Abstract
Importance
Sentinel lymph node biopsy (SLNB) provides prognostic information for melanoma; however, a survival benefit has not been demonstrated.
Objective
To assess the association of SLNB with survival for head and neck melanoma (HNM).
Design
Propensity score-matched retrospective cohort study using the Surveillance Epidemiology and End Results (SEER) database to compare patients with HNM initially treated with SLNB versus nodal observation.
Setting
United States population
Patients
Melanoma arising in head and neck subsites meeting current recommendations for SLNB, treated during the years 2004-2011 with either a) SLNB +/− neck dissection, or b) no SLNB or neck dissection. Intervention: SLNB +/− neck dissection
Main Outcome
Disease-specific survival (DSS) estimates based on the Kaplan-Meier method, and Cox proportional-hazards modeling to compare survival outcomes between matched-pair cohorts
Results
7266 HNM patients meeting study criteria were identified from the SEER database. Matching of treatment cohorts was performed utilizing propensity scores modeled on 10 covariates known to be associated with SLNB treatment or melanoma survival. Cohorts were stratified by tumor thickness (thin: >0.75-1mm Breslow depth, intermediate: >1-4mm, and thick: >4mm) and exactly-matched within five age categories. In the intermediate-thickness cohort, 2808 HNM patients were matched and balanced by propensity score for SLNB treatment; the 5-year DSS estimate for those treated by SLNB was 89% vs. 88% for nodal observation (log-rank p=0.30). The hazard ratio for melanoma-specific death was 0.87 for those undergoing SLNB (95% CI 0.66-1.14, p=0.31). In each of the other cohorts analyzed, including the thin, thick, and overall cohorts, no significant difference in DSS was demonstrated.
Conclusions
This SEER cohort analysis demonstrates no significant association between SLNB and improved disease survival for patients with HNM.
Introduction
There were an estimated 68,000 new melanoma cases in the US in 2010, and the incidence appears to be increasing.1 At diagnosis, 82% of patients have localized disease, while 11% already have spread to regional sites.2 Any regional lymph node metastasis in melanoma decreases survival.3 Sentinel lymph node biopsy (SLNB) for melanoma was introduced in the early 1990's to identify the presence of occult regional metastatic disease.4 The procedure has been shown to provide important prognostic information,3,5 and obviates the need for elective regional node dissection in the majority of patients.6 Patients with a positive SLNB may be offered more aggressive treatment, including regional node dissection, other adjuvant therapy, or enrollment in clinical trials. Though SLNB identifies patients with microscopic nodal disease, improves regional disease control,7 and may facilitate the escalation of treatment, it is still controversial whether there is any therapeutic benefit of the procedure, especially in terms of survival.8-10 In fact, the only randomized-controlled trial (RCT) to evaluate the question of a survival benefit demonstrated no difference in disease-specific survival (DSS) for those treated with SLNB for intermediate-thickness melanoma.11,12 A criticism of the trial has been that it was under-powered to detect a small but clinically significant survival effect.13
Another adequately-powered RCT to assess survival of SLNB for melanoma is likely impossible, due to the size of study necessary to detect a small treatment difference, and that the SLNB intervention is now widely practiced as the standard-of-care for the diagnostic information it provides, so enrolling patients into a randomized study to assess therapeutic effect is unlikely to succeed. The Surveillance Epidemiology and End Results (SEER) database provides prospectively collected and updated patient data from 18 registries, representing nearly 26% of the population of the United States. Though an RCT is ideal for comparing the effects of treatment since it controls for confounding factors through the randomization of treatment assignment, an observational study can approximate an RCT if bias in treatment assignment is controlled, using methods such as a propensity score matched-pairs design.
18% of new melanoma cases occur in the head and neck.14 There are multiple lines of evidence suggesting head and neck melanoma (HNM) behaves differently from melanoma in other skin sites. For example, HNM has worse survival than trunk or extremity melanoma.14-16 The decision to perform SLNB for HNM poses unique considerations. A lower rate of SLNB positivity in HNM has been reported (10% versus 17-19% for trunk/extremity in one study).14-16 In addition, HNM patients with a negative SLNB have worse survival and less of an absolute survival difference from positive SLNB patients than in other body sites.16 A higher false negative rate for SLNB in HNM also has been reported in several studies.16-18 Therefore, the prognostic information from performing SLNB may not be as valuable for HNM as in other body sites, and the relative benefit to patients of undergoing the surgical procedure should be carefully evaluated.
Given the small sample size of the previously conducted RCT, and the lack of other published studies on this important clinical question, particularly for melanoma in the head and neck, we sought to use a large, national population-based data set (SEER) to compare the survival of patients treated with SLNB for HNM to those who did not undergo any initial treatment of the regional lymphatics, controlling for bias in treatment selection. The hypothesis was that SLNB as part of initial treatment for melanoma arising in the head and neck has no association with melanoma-specific survival relative to observation.
Methods
Data from the SEER Program of the National Cancer Institute Public Use Dataset were extracted for patients with a primary diagnosis of invasive melanoma (ICD-O-3 code 8720-8723, 8726, 8730, 8740-8745, 8760, 8761, 8770-8774, and 8780). The SEER data is obtained from population-based prospective tumor registries in the following areas from 2004-2011: the metropolitan areas of San Francisco/Oakland, Detroit, Atlanta, and Seattle; Los Angeles county; the San Jose-Monterey area; rural Georgia; the Alaska Native Registry; Greater California; Greater Georgia; and the states of Connecticut, Kentucky, Iowa, Louisiana, New Jersey, New Mexico, Utah, and Hawaii. The data was accessed from SEER on May 9, 2014.
The intention of the selection criteria for this study was to include patients who would have been offered a SLNB in the treatment of their melanoma based on current national consensus guidelines in the United States. The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Melanoma19 currently recommends SLNB for patients with stage IB-II melanoma (i.e. 0.76-1 mm thick with ulceration or ≥1 mitosis/mm2, or >1mm thick with or without ulceration). The NCCN guidelines suggest that SLNB be considered for patients with stage IA melanoma with tumor thickness >0.75 mm; for other stage IA melanomas, it is controversial whether SLNB should be offered, and for which adverse factors (such as positive deep margins, lymphovascular invasion, mitoses, or Clark level IV or V). Patients were selected for this study if they qualified for SLNB based on these recommendations, limiting selection of stage IA melanomas to those >0.75mm depth.
