Abstract
Bucillamine is a disease-modifying antirheumatic drug that is structurally similar to D-penicillamine. The major renal side effect of bucillamine and D-penicillamine is proteinuria caused by membranous nephropathy (MN). In addition to MN, combined crescent formation has been occasionally reported in D-penicillamine-induced MN, while crescent formation has been rarely reported in bucillamine-treated cases. Here, we describe a 76-year-old female who presented with nephrotic syndrome and rapidly progressive glomerulonephritis. She was receiving bucillamine as initial treatment for recently diagnosed rheumatoid arthritis, and renal biopsy showed MN with crescent formation. To the best of our knowledge, this is the first report of bucillamine-induced MN with crescent formation in the English literature.
Key words: Bucillamine, Rheumatoid arthritis, Membranous nephropathy, Crescent formation, Nephrotic syndrome, Rapidly progressive glomerulonephritis
Introduction
Bucillamine is a disease-modifying antirheumatic drug, which was developed in Japan in 1975 and is widely used in Asian countries. The chemical structure of bucillamine is similar to that of D-penicillamine, and the pharmacological effects of both drugs are believed to be linked to the sulfhydryl (SH) bonds [1]. Therefore, the 2 drugs have similar mechanisms of action and side effects, such as skin rash, pruritus, gastrointestinal disturbances, and proteinuria. Proteinuria gradually increases in these cases, and renal biopsy commonly shows membranous nephropathy (MN) [2, 3, 4, 5, 6]. Discontinuation of the drug achieves resolution of proteinuria without residual renal damage in these MN [2, 3, 4, 6].
On the other hand, D-penicillamine can also cause rapidly progressive glomerulonephritis (RPGN) and crescent formation associated with drug-induced antineutrophil cytoplasmic antibody (ANCA). These cases show a rapid clinical course and require both discontinuation of the drug and aggressive treatment [7]. Despite the structural similarity between bucillamine and D-penicillamine, RPGN or crescent formation has seldom been reported in bucillamine-treated cases, and this deviation has remained unexplainable. Here, we report a rare case of bucillamine-induced MN with crescent formation, which presented with nephrotic syndrome (NS) and RPGN.
Case Presentation
A 76-year-old female, with a history of hypertension that resolved with mild sodium restriction, began experiencing stiffness and spontaneous pain in her distal interphalangeal joints in December 2011. In January 2012, rheumatoid arthritis was diagnosed and bucillamine (300 mg/day) was administrated as a monotherapy. At the 1-month follow-up, the arthritis had resolved, renal function was normal, her serum creatinine was 0.52 mg/dl [estimated glomerular filtration rate (eGFR) 84.6 ml/min/1.73 m2] [8], and urinalysis was negative for hematuria and proteinuria.
In mid-March 2012, the patient presented with complaints of edema and abnormal weight gain. She was afebrile with no recent infectious episode. Her blood pressure was 130/77 mm Hg, and she had periorbital and lower-extremity edema. Her laboratory findings were as follows: total protein 4.8 g/dl, albumin 1.9 g/dl, creatinine 0.63 mg/dl (eGFR 68.6 ml/min/1.73 m2), C-reactive protein 0.04 mg/dl, antistreptolysin O antibody 28 IU/ml, normal total hemolytic complement (CH50) and its fraction (C3, C4, CH50), and negative circulating immune complex. Urinalysis revealed hematuria (30–49/high-power field, with many red blood cell casts) and proteinuria (4.67 g/day in pooled urine). Antinuclear antibody titer 40×, rheumatoid factor 29 IU/ml, anticyclic citrullinated peptide antibody >300 U/ml, matrix metalloproteinase-3 85.5 ng/ml, and tests for other specific antibodies were negative: enzyme-linked immunosorbent assay myeloperoxidase (MPO)-ANCA <10 EU/ml, proteinase 3-ANCA <10 EU/ml, and antiglomerular basement membrane antibody <10 EU/ml. ANCA by indirect immunofluorescence (IF) was also negative. Hepatitis B surface antigen and hepatitis C antibody were negative. Chest computed tomography excluded alveolar hemorrhage, interstitial pneumonia or pleurisy.
