Abstract
Objective
We sought to investigate prognostic implications of the relationships of estimated left ventricular (LV) myocardial energy expenditure (MEE) with LV systolic dysfunction, body composition, and inflammation in a population-based sample of adults without overt congestive heart failure.
Methods
Echocardiography was used to assess LV ejection fraction (EF) and MEE. Body composition was evaluated by bioelectric impedance. Dietary recall was used to assess 24-hour calorie intake. Participants in the Strong Heart Study without prior congestive heart failure and with all needed data available (n = 3087) were divided based on LV EF (>55%, 54%–45%, or <45%).
Results
Participants with EF less than 45% were older and they had lower body mass index, adipose mass, fat-free mass, and 24-hour calorie intake than participants with normal EF (≥55%), and had greatest reductions of body mass index and physical activity in a time interval of 3.5 years, on average, elapsed between an initial clinical assessment and the evaluation at the time of the echocardiographic examination (P < .01). Lower EF was associated with male sex, hypertension, diabetes, coronary heart disease, and higher fibrinogen, C-reactive protein, and plasma creatinine levels (all P < .01). MEE was higher with lower EF (all P < .001). In Cox regression models, during approximately 8 years of observation, MEE comprised between 97 and 123 cal/min and MEE greater than 123 cal/min were associated with 2.5-fold and additional 3.3-fold higher rates of cardiac death, respectively, compared with MEE less than 97 cal/min, independently of EF, body composition, and other covariates. However, lower adipose mass predicted increased risk of cardiac death independent of MEE and EF.
Conclusion
In a population-based sample of adults including ambulatory individuals with depressed LV systolic function but without overt congestive heart failure, depressed EF was associated independently with higher MEE, lower adipose mass, and higher fibrinogen. However, increased MEE and lower adipose mass predicted cardiac death independently of EF and other covariates.
In contrast to the association of obesity with increased cardiovascular (CV) risk in the general population and in clinically healthy hypertensive adults,1,2 in patients with overt systolic congestive heart failure (CHF), mortality is higher with lower body mass index (BMI).3–6 On one hand, overt systolic CHF is associated with elevated cytokine levels and metabolic imbalance, which contribute to weight loss and ultimately cachexia.7 On the other hand, lower skeletal muscle energy requirement as a result of reduced physical activity may predict lower metabolic needs in patients with symptomatic left ventricular (LV) systolic dysfunction.8 The relationship of lower BMI with untoward outcomes9 in patients with clinically overt LV systolic dysfunction may reflect, at least in part, the relationships of LV systolic dysfunction with inflammation and with increased cardiac energy expenditure.10 However, in individuals from population source, the relations of LV dysfunction without overt CHF to body composition and cardiac energy demand and their impact on cardiac outcomes remain elusive. Indeed, the relation of LV systolic dysfunction to myocardial energy expenditure (MEE) has not been assessed in population-based samples. Moreover, BMI is only an indirect estimate of body composition. Therefore, in a population-based sample of adults without CHF, we evaluated prognostic implications of MEE and severity of LV systolic dysfunction in relation to body composition, markers of inflammation.
METHODS
Population
The Strong Heart Study (SHS) is a population-based survey of the prevalence and incidence of CV risk factors and of CV morbidity and mortality in 13 Native American communities in Arizona, Oklahoma, and South and North Dakota.11–16 Tribal members aged 45 to 74 years were recruited for an initial examination in 1989 to 1992 (overall rate 62%). Extensive characterization of participants included standardized anthropometric, clinical, and laboratory data that have been previously described.11 The return rate for those alive at the second examination (1993–1995) was 88%,15 97% of whom (n = 3501) underwent echocardiographic examination.17 Hypertension was defined by systolic blood pressure (BP) greater than or equal to 140 mm Hg and/or diastolic BP greater than or equal to 90 mm Hg, or use of antihypertensive medications. Diabetes was defined by American Diabetes Association criteria.18 Participants with CHF, given the diagnosis based on review of medical records by the following criteria, were excluded: hospitalization, without end-stage renal disease or noncardiac disease leading to massive fluid overload, with at least two major criteria (paroxysmal nocturnal dyspnea or orthopnea, jugular venous distension, rales, cardiomegaly, radiographic pulmonary edema, S3 gallop, hepatojugular reflux, or weight loss > 4.5 kg in response to diuretics) or one major and two minor criteria (ankle edema, night cough, dyspnea on exertion, hepatomegaly, or pleural effusion).19 Coronary heart disease was defined by standard criteria based on medical record review or electrocardiogram findings as previously reported.13 Smoking was assessed by self-report. Anthropometry included BMI and body surface area calculated by standard formulae. Fat-free mass and adipose mass were estimated using an impedance meter and equations based on total water validated in the American Indian population, as previously described.20 A 24-hour recall was performed to assess 24-hour food intake, energy, and macronutrient dietary content in kcal/day.21
This study evaluated 3032 SHS participants free of CHF, with less than 3+ mitral regurgitation, who had needed echocardiographic measurements at the second SHS examination. Observation for cardiac mortality (n = 127) extended from the date of the echocardiogram to December 2002 (loss to mortality follow-up 0.2%). Methods to identify events and their cause were previously described.15 Cause of death was investigated through autopsy reports, medical records, and informant interviews, which were examined independently by physician members of the SHS Mortality Review Committee,15 with estimated concordance in diagnosis greater than 90%. Definite cardiac deaths comprised deaths caused by myocardial infarction, coronary heart disease, sudden death, and CHF.