Patients were also required to have had at least an excision of the primary melanoma. Those with distant metastatic disease, regional disease, in-transit metastasis, or satellitosis at presentation, as well as patients who underwent a neck dissection without SLNB, or underwent needle biopsy of lymph nodes without SLNB, were excluded. Patients under 18 years old were excluded. Patients >85 years old were also excluded, as they are less likely to undergo SNLB and their survival is inferior; it was not possible to control this bias by matching.
Two treatment groups were considered: a control group, which had no SLNB or other lymph node dissection performed, and the intervention group, which underwent a SLNB, and possibly additional lymph node dissection. The SEER database recorded whether a SLNB was performed for melanoma beginning in 1998, in the variables “Scope of regional lymph nd surg (1998-2002)” and “Rx Summ—Scope Reg LN Sur (2003+)”. However, it has been recognized that the receipt of SLNB vs. neck dissection may have been inconsistently coded in the years 1998-2002, with patients undergoing both procedures coded only as having undergone neck dissection by coding rules.13 In addition, SEER introduced the “CS Site-Specific Factor 3” variable for melanoma in 2004, which identifies lymph node metastases as clinically occult versus clinically evident, allowing for consistently accurate inclusion for this study. Consequently, the years of inclusion for the study are 2004-2011.
To control for confounding factors and minimize bias in the initial treatment selection from influencing the observed outcome, the two cohorts were matched using propensity scores and the prognostic factors available at the time of diagnosis. Propensity score computation should include those variables that affect the outcome or include those variables that affect both treatment selection and the outcome.20 Variable selection for the propensity model was limited to the information available within the SEER database during all of the years of the study, and included variables that have been shown to be related to treatment with a SLNB or to survival, which include age,21-25 race,21 gender,26-28 marital status,21 scalp location, 14,22,29,30 tumor registry,21 histology,2 thickness,3,31 and ulceration.31 The variables incorporate patient-specific, tumor-specific, and regional characteristics, which form a theoretical basis for bias in treatment selection. In addition, we used a SEER variable indicating whether the case was the patient's first malignancy or not, as we hypothesized that this may have some influence on the choice of treatment.
Dichotomous variables were created for marital status, race (white or other), gender, tumor location (face vs. scalp/neck), any prior malignancy (yes or no), and ulceration. There were 18 tumor registries which contributed patients to this study; these were dichotomized according to whether they were above or below the median proportion of melanoma cases in the registry undergoing SLNB. Histologic type was grouped in categories for the major melanoma histologic types (superficial spreading, nodular, desmoplastic, lentigo maligna, not-otherwise-specified, and other). Age and Breslow depth were considered as continuous variables. They were separately also stratified into categories, with stratification of Breslow depth based on the American Joint Committee on Cancer TNM classification system, and age as 18-44, 45-54, 55-64, 65-74, 75-84.
The initial data analysis evaluated treatment selection, focusing on whether SLNB was performed. To control for confounding factors and minimize bias in the initial treatment selection from influencing the observed outcome, the two cohorts were matched using propensity scores calculated by logistic regression using the covariates discussed above. A greedy nearest-neighbor matching algorithm with calipers set at 0.1 standard deviations of the logit of the propensity score was used to create matched pairs. In addition, cases were required to be matched exactly within age categories, defined as: 18-44, 45-54, 55-64, 65-74, and 75-84. This requirement created perfect balance between the control and treatment groups within each age category. The balance of the matched model was assessed, and further refinements of the matching model were made iteratively. The standardized difference of the means for each covariate, as well as for interaction terms of the covariates, were computed. Some authors suggest a difference of >25% be used to represent meaningful imbalance amongst the treatment groups; others suggest using >10% as a balancing measure.32,33 Dot-plots of the propensity score and standardized differences of covariates were generated for comparisons. The balance was also checked through statistical tests, including Hansen-Bower's omnibus imbalance test statistic,34 or a comparison of the L1 balance measure pre- and post-matching, which is calculated by an automatic binning of all covariates and comparing frequencies in a multivariate contingency table of the treatment versus non-treatment groups.35 The propensity score modeling and matching was repeated for subsequent cohorts based on Breslow depth, defined as Thin (>0.75-1 mm), Intermediate (>1-4 mm), and Thick (>4mm). The propensity score calculation and matching was performed with the software extension Propensity Score Matching for SPSS, version 3.02.36, which utilizes the R software programs Matchit, RItools, and cem.34,35,37-39
The baseline covariates were analyzed for the overall study population, and the treatment groups pre- and post-matching, comparing prevalence for categorical variables and the means and standard deviation for continuous variables. Survival was the main outcome measure, which was only assessed once the treatment groups had been matched and balanced optimally. Survival estimates were created by the Kaplan-Meier method and compared using the log-rank test. Assessment of the effect of covariates on survival was accomplished on the paired data using a Cox proportional hazards model utilizing a robust sandwich variance estimator to compare the matched-pair survival durations. Formal sensitivity analysis was performed as described elsewhere.40 Statistical analyses were performed using SPSS Version 21 (SPSS, Inc., an IBM Company, Chicago, Illinois), SAS 9.3 (SAS Institute Inc., Cary, NC) and R (http://www.R-project.org/). The data used in this study is public access and de-identified, and therefore this study was granted exemption from institutional review board oversight.