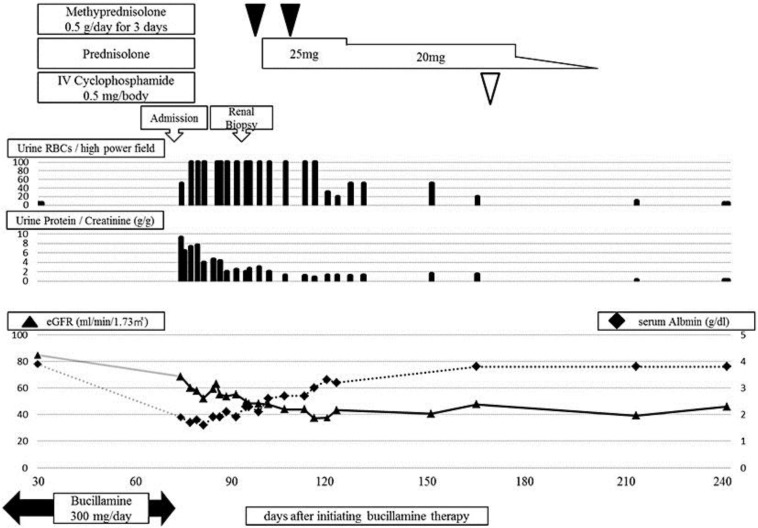
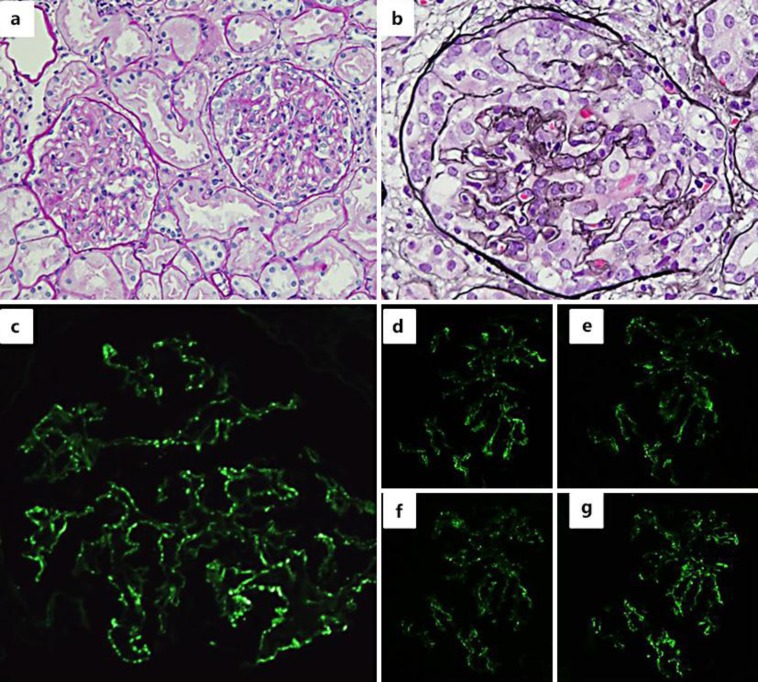
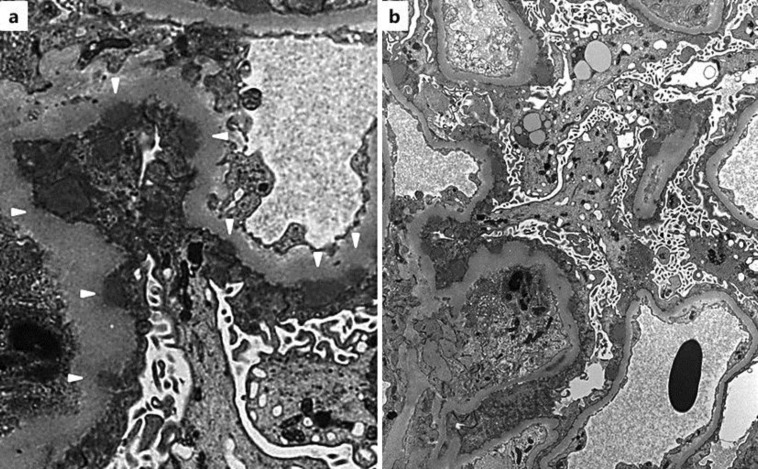
After the patient's admission, bucillamine was stopped and her clinical course was monitored (fig. 1). Her proteinuria improved to 1.35 g/day, and her serum albumin level gradually recovered within 2 weeks. However, the renal dysfunction progressed, and hematuria worsened despite partial resolution of NS. Therefore, an ultrasound-guided percutaneous renal biopsy was performed. The renal biopsy specimen contained 23 glomeruli, 5 of which demonstrated global sclerosis. On light microscopy, slight endocapillary proliferation with inflammatory cell infiltration was observed (fig. 2a), and crescent formation was seen in 7 glomeruli (fig. 2a, b). Neither arteritis nor peritubular capillaritis was seen. IF study revealed fine granular deposits of immunoglobulin G (IgG) (3+; fig. 2c), C3c (3+), and C1q (1+) along the capillary walls. Staining for IgG subclass showed positive staining for IgG1 (3+), IgG2 (1–2+), IgG3 (1–2+), and IgG4 (3+) (fig. 2d–g). Electron microscopy (EM) showed segmental subepithelial deposits along the glomerular basement membrane (fig. 3a) with extensive foot process effacement, microvillus transformation, and vacuolar degeneration of podocytes (fig. 3b). On the basis of the above findings, we determined the diagnosis as MN stage 1 [9] with crescent formation.
Fig. 1.
Clinical course after the initiation of bucillamine therapy.
Fig. 2.
Light microscopy findings of the renal biopsy. a Mild intracapillary proliferation and small cellular crescents. Periodic acid Schiff staining. ×40. b Global cellular crescent. Periodic acid methenamine silver staining. ×100. IF findings: fine granular staining of IgG (c), IgG1 (d), IgG2 (e), IgG3 (f), and IgG4 (g) on the glomerular capillary loop.
Fig. 3.
EM findings. a Subepithelial electron-dense deposits (white arrow heads). Original magnification ×6,000. b Extensive foot process effacement, microvillus transformation, and vacuolar degeneration of podocytes. Original magnification ×2,000.
The severe hematuria persisted, and eGFR rapidly declined to approximately 50.0 ml/ min/1.73 m2. Therefore, we made the diagnosis of RPGN on the basis of the clinical course and the renal histopathological findings. The patient underwent a 3-day course of methylprednisolone 0.5 g/day twice, which was followed by oral prednisolone 25 mg/day (fig. 1). The rapid decline of eGFR stopped and the hematuria resolved after the initiation of active treatment. Since the patient developed psychiatric symptoms after prednisolone treatment, her medications including prednisolone were stopped, and she received intravenous cyclophosphamide (0.5 g) in June 2012. In the subsequent 2 years, she showed no recurrence of hematuria or proteinuria; however, the declined eGFR has persisted with no recovery.
