Abstract
Increased ‘anaerobic' glucose metabolism is observed after traumatic brain injury (TBI) attributed to increased glycolysis. An alternative route is the pentose phosphate pathway (PPP), which generates putatively protective and reparative molecules. To compare pathways we employed microdialysis to perfuse 1,2-13C2 glucose into the brains of 15 TBI patients and macroscopically normal brain in six patients undergoing surgery for benign tumors, and to simultaneously collect products for nuclear magnetic resonance (NMR) analysis. 13C enrichment for glycolytic 2,3-13C2 lactate was the median 5.4% (interquartile range (IQR) 4.6–7.5%) in TBI brain and 4.2% (2.4–4.4%) in ‘normal' brain (P<0.01). The ratio of PPP-derived 3-13C lactate to glycolytic 2,3-13C2 lactate was median 4.9% (3.6–8.2%) in TBI brain and 6.7% (6.3–8.9%) in ‘normal' brain. An inverse relationship was seen for PPP-glycolytic lactate ratio versus PbtO2 (r=−0.5, P=0.04) in TBI brain. Thus, glycolytic lactate production was significantly greater in TBI than ‘normal' brain. Several TBI patients exhibited PPP–lactate elevation above the ‘normal' range. There was proportionally greater PPP-derived lactate production with decreasing PbtO2. The study raises questions about the roles of the PPP and glycolysis after TBI, and whether they can be manipulated to achieve a better outcome. This study is the first direct comparison of glycolysis and PPP in human brain.
Keywords: 13C-labeling, glycolysis, lactate, pentose phosphate pathway, traumatic brain injury (human)
Introduction
An increase in the proportion of glucose undergoing ‘anaerobic' (non-oxygen consuming) metabolism is observed after traumatic brain injury (TBI).1, 2, 3 This has been postulated to provide the energy in the form of adenosine triphosphate (ATP), produced via glycolysis, needed to restore ionic and neurochemical gradients, which become disturbed after TBI.4,5 Upregulation of the pentose phosphate pathway (PPP) has also been suggested as a possible contributor to increased anaerobic glucose metabolism after TBI, based on rat models, and indirect (arteriovenous-jugular difference) studies in human patients.6, 7, 8
Glycolysis consists of the Embden–Meyerhof pathway from glucose to pyruvate, which does not use oxygen, and generates 2 moles of ATP per mole of glucose. Pyruvate can then be metabolized via the mitochondrial tricarboxylic acid cycle (TCA) cycle coupled to mitochondrial electron transport chains, which utilize oxygen in oxidative phosphorylation. The theoretical overall yield is 36 moles of ATP per mole of glucose. Alternatively, pyruvate can be converted to lactate, which recycles NADH (reduced nicotinamide-adenine dinucleotide) to NAD+, enabling further glucose molecules to be processed by glycolysis.
The PPP, also termed the hexose monophosphate shunt, is a biosynthetic pathway that constitutes a complex detour bypassing some of the steps of glycolysis in the metabolism of glucose. The key enzyme for the PPP, glucose-6-phosphate dehydrogenase, which is responsible for the rate-limiting step, is present in most tissues and cell types, and is regarded as a ‘housekeeping' enzyme.9,10 The PPP does not consume or produce ATP and does not require molecular oxygen. In the early ‘oxidative phase' of the PPP, during which the first carbon of the glucose skeleton is lost as carbon dioxide, nicotinamide adenine dinucleotide phosphate (NADP+) is converted to NADPH. The latter is a reducing agent that participates in reductive biosynthetic reactions, such as lipid and steroid synthesis, and in the production of the reduced form of glutathione and thioredoxin. Glutathione and thioredoxin are cofactors for glutathione peroxidase enzymes and peroxiredoxins respectively, both of which scavenge hydroperoxides, thereby combatting oxidative stress. Among the many intermediates of the later ‘non-oxidative' phase of the PPP is ribose 5-phosphate, used for nucleic acid synthesis. Hence, the proportion of glucose metabolized via the PPP is greatest in tissues with high lipid- and steroid-synthetic roles (for example, liver and lactating mammary glands), also in cells with a high oxidative load (for example, red blood cells), and is thought to be one of the mechanisms supporting high cell proliferation rates in cancer.10 PPP activity after TBI has been postulated as having a protective role, promoting synthesis of nucleic acids and fatty acids for tissue repair and combatting the oxidative stress that results from injured cells.6,11
Our aim was to evaluate glycolysis and the PPP as routes of glucose metabolism, directly in TBI patients' brains. Our novel approach was to perfuse the brain using a microdialysis catheter with 1,2-13C2 glucose and measure the ensuing labeling patterns in lactate collected in the emerging microdialysates by analyzing them using high-resolution nuclear magnetic resonance (NMR) spectroscopy. For comparison, we also carried out the same technique in the ‘normal' brain, and in muscle as a non-CNS tissue. The present study is the first direct comparison of glucose metabolism via glycolysis and PPP in human brain.
Materials and Methods
Patients
The Cambridge Central Local Research Ethics Committee approved this study. Informed consent was obtained from all participants in the control ‘normal' brain and muscle groups and assent from the relatives of those patients who had suffered a TBI. The study was carried out in conformation with the spirit and the letter of the Declaration of Helsinki.
TBI brain microdialysis patients
The TBI patients were defined as those experiencing cranial trauma with consistent computed tomography (CT) scan findings and requiring invasive intracranial monitoring as part of their clinical management. Patients were managed according to local TBI-management protocols, which include endotracheal intubation, ventilation, sedation, and muscular paralysis.12
Control ‘normal' brain microdialysis patients
Patients undergoing a craniotomy for the resection of a benign lesion whereby a microdialysis catheter could be safely placed into radiographically normal brain were selected as control subjects. The microdialysis catheter was placed via the craniotomy and tunneled under the scalp. The control patients were awake for the duration of microdialysis perfusion.
Muscle microdialysis patients
Patients undergoing resections of acoustic neuromas that required thigh fat and fascia harvesting for dural closure were recruited for studying muscle, for comparison with the brain. During the procedure to harvest fat and fascia, a microdialysis catheter was placed under direct vision into quadriceps muscle. The muscle microdialysis subjects were under general anesthesia for the duration of the microdialysis perfusion, which was performed at the same time of day for each patient minimizing the effect of diurnal variation.
Microdialysis Technique
CMA 71 microdialysis catheters (membrane length 10 mm, nominal molecular weight cutoff 100 kDa) (M Dialysis AB, Stockholm, Sweden) were placed either via a triple lumen cranial access device (Technicam, Newton Abbot, UK) or during a craniotomy for a traumatic lesion, along with an intracranial pressure monitor (Codman, Raynham, MA, USA) and a Licox brain tissue oxygen sensor (GMS, Kiel-Mielkendorf, Germany) into the frontal lobe. For TBI patients with a diffuse injury, the right frontal region was chosen; if there was greater injury to one hemisphere rather than the other, the most injured side was chosen. The catheter was neither placed within nor immediately adjacent to the lesion itself. The CMA 71 catheters were used for control ‘normal' brain subjects and were placed via the cranial opening at the end of their neurosurgical procedure. CMA 60 catheters (membrane length 30 mm, nominal molecular weight cutoff 20 kDa) were used for muscle subjects and placed under direct vision into right quadriceps muscle following fat and fascia harvesting under general anesthesia.