Results
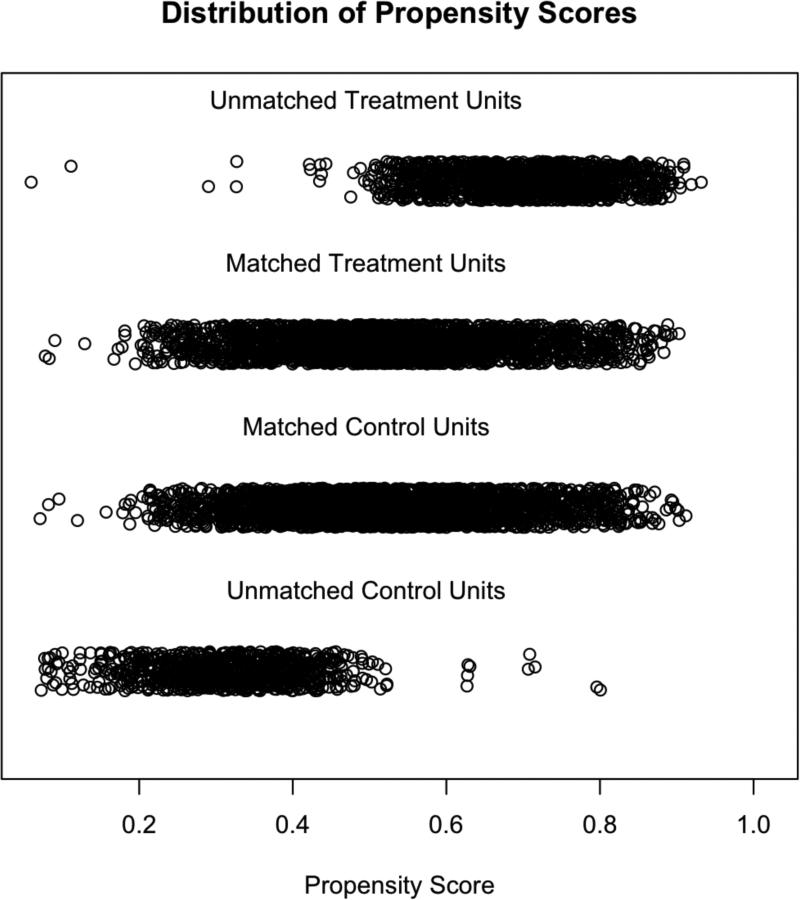
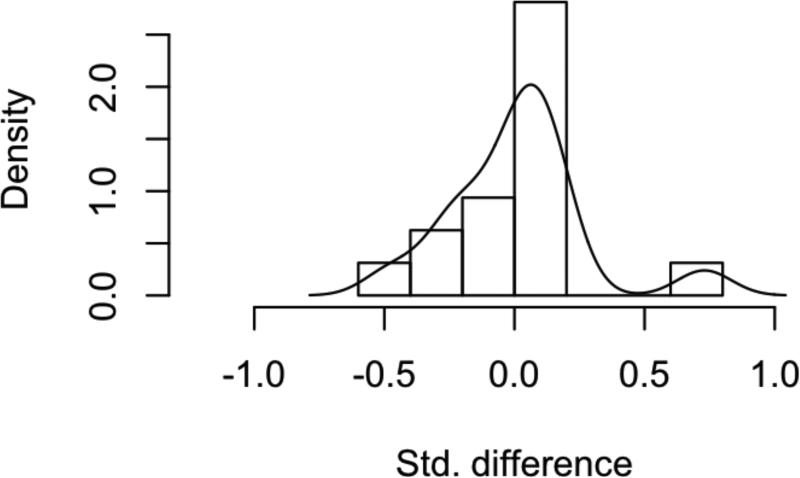
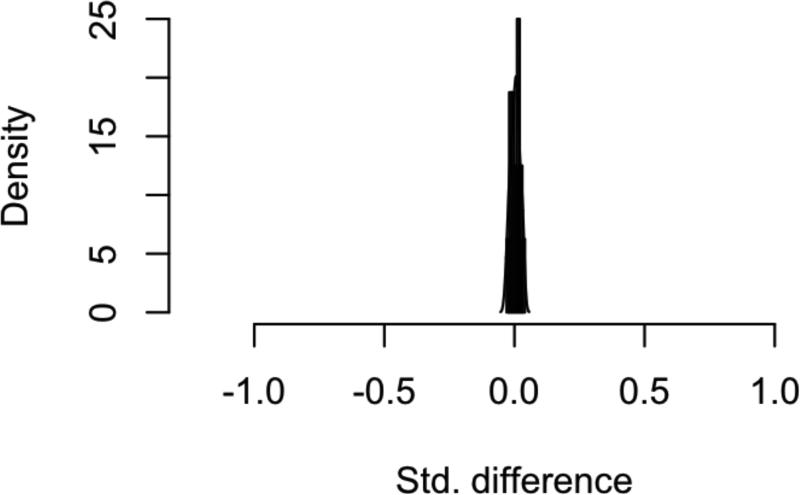
7266 patients were initially identified from the SEER database with HNM meeting the study criteria (Table 1). Of these patients, 46% did not undergo SLNB and 54% did. When stratified based on intervention with SLNB or not, the treatment groups were significantly different from one another for every variable under consideration. Propensity-scores for undergoing SLNB were calculated by logistic regression on ten covariates, and 1:1 matching of observation and treatment cases was completed, resulting in 2551 matched pairs (Figure 1). Covariate balance within the groups was achieved, as demonstrated by the improvement in all standardized differences for each covariate, and all absolute values of the standardized differences of the covariates being less than 0.1 (Figure 2). The characteristics of the observation and treatment groups and standardized differences were compared after matching (Table 1). In addition, the distributions for the continuous variables age and Breslow depth were compared (Supplemental data – eFigures 1-2). Bower-Hansen's omnibus balance statistic suggested no significant imbalance between the treatment cohort (Χ2=5.09, p=.985), and the L1 measure decreased pre- and post-matching (.850 to .847).
Table 1.