Echocardiography
Echocardiographic methods have been previously described.17,20,22 A standard echocardiographic protocol was used to obtain parasternal views with optimal orientation to maximize LV internal diameters. This method has been shown to allow reliable estimation of LV structure and function.23 All echocardiograms were recorded on videotapes and sent to the echocardiography reading center for central evaluation by trained blinded readers and subsequently over-read by highly experienced readers as final arbiters.
Echocardiographic measurements
Measurements of LV internal dimension and wall thickness were obtained at end diastole and end systole following American Society of Echocardiography recommendations,24 allowing reliable estimation of LV mass23 by an anatomically validated formula25; LV mass was indexed for height to the power of the LV mass-body height allometric relationship (height2.7).26 Relative wall thickness was calculated as posterior wall thickness/LV radius and used as a measure of LV concentricity. Ejection fraction (EF) was calculated from LV linear dimensions, as has been shown to be prognostically relevant.9 Stroke volume was derived by Doppler method22; LV internal diastolic diameter and stroke volume were indexed by body surface area to exclude impact of body weight. The ratio of circumferential end-systolic stress/end-systolic volume index, calculated from LV internal end-systolic diameter27 was used as indicator of LV chamber contractility.28
Noninvasive estimation of LV workload and biomechanical energy expenditure
Pioneering studies by Starling et al29,30 and Bing et al31 demonstrated that LV external work is proportional to mean aortic pressure and cardiac output. Sarnoff et al32 subsequently clarified the primary role of the systolic tension (or stress) applied to the LV in determining myocardial oxygen consumption and, therefore, energy expenditure. However, invasive methods are not applicable to epidemiologic studies. As described previously33: (1) assuming that end-systolic stress34 is a representative measure of the systolic tension applied to the myocardium during the ejection phase; (2) using Doppler echocardiography to estimate the mass moved by the myocardium (ie, the stroke volume22); and (3) transaortic Doppler flow to measure the period during which the tension is applied to the myocardium during LV ejection (LV ejection time), MEE was calculated as: circumferential end-systolic stress (kdyne/cm2) × ejection time (seconds) × stroke volume (cm3) × 4.2 × 10−7; and expressed as kcal/systole.33
In a reference group of 89 normal-weight men and women (age 57 ±7 years, BMI 22.8 ±1.8 kg/m2, LV mass 124 ±28 g, heart rate 65 ± 9 beat/min) without clinically overt hypertension (BP 111/68 ± 12/7 mm Hg), diabetes, or preclinical echocardiographic CV disease and without aortic stenosis or insufficiency of any degree, or more than mild mitral regurgitation, MEE was 1.07 ± 0.43 cal/beat or 69 ± 27 cal/min, which yields an estimate of potential oxygen/ g/min of LV mass of 0.12 ± 0.04 mL. This value is similar to those measured invasively by Vaz et al35 (0.12 ±0.02 and 0.14 ±0.02 mL O2/g/min) in 9 young men (22 ± 1 years, 22.8 ± 1.1 kg/m2, BP 131/69 ± 2/1 mm Hg, LV mass 174 ± 13 g, heart rate 58 ± 4 beat/min) and 12 older men (65 ± 4 years, 26 ± 0.8 kg/m2, BP 138/66 ± 4/3 mm Hg, LV mass 158 ± 14 g, heart rate 66 ± 3 beat/min).