Catheters in the TBI patients and the normal brain control subjects were perfused with CNS Perfusion Fluid (M Dialysis, AB), composed of NaCl (147 mmol/L), KCl (2.7 mmol/L), CaCl2 (1.2 mmol/L), and MgCl2 (0.85 mmol/L) in water, supplemented with 4 mmol/L 1,2-13C2 glucose (see below for details). Muscle microdialysis catheters were perfused with T1 Perfusion Fluid (M Dialysis AB), composed of NaCl (147 mmol/L), KCl (4 mmol/L), and CaCl2 (2.3 mmol/L) in water, supplemented with 4 mmol/L 1,2-13C2 glucose. The concentration (4 mmol/L) of the 13C-labeled substrate was chosen to be within the range found in brain and muscle microdialysates in unlabeled studies, to minimize perturbation.13, 14, 15 1,2-13C2 glucose (isotopic enrichment 99%, chemical purity 99%) was obtained from Cambridge Isotope Laboratories (Tewksbury, MA, USA) and was formulated in CNS perfusion fluid or T1 perfusion fluid by the Manufacturing Unit, Department of Pharmacy, Ipswich Hospital NHS Trust (Ipswich, UK), who then tested the formulations to verify purity, sterility, freedom from endotoxins, and absence of pyrogenicity, to comply with current regulations, before releasing them for use in patients. Microdialysate collection vials from the TBI patients were changed hourly and analyzed at the bedside using an ISCUS analyzer (M Dialysis AB) employing enzymatic colorimetric assays for glucose, lactate, pyruvate, glutamate, and glycerol. Microdialysate vials from the ‘normal' brain and from muscle were also analyzed with the ISCUS analyzer at 4- and 2-hour intervals, respectively. Intracranial pressure, cerebral perfusion pressure, and brain tissue oxygen tension (PbtO2) data from TBI patients were recorded at the bedside using ICM+ software (ICM+, University of Cambridge, UK). Microdialysate samples were stored at 4 °C (or at −80 °C if storage for longer than a few days was necessary) before the NMR analysis.
NMR Analysis
Brain microdialysate samples were pooled into 24-hour periods for each individual patient. For NMR analysis, 20 μL of deuterium oxide (D2O) and 50 μL internal reference standard from a stock solution of 24.0 mmol/L 3-(trimethylsilyl)-1-propanesulfonic acid sodium salt (also termed 2,2-dimethyl-2-silapentane-5-sulfonate sodium salt or 4,4-dimethyl-4-silapentane-1-sulfonate sodium salt; DSS; Sigma-Aldrich, Poole, Dorset, UK) in the CNS perfusion fluid (M Dialysis) was added to 180 μL of the pooled microdialysate sample. Muscle microdialysate samples were pooled into 8-hour periods. Twenty microliters of D2O and 70 microliters from a stock solution of 2.84 mmol/L DSS was added to 120 μL of the pooled microdialysate sample. Pooled samples, after addition of D2O and DSS, were stored at −80°. For NMR analysis, samples were transferred into 3 mm NMR tubes (Hilgenberg GmbH, Malsfeld, Germany).
13C and 1H NMR spectra were acquired on a Bruker Avance III HD 500 MHz spectrometer (Bruker BioSpin GmbH, Karlsruhe, Germany) with a dual 1H/13C cryoprobe (CP DUL500C/H, Bruker BioSpin GmbH). 13C and 1H NMR spectra were acquired and processed using the TopSpin software (Bruker GmbH). 1H spectra were acquired using the pulse program noesypr1d, a 1D Nuclear Overhauser Effect Spectroscopy (NOESY) experiment using presaturation to suppress the water signal. Acquisition parameters included 32 scans with a d1 (relaxation delay) of 2 seconds. 13C spectra were acquired using the pulse program zgpg30, which has a 30-degree flip angle on the carbon channel, with a d1 of 3 seconds, using 4,096 (4 k) scans and digitizing 64 K points. Power-gated broadband 1H decoupling is achieved using the ‘WALTZ-16' supercycle. The receiver gain is set to a constant value in each experiment. Metabolite signals in the samples were identified by comparison of their chemical shifts (p.p.m., reference DSS) to values from NMR databases (BMRB—Biological Magnetic Resonance Bank, University of Wisconsin16; HMDB—Human Metabolome Database, Genome Alberta17) and to those of our own standards. To confirm the identity of peaks, in selected cases, 1H–13C heteronuclear single quantum correlation spectra were acquired on standards and patients' microdialysate samples.
To enable quantification of the signals in the 13C and 1H one-dimensional spectra, calibration was carried out with a series of known concentrations of standards including lactate, 3-13C lactate, glucose, 1,2-13C2 glucose, and glutamine (from Sigma-Aldrich and Cambridge Isotope Laboratories). These standards were prepared in CNS perfusion fluid with the same fixed concentration of D2O and DSS as for the preparation of brain microdialysate samples (see above) as an internal standard and run under identical NMR conditions to the pooled microdialysates. Peak areas for DSS, glucose, lactate, and glutamine signals identified on the 13C and 1H spectra were determined using combined Gaussian and Lorentzian line-shape fitting. Peak areas relative to the DSS internal standard were used with reference to calibration curves for each signal derived from standards (see above) of known concentrations, which showed linear relationships between peak areas (ratio to DSS internal standard) and concentrations. Fractional enrichment (%) is defined as 100 × [13C]/([13C]+[12C]) where square brackets indicate concentrations of the relevant species. [13C] was determined from the 13C NMR spectra and [12C] from the 1H spectra, using the calibration method above. The natural abundance of 13C is 1.1% of all carbon atoms, and 13C results presented for lactate (see Results section) were expressed after subtracting this natural background from the 13C singlet signals. 13C doublet signals were not background-subtracted because the probability of two 13C atoms occurring next to each other naturally is 0.01%.
Interpretation of the NMR results was based on biosynthetic pathways summarized schematically in Figure 1, which shows the lactate-labeling patterns corresponding to glycolysis, which produces 2,3-13C2 lactate, and the PPP, which produces 3-13C lactate.
Figure 1.
Simplified schematic of steps in glycolysis and the pentose phosphate pathway (PPP) showing 13C labeling patterns resulting from 1,2-13C2 glucose substrate. Red fills indicate 13C atoms. Glc-6-P, glucose-6-phosphate; 6PGL, 6-phosphogluconolactone; F6P, fructose-6-phosphate; G3P, glyceraldehyde-3-phosphate; PYR, pyruvate. Figure originally published in Carpenter et al.34 under a Creative Commons Attribution License.
Statistical Analysis
Statistical analyses performed using SPSS21 for Mac (IBM SPSS Statistics, NY, USA) included non-parametric tests (Mann–Whitney U-test and Kruskal–Wallis one-way analysis of variance) with a preselected P value of 0.05 for statistical significance. Results are presented as the median values with the interquartile range (IQR) given in parentheses. Relationships between PbtO2 and the 13C labeling results were explored using linear regression, with Pearson's correlation coefficient r and analysis of variance P values.
Results
Demography
Fifteen severe TBI patients (10 male and 5 female patients) aged 16–59 years (median 27 years) were studied using 1,2-13C2 glucose (4 mmol/L) perfusion via the microdialysis catheter, with simultaneous collection of microdialysates for NMR analysis. For comparison, macroscopically normal-appearing brain was studied using the same 13C-labeled microdialysis method in six patients (age range 59–73 years; three male and three female patients) undergoing surgery for benign brain tumors. For a non-CNS tissue comparison, thigh (quadriceps) muscle was similarly studied in four patients (age range 20–61 years; three male and one female patients) who underwent surgery for acoustic neuroma resection. A further seven patients (two ‘normal' brain and five muscle) were studied by microdialysis but with plain unsupplemented perfusion fluid without 1,2-13C2 glucose. The 15 TBI patients who received 1,2-13C2 glucose (see above) were also studied for a baseline period with plain unsupplemented perfusion fluid (without 1,2-13C2 glucose). Demographic details of all patients are presented in Supplementary Table 1.