Characteristics of SEER Head and Neck Melanoma by SLNB Treatment Pre- and Post- Matchinga
| HN Melanoma Pre-Matching (n=7266) | HN Melanoma Post-Matching (n=5102) | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Subcategory | No SNB 3381(46) | Yes SNB 3885(54) | pb | dc | No SNB 2551(50) | Yes SNB 2551(50) | dc |
| Age Categories | 15-44 | 229 (30) | 547 (70) | <.001 | 221 (50) | 221 (50) | ||
| 45-54 | 257 (31) | 574 (69) | 247 (50) | 247 (50) | ||||
| 55-64 | 534 (38) | 881 (62) | 489 (50) | 489 (50) | ||||
| 65-74 | 861 (47) | 985 (53) | 719 (50) | 719 (50) | ||||
| 75-84 | 1500 (63) | 898 (37) | 875 (50) | 875 (50) | ||||
| Age | 68.8 ± 13.7 | 61.6 ± 15.1 | <.001 | .475 | 66.2 ± 14.3 | 66.2 ± 13.8 | .006 | |
| Marital Status | Married | 1874 (41) | 2683 (69) | <.001 | .295 | 1637 (51) | 1601 (49) | .031 |
| Not | 1507 (56) | 1202 (44) | 914 (49) | 950 (51) | ||||
| Race | White | 3259 (46) | 3841 (54) | <.001 | .234 | 2516 (50) | 2517 (50) | .004 |
| Other | 122 (74) | 44 (26) | 35 (51) | 34 (49) | ||||
| Sex | Female | 888 (49) | 935 (51) | .03 | .051 | 617 (49) | 637 (51) | .018 |
| Male | 2493 (46) | 2950 (54) | 1934 (50) | 1914 (50) | ||||
| Breslow Thickness | <=1mm | 1184 (63) | 694 (37) | <.001 | 869 (65) | 473 (35) | ||
| >1 to 2mm | 1088 (40) | 1658 (60) | 794 (42) | 1098 (58) | ||||
| >2 to 4mm | 636 (40) | 957 (60) | 484 (43) | 630 (57) | ||||
| >4mm | 473 (45) | 576 (55) | 404 (54) | 350 (46) | ||||
| Breslow | 2.2 ± 2.0 | 2.4 ± 1.9 | <.001 | .108 | 2.3 ± 2.1 | 2.4 ± 1.8 | .010 | |
| H&N Site | Face | 2124 (50) | 2159 (50) | <.001 | .146 | 1520 (50) | 1514 (50) | .005 |
| Scalp/Neck | 1257 (42) | 1726 (58) | 1031 (50) | 1037 (50) | ||||
| Tumor Registry | <Median | 1498 (51) | 1446 (49) | <.001 | .147 | 1066 (51) | 1041 (49) | .020 |
| >Median | 1883 (44) | 2439 (56) | 1485 (50) | 1510 (50) | ||||
| Major Histology Groups | Desmoplastic | 198 (45) | 243 (55) | <.001 | .016 | 165 (52) | 149 (48) | .026 |
| LM | 378 (59) | 262 (41) | .177 | 222 (50) | 220 (50) | .003 | ||
| NOS | 1488 (47) | 1667 (53) | .022 | 1132 (51) | 1107 (49) | .020 | ||
| Nodular | 418 (39) | 646 (61) | .115 | 353 (48) | 377 (52) | .025 | ||
| SS | 687 (46) | 823 (54) | .021 | 518 (50) | 528 (50) | .010 | ||
| Other | 212 (46) | 244 (54) | .000 | 161 (49) | 170 (51) | .015 | ||
| Ulceration | No | 2729 (48) | 2935 (52) | <.001 | .120 | 2013 (50) | 1987 (50) | .024 |
| Yes | 652 (41) | 950 (59) | 538 (49) | 564 (51) | ||||
| 1st Malignancy | No | 915 (55) | 754 (45) | <.001 | .194 | 645 (51) | 621 (49) | .024 |
| Yes | 2466 (44) | 3131 (56) | 1906 (50) | 1930 (50) | ||||
Reported as frequency (percent) for nominal variables, and mean +− standard deviation (range) for continuous variables
Chi-square and p-value for dichotomous variables; two-sample t-test and p-value for continuous variables; <0.05 considered significant.
Standardized difference of the means (absolute value); <0.1 considered to represent good balance.
Abbreviations: SLNB=sentinel lymph node biopsy, HN= Head and Neck, LM=lentigo maligna, NOS=Melanoma NOS, SS=superficial spreading
Figure 1. Propensity Score Distribution.
Dot-plot demonstrating the distribution of propensity scores for SLNB (treatment) or no SLNB (control) in HNM patients, stratified into matched and unmatched categories.
Figure 2. Assessment of Balance Following Propensity-Score Matching.
Histogram of standardized difference in means for all covariates and interaction terms A) before and B) after matching in the HNM cohort.
However, because of remaining differences in the percentage of SLNB in each of the Breslow Thickness categories, and the known differences in survival based on Breslow depth, each Breslow category separately underwent propensity-score matching and analysis, as for the overall study group. The cohorts Thin, Intermediate, and Thick were each well balanced on all of the remaining covariates following the matching procedure, and the characteristics of each separate cohort were analyzed (for data on the Intermediate cohort, see eTable 1 in Supplemental Materials).
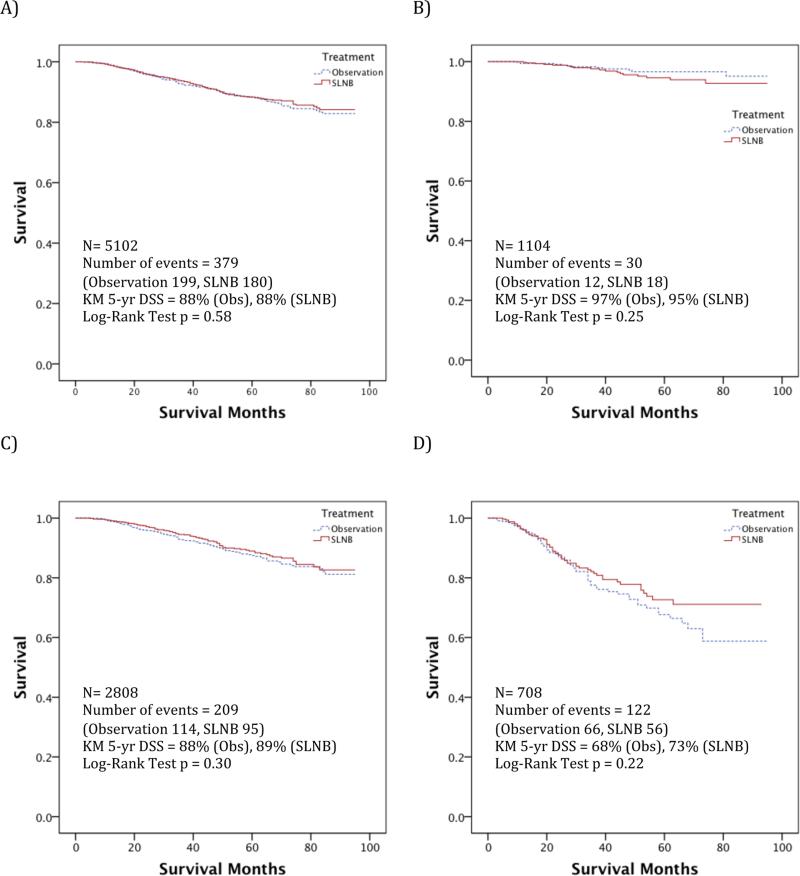
Disease-specific survival was compared in each of the matched cohorts. In the Intermediate cohort, including 2808 patients, the 5-year DSS estimate was 89% vs. 88% for SLNB vs. observation, respectively (Log-rank test p=0.30). In each of the other cohorts analyzed, including for thin or thick melanoma, no significant difference in DSS was demonstrated either (Figure 3). The 5-year DSS estimate for Thin melanoma was 96%, and for Thick melanoma 70%.