Discussion
To the best of our knowledge, this is the first report describing bucillamine-induced MN with crescent formation that presented with NS and RPGN. Acute renal injury, which was caused by NS, such as prerenal azotemia or acute tubular injury, might have coexisted in our patient [10]. However, even after the partial resolution of proteinuria and recovery of the serum albumin level, heavy hematuria persisted, and her renal dysfunction rapidly progressed (fig. 1). On the basis of these findings, we concluded that the patient's clinical course was different from acute renal injury caused by NS or from usual bucillamine-induced MN [2, 3, 4]. Therefore, we finally performed a renal biopsy to make the accurate diagnosis, and the renal biopsy revealed MN with crescent formation, which was consistent with NS and RPGN.
In the present case, the patient showed a favorable clinical course without relapse of either NS or RPGN after the discontinuation of bucillamine therapy. Since MN is a well-documented side effect of bucillamine [2, 3, 4], it seemed suitable to diagnose that this NS was caused by bucillamine-induced MN. The mechanism of subepithelial immune complex deposition is believed to be similar to MN induced by D-penicillamine, and the mechanism is explained as follows: D-penicillamine has the capacity to convert large circulating immune complexes to small ones. Since small immune complexes tend to circulate longer, delayed removal of immune complexes results in glomerular depositions [11]. In contrast, the etiology of RPGN and crescent formation in the present case remained unclear. A combination of MN and crescent formation has been rarely reported, and the reported cases are limited to lupus nephritis or dual glomerulopathy with superimposed antiglomerular basement membrane disease, ANCA-associated vasculitis, and pauci-immune glomerulonephritis of unknown etiology [12]. However, the concurrence of these primary diseases was deniable from laboratory findings, histopathological findings, and the clinical course without relapse in the present case. Therefore, on the basis of exclusion, we speculated that bucillamine was the cause of MN as well as the crescent formation.