Baseline Results Compared with 1,2-13C2 Glucose Perfusion Period
Microdialysate measurements (using the ISCUS analyzer) of glucose, lactate, pyruvate, glutamate, and glycerol are shown in Figure 2. These were acquired in the 15 TBI patients during periods when the microdialysis catheter was perfused with plain unsupplemented CNS perfusion fluid and also during the 24-hour period of perfusion with 1,2-13C2 glucose (4 mmol/L). In ‘normal' brain, the ISCUS analysis was performed for two patients who received plain unsupplemented perfusion fluid and for six patients who received 24-hour perfusion with 1,2-13C2 glucose (4 mmol/L). In muscle, the ISCUS analysis was performed for five patients who received plain unsupplemented perfusion fluid, and for four patients who received 8-hour perfusion with 1,2-13C2 glucose (4 mmol/L). The 8-hour period for muscle was necessitated because of clinical practicalities of limb movement—microdialysis was limited to periods during the acoustic neuroma surgery.
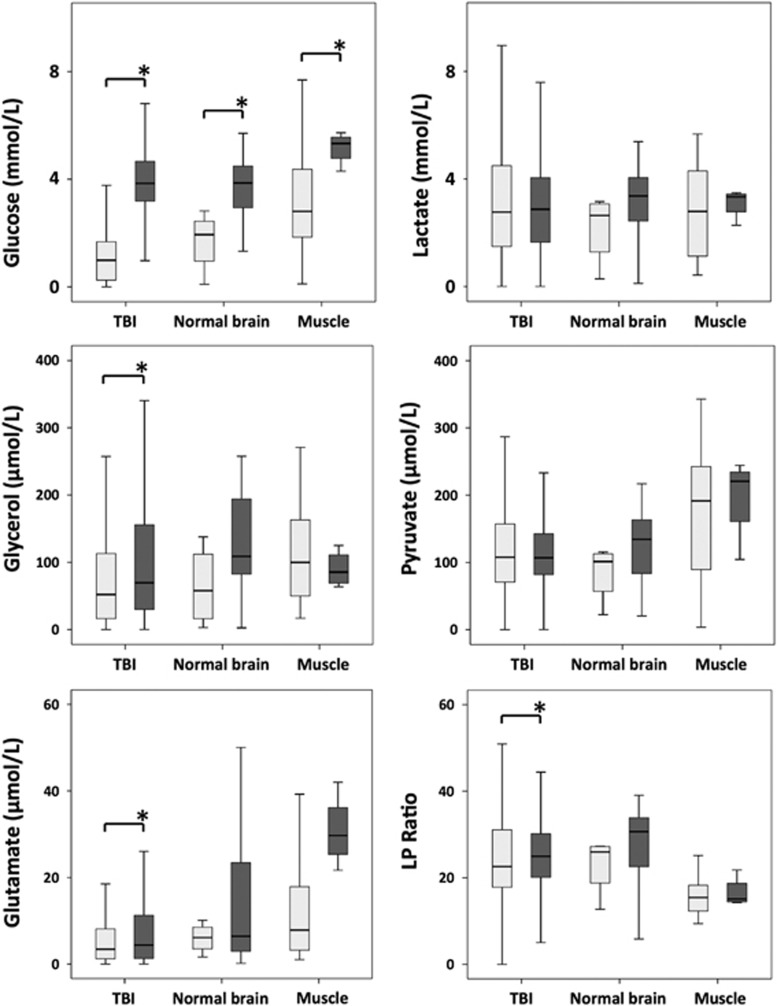
Figure 2.
ISCUS clinical microdialysis analyzer measurements: at baseline (light gray bars) with plain unsupplemented perfusion fluid and during 24-hour perfusion (brain: TBI or normal) or 8-hour perfusion (muscle) with 1,2-13C2 glucose (4 mmol/L; dark gray bars). LP ratio is the lactate/pyruvate ratio. *P<0.05 (Mann–Whitney) for baseline versus perfusion with 1,2-13C2 glucose. Box and whisker plots represent pooled data. Numbers of patients with baseline (unsupplemented) measurements were 15 TBI, 2 ‘normal' brain, and 5 muscle. Numbers of patients who received 1,2-13C2 glucose were 15 TBI, 6 ‘normal' brain, and 4 muscle. ‘Normal' brain was macroscopically normal-appearing brain in patients who underwent surgery for benign brain tumors. Muscle was leg quadriceps in patients undergoing acoustic neuroma resection surgery.
Significant increases (P<0.05) in microdialysate glucose concentration (measured on the ISCUS analyzer) between baseline (unsupplemented) and the 1,2-13C2 glucose perfusion period were seen for all three groups, as follows (medians): TBI brain (from 1.0 to 3.8 mmol/L), ‘normal' brain (from 1.9 to 3.9 mmol/L), and muscle (from 2.8 to 5.3 mmol/L). The only other statistically significant, but not thought to be biologically significant, changes in ISCUS results between baseline and the 1,2-13C2 glucose perfusion period were for lactate/pyruvate ratio in TBI brain (from 22.2 to 24.8), for glycerol in TBI brain (from 52.0 to 69.6 μmol/L), and for glutamate in TBI brain (from 3.4 to 4.4 μmol/L) and muscle (from 7.9 to 29.7 μmol/L). Concentrations of 1,2-13C2 glucose (median and IQR) measured with 13C NMR in the microdialysates from TBI brain (24-hour perfusion period), normal brain (24-hour perfusion period), and muscle (8-hour perfusion period) were, respectively, 2.14 (1.45–2.43), 0.93 (0.83–1.83), and 0.95 (0.59–1.25) mmol/L, with TBI significantly different from normal brain (P=0.008) or muscle (P=0.016).
Throughout the 24-hour period, during which the cerebral microdialysis catheter was perfused with 1,2-13C2 glucose, the median serum glucose concentration measured in TBI patients was 7.3 mmol/L and all values were within the range 4–12 mmol/L except for one time point in patient 11 in which glucose was 1 mmol/L (Supplementary Figure 1). The median serum lactate concentration was 1.1 mmol/L (min 0.7, max 3.0 mmol/L; Supplementary Figure 1).