Figure 3. Survival Analysis Stratified by Breslow Depth.
Unadjusted melanoma disease-specific survival curves comparing SLNB intervention versus observation for HNM age 18-84, A) Overall, B) Thin, C) Intermediate, and D) Thick matched-pair cohorts. SLNB=Sentinel lymph node biopsy; Obs=Observation; N=Number of cases; KM= Kaplan-Meier; DSS= Disease-specific survival.
The effect of SLNB on DSS was assessed in a Cox proportional hazards model in each matched-pair cohort (Table 2). For the Intermediate cohort, the hazard ratio of death from melanoma in the SLNB group was 0.87 (95% CI 0.66-1.14, p=0.31). Similarly, the disease-specific hazard ratio for the SLNB group in the Thick cohort was 0.80 (95% CI 0.56-1.15, p=0.23). There was also no statistically significant association between SLNB and DSS in the overall group, or for Thin melanomas.
Table 2.
Head and Neck Melanoma Disease-Specific Mortality Hazards for SLNB Compared to Nodal Observation in Propensity-Score Matched-Pair Cohorts
| Cohorta | Number of matched pairs | DSS |
||
|---|---|---|---|---|
| HRb | CIc | pd | ||
| All | 2551 | 0.94 | 0.77-1.15 | 0.57 |
| Thin | 552 | 1.53 | 0.75-3.13 | .24 |
| Intermediate | 1404 | 0.87 | 0.66-1.14 | .31 |
| Thick | 354 | 0.80 | 0.56-1.15 | .23 |
Each cohort underwent separately performed propensity-score matching
Based on Cox proportional-hazards model with a robust sandwich covariance estimator
95% confidence interval for the hazard ratio
p-value for Cox proportional hazards test, where values <0.05 are considered significant
Abbreviations: SLNB=sentinel lymph node biopsy; HR=hazard ratio; CI=confidence interval; DSS=melanoma disease-specific survival; Thin=Breslow depth 0.75-1mm; Intermediate=Breslow depth 1-4mm; Thick=Breslow depth >4mm
For the patients who underwent SLNB for HNM, 7.4% overall had microscopic nodal metastases identified (Table 3). This rate varied significantly by Breslow thickness, from 2.9% for Thin, 7.1% for Intermediate, and 11% for Thick melanomas. The rate of positive nodes following SLNB also varied significantly by age category in the Intermediate melanoma cohort, varying in frequency from 11-14% for ages 18-64, but decreasing to 6% for ages 65-74, and to 4% for ages 75-84. The rate of positive nodes in the Intermediate cohort were also significantly less for those with melanoma of the face; desmoplastic or lentigo maligna histology; or who were married. Those who had a positive occult nodal metastasis identified by SLNB were more likely to be treated with a neck dissection in the Intermediate and Thick cohorts, and more likely to receive radiation in the Intermediate cohort, but not the Thick cohort. Having a positive SLNB result was associated with melanoma specific-death.
Table 3.
Microscopic Nodal Disease Comparison Amongst SLNB Propensity-Score Matched-Pair Cohorts
| Cohort | Number of SLNB Performed | SLNB Resultb |
||
|---|---|---|---|---|
| Negative | Positive | pc | ||
| Alla | 2551 | 2363 (92.6) | 188 (7.4) | |
| Thina | 552 | 536 (97.1) | 16 (2.9) | |
| Intermediatea | 1404 | 1304 (92.9) | 100 (7.1) | .001 |
| Thicka | 354 | 315 (89) | 39 (11) | |
| Age 18-44 | 221 | 193 (87.3) | 28 (12.7) | |
| 45-54 | 247 | 221 (89.5) | 26 (10.5) | |
| 55-64 | 489 | 447 (91.4) | 42 (8.6) | .001 |
| 65-74 | 719 | 677 (94.2) | 42 (5.8) | |
| 75-84 | 875 | 825 (94.3) | 50 (5.7) | |
| Intermediatea | ||||
| 18-44 | 95 | 83 (87.4) | 12 (12.6) | |
| 45-54 | 105 | 90 (85.7) | 15 (14.3) | |
| 55-64 | 235 | 209 (88.9) | 26 (11.1) | .001 |
| 65-74 | 398 | 375 (94.2) | 23 (5.8) | |
| 75-84 | 571 | 547 (95.8) | 24 (4.2) | |
| Thicka | ||||
| 18-44 | 14 | 13 (92.9) | 1 (7.1) | |
| 45-54 | 27 | 25 (92.6) | 2 (7.4) | |
| 55-64 | 62 | 52 (83.9) | 10 (16.1) | .591 |
| 65-74 | 103 | 94 (91.3) | 9 (8.7) | |
| 75-84 | 148 | 131 (88.5) | 17 (11.7) | |
| Intermediatea | ||||
| Face | 849 | 803 (94.6) | 46 (5.4) | .003 |
| Scalp/Neck | 555 | 501 (90.3) | 54 (9.7) | |
| Intermediatea | ||||
| Desmoplastic | 87 | 85 (97.7) | 2 (2.3) | |
| Lentigo maligna | 128 | 127 (99.2) | 1 (0.8) | |
| Nodular | 225 | 204 (90.7) | 21 (9.3) | .019 |
| Superficial spreading | 265 | 245 (92.5) | 20 (7.5) | |
| Melanoma NOS | 613 | 563 (91.8) | 50 (8.2) | |
| Other | 86 | 80 (93.0) | 6 (7.0) | |
| Intermediatea | ||||
| Not ulcerated | 1080 | 1003 (92.9) | 77 (7.1) | 1.00 |
| Ulcerated | 324 | 301 (92.9) | 23 (7.1) | |
| Intermediatea | ||||
| Married | 894 | 842 (94.2) | 52 (5.8) | .013 |
| Not Married | 510 | 462 (90.6) | 48 (9.4) | |
| Radiation | 86 | 58 (67.4) | 28 (32.6) | .001 |
| Intermediatea | 35 | 25 (71.4) | 10 (28.6) | .001 |
| Thicka | 38 | 31 (81.6) | 7 (18.4) | .164 |
| Regional Node Dissection | 441 | 307 (69.6) | 134 (30.4) | .001 |
| Intermediatea | 239 | 169 (70.7) | 70 (29.3) | .001 |
| Thicka | 81 | 53 (65.4) | 28 (34.6) | .001 |
| Intermediatea | ||||
| Melanoma survival | 1309 | 1225 (93.6) | 84 (6.4) | .001 |
| Melanoma death | 95 | 79 (83.2) | 16 (16.8) | |
Each cohort underwent separately performed propensity-score matching
Reported as Number (row percentage)
p-value for Chi-square comparison, where values <0.05 are considered significant
Abbreviations: SLNB=sentinel lymph node biopsy; Thin=Breslow depth >0.75-1mm; Intermediate=Breslow depth >1-4mm; Thick=Breslow depth >4mm
Discussion
Our findings based on propensity score matched-pair cohorts of HNM patients suggest there is no significant association between SLNB and DSS for HNM patients aged 18-84 with Breslow depths 0.76mm and above. The hazard ratio of 0.87 for Intermediate melanoma and 0.80 for Thick melanoma indicates that undergoing SLNB may be associated with decreased odds of death from melanoma. However, there is no statistically significant relationship identified for either of these cohorts in this SEER analysis.