Laboratory Data
Participants were examined in the morning after at least a 12-hour overnight fast. Methods for assessment of fibrinogen and high-sensitivity C-reactive protein were published previously.36
Statistical Analysis
Data in tables are mean ± SD or percent. For continuous variables, differences among EF groups were tested using analysis of variance. Pairwise comparisons were tested by Dunnett’s T3 post hoc test based on Studentized maximum modulus, which does not assume equal variance among groups. For proportions, random distribution was tested by χ2 analysis. Simple bivariate correlations were tested using Pearson’s method. Multiple linear regressions were used to test independent correlates of continuous variables using a stepwise procedure (P < .05 to enter and P > .1 to remove variables). Cox regression models were used to identify independent predictors of cardiac death among MEE, BMI, adipose and fat-free mass, and LV dysfunction, using a standard set of covariates (age, sex, diabetes, hypertension, low-density lipoprotein [LDL] and high-density lipoprotein cholesterol, fibrinogen, high sensitivity C-reactive protein, smoking, plasma creatinine). Backward stepwise selection of variables was used. Removal testing was based on the probability of the likelihood ratio based on the maximum partial likelihood estimates. Two-tailed P less than .05 was used to reject null hypotheses.
RESULTS
Of the study sample, 86% had normal EF (≥55%), about 11% had mildly reduced EF (45%–54%), and 4% had moderate to severely reduced EF (≤44%). Participants with moderately or severely reduced EF were slightly older than those with normal EF; proportions of men and prevalence of arterial hypertension, diabetes, or history of coronary heart disease were higher with lower EF (Table 1) (all P < .01 for trends). After adjusting for age, sex, risk factors, and coronary heart disease, BMI and adipose mass did not differ significantly across EF classes; fat-free mass was higher in participants with mildly reduced EF compared with those with normal EF, but did not differ significantly among other groups. Total calorie intake differed significantly among the EF groups, being lowest in participants with lowest EF. Lower EF tended to be associated with greater reduction in BMI (P = .09 for trend) and body fat as percent of body weight (P = .02 for trend) between the first (a mean of 3.5 years earlier) and the second SHS examinations. The proportion of participants who reported reduction of physical activity from the first SHS examination tended to be higher with lower EF (P < .05 for trend). Fibrinogen and high-sensitivity C-reactive protein were higher in the participants with moderately to severely reduced EF than in those with normal EF. Cardiac death was significantly more common with worse LV systolic function. Plasma creatinine level was higher with lower EF (1.80 ± 2.16 vs 1.17 ± 1.26 vs 0.95 ± 0.65 mg/dL, all P < .01). Total cholesterol and LDL cholesterol were nonsignificantly lower with lower EF; HDL cholesterol was comparable in the 3 groups (data not shown).
Table 1.
Demographics and comorbidity of the study population stratified by ejection fraction
| EF ≥ 55% | EF 54%–45% | EF < 45% | |
|---|---|---|---|
| N | 2606 | 317 | 109 |
| Age, y | 60 ± 8 | 60 ± 8 | 62 ± 8* |
| Men | 33% | 62%* | 59%* |
| Coronary heart disease, % | 4 | 12* | 30* |
| Type 2 diabetes, % | 47 | 52* | 59* |
| Arterial hypertension, % | 43 | 56* | 66* |
| BMI, kg/m2 | 31.0 ± 5.8 | 31.3 ± 5.9 | 30.2 ± 5.7 |
| Adipose mass, kg | 31.2 ± 11.1 | 31.7 ± 11.2 | 28.4 ± 10.5 |
| Fat-free mass, kg | 52.2 ± 7.6 | 53.9 ± 7.5 | 53.2 ± 12.6 |
| Intake, kcal/day | 1790 ± 892 | 1890 ± 959*† | 1535 ± 902* |
| Decrease in physical activity from first SHS examination | 21% | 19% | 30% |
| BMI change from first SHS examination, percent change | 0.9 ± 8.3 | 0.5 ± 7.3 | −0.3 ± 9.8 |
| Change in adipose mass, as percent of body weight, from first SHS examination, % | 0.05 ± 14.3 | 0.05 ± 14.4† | −3.7 ± 14.6* |
| Fibrinogen, mg/dL | 359 ± 78 | 364 ± 79 | 378 ± 80* |
| C-Reactive protein, mg/L | 6.4 ± 9.8 | 7.4 ± 10.4† | 10.1 ± 11.8* |
| Cardiac death, % | 3 | 6* | 19* |
BMI, Body mass index; EF, ejection fraction; SHS, Strong Heart Study.