NMR Results for Lactate Production by Glycolysis and PPP, and Relationship with Brain Tissue Oxygen
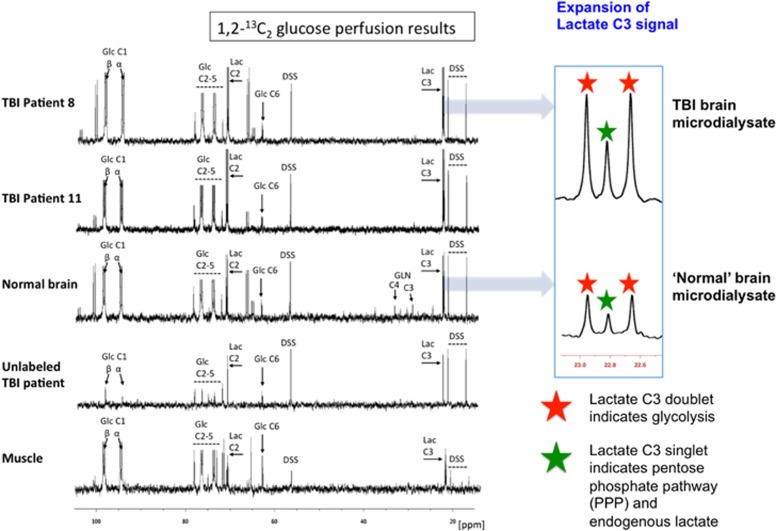
Illustrative examples of 13C NMR spectra of the microdialysates are shown in Figure 3. As a result of 1,2-13C2 glucose perfusion, TBI brain and ‘normal' brain microdialysates included a clearly visible doublet (red stars in expansion of 13C spectra) for the lactate C3 methyl group, and likewise a double for lactate C2, indicating glycolysis-derived 2,3-13C2 lactate, and a smaller C3 singlet (green stars in expansion of 13C spectra) representing PPP-derived 3-13C lactate plus endogenous lactate. Qualitatively similar-appearing spectra were seen for muscle microdialysates resulting from 1,2-13C2 glucose perfusion. Unlabeled TBI microdialysates (with plain unsupplemented perfusion fluid) showed singlets for endogenous lactate C3 and C2 (Figure 3).
Figure 3.
Illustrative examples of 13C NMR spectra for microdialysates from patients who received perfusion with 1,2-13C2 glucose (4 mmol/L): TBI brain (uppermost two spectra, 24-hour perfusion), ‘normal' brain (third spectrum, 24-hour perfusion), and muscle (bottom spectrum, 8-hour perfusion). An example of brain microdialysate from an unlabeled TBI patient with plain (unsupplemented) perfusion fluid is shown for comparison (fourth spectrum). Examples of expansion of the lactate C3 signal are shown for TBI brain and ‘normal' brain. Red stars indicate lactate C3 doublet (due to glycolytic 2,3-13C2 lactate) and green stars indicate C3 singlet (due to pentose phosphate pathway-derived 3-13C lactate plus endogenous lactate). Glucose (Glc), lactate (Lac), 4,4-dimethyl-4-silapentane-1-sulfonate sodium salt (DSS, the internal reference standard). Spectra were run from –20 to +250 p.p.m. The main reference DSS signal at 0 p.p.m. is not shown in the range illustrated.
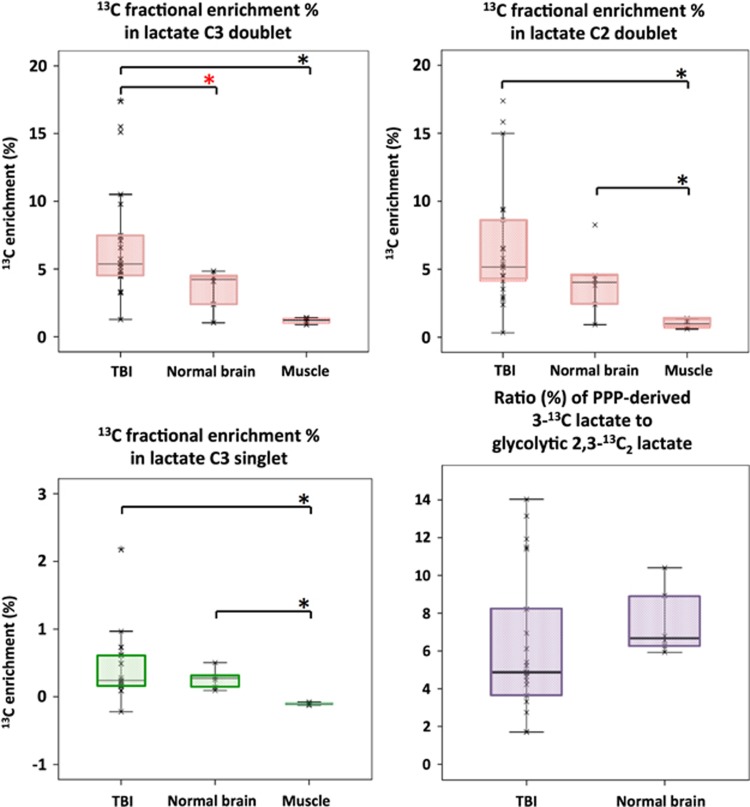
Natural abundance of 13C was assumed to be 1.1%, and 13C fractional enrichment values for lactate were expressed after subtracting this naturally occurring 13C background from the 13C singlet signals. In TBI brain microdialysates, 13C fractional enrichment (%) in 2,3-13C2 lactate, indicative of glycolysis, was median 5.4% (IQR 4.6–7.5%) measured using the C3 doublet and this was significantly greater than in ‘normal' brain (Figure 4). Fractional enrichment was based on quantification of the lactate C3 doublet signal (13C spectrum) to measure 13C at the C3 position and the lactate methyl protons (attached to C3) triplet signal (1H spectrum) to quantify 12C at the C3 position. Similar 13C fractional enrichment to that of lactate C3 doublet was obtained using the lactate C2 doublet, as expected (Figure 4). In muscle, 13C fractional enrichment in 2,3-13C2 lactate was lower than in TBI and ‘normal' brain (Figure 4).
Figure 4.
Microdialysate NMR measurements of 13C labeling: results from perfusion for 24-hour- (brain: TBI or ‘normal') or 8-hour perfusion (muscle) with 1,2-13C2 glucose (4 mmol/L). Red asterisks denote P<0.01 for TBI versus ‘normal' brain (Mann–Whitney); other comparisons asterisked in black denote P<0.05. Individual data points are shown by × symbols. Number of patients: 15 TBI, 6 ‘normal' brain, and 4 muscle.
On the basis of the lactate C3 methyl group, 13C fractional enrichment (%) in 3-13C lactate indicative of the PPP in TBI brain microdialysates was median 0.24% (IQR 0.17–0.61% Figure 4), which was thus much less than the 13C fractional enrichment in glycolytic 2,3-13C2 lactate (above). The ratio in TBI brain microdialysates of PPP-derived 3-13C lactate to glycolytic 2,3-13C2 lactate was median 4.9% (IQR 3.6–8.2%) (Figure 4). Four of the fifteen TBI patients exhibited ratios of PPP-derived to glycolytic lactate (11.4, 11.5, 11.9, and 14.0%) that were greater than the range observed in the present study in normal brain (min 5.9, max 10.4%).
In muscle, microdialysis perfusion with 1,2-13C2 glucose (4 mmol/L, for 8 hours) resulted in lactate C3 doublet and C2 doublet, indicating glycolytic 2,3-13C2 lactate, with 13C enrichment at the C3 median 1.2% (range 1.0–1.4%). However, there was no 13C enrichment above background natural abundance for lactate C3 singlet; therefore, PPP production of lactate was not detected in muscle.