However, only 2 cases of crescent formation caused by bucillamine have been reported (table 1) [13, 14]. In case 1, 18 months after the initiation of bucillamine therapy, although ANCA was negative, the patient showed severe hematuria, proteinuria, and a rapid decline in renal function. The biopsy showed crescentic glomerulonephritis with arteritis. IF study was negative, and EM study revealed no electron-dense deposit [13]. In case 2, proteinuria without hematuria appeared 7 months after the initiation of bucillamine therapy. The proteinuria gradually increased to a nephrotic range, and ANCA was not examined. The biopsy showed crescentic glomerulonephritis. IF study was negative, and EM study revealed slight mesangial deposits [14]. Taken together, the clinical courses and histopathological findings of the 2 reported cases and the present case are different from the each other. Therefore, it was difficult to surmise the etiology of bucillamine-induced crescent formation from these cases.
Table 1.
Reported cases of bucillamine-induced crescent formation
| Year | Initial diagnosis | Clinical manifestation | IF study | EM study | ANCA | Outcome | Treatment | Reference |
| 1993 | RA | RPGN | negative | no EDD | negative | improved | prednisolone, cyclophosphamide | 13 |
| 1993 | RA | NS without hematuria | negative | mesangial EDD | NR | improved | prednisolone | 14 |
| 2012 | RA | RPGN, NS | IgG, C3, C1q | subepithelial EDD | negative | improved | prednisolone, cyclophosphamide | present case |
RA = Rheumatoid arthritis; EDD = electron-dense deposit; NR = not reported.
By contrast, there have been a few cases of MN with crescent formation reported in MN induced by D-penicillamine (table 2) [15, 16, 17, 18]. In those cases, MPO-ANCA or perinuclear ANCA were positive in 3 out of the 4 cases that were tested for ANCA. Furthermore, 1 case had a pulmonary lesion consistent with systemic vasculitis. Therefore, the crescent formation seemed to be caused by concurrent drug-induced ANCA-associated vasculitis. The pathogenesis responsible for D-penicillamine to induce ANCA is still far from fully understood. However, induction is considered to be triggered by the interaction between MPO and SH bonds, and this mechanism is believed to be common in other SH bond-containing drugs known to induce vasculitis, such as propylthiouracil [7, 19].
Table 2.
Reported cases of MN induced by D-penicillamine associated with crescent formation evaluated for ANCA
| Year | Initial diagnosis | Clinical manifestation | IF study (capillary wall) | MN stage | ANCA | Outcome | Treatment | Reference |
| 1993 | RA | RPGN | Granular IgG, IgA, C3 | NR | negative | improved | prednisolone, cyclophosphamide, plasma exchange | 15 |
| 1995 | RA | PRS | Granular IgG, C3 | NR | P-ANCA+, MPO-ANCA+ | ESRD | prednisolone, cyclophosphamide, plasma exchange | 16 |
| 1996 | RA | RPGN | Granular IgG, C3 | II | P-ANCA + | improved | prednisolone, cyclophosphamide, azathioprine | 17 |
| 1997 | SSc | RPGN | Granular IgG, C3 | II–III | P-ANCA+, MPO-ANCA+ | improved | prednisolone, cyclophosphamide, hemodialysis | 18 |
RA = rheumatoid arthritis; NR = not reported; PRS = pulmonary renal syndrome; P-ANCA = perinuclear ANCA; ESRD = end-stage renal disease; SSc = systemic sclerosis.
