;249(4964):67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- Born W., Happ M. P., Dallas A., Reardon C., Kubo R., Shinnick T., Brennan P., O'Brien R. Recognition of heat shock proteins and gamma delta cell function. Immunol Today. 1990 Feb;11(2):40–43. doi: 10.1016/0167-5699(90)90015-2. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Grillot D., Rénia L., Müller I., Corradin G., Louis J. A., Mazier D., Lambert P. H. Peptide-primed CD4+ cells and malaria sporozoites. Immunol Lett. 1990 Aug;25(1-3):59–63. doi: 10.1016/0165-2478(90)90092-5. [DOI] [PubMed] [Google Scholar]
- Goodier M., Fey P., Eichmann K., Langhorne J. Human peripheral blood gamma delta T cells respond to antigens of Plasmodium falciparum. Int Immunol. 1992 Jan;4(1):33–41. doi: 10.1093/intimm/4.1.33. [DOI] [PubMed] [Google Scholar]
- Goodman T., Lefrancois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989 Nov 1;170(5):1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Hiromatsu K., Yoshikai Y., Matsuzaki G., Ohga S., Muramori K., Matsumoto K., Bluestone J. A., Nomoto K. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992 Jan 1;175(1):49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Itohara S., Mombaerts P., Lafaille J., Iacomini J., Nelson A., Clarke A. R., Hooper M. L., Farr A., Tonegawa S. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993 Feb 12;72(3):337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Bal V., Mendez-Samperio P., Mehlert A., So A., Rothbard J., Jindal S., Young R. A., Young D. B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1(2):191–196. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- Matsuzaki G., Yoshikai Y., Harada M., Nomoto K. Autoreactive T cells from normal mice recognize mycobacterial 65 kd heat-shock protein from Mycobacterium bovis. Int Immunol. 1991 Feb;3(2):215–220. doi: 10.1093/intimm/3.2.215. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A. R., Rudnicki M. A., Iacomini J., Itohara S., Lafaille J. J., Wang L., Ichikawa Y., Jaenisch R., Hooper M. L. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992 Nov 19;360(6401):225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 Mar 6;68(5):869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Nardin E. H., Nussenzweig V., Nussenzweig R. S., Collins W. E., Harinasuta K. T., Tapchaisri P., Chomcharn Y. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 1982 Jul 1;156(1):20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Rodrigues M. M., Cordey A. S., Arreaza G., Corradin G., Romero P., Maryanski J. L., Nussenzweig R. S., Zavala F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991 Jun;3(6):579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- Rosat J. P., MacDonald H. R., Louis J. A. A role for gamma delta + T cells during experimental infection of mice with Leishmania major. J Immunol. 1993 Jan 15;150(2):550–555. [PubMed] [Google Scholar]
- Tsuji M., Romero P., Nussenzweig R. S., Zavala F. CD4+ cytolytic T cell clone confers protection against murine malaria. J Exp Med. 1990 Nov 1;172(5):1353–1357. doi: 10.1084/jem.172.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderberg J. P., Nussenzweig R. S., Most H. Further studies on the Plasmodium berghei-Anopheles stephensi--rodent system of mammalian malaria. J Parasitol. 1968 Oct;54(5):1009–1016. [PubMed] [Google Scholar]
- Vinetz J. M., Kumar S., Good M. F., Fowlkes B. J., Berzofsky J. A., Miller L. H. Adoptive transfer of CD8+ T cells from immune animals does not transfer immunity to blood stage Plasmodium yoelii malaria. J Immunol. 1990 Feb 1;144(3):1069–1074. [PubMed] [Google Scholar]
- Wand-Württenberger A., Schoel B., Ivanyi J., Kaufmann S. H. Surface expression by mononuclear phagocytes of an epitope shared with mycobacterial heat shock protein 60. Eur J Immunol. 1991 Apr;21(4):1089–1092. doi: 10.1002/eji.1830210437. [DOI] [PubMed] [Google Scholar]