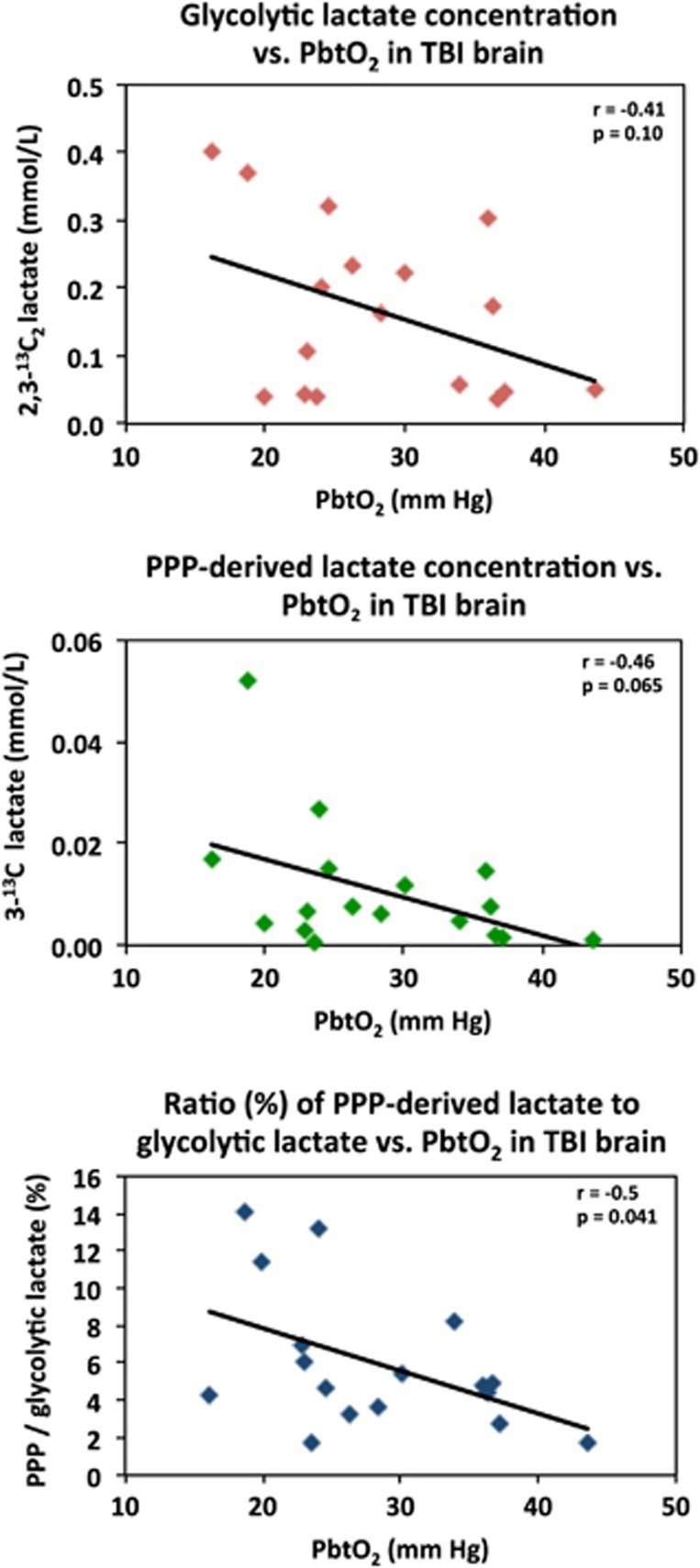
The concentration of 2,3-13C2 lactate (indicative of glycolysis) showed a nonsignificant inverse trend (r=−0.4, P=0.1) with brain tissue oxygen concentrations (PbtO2 expressed in mm Hg) measured in the vicinity of the microdialysis catheter in TBI brain. A significant inverse correlation (r=−0.5, P=0.04) was seen for the ratio of PPP-lactate to glycolytic lactate versus PbtO2 (Figure 5). Local tissue oxygen concentration was not measured in normal brain or muscle.
Figure 5.
Relationships in TBI brain for glycolytic lactate and pentose phosphate pathway (PPP)-derived lactate versus PbtO2. Concentrations of glycolytic 2,3-13C2 lactate (upper panel) and PPP-derived 3-13C lactate (middle panel) plotted versus PbtO2. Lower panel: ratio (%) of PPP-derived 3-13C lactate to glycolytic 2,3-13C2 lactate, plotted versus PbtO2. Each data point represents the results of NMR analysis of the combined contents of 24 × 1 hours of microdialysate collection vials from one microdialysis catheter, plotted against the corresponding PbtO2 concentration expressed in mm Hg, measured using a Licox oxygen probe placed alongside the microdialysis catheter in the brain. Lines are fitted by linear regression (statistics shown are Pearson's correlation coefficient r and analysis of variance P value). Data are from 13 TBI patients. Four of these thirteen had a second period of monitoring, making 17 data points in total for each correlation.
Five TBI patients had a second 24-hour period of 1,2-13C2 glucose (4 mmol/L) microdialysis perfusion. There were no significant differences in the production of glycolytic lactate or PPP-derived lactate between these two time periods.
Discussion
This study has shown that 1,2-13C2 glucose infusion via the microdialysis catheter results in 13C-labeling in lactate (Figure 3) in the emerging microdialysates, enabling us to compare glycolysis and PPP as routes by which lactate is derived. This is the first time this comparison has been performed directly in human brain.
Glucose Metabolism via Glycolysis and the Pentose Phosphate Pathway
Clear evidence for glycolysis being the main route of lactate production from glucose, as expected, was seen as diagnostic doublet signals for both C3 and C2 of lactate (Figure 3) indicating 2,3-13C2 lactate, a hallmark of the pathway (Figure 1). These doublets were seen for all patients, in TBI brain, ‘normal' brain and muscle. This is in accord with the recognized glycolysis pathway consisting of the Embden–Meyerhof pathway from glucose to pyruvate, followed by conversion to lactate by the action of lactate dehydrogenase (Figure 1). This 13C enrichment was significantly higher in the TBI brain, suggesting greater glycolytic activity than in ‘normal' brain and muscle. The doublet signals provide a distinctive signature that in effect avoids the issue of natural abundance 13C background (1% of all carbon atoms) because the probability of two endogenous 13C atoms occurring next to each other naturally is 0.01%.
The PPP loses the first carbon of 1,2-13C2 glucose as carbon dioxide and thus gives rise to 3-13C lactate with a singlet signal for C3 (Figure 1). Whereas 13C singlet signals for lactate C3 were clearly visible in all patients' microdialysates (Figure 3), these were smaller than the C3 doublet. Furthermore, it emerged that much of the C3 singlet signal intensity (peak area) was because of endogenous natural abundance 13C. Small 13C fractional enrichments for lactate C3 singlet above this background were seen in microdialysates from TBI brain and ‘normal' brain, indicating PPP-derived lactate, but were negligible in muscle. Although there was no statistically significant difference in 13C fractional enrichment for lactate C3 between ‘normal' brain and TBI brain, the higher upper range of the latter suggests that in certain TBI individuals lactate production via the PPP is elevated above that in ‘normal' brain. In the four TBI patients with the most elevated PPP lactate, the ratio (%) of PPP lactate to glycolytic lactate was 11.4–14.0%.
Our finding of increased PPP activity in some patients as a result of brain injury is supported by experimental studies. In rat models relevant to TBI, Bartnik et al.6,7 infused 1,2-13C2 glucose intravenously after fluid percussion injury and cortical contusion injury, and performed NMR analysis of brain tissue extracts.6,7 They found an increase in the proportion of 3-13C lactate (indicative of PPP) relative to 2,3-13C2 lactate (indicative of glycolysis) in brain-injured rats compared with control rats, although with glycolysis remaining dominant. In humans, Dusick et al.8 infused 1,2-13C2 glucose intravenously, with 13C labeling in lactate assessed by arteriovenous-jugular difference.8 This study in TBI patients and controls, although it did not sample the brain directly and could only yield information on brain lactate labeling during periods when the brain was a net exporter of lactate into the vasculature, led to similar conclusions to the rat studies above.