One interpretation of such hazard ratios is that for two identical patients with an intermediate-thickness melanoma with baseline equal probability of dying, the patient undergoing nodal observation has a 54% chance of dying from melanoma before the one having a SLNB. However, death from melanoma is a relatively uncommon event in this population, occurring in only 88% at 5-years, as indicated by the Kaplan-Meier survival estimates. So not only is no statistically significant association between DSS and SLNB seen in this analysis, but the estimated size of such an association is predicted to be a small change in the probability of an uncommon event, and therefore the clinical significance of such an association would be minimal.
Our results are consistent with previous studies. The Multicenter Selective Lymphadenectomy Trial-1 (MSLT-1), a RCT comparing SLNB to observation in 1270 patients with melanoma 1.2-3.5mm Breslow thickness of all body sites, recently published final results with 10-year survival data.11 Of patients in this study, 17% had HNM. In the main study outcome, they did not demonstrate a significant difference in melanoma disease-specific survival between the treatment arms. However, they did demonstrate a trend towards reduced risk of death from melanoma with SLNB, with a hazard ratio of 0.84, which is similar to the effect size seen in our analysis. We found a similar 5-year DSS for intermediate-thickness HNM to the 87% seen in the MSLT-1 trial.12 It has been suggested that the MSLT-1 trial was underpowered to detect a small but clinically significant survival difference.13 Even though this study evaluated more cases than the MSLT-1 trial, based on the small estimated treatment effect, it is likely that it too is underpowered to identify a significant association. If we were to design an RCT to validate this small difference in survival between SLNB and observation in a similar design as the MSLT-I trial, with 5-years follow-up and a power of 90%, based on an estimated hazard ratio of 0.80, it would require randomization of 6500 patients (assigning 60% to SLNB and 40% to observation, as in the MSLT-1).
To our knowledge, no other observational studies have used SEER to compare the survival of patients who undergo SLNB. Other than the aforementioned prospective RCT, only one other study has specifically studied the survival effect of SLNB compared to nodal observation. A single-institution retrospective case series from Germany examined 673 primary melanoma patients with Breslow depth >1mm, 377 treated prior to 2000 without SLNB and 296 after 2000 who underwent SLNB when the practice-pattern at the institution changed.41 The measured patient characteristics across the time periods were reported to be otherwise similar; however, there was no other attempt to control for differences between the cohorts, except by the temporal separation of the patients into different treatment paradigms. For the cohort treated with SLNB, they reported significantly improved recurrence-free survival, distant-metastasis-free survival, and overall survival, and a trend towards improved melanoma-disease-specific survival. Only 10% of the patients in that study had melanoma in a head and neck site.
We note that a previous study attempted to analyze the SEER data from 1998-2002 to determine the association of SLNB with survival for melanoma of all body sites.13 However, the study was not published due to issues with the coding algorithm by which SEER indicated SLNB status. We likewise recognized there to be an issue with SEER-data from 1998-2002, and avoided the potential mis-categorization of patients who underwent SLNB + completion lymphadenectomy by excluding the patients between 1998-2002.
The critical assumption in using propensity-score matched observational data to make inferences about treatment effects is that one has accounted for all possible variables which influence treatment assignment.42 If this assumption is true, then after achieving evenly matched and balanced cohorts, one can attribute the difference in outcome to the intervention, as one would in a randomized controlled experiment. However, in reality it is improbable that every possible variable influencing the treatment assignment is measured and accounted for.
There could reasonably be many such factors that one could speculate might be confounding the results of this study: one would be treatment in a highly experienced center with specialists in the field; another might be access to adjuvant treatment in clinical trials, which incorporate performance of SLNB as inclusion criteria. Another possible confounder is comorbidity, which would influence the performance of a surgical procedure (such as SLNB) and the aggressiveness of other treatments for melanoma. Comorbidity information is not collected in the SEER database, and its absence from inclusion in the propensity model is a limitation of this study.