In the present case, on the basis of previous knowledge, we speculate 3 possible hypotheses to explain crescent formation. The first one is bucillamine-induced vasculitis. As mentioned above, based on its chemical structure containing SH bonds, bucillamine may have the capacity to induce ANCA as D-penicillamine. However, there have been no previous reports of drug-induced ANCA in bucillamine-treated cases. Moreover, MPO-ANCA, which is the majority of ANCA induced by D-penicillamine [7], was negative in the present case. Therefore, an association of drug-induced ANCA seems to be less likely in the present case. Though, in propylthiouracil-induced ANCA, both MPO-ANCA and atypical ANCA have been reported to be positive [19], and atypical ANCA might be associated with crescent formation in the present case. The second possibility is crescent formation caused by subepithelial deposits. The IF study of IgG subclasses revealed a deposition of IgG1, IgG2, and IgG3 subclasses, in addition to the deposition of IgG4 subclass. The deposition of IgG4 subclass, which is the predominant deposition in idiopathic MN [20], reflects Th2-type immune reaction. By contrast, the deposition of other IgG subclasses reflects Th1-type immune reaction, which may relate to glomerular inflammation and crescent formation [21]. Moreover, the deposition of C1q suggested that the classical complement pathway was activated in the present case [22]. Therefore, the immunological background differs from primary MN in the present case, and the difference may relate to the infiltration of inflammatory cells and crescent formation. However, since bucillamine suppresses the Th1-type immune response [23], it is difficult to explain this discrepancy. The third possibility is crescent formation caused by severe podocyte damage, which is known as a pseudocrescent [24]. Only a limited number of drugs are known to cause direct podocyte damage, such as doxorubicin, puromycin, and bisphosphonates. Bucillamine has been reported to cause podocyte degeneration unaccompanied by immune complex deposition in a murine model [25]. This is a unique action of bucillamine that is not shared by D-penicillamine. However, the light microscopy findings of inflammatory cell infiltration in the crescents and prominent periglomerular inflammation are somewhat inconsistent histological features with pseudocrescent formation [24]. Altogether, the etiology of bucillamine-induced crescent formation in the present case is uncertain. However, one or more of the above hypotheses might serve a function and form the present complex histopathology.
We emphasize that the diagnosis of bucillamine-induced MN with crescent formation was made on the basis of exclusion. Unlike the typical bucillamine-induced MN, the patient revealed a rapid clinical course and permanent renal damage. As a conclusion, it is important to be aware that bucillamine-induced nephropathy may not always take a benign clinical course.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Wood PL, Khan MA, Moskal JR, et al. Mechanism of action of the disease-modifying anti-arthritic thiol agents D-penicillamine and sodium aurothiomalate: restoration of cellular free thiols and sequestration of reactive aldehydes. Eur J Pharmacol. 2008;580:48–54. doi: 10.1016/j.ejphar.2007.10.066. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida A, Morozumi K, Suganuma T, et al. Clinicopathological findings of bucillamine-induced nephrotic syndrome in patients with rheumatoid arthritis. Am J Nephrol. 1991;11:284–288. doi: 10.1159/000168323. [DOI] [PubMed] [Google Scholar]
- 3.Obayashi M, Uzu T, Harada T, et al. Clinical course of bucillamine-induced nephropathy in patients with rheumatoid arthritis. Clin Exp Nephrol. 2003;7:275–278. doi: 10.1007/s10157-003-0252-0. [DOI] [PubMed] [Google Scholar]
- 4.Hoshino J, Ubara Y, Hara S, et al. Outcome and treatment of bucillamine-induced nephropathy. Nephron Clin Pract. 2006;104:c15–c19. doi: 10.1159/000093254. [DOI] [PubMed] [Google Scholar]