Significance of the PPP
The PPP is a complex biosynthetic network generating many other species besides lactate (Figure 1) and the balance between these species can potentially shift depending on the local biology and pathology. Some PPP-derived species are potentially reparative, for example, ribose (for nucleic acid synthesis) and NADPH (to provide reducing equivalents for fatty acid synthesis), and neuroprotective for example, NADPH, used for producing the reduced form of glutathione and thioredoxin, which are cofactors for glutathione peroxidases and peroxiredoxins, respectively, enzymes that combat oxidative stress. Accordingly, Herrero-Mendez et al.18 demonstrated that if one of the key regulatory enzymes of glycolysis, phosphofructokinase, is activated in neurons so that more glucose is funneled through glycolysis at the expense of the PPP, apoptosis soon ensues because of oxidative stress.18 The PPP, which does not involve molecular oxygen and does not generate ATP, can thus be regarded as sacrificing some of the cells' supply of glucose molecules, which might otherwise have been used for ATP synthesis via glycolysis and the TCA cycle, for the sake of generating more reducing power (NADPH) and the ability to protect, repair, or build cells.
NADPH is involved in many other biochemical reactions, for example, NADPH is a cofactor for NADPH oxidase and nitric oxide synthase and, if these become dysregulated, oxidative stress may ensue, with adverse consequences. Zuurbier et al.19 reported that inhibition of the so-called ‘oxidative' phase of the PPP (the early steps of the PPP responsible for NADPH production), by means of administering 6-aminonicotinamide, was cardioprotective in an ischemia-reperfusion rat heart model.19 Interestingly, when Tyson et al.20 administered 6-aminonicotinamide to inhibit the PPP in rats in a study of brain using intravenous 2-13C glucose as the substrate, they found that despite achieving PPP inhibition, evidenced by a build-up of 6-phosphogluconate, there appeared to be a feedback effect of PPP inhibition on glycolysis, such that both pathways decreased in a constant ratio.20
Role of Brain Tissue Oxygen Concentration in Brain Metabolism
In the literature, ‘hyperglycolysis' and the elevation of lactate production in the injured brain have been attributed to hypoxia and/or mitochondrial dysfunction, although the exact nature of the latter remains unclear. In a combined positron emission tomography (oxygen-15 and fluorodeoxyglucose (18 F)) and microdialysis study, Vespa et al.2 reported that ‘metabolic crisis' (defined as lactate/pyruvate ratio >40) occurred in 7 out of 19 TBI patients studied, although only one case showed regional ischemia judged by positron emission tomography.2 In a microdialysis study of 24 TBI patients in conjunction with PbtO2 measurement and perfusion CT, Sala et al.21 concluded that the majority of lactate production was ‘glycolytic' (rather than hypoxic), albeit without evidence from carbon labeling.21
In the present study, we measured PbtO2 alongside the microdialysis catheter in the TBI patients and found that there was a nonsignificant trend towards greater glycolytically generated 2,3-13C2 lactate with decreasing PbtO2. It is important to note that there were only three cases that could be described as hypoxic (defined as PbtO2<20 mm Hg), and the remaining 14 data points ranged from 22 to 43 mm Hg. Interestingly, the ratio of PPP-derived lactate to glycolytic lactate showed a significant inverse correlation with PbtO2 (r=−0.5, P=0.04; Figure 5). Thus, although PPP-derived lactate was always much less than glycolytic lactate, the balance between the two appeared to shift towards the PPP with lower PbtO2. Similar to glycolysis, the PPP does not utilize oxygen. Studies in adult rats and in brain slices have also suggested that hypoxia increases the PPP.22 In contrast, Brekke et al.23 found evidence for a decrease in PPP in neonatal rats after hypoxic–ischemic injury, which the authors discussed as paradoxical.23 Moreover, they postulated that the apparent inability to upregulate the PPP in this situation might render the neonatal rats vulnerable. An analogous situation might conceivably exist in human TBI, whereby low ability to upregulate the PPP might result in increased risk of damage in those individuals' brain tissue (either globally or locally). Therapeutic upregulation of the PPP in this context might be beneficial. Apart from hypoxia (mentioned above), a few other upregulators of the PPP have been identified. Insulin has been shown to increase the expression of glucose-6-phosphate dehydrogenase mRNA in primary rat hepatocytes.24 Dehydroascorbate, the oxidized form of vitamin C, has been shown to stimulate the activity of several PPP enzymes, increase glutathione levels, and inhibit hydrogen peroxide-induced changes in mitochondrial transmembrane potential in Jurkat cells (a cancer cell line).25 Although these two latter studies were not performed in the brain, they nevertheless illustrate the principle that the PPP can be deliberately upregulated with measurable biochemical and biological effects.
Significance of Lactate in the Brain
Lactate has been conventionally regarded as a waste product of glucose metabolism, although a more recent idea is that it can act as a brain fuel—neurons take up lactate (produced from glucose by glia), metabolize lactate to pyruvate, which is transported into the mitochondria and converted to acetyl CoA, which enters the tricarboxylic acid (TCA) cycle. This has become known as the astrocyte–neuron lactate shuttle hypothesis.26 Animal studies have provided evidence for brain utilization of intravenously administered 3-13C lactate via the TCA cycle.27 In the human brain, direct evidence for utilization of lactate was first obtained in a cerebral microdialysis study in TBI patients.28 In the latter study, administration of 3-13C lactate via the microdialysis catheter, and simultaneous collection of the emerging microdialysates, with 13C NMR analysis, revealed 13C labeling in glutamine consistent with lactate utilization via the TCA cycle.28 Recently, an intravenous lactate supplementation study in TBI patients revealed evidence for a beneficial effect judged by surrogate end points.29 Studies of brain microdialysates (without supplementation) in 223 patients show a statistical association between high extracellular lactate and unfavorable outcome.30 Taken together, available evidence suggests that where neurons are too damaged to utilize the lactate produced from glucose by astrocytes, that is, uncoupling of neuronal and glial metabolism, high extracellular levels of lactate would accumulate, explaining one potential mechanism behind the association between high extracellular lactate and poor outcome.
Fate of Lactate
Regarding the possible fate of labeled lactate produced from 1,2-13C2 glucose, a likely scenario is that some of the labeled lactate is processed via the TCA cycle, and some of it exported into the bloodstream. Because we were microdosing the brain with 1,2-13C2 glucose locally via the microdialysis catheter, it seemed highly unlikely that any of the ensuing doubly labeled lactate would have been detectable in the bloodstream, given the inevitable dilution both with endogenous unlabeled lactate and with the volume of blood; therefore, 13C NMR analyses of blood were not performed.
Previously, using 3-13C lactate as the substrate, we detected singly labeled glutamine (and/or glutamate) in TBI brain microdialysates, indicating the utilization of lactate via the TCA cycle.28 In the present study, if 2,3-13C2 lactate (produced from the substrate 1,2-13C2 glucose) entered the TCA cycle in brain cells, this would in theory have resulted in doublets in the 13C NMR spectra, because of 4,5-13C2 glutamine and 2,3-13C2 glutamine (and/or glutamate in each case) on the first turn of the cycle, and, on the second turn, doublets because of 1,213C2 glutamine and a singlet because of 3-13C glutamine.23,31 Experimental models of brain injury in rodents, using 1,2-13C2 glucose as the substrate, with analysis of brain tissue extracts representing total intracellular and extracellular molecules, confirm double labeling in glutamate and glutamine.6,7,31 In our present study, small singlet signals, but not doublets, corresponding to glutamine were seen in the 13C NMR spectra of some of the microdialysates from ‘normal' brain (4/6 patients) and TBI (2/15 patients), verified by 2D NMR methods, including 1H-13C heteronuclear single quantum correlation (Supplementary Figure 2). In theory, pyruvate cycling,32 which breaks the 13C–13C bond might explain the glutamine singlets. In addition, in theory, PPP-derived 3-13C lactate might enter the TCA cycle forming singly labeled glutamine (and/or glutamate), although accompanied by doubly labeled glutamine from glycolytic 2,3-13C2 lactate, as found in vitro by Brekke et al.33 However, in the present study, quantification of the small glutamine singlets revealed no significant 13C enrichment above natural abundance.