P < .01 vs normal EF group.
P < .01 vs lowest EF group.
Data regarding BMI, BMI change from first SHS examination, adipose masse, fat-free mass, calorie intake, fibrinogen, and high-sensitivity C-reactive protein are adjusted for age, sex, hypertension, diabetes, and baseline cardiovascular disease.
LV Dysfunction and MEE
More severe LV systolic dysfunction was associated with progressively higher LV mass index and higher LV internal diameter, with lower relative wall thickness, higher end-systolic stress, lower Doppler stroke index, and lower end-systolic stress/end-systolic volume index, index of LV chamber contractility (Table 2). LV ejection time and the average cardiac cycle length were shorter with more severe LV systolic dysfunction. Because of effects of higher wall stress and heart rate, which more than offset the lower LV ejection time and stroke volume, MEE increased stepwise with more severe LV systolic dysfunction.
Table 2.
Echocardiographic findings in relation to left ventricular ejection fraction
| EF ≥ 55% | EF 54%–45% | EF 44%–35% | |
|---|---|---|---|
| LV mass index, g/m2.7 | 41 ± 10 | 46 ± 12*† | 57 ± 15* |
| LV internal diastolic diameter, cm/m2 | 1.85 ± 0.20 | 1.97 ± 0.21*† | 2.19 ± 0.21* |
| Relative wall thickness | 0.36 ± 0.05 | 0.33 ± 0.05*† | 0.32 ± 0.05* |
| ESS, kdyne/cm2 | 114 ± 31 | 151 ± 32*† | 201 ± 32* |
| Doppler stroke index, mL/m2 | 38 ± 7 | 36 ± 7*† | 34 ± 8* |
| LV ejection time, ms | 328 ± 37 | 321 ± 38*† | 309 ± 38* |
| RR, ms | 912 ± 134 | 889 ± 150*† | 825 ± 120* |
| ESS/ESVi, 104 kdyne/cm3 | 7.1 ± 1.5 | 5.6 ± 1.6*† | 4.4 ± 1.6* |
| MEE, kcal/min | 0.095 ± 0.040 | 0.122 ± 0.040*† | 0.150 ± 0.040* |
EF, Ejection fraction; ESS, end-systolic stress; ESVi, end-systolic volume index; LV, left ventricular; MEE, myocardial work energy expenditure; RR, cardiac cycle duration.
P < .01 vs normal EF group.
P < .01 vs lowest EF group.
Data are adjusted for age, sex, hypertension, diabetes, and baseline cardiovascular disease.
Clinical Correlates of MEE
MEE was higher in men than in women (0.110 ± 0.043 vs 0.094 ± 0.037 kcal/min) and was related to higher BMI (r = .20), fat-free body mass (r = 0.33), and adipose mass (r = 0.13) and weakly to older age (all P < .01). MEE was higher in the presence of hypertension (0.113 ± 0.044 vs 0.090 ± 0.033 kcal/min), diabetes (0.103 ± 0.041 vs 0.097 ± 0.039 kcal/min), and history of coronary heart disease (0.119 ± 0.052 vs 0.099 ± 0.039 kcal/min) (all P < .01), and was weakly but positively related to stress-corrected LV midwall shortening (r = 0.08, P < .01), an index of myocardial contractility.
Predictors of Cardiac Death
In a Cox multiple regression model adjusting for age, sex, risk factors, inflammation, and concomitant coronary heart disease, lower BMI was associated with higher risk of cardiac death independent of LV systolic dysfunction (used as categorical variable to avoid excessive colinearity with MEE) (Table 3, Model 1). In a subsequent multivariate model (Table 3, Model 2), the percent change in BMI between the first and second SHS examinations had a borderline significant relationship with cardiac mortality. When fat-free mass was added and adipose mass replaced BMI in a subsequent Cox regression model (Table 3, Model 3), lower adipose mass predicted higher cardiac mortality independent of covariates and degree of LV dysfunction. In a further model (Table 3, Model 4) in which MEE was added, cardiac death was independently predicted by higher fibrinogen, lower adipose mass, and higher MEE. When variables used to compute MEE (end-systolic stress, Doppler stroke volume, and LV ejection time) were included separately in another subsequent multivariate model, end-systolic stress and ejection time emerged as the components of MEE significantly related to cardiac mortality (adjusted hazard ratios = 1.010/kdyne/cm2 of end-systolic stress; 95% confidence interval [CI] 1.007–1.014) and =1.005/msec of ejection time (95% CI 1.0003–1.010) in addition to fibrinogen and adipose mass (both P < .01).