There are several other limitations of our analysis based on SEER data. The presence of mitoses was not recorded in SEER during the period of the study, and this information might have helped stratify patients, especially in the cohort with Breslow depth between 0.75-1mm. In addition, the SEER data only records staging at diagnosis and initial treatment, so information is not available regarding subsequent development of lymph node metastasis. Finally, while the SEER registries have well-managed data collection systems and quality control practices, the accuracy and quality control for the clinical and pathologic source information is less well defined, and inaccuracies can enter the system at many different points.43,44
The strengths of the current study include the large, geographically diverse sample likely to be representative of the U.S. population; the inclusion of patients regardless of the type of institution at which they were treated; the well-established, high-quality data captured by SEER registries; and, the carefully designed and conducted methodology to control for potential sources of bias impacting SLNB intervention through propensity score matching. This study is the largest sample of head and neck melanoma patients to assess the association of SLNB on survival. With these strengths, this study has not identified a statistically significant association between treatment including SLNB and improved DSS for HNM; furthermore, we suggest that the size of the association is unlikely to be clinically relevant for patients. It is unlikely that another RCT with adequate power will ever be designed to answer this question. Therefore, observational data analyses such as that presented here may remain the best level of evidence regarding this important clinical question for HNM. While the potential limitations associated with unmeasured confounders must be considered, our results do not provide evidence to warrant further study to assess the therapeutic survival benefit of SLNB for HNM.
Conclusions
This SEER propensity-score matched cohort analysis fails to demonstrate an association with improved survival for patients with HNM who undergo SLNB rather than nodal observation.
Supplementary Material
Footnotes
Abstract accepted for Oral Presentation at AHNS 2014 Annual Meeting and 5th World Congress, July 26-30, New York, NY – Abstract ID# 58306
Conflict of Interest/Disclosures: None
Author Contributions: Drs. Sperry and Pagedar had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Sperry, Charlton, Pagedar. Acquisition of data: Sperry. Analysis and interpretation of data: Sperry, Pagedar. Drafting of the manuscript: Sperry, Pagedar. Critical revision of the manuscript for important intellectual content: Sperry, Charlton, Pagedar. Statistical analysis: Sperry, Pagedar. Study supervision: Pagedar.
Previous Presentation: None
Additional Contributions: None
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60(5):277–300. doi: 10.3322/caac.20073. doi:10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Pollack LA, Li J, Berkowitz Z, et al. Melanoma survival in the United States, 1992 to 2005. Journal of the American Academy of Dermatology. 2011;65(5):S78.e1–S78.e10. doi: 10.1016/j.jaad.2011.05.030. doi:10.1016/j.jaad.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong S-J, et al. Final version of 2009 AJCC melanoma staging and classification. Journal of Clinical Oncology. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. doi:10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morton DL, Wen DR, Wong JH. Technical details of intraoperative lymphatic mapping for early stage melanoma. Archives of Surgery. 1992;(127):392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 5.Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. Journal of Clinical Oncology. 1999;17(3):976–976. doi: 10.1200/JCO.1999.17.3.976. [DOI] [PubMed] [Google Scholar]
- 6.Morton DL, Hoon DSB, Cochran AJ, et al. Lymphatic Mapping and Sentinel Lymphadenectomy for Early-Stage Melanoma. Transactions of the Meeting of the American Surgical Association. 2003;121(4):231–243. doi:10.1097/01.sla.0000086543.45557.cb. [Google Scholar]
- 7.Gutzmer R, Ghazal Al M, Geerlings H, Kapp A. Sentinel node biopsy in melanoma delays recurrence but does not change melanoma-related survival: a retrospective analysis of 673 patients. Br J Dermatol. 2005;153(6):1137–1141. doi: 10.1111/j.1365-2133.2005.06941.x. doi:10.1111/j.1365-2133.2005.06941.x. [DOI] [PubMed] [Google Scholar]
- 8.Medalie NS, Ackerman AB. Sentinel lymph node biopsy has no benefit for patients with primary cutaneous melanoma metastatic to a lymph node: an assertion based on comprehensive, critical analysis: part I. Am J Dermatopathol. 2003;25(5):399–417. doi: 10.1097/00000372-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 9.McMasters KM. What Good is Sentinel Lymph Node Biopsy for Melanoma if it does not Improve Survival? Ann Surg Oncol. 2004;11(9):810–812. doi: 10.1245/ASO.2004.07.919. doi:10.1245/ASO.2004.07.919. [DOI] [PubMed] [Google Scholar]
- 10.Freeman SR, Gibbs BB, Brodland DG, Zitelli JA. Prognostic Value of Sentinel Lymph Node Biopsy Compared with that of Breslow Thickness: Implications for Informed Consent in Patients with Invasive Melanoma. Dermatol Surg. 2013;39(12):1800–1812. doi: 10.1111/dsu.12351. doi:10.1111/dsu.12351. [DOI] [PubMed] [Google Scholar]
- 11.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609. doi: 10.1056/NEJMoa1310460. doi:10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-Node Biopsy or Nodal Observation in Melanoma. N Engl J Med. 2006;355(13):1307–1317. doi: 10.1056/NEJMoa060992. doi:10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 13.Gimotty PA, Yoon F, Hammond R, Rosenbaum P, Guerry D. Therapeutic Effect of Sentinel Lymph Node Biopsy in Melanoma Remains an Open Question. Journal of Clinical Oncology. 2009;27(26):4236–4238. doi: 10.1200/JCO.2009.23.4518. doi:10.1200/JCO.2009.23.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lachiewicz AM, Berwick M, Wiggins CL, Thomas NE. Survival differences between patients with scalp or neck melanoma and those with melanoma of other sites in the Surveillance, Epidemiology, and End Results (SEER) program. Arch Dermatol. 2008;144(4):515–521. doi: 10.1001/archderm.144.4.515. doi:10.1001/archderm.144.4.515. [DOI] [PubMed] [Google Scholar]
- 15.Fadaki N, Li R, Parrett B, et al. Is head and neck melanoma different from trunk and extremity melanomas with respect to sentinel lymph node status and clinical outcome? Ann Surg Oncol. 2013;20(9):3089–3097. doi: 10.1245/s10434-013-2977-7. doi:10.1245/s10434-013-2977-7. [DOI] [PubMed] [Google Scholar]
- 16.Ghazal Al P, Gutzmer R, Satzger I, et al. Lower prevalence of lymphatic metastasis and poorer survival of the sentinel node-negative patients limit the prognostic value of sentinel node biopsy for head or neck melanomas. Melanoma Research. 2013 doi: 10.1097/CMR.0000000000000042. doi:10.1097/CMR.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 17.McMasters KM, Noyes RD, Reintgen DS, et al. Lessons learned from the Sunbelt Melanoma Trial. J Surg Oncol. 2004;86(4):212–223. doi: 10.1002/jso.20084. doi:10.1002/jso.20084. [DOI] [PubMed] [Google Scholar]
- 18.Saltman BE, Ganly I, Patel SG, et al. Prognostic implication of sentinel lymph node biopsy in cutaneous head and neck melanoma. Head Neck. 2010;32(12):1686–1692. doi: 10.1002/hed.21390. doi:10.1002/hed.21390. [DOI] [PubMed] [Google Scholar]
- 19.NCCN Clinical Practice Guidelines in Oncology - Melanoma [October 29, 2013];nccnorg. Available at: http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf.