Microdialysis only samples the extracellular compartment and therefore cannot measure the intracellular distribution of 13C. Even so, our 13C-labeled microdialysis method provides a useful means for measuring ‘early' glucose metabolism (glycolysis and PPP) as sufficient labeled lactate is exported into the extracellular compartment to allow detection. However, in the subsequent metabolism of lactate, the downstream dilution of 13C label with endogenous unlabeled intermediates probably explains why we did not observe significant 13C enrichment of extracellular metabolites derived from the TCA cycle. In our previous study, when 3-13C lactate (4 mmol/L) or 2-13C acetate (4 mmol/L) was perfused via the microdialysis catheter in the TBI brain, labeling in glutamine consistent with TCA cycle was seen in the emerging microdialysates.28 However, in the same study, perfusion with 1-13C glucose (2 mmol/L) resulted in negligible labeling in glutamine, consistent with the present study.
Strengths and Limitations
This 13C-labeled microdialysis method, performed in parallel with local brain tissue oxygen measurement, provides a method of measuring glycolytic conversion of glucose into lactate and at the same time distinguishing hypoxic from non-hypoxic glycolysis, as well as evaluating the contribution of the PPP. Even though our evidence indicates a minor PPP-derived lactate (3-13C lactate), it must be remembered that the PPP is a complex network of biosynthetic reactions (Figure 1) and it is conceivable that if heavy recycling is operating within the PPP, less lactate might emerge, although with elevated production of NADPH and intermediates utilized in neuroprotection and repair of cells.
The strategy of double labeling (using 1,2-13C2 glucose as the substrate) provides a characteristic signature that appears in glycolytic lactate (2,3-13C2 lactate) showing that the 13C–13C covalent bond stays intact. Moreover, the doublet signal is essentially free from endogenous lactate because the probability of two 13C atoms being adjacent to each other naturally is 0.01%. Similar to all cerebral microdialyses, the 13C-labeled microdialysis method is invasive, so is only suitable for severe TBI patients or those requiring brain surgery (for example, for tumors), and it is a focal technique.
This 13C-labeling method has the potential to be a useful adjunct to existing methodologies of positron emission tomography and unlabeled microdialysis in monitoring and studying TBI patients. The 13C-labeled microdialysis method does not involve disruption of the patient's standard medical care on the neurocritical care unit and does not involve radioactivity or moving the patient to a scanner. The 1,2-13C2 glucose microdialysis method is reasonably inexpensive and convenient to perform because 1 or 2 g of the labeled material are enough to formulate enough perfusion fluid for multiple patients, and the formulation can be stored in individual sterile ready-to-use sealed glass vials in a refrigerator. The concentration (4 mmol/L) of 1,2-13C2 glucose administered via microdialysis locally into the brain corresponds to the upper end of the ‘normal' range found in brain extracellular fluid.13 Whereas conventional microdialysis performed with unsupplemented perfusion fluid allows us to measure endogenous extracellular lactate, the unsupplemented method cannot inherently distinguish between ‘old' lactate and recently synthesized lactate. An advantage of the 1,2-13C2 glucose microdialysis method is that it enables the measurement of labeled lactate production within a known timeframe—at present this is 24 hours because of current practical NMR considerations (see below).
A current limitation to our 13C-labeled microdialysis method is the amount of material necessary for performing NMR analysis. We have combined brain microdialysates for a 24-hour period for each patient to facilitate 13C NMR spectroscopy within a convenient acquisition time on the spectrometer. Our Bruker Avance 500 MHz spectrometer is equipped with a cryoprobe, as used in the present study and in our previous study,28 which is more sensitive than non-cryo probe technology. Even so, the practical limit with our present equipment limits us in terms of how short a time frame we can analyze in terms of microdialysis perfusion. Having better time resolution for the microdialysis labeling results would be useful from a biochemical perspective allowing time course evaluation. 1,2-13C2 glucose microdialysis may have future potential for metabolic kinetic modeling of local glucose consumption rates and glycolytic- and PPP-production rates of lactate. Commercially available NMR microcryoprobes, which take smaller volumes, may be useful in future. Analysis with mass spectrometry might also be useful in future, enabling smaller volumes to be analyzed, although at the expense of at least some of the detailed information on precise intramolecular position of label.
Conclusion
Here we have shown that 13C-labeled microdialysis can be used to interrogate glucose metabolism via glycolysis and the PPP. The major pathway, glycolytic lactate production, was significantly greater in the TBI brain than in the normal brain. The minor pathway, PPP-derived lactate production, was statistically not significantly different in the TBI brain than in normal brain. However, several of the TBI individuals showed PPP-derived lactate elevation above the range observed in the normal brain. There was a shift in glucose metabolism from glycolysis to PPP with decreasing brain tissue oxygen concentrations. The findings raise interesting questions about the roles of the PPP and glycolysis after TBI, and whether they can be manipulated to enhance the potentially reparative and antioxidant role of the PPP and achieve a better outcome for the patient. The 13C methodology developed here provides a means of distinguishing recently synthesized lactate and its biosynthetic origin, and at the same time measuring local oxygen tension alongside. 13C-labeled microdialysis with 1,2-13C2 glucose as substrate may thus find a methodological role in studies of hyperoxia or strategies to optimize perfusion and mitochondrial function. This is the first time that a comparison between glycolysis and the PPP has been carried out directly in the human brain.
Acknowledgments
We thank Mr R. Kirollos, Mr R. Macfarlane, and Mr R. Mannion for assistance in placing microdialysis catheters into the control subjects. We thank Dr R.J. Shannon for assistance with microdialysate analysis. We thank Mr John Harwood (Ipswich Hospital NHS Trust) for supervising the formulation of the 13C substrate.
J.D.P. and P.J.H. are the Directors of Technicam.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Medical Research Council (Grant Nos. G0600986 ID79068 and G1002277 ID98489) and the National Institute for Health Research Biomedical Research Centre, Cambridge (Neuroscience Theme; Brain Injury and Repair Theme). I.J. was supported by the Medical Research Council (Grant no. G1002277 ID 98489) and the National Institute for Health Research Biomedical Research Centre, Cambridge; K.L.H.C. was supported by the National Institute for Health Research Biomedical Research Centre, Cambridge (Neuroscience Theme; Brain Injury and Repair Theme); C.G. was supported by the the Canadian Institute of Health Research; A.H.—Medical Research Council/Royal College of Surgeons of England Clinical Research Training Fellowship (Grant no. G0802251) and Raymond and Beverly Sackler Fellowship; D.K.M. and J.D.P. were supported by the National Institute for Health Research Senior Investigator Awards; P.J.H. was supported by the National Institute for Health Research Professorship, Academy of Medical Sciences/Health Foundation Senior Surgical Scientist Fellowship.