Table 3.
Predictors of cardiac death: Cox proportional hazard models
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| BMI, kg/min | 0.97 (0.93–1.001) | Not used | Not used | Not used |
| Percent change in BMI from first SHS examination | Not used | 0.98 (P = .1)(0.96–1.006) | Not used | Not used |
| Adipose mass, kg | Not used | Not used | 0.97* (0.95–0.98) | 0.96* (0.94–0.98) |
| Fat-free mass, kg | Not used | Not used | 1.02 (1.004–1.04) | NS |
| EF < 55 vs >55% | 2.1 (1.4–3.2) | 2.1 (1.4–3.2) | 1.9 (1.2–2.9) | NS |
| Fibrinogen, mg/dL | 1.004 (1.002–1.006) | 1.004 (1.002–1.006) | 1.004 (1.002–1.006) | 1.004 (1.002–1.006) |
| MEE, cal/min | Not used | Not used | Not used | 1.010* (1.007–1.014) |
BMI, Body mass index; EF, ejection fraction; MEE, myocardial work energy expenditure; NS, not significant; SHS, Strong Heart Study.
P < .01.
Data regarding BMI, BMI change from first SHS examination, adipose masse, fat-free mass, calorie intake, fibrinogen, and high-sensitivity C-reactive protein are adjusted for age, sex, hypertension, diabetes, and baseline cardiovascular disease.
Elevated MEE as Independent Predictor of Cardiac Death
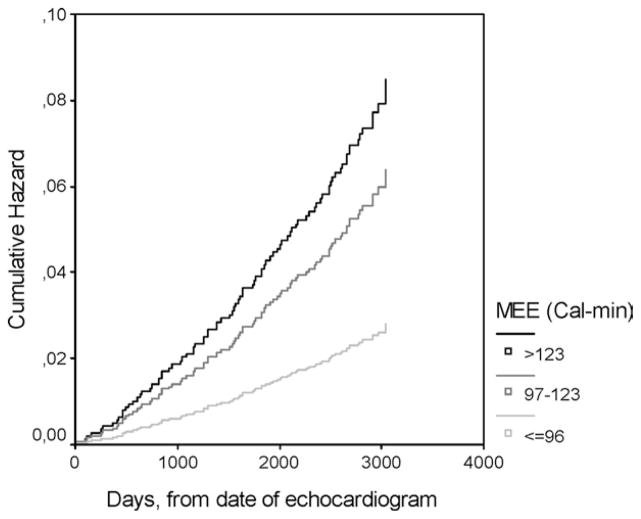
In a Cox multiple regression model adjusting for baseline age, sex, risk factors (hypertension, diabetes, LDL cholesterol > 130 mg/dL, HDL cholesterol < 50 mg/dL, smoking), inflammation (fibrinogen > 400 mg/dL), renal dysfunction (plasma creatinine > 1.2 mg/dL), overt coronary heart disease, weight loss between first and second SHS examinations greater than 10 kg, EF less than 55%, LV hypertrophy, and uncontrolled systolic BP (≥140 mm Hg), MEE between 97 and 123 cal/min (ie, between 1 and 2 SD above the mean in the reference group) was associated with 2.5-fold higher hazard of incident cardiac death (95% CI 1.4–4.4); MEE greater than 123 cal/min (ie, 2 SD above the mean in the reference group) was associated with a 3.3-fold higher hazard of cardiac death (95% CI 1.7–5.6) compared with participants with lower MEE (Figure 1); older age, diabetes, higher plasma creatinine levels (all P < .001), and LDL cholesterol above 130 mg/dL (P = .02) were additional independent predictors of fatal events, whereas other variables, including normal versus abnormal EF and weight loss did not enter the multivariate Cox model.
Figure 1.
Cardiac death was more frequent in participants with myocardial energy expenditure (MEE) 1 to 2 SD (heavy gray medium-thick line) or greater than or equal to 2 SD (black line) above mean MEE in reference population (69 ± 27 cal/min), compared with group with MEE less than 1 SD above normal mean (thin light-gray line) (hazard ratio [HR] 2.28, 95% confidence interval [CI] 1.30–4.02 and HR = 3.02, 95% CI 1.74–5.26, respectively). Data are adjusted for baseline age, sex, hypertension, diabetes, overt coronary heart disease, systolic blood pressure greater than or equal to 140 mm Hg, current or previous smoking, ejection fraction less than 55%, left ventricular hypertrophy, low-density lipoprotein cholesterol greater than 130 mg/dL, high-density lipoprotein cholesterol less than 50 mg/dL, fibrinogen greater than 400 mg/dL, and greater than 10-kg weight loss between first and second Strong Heart Study examinations.