- 20.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26(4):734–753. doi: 10.1002/sim.2580. doi:10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 21.Cormier JN, Xing Y, Ding M, et al. Population-based assessment of surgical treatment trends for patients with melanoma in the era of sentinel lymph node biopsy. J Clin Oncol. 2005;23(25):6054–6062. doi: 10.1200/JCO.2005.21.360. doi:10.1200/JCO.2005.21.360. [DOI] [PubMed] [Google Scholar]
- 22.Stitzenberg KB, Thomas NE, Beskow LM, Ollila DW. Population-based analysis of lymphatic mapping and sentinel lymphadenectomy utilization for intermediate thickness melanoma. J Surg Oncol. 2006;93(2):100–107. doi: 10.1002/jso.20403. doi:10.1002/jso.20403. [DOI] [PubMed] [Google Scholar]
- 23.Sondak VK, Taylor JMG, Sabel MS, et al. Mitotic Rate and Younger Age Are Predictors of Sentinel Lymph Node Positivity: Lessons Learned From the Generation of a Probabilistic Model. Ann Surg Oncol. 2004;11(3):247–258. doi: 10.1245/aso.2004.03.044. doi:10.1245/ASO.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 24.Balch CM, Soong S-J, Gershenwald JE, et al. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann Surg Oncol. 2013;20(12):3961–3968. doi: 10.1245/s10434-013-3100-9. doi:10.1245/s10434-013-3100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao C, Martin RCG, II, Ross MI, et al. Correlation Between Prognostic Factors and Increasing Age in Melanoma. Ann Surg Oncol. 2004;11(3):259–264. doi: 10.1245/aso.2004.04.015. doi:10.1245/ASO.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Kemeny MM, Busch E, Stewart AK, Menck HR. Am J Surg. 6. Vol. 175. 437-44: 1998. Superior survival of young women with malignant melanoma. discussion 444-5. [DOI] [PubMed] [Google Scholar]
- 27.Stidham KR, Johnson JL, Seigler HF. Survival superiority of females with melanoma. A multivariate analysis of 6383 patients exploring the significance of gender in prognostic outcome. Arch Surg. 1994;129(3):316–324. doi: 10.1001/archsurg.1994.01420270094020. [DOI] [PubMed] [Google Scholar]
- 28.MacKie RM, Hole D, Hunter JA, et al. Cutaneous malignant melanoma in Scotland: incidence, survival, and mortality, 1979-94. The Scottish Melanoma Group. BMJ. 1997;315(7116):1117–1121. doi: 10.1136/bmj.315.7116.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Brien CJ, Coates AS, Petersen-Schaefer K, et al. Experience with 998 cutaneous melanomas of the head and neck over 30 years. Am J Surg. 1991;162(4):310–314. doi: 10.1016/0002-9610(91)90138-4. [DOI] [PubMed] [Google Scholar]
- 30.Golger A, Young DS, Ghazarian D, Neligan PC. Epidemiological features and prognostic factors of cutaneous head and neck melanoma: a population-based study. Arch Otolaryngol Head Neck Surg. 2007;133(5):442–447. doi: 10.1001/archotol.133.5.442. doi:10.1001/archotol.133.5.442. [DOI] [PubMed] [Google Scholar]
- 31.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 32.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behavioral Research. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. doi:10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuart EA. Matching methods for causal inference: A review and a look forward. Statistical science : a review journal of the Institute of Mathematical Statistics. 2010;25(1):1. doi: 10.1214/09-STS313. doi:10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen BB, Bowers J. Covariate Balance in Simple, Stratified and Clustered Comparative Studies. Statistical Science. 2008;23(2):219–236. [Google Scholar]
- 35.Iacus SM, King G, Porro G. Causal Inference without Balance Checking: Coarsened Exact Matching. Political Analysis. 2012;20(1):1–24. doi:10.1093/pan/mpr013. [Google Scholar]
- 36.Thoemmes F. Propensity score matching in SPSS. [November 4, 2013];arXiv: 12016385. 2012 Available at: http://arxiv.org/abs/1201.6385.
- 37.Ho DE, Imai K, King G, Stuart EA. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. Journal of Statistical Software. 2011;42(8) [Google Scholar]
- 38.Ho DE, Imai K, King G, Stuart EA. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Analysis. 2007;15(3):199–236. doi:10.1093/pan/mpl013. [Google Scholar]
- 39.Hansen BB. Full Matching in an Observational Study of Coaching for the SAT. Journal of the American Statistical Association. 2004;99(467):609–618. doi:10.1198/016214504000000647. [Google Scholar]
- 40.Love TE. Spreadsheet-based sensitivity analysis calculations forward matched samples. [December 31, 2013];Center for Health Care Research & Policy, Case Western Reserve University. 2008 Available at: http://www.chrp.org/propensity.
- 41.Satzger I, Meier A, Hoy L, et al. Sentinel Node Dissection Delays Recurrence and Prolongs Melanoma-Related Survival: An Analysis of 673 Patients from a Single Center with Long-Term Follow-Up. Ann Surg Oncol. 2010;18(2):514–520. doi: 10.1245/s10434-010-1318-3. doi:10.1245/s10434-010-1318-3. [DOI] [PubMed] [Google Scholar]
- 42.Austin PC. A tutorial on the use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2013 doi: 10.1002/sim.5984. doi:10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peabody JW, Luck J, Jain S, Bertenthal D, Glassman P. Assessing the accuracy of administrative data in health information systems. Med Care. 2004;42(11):1066–1072. doi: 10.1097/00005650-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Nathan H, Pawlik TM. Limitations of Claims and Registry Data in Surgical Oncology Research. Ann Surg Oncol. 2007;15(2):415–423. doi: 10.1245/s10434-007-9658-3. doi:10.1245/s10434-007-9658-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.