Supplementary Material
References
- Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, et al. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu H-M, Huang S-C, Martin NA, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-M, Huang S-C, Vespa P, Hovda DA, Bergsneider M. Redefining the pericontusional penumbra following traumatic brain injury: evidence of deteriorating metabolic derangements based on positron emission tomography. J Neurotrauma. 2013;30:352–360. doi: 10.1089/neu.2012.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Katayama Y, Hovda DA, Yoshino A, Becker DP. Administration of excitatory amino acid antagonists via microdialysis attenuates the increase in glucose utilization seen following concussive brain injury. J Cereb Blood Flow Metab. 1992;12:12–24. doi: 10.1038/jcbfm.1992.3. [DOI] [PubMed] [Google Scholar]
- Bartnik BL, Sutton RL, Fukushima M, Harris NG, Hovda DA, Lee SM. Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J Neurotrauma. 2005;22:1052–1065. doi: 10.1089/neu.2005.22.1052. [DOI] [PubMed] [Google Scholar]
- Bartnik BL, Lee SM, Hovda DA, Sutton RL. The fate of glucose during the period of decreased metabolism after fluid percussion injury: a 13C NMR study. J Neurotrauma. 2007;24:1079–1092. doi: 10.1089/neu.2006.0210. [DOI] [PubMed] [Google Scholar]
- Dusick JR, Glenn TC, Lee WNP, Vespa PM, Kelly DF, Lee SM, et al. Increased pentose phosphate pathway flux after clinical traumatic brain injury: a [1,2-13C2]glucose labeling study in humans. J Cereb Blood Flow Metab. 2007;27:1593–1602. doi: 10.1038/sj.jcbfm.9600458. [DOI] [PubMed] [Google Scholar]
- Pandolfi P, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995;14:5209. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012;53:421–436. doi: 10.1016/j.freeradbiomed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Ben-Yoseph O, Boxer PA, Ross BD. Assessment of the role of the glutathione and pentose phosphate pathways in the protection of primary cerebrocortical cultures from oxidative stress. J Neurochem. 1996;66:2329–2337. doi: 10.1046/j.1471-4159.1996.66062329.x. [DOI] [PubMed] [Google Scholar]
- Helmy A, Vizcaychipi M, Gupta AK. Traumatic brain injury: intensive care management. Br J Anaesth. 2007;99:32–42. doi: 10.1093/bja/aem139. [DOI] [PubMed] [Google Scholar]
- Reinstrup P, Ståhl N, Mellergård P, Uski T, Ungerstedt U, Nordström C. Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery. 2000;47:701–709. doi: 10.1097/00006123-200009000-00035. [DOI] [PubMed] [Google Scholar]
- Rosdahl H, Ungerstedt U, Jorfeldt L. Interstitial glucose and lactate balance in human skeletal muscle and adipose tissue studied by microdialysis. J Physiol. 1993;471:637–657. doi: 10.1113/jphysiol.1993.sp019920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz MK, Wang LP, Tange M, Bjerre P. Cerebral microdialysis monitoring: determination of normal and ischemic cerebral metabolisms in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2000;93:808–814. doi: 10.3171/jns.2000.93.5.0808. [DOI] [PubMed] [Google Scholar]
- Ulrich EL, Akutsu H, Doreleijers JF, Harano Y, Ioannidis YE, Lin J, et al. BioMagResBank Nucleic Acids Res 200736D402–D408.Available from: http://www.bmrb.wisc.edu [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, et al. HMDB: the Human Metabolome Database Nucleic Acids Res 200735D521–D526.(cited 11 October 2013). Available from http://www.hmdb.ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Mendez A, Almeida A, Fernández E, Maestre C, Moncada S, Bolaños JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C–Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- Zuurbier CJ, Eerbeek O, Goedhart PT, Struys EA, Verhoeven NM, Jakobs C, et al. Inhibition of the pentose phosphate pathway decreases ischemia-reperfusion-induced creatine kinase release in the heart. Cardiovasc Res. 2004;62:145–153. doi: 10.1016/j.cardiores.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Tyson RL, Perron J, Sutherland GR. 6-Aminonicotinamide inhibition of the pentose phosphate pathway in rat neocortex. Neuroreport. 2000;11:1845–1848. doi: 10.1097/00001756-200006260-00009. [DOI] [PubMed] [Google Scholar]
- Sala N, Suys T, Zerlauth J-B, Bouzat P, Messerer M, Bloch J, et al. Cerebral extracellular lactate increase is predominantly nonischemic in patients with severe traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1815–1822. doi: 10.1038/jcbfm.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domańska-Janik K. Hexose monophosphate pathway activity in normal and hypoxic rat brain. Resuscitation. 1988;16:79–90. doi: 10.1016/0300-9572(88)90073-1. [DOI] [PubMed] [Google Scholar]
- Brekke EMF, Morken TS, Widerøe M, Håberg AK, Brubakk A-M, Sonnewald U. The pentose phosphate pathway and pyruvate carboxylation after neonatal hypoxic-ischemic brain injury. J Cereb Blood Flow Metab. 2014;34:724–734. doi: 10.1038/jcbfm.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar I, Szeszel-Fedorowicz W, Salati LM. Arachidonic acid inhibits the insulin induction of glucose-6-phosphate dehydrogenase via p38 MAP kinase. J Biol Chem. 2005;280:40660–40667. doi: 10.1074/jbc.M505531200. [DOI] [PubMed] [Google Scholar]
- Puskas F, Gergely P, Banki K, Perl A. Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate, the oxidized form of vitamin C. FASEB J. 2000;14:1352–1361. doi: 10.1096/fj.14.10.1352. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson RL, Gallagher CN, Sutherland GR. 13C-Labeled substrates and the cerebral metabolic compartmentalization of acetate and lactate. Brain Res. 2003;992:43–52. doi: 10.1016/j.brainres.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Gallagher CN, Carpenter KLH, Grice P, Howe DJ, Mason A, Timofeev I, et al. The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain. 2009;132:2839–2849. doi: 10.1093/brain/awp202. [DOI] [PubMed] [Google Scholar]
- Bouzat P, Sala N, Suys T, Zerlauth J-B, Marques-Vidal P, Feihl F, et al. Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Med. 2014;40:412–421. doi: 10.1007/s00134-013-3203-6. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Carpenter KLH, Nortje J, Al-Rawi PG, O'Connell MT, Czosnyka M, et al. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain. 2011;134:484–494. doi: 10.1093/brain/awq353. [DOI] [PubMed] [Google Scholar]
- Bartnik BL, Hovda DA, Lee PWN. Glucose metabolism after traumatic brain injury: estimation of pyruvate carboxylase and pyruvate dehydrogenase flux by mass isotopomer analysis. J Neurotrauma. 2007;24:181–194. doi: 10.1089/neu.2006.0038. [DOI] [PubMed] [Google Scholar]
- Cruz F, Cerdán S. Quantitative 13C NMR studies of metabolic compartmentation in the adult mammalian brain. NMR Biomed. 1999;12:451–462. doi: 10.1002/(sici)1099-1492(199911)12:7<451::aid-nbm571>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Brekke EMF, Walls AB, Schousboe A, Waagepetersen HS, Sonnewald U. Quantitative importance of the pentose phosphate pathway determined by incorporation of 13C from [2-13C]- and [3-13C]glucose into TCA cycle intermediates and neurotransmitter amino acids in functionally intact neurons. J Cereb Blood Flow Metab. 2012;32:1788–1799. doi: 10.1038/jcbfm.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KLH, Jalloh I, Gallagher CN, Grice P, Howe DJ, Mason A, et al. (13)C-labelled microdialysis studies of cerebral metabolism in TBI patients. Eur J Pharm Sci. 2014;57:87–97. doi: 10.1016/j.ejps.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.