DISCUSSION
Our study provides a number of original findings on prognostic implications of estimated MEE in relation to LV dysfunction, body composition, and inflammation in a population-based sample of middle-age and elderly adults with a broad range of LV EF, but without overt CHF. More severe LV systolic dysfunction was associated with higher MEE and lower adipose mass. However, MEE was a powerful predictor of cardiac death independent of established prognostic indicators including impaired EF, major CV risk factors, body composition, comorbidities, renal function, and fibrinogen. Stepwise higher rates of cardiac death occurred at MEE values above 97 and 123 cal/min.
The fact that MEE predicted cardiac mortality in individuals from a population source and independent of the severity of LV systolic dysfunction, body composition, weight loss, and fibrinogen suggests that CV metabolic need is an important determinant of cardiac outcome before the clinical syndrome of systolic CHF is fully manifested. Higher myocardial energy requirement in overloaded hearts is expected based on experimental data on myocardial bioenergetics.10 In our study, higher MEE with lower EF was primarily caused by increased LV wall stress, which offset potential reduction of external LV mechanical work by lower stroke volume, shorter ejection time, and lower LV chamber contractility associated with lower EF. In fact, end-systolic stress was the strongest determinant of MEE in our study and it emerged as a prognostic determinant when components of MEE were tested in separate Cox models. However, MEE integrates a number of physiologic factors contributing to myocardial energetic requirement in addition to wall stress, ie, stroke volume and LV ejection time. A direct association between MEE and myocardial contractility, estimated by the stress-corrected LV midwall shortening, was found, at least in part driven by the association of higher stress-corrected midwall shortening with higher stroke volume, a determinant of MEE.
In our study, lower EF was also associated with tendency toward lower adipose mass; our analyses revealed that the latter predicted cardiac death independent of EF and MEE whereas BMI did not. Therefore, estimation of body composition by bio-impedance appears to provide greater independent prognostic information than BMI. On the other hand, the association of lower EF with higher MEE is consistent with increased total energy requirements previously documented in patients with systolic CHF.37 Increased MEE may offset lower metabolic requirements predicted by reduced physical activity in those with LV systolic dysfunction. Thus, MEE may be a hitherto underappreciated component of metabolic needs in patients with LV systolic dysfunction contributing to change in body composition37; of note, in our study, participants with lowed EF had greatest weight and adipose mass losses between the first and the second SHS examinations.
Increased MEE with systolic LV dysfunction may be mediated by sympathetic activity,38 which is exaggerated with LV systolic dysfunction.39 Faster heart rate in our participants with EF less than 45% suggests sympathetic overactivity with moderately or severely depressed LV systolic function. However, our findings are made more generally applicable because they were obtained in a population-based sample of adults, from which severe LV dysfunction with acute CHF was excluded, thereby largely excluding cardiac cachexia.
Our method to estimate MEE, obtained from standard echocardiographic measures, yielded theoretic values of myocardial oxygen consumption in a normal reference subgroup of our study population consistent with those measured invasively by Vaz et al35 in a group of healthy individuals. In terms of physiologic correlations, MEE was higher with men, with hypertension, with higher body size, and higher myocardial contractility; although those correlations were overall weak, they reinforced the fact that our index of MEE behaves consistently with physiologic models. However, our method may not have the precision of invasive measurements and should be considered as a tool for clinical-epidemiologic context.
Conclusions
In a population-based sample of adults with high prevalence of obesity, diabetes, and traditional CV risk factors, but without overt CHF, depressed LV EF was associated with lower adipose mass, and with higher fibrinogen and higher MEE. Higher MEE and lower adipose mass predicted cardiac death independently, and more strongly than EF, suggesting that metabolic factors and inflammation may explain in substantial proportion the prognostic impact of depressed LV systolic chamber function in population.
Acknowledgments
Supported by cooperative agreement grants U01-HL41642, HL41652, HL41654, and HL65521 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland, and grant M10RR0047 (General Clinical Research Center) from the National Institutes of Health, Bethesda, Maryland.
The authors thank the SHS participants, staff, and coordinators. The views expressed in this article are those of the authors and do not necessarily reflect those of Indian Health Service.