- 5.Habib GS, Saliba W, Nashashibi M, et al. Penicillamine and nephrotic syndrome. Eur J Intern Med. 2006;17:343–348. doi: 10.1016/j.ejim.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Hall CL, Jawad S, Harrison PR, et al. Natural course of penicillamine nephropathy: a long term study of 33 patients. Br Med J (Clin Res Ed) 1988;296:1083–1086. doi: 10.1136/bmj.296.6629.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bienaimé F, Clerbaux G, Plaisier E, et al. D-Penicillamine-induced ANCA-associated crescentic glomerulonephritis in Wilson disease. Am J Kidney Dis. 2007;50:821–825. doi: 10.1053/j.ajkd.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 9.Chrug J, Ehrenreich T. Membranous nephropathy. Perspect Nephrol Hypertens. 1973. pp. 1443–1448. [PubMed]
- 10.James SH, Lien YH, Ruffenach SJ, et al. Acute renal failure in membranous glomerulonephropathy: a result of superimposed crescentic glomerulonephritis. J Am Soc Nephrol. 1995;6:1541–1546. doi: 10.1681/ASN.V661541. [DOI] [PubMed] [Google Scholar]
- 11.Ueda S, Porter GA. Gold salts, D-penicillamine and allopurinol. In: de Broe M, Porter GA, editors. Clinical Nephrotoxins. ed 3. Berlin: Springer; 2008. pp. 459–480. [Google Scholar]
- 12.Rodriguez EF, Nasr SH, Larsen CP, et al. Membranous nephropathy with crescents: a series of 19 cases. Am J Kidney Dis. 2014;64:66–73. doi: 10.1053/j.ajkd.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida A, Morozumi K, Takeda A, et al. A case of rapidly progressive glomerulonephritis associated with bucillamine-treated rheumatoid arthritis. Am J Kidney Dis. 1992;20:411–413. doi: 10.1016/s0272-6386(12)70309-8. [DOI] [PubMed] [Google Scholar]
- 14.Isozaki T, Kimura M, Ikegaya N, et al. Bucillamine (a new therapeutic agent for rheumatoid arthritis) induced nephrotic syndrome: a report of two cases and review of the literature. Clin Investig. 1992;70:1036–1042. doi: 10.1007/BF00180315. [DOI] [PubMed] [Google Scholar]
- 15.Almirall J, Alcorta I, Botey A, et al. Penicillamine-induced rapidly progressive glomerulonephritis in a patient with rheumatoid arthritis. Am J Nephrol. 1993;13:286–288. doi: 10.1159/000168636. [DOI] [PubMed] [Google Scholar]
- 16.Gaskin G, Thompson EM, and Pusey CD. Goodpasture-like syndrome associated with anti-myeloperoxidase antibodies following penicillamine treatment. Nephrol Dial Transplant. 1995;10:1925–1928. [PubMed] [Google Scholar]
- 17.Mathieson PW, Peat DS, Short A, et al. Coexistent membranous nephropathy and ANCA-positive crescentic glomerulonephritis in association with penicillamine. Nephrol Dial Transplant. 1996;11:863–866. doi: 10.1093/oxfordjournals.ndt.a027416. [DOI] [PubMed] [Google Scholar]
- 18.Karpinski J, Jothy S, Radoux V, et al. D-penicillamine-induced crescentic glomerulonephritis and antimyeloperoxidase antibodies in a patient with scleroderma. Case report and review of the literature. Am J Nephrol. 1997;17:528–532. doi: 10.1159/000169183. [DOI] [PubMed] [Google Scholar]
- 19.Yu F, Chen M, Gao Y, et al. Clinical and pathological features of renal involvement in propylthiouracil-associated ANCA-positive vasculitis. Am J Kidney Dis. 2007;49:607–614. doi: 10.1053/j.ajkd.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Nagahama K, Matsushita H, Hara M, et al. Bucillamine induces membranous glomerulonephritis. Am J Kidney Dis. 2002;39:706–712. doi: 10.1053/ajkd.2002.31987. [DOI] [PubMed] [Google Scholar]
- 21.Tipping PG, Kitching AR. Glomerulonephritis, Th1 and Th2: what's new? Clin Exp Immunol. 2005;142:207–215. doi: 10.1111/j.1365-2249.2005.02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma H, Sandor DG, Beck LH. The role of complement in membranous nephropathy. Semin Nephrol. 2013;33:531–542. doi: 10.1016/j.semnephrol.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morinobu A, Wang Z, Kumagai S. Bucillamine suppresses human Th1 cell development by a hydrogen peroxide-independent mechanism. J Rheumatol. 2000;27:851–858. [PubMed] [Google Scholar]
- 24.Albaqumi M, Barisoni L. Current views on collapsing glomerulopathy. J Am Soc Nephrol. 2008;19:1276–1281. doi: 10.1681/ASN.2007080926. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara Y, Tsuchiya H, Sakai N, et al. Proximal tubules and podocytes are toxicity targets of bucillamine in a mouse model of drug-induced kidney injury. Eur J Pharmacol. 2011;670:208–215. doi: 10.1016/j.ejphar.2011.08.051. [DOI] [PubMed] [Google Scholar]