References
- 1.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of US adults. N Engl J Med. 1999;341:1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 2.de Simone G, Wachtell K, Palmieri V, Hille DA, Beevers G, Dahlof B, et al. Body build and risk of cardiovascular events in hypertension and left ventricular hypertrophy: the LIFE (losartan intervention for endpoint reduction in hypertension) study. Circulation. 2005;111:1924–31. doi: 10.1161/01.CIR.0000161799.91577.0A. [DOI] [PubMed] [Google Scholar]
- 3.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91:891–4. doi: 10.1016/s0002-9149(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 4.Davos CH, Doehner W, Rauchhaus M, Cicoira M, Francis DP, Coats AJ, et al. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail. 2003;9:29–35. doi: 10.1054/jcaf.2003.4. [DOI] [PubMed] [Google Scholar]
- 5.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 6.Gustafsson F, Kragelund CB, Torp-Pedersen C, Seibaek M, Burchardt H, Akkan D, et al. Effect of obesity and being overweight on long-term mortality in congestive heart failure: influence of left ventricular systolic function. Eur Heart J. 2005;26:58–64. doi: 10.1093/eurheartj/ehi022. [DOI] [PubMed] [Google Scholar]
- 7.Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–34. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 8.Starling RD, Toth MJ, Carpenter WH, Matthews DE, Poehlman ET. Energy requirements and physical activity in free-living older women and men: a doubly labeled water study. J Appl Physiol. 1998;85:1063–9. doi: 10.1152/jappl.1998.85.3.1063. [DOI] [PubMed] [Google Scholar]
- 9.Devereux RB, Roman MJ, Palmieri V, Liu JE, Lee ET, Best LG, et al. Prognostic implications of ejection fraction from linear echocardiographic dimensions: the Strong Heart Study. Am Heart J. 2003;146:527–34. doi: 10.1016/S0002-8703(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 10.Hasenfuss G, Mulieri LA, Holubarsch C, Blanchard EM, Just H, Alpert NR. Myocardial adaptation to stress from the viewpoint of adaptation and development. Basic Res Cardiol. 1993;88:91–102. [PubMed] [Google Scholar]
- 11.Lee ET, Welty TK, Fabsitz RR, Cowan LD, Le NA, Oopik AJ, et al. The Strong Heart Study–study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–55. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 12.Welty TK, Lee ET, Yeh J, Cowan LD, Go O, Fabsitz RR, et al. Cardiovascular disease risk factors among American Indians: the Strong Heart Study. Am J Epidemiol. 1995;142:269–87. doi: 10.1093/oxfordjournals.aje.a117633. [DOI] [PubMed] [Google Scholar]
- 13.Howard BV, Lee ET, Cowan LD, Fabsitz RR, Howard WJ, Oopik AJ, et al. Coronary heart disease prevalence and its relation to risk factors in American Indians: the Strong Heart Study. Am J Epidemiol. 1995;142:254–68. doi: 10.1093/oxfordjournals.aje.a117632. [DOI] [PubMed] [Google Scholar]
- 14.Howard BV, Robbins DC, Sievers ML, Lee ET, Rhoades D, Devereux RB, et al. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2000;20:830–5. doi: 10.1161/01.atv.20.3.830. [DOI] [PubMed] [Google Scholar]
- 15.Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, et al. Rising tide of cardiovascular disease in American Indians: the Strong Heart Study. Circulation. 1999;99:2389–95. doi: 10.1161/01.cir.99.18.2389. [DOI] [PubMed] [Google Scholar]
- 16.Lee ET, Howard BV, Wang W, Welty TK, Galloway JM, Best LG, et al. Prediction of coronary heart disease in a population with high prevalence of diabetes and albuminuria: the Strong Heart Study. Circulation. 2006;113:2897–905. doi: 10.1161/CIRCULATIONAHA.105.593178. [DOI] [PubMed] [Google Scholar]
- 17.Devereux RB, Roman MJ, de Simone G, O’Grady MJ, Paranicas M, Yeh JL, et al. Relations of left ventricular mass to demographic and hemodynamic variables in American Indians: the Strong Heart Study. Circulation. 1997;96:1416–23. doi: 10.1161/01.cir.96.5.1416. [DOI] [PubMed] [Google Scholar]
- 18.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 19.Devereux RB, Roman MJ, Liu JE, Welty TK, Lee ET, Rodeheffer R, et al. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol. 2000;86:1090–6. doi: 10.1016/s0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 20.Bella JN, Devereux RB, Roman MJ, O’Grady MJ, Welty TK, Lee ET, et al. Relations of left ventricular mass to fat-free and adipose body mass: the Strong Heart Study investigators. Circulation. 1998;98:2538–44. doi: 10.1161/01.cir.98.23.2538. [DOI] [PubMed] [Google Scholar]
- 21.Zephier EM, Ballew C, Mokdad A, Mendlein J, Smith C, Yeh JL, et al. Intake of nutrients related to cardiovascular disease risk among three groups of American Indians: the Strong Heart dietary Study. Prev Med. 1997;26:508–15. doi: 10.1006/pmed.1997.0164. [DOI] [PubMed] [Google Scholar]
- 22.Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Wood EA, Howard BV, et al. Relations of Doppler stroke volume and its components to left ventricular stroke volume in normotensive and hypertensive American Indians: the Strong Heart Study. Am J Hypertens. 1997;10:619–28. doi: 10.1016/s0895-7061(97)00059-9. [DOI] [PubMed] [Google Scholar]
- 23.Palmieri V, Dahlöf B, DeQuattro V, Sharpe N, Bella JN, de Simone G, et al. Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE study. J Am Coll Cardiol. 1999;34:1625–32. doi: 10.1016/s0735-1097(99)00396-4. [DOI] [PubMed] [Google Scholar]
- 24.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 26.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–60. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 27.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic- angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 28.Ganau A, Devereux RB, Pickering TG, Roman MJ, Schnall PL, Santucci S, et al. Relation of left ventricular hemodynamic load and contractile performance to left ventricular mass in hypertension. Circulation. 1990;81:25–36. doi: 10.1161/01.cir.81.1.25. [DOI] [PubMed] [Google Scholar]
- 29.Starling EH, Evans LL. The respiratory exchanges of the heart in the diabetic animal. J Physiol. 1914;49:67. doi: 10.1113/jphysiol.1914.sp001690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starling EH, Visscher MB. The regulation of energy output of the heart. J Physiol. 1926;62:243. doi: 10.1113/jphysiol.1927.sp002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bing RJ, Hammond MM, Handelsman JC, Powers SR, Spencer FC, Eckenhoff JE, et al. The measurement of coronary blood flow, oxygen consumption, and efficiency of the left ventricle in man. Am Heart J. 1949;38:1–24. doi: 10.1016/0002-8703(49)90788-7. [DOI] [PubMed] [Google Scholar]
- 32.Sarnoff SJ, Braunwald E, Welch GH, Jr, Case RB, Stainsby WN, Macruz R. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol. 1958;192:156. doi: 10.1152/ajplegacy.1957.192.1.148. [DOI] [PubMed] [Google Scholar]
- 33.Palmieri V, Bella JN, Arnett DK, Oberman A, Kitzman DW, Hopkins PN, et al. Associations of aortic and mitral regurgitation with body composition and myocardial energy expenditure in adults with hypertension: the hypertension genetic epidemiology network study. Am Heart J. 2003;145:1071–7. doi: 10.1016/S0002-8703(03)00099-1. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu G, Hirota Y, Kita Y, Kawamura K, Saito T, Gaasch WH. Left ventricular midwall mechanics in systemic arterial hypertension: myocardial function is depressed in pressure-overload hypertrophy. Circulation. 1991;83:1676–84. doi: 10.1161/01.cir.83.5.1676. [DOI] [PubMed] [Google Scholar]
- 35.Vaz M, Rajkumar C, Wong J, Mazzeo RS, Turner AG, Cox HS, et al. Oxygen consumption in the heart, hepatomesenteric bed, and brain in young and elderly human subjects, and accompanying sympathetic nervous activity. Metabolism. 1996;45:1487–92. doi: 10.1016/s0026-0495(96)90177-8. [DOI] [PubMed] [Google Scholar]
- 36.Palmieri V, Tracy RP, Roman MJ, Liu JE, Best LG, Bella JN, et al. Relation of left ventricular hypertrophy to inflammation and albuminuria in adults with type 2 diabetes: the Strong Heart Study. Diabetes Care. 2003;26:2764–9. doi: 10.2337/diacare.26.10.2764. [DOI] [PubMed] [Google Scholar]
- 37.Aquilani R, Opasich C, Verri M, Boschi F, Febo O, Pasini E, et al. Is nutritional intake adequate in chronic heart failure patients? J Am Coll Cardiol. 2003;42:1218–23. doi: 10.1016/s0735-1097(03)00946-x. [DOI] [PubMed] [Google Scholar]
- 38.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–9. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 39.Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–9. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]