Abstract
Chronic muscle pain remains a significant source of suffering and disability despite the adoption of pharmacologic and physical therapies. Muscle pain is mediated by free nerve endings distributed through the muscle along arteries. These nerves project to the superficial dorsal horn and are transmitted primarily through the spinothalamic tract to several cortical and subcortical structures, some of which are more active during the processing of muscle pain than other painful conditions. Mechanical forces, ischemia, and inflammation are the primary stimuli for muscle pain, which is reflected in the array of peripheral receptors contributing to muscle pain-ASIC, P2X, and TRP channels. Sensitization of peripheral receptors and of central pain processing structures are both critical for the development and maintenance of chronic muscle pain. Further, variations in peripheral receptors and central structures contribute to the significantly greater prevalence of chronic muscle pain in females.
Introduction
Chronic muscle pain affects between 11–24% of the world’s population with the majority of people experiencing musculoskeletal pain at some time in their life (Cimmino et al. 2011). Older, sedentary, unemployed, less-well educated individuals with anxiety are more likely to suffer from chronic muscle pain (Ahacic and Kåreholt 2010; Azevedo et al. 2012). Those who suffer from chronic muscle pain often report decreased productivity and a significant portion have had to change jobs or quit working entirely as a result of their pain (Miranda et al. 2010). In the U.S. alone, all forms of chronic pain are estimated to incur an economic burden of $500 billion dollars annually.
The two primary diseases associated with chronic muscle pain are myofascial pain syndrome (MPS) and fibromyalgia (FM). Myofascial pain syndrome is characterized by regional muscle pain with areas of focal tenderness to mechanical pressure. These trigger points are palpable, taut masses typically found within the muscle belly. Affected muscles are stiff and contracted, which can put stresses on adjacent or antagonist muscles that lead to the development of secondary trigger points. MPS is often associated with anxiety and depression (Bennett 2007; Vázquez-Delgado et al. 2009). FM is the most extreme example of chronic muscle pain. Pain is widespread and, like MPS, patients often report areas of local tenderness to palpation. Along with pain, FM patients also report fatigue, depression, insomnia, and cognitive impairment (Mease 2005; Staud 2007). The combination of these symptoms, particularly pain and fatigue, leads FM patients to report significantly more disability and poorer physical fitness than other chronic pain conditions (Verbunt et al. 2008; Valkeinen et al. 2008). However, many other conditions, such as whiplash injury, neck pain, and chronic low back pain, also have subpopulations that can be considered as chronic muscle pain that is localized to one or two regions.
Treatment of chronic muscle pain has only been partially effective. Pharmacologic approaches have shown some usefulness (Mease 2005). Non-steroidal anti-inflammatory drugs, some anti-depressants (tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors), and some anti-epileptics (gabapentin, pregabalin) have some benefit for pain and mixed effects on the attendant depression, insomnia, cognitive impairment, and fatigue (Mease 2005; Bennett 2007). Non-pharmacological approaches show some effectiveness in both MPS and FM. MPS patients benefit from gentle stretching of muscles, from palpitation of trigger points to reduce stiffness, and from ergonomic adjustments to reduce muscle overuse (Bennett 2007; Thompson 2012). In FM, regular moderate exercise improves pain, fatigue, mood, and loss of sleep, though exercise can acutely exacerbate pain in patients that are unaccustomed to exercise (Staud 2007; Thompson 2012). Chronic muscle pain, with its substantial suffering and poor treatment options, remains a significant health burden worldwide.
The current chapter will review the peripheral mechanisms involved in chronic muscle pain. We will describe the anatomy of sensory fibers and their innervation, peripheral ion channels involved in transmitting nociceptive information from muscle, animal models of muscle pain, and changes that occur in nociceptors. We will also briefly touch on central pathways and sex differences in these models.
Anatomy
Sensory fibers innervating the muscle are classified into four groups based on size and myelination (Lloyd and Chang 1948). Group I and II fibers are large, myelinated fibers and play a role in proprioception. Group III and IV fibers are small myelinated and unmyelinated fibers, respectively, corresponding to A∂ and C fibers of the skin. Group III and IV, but not I and II, respond to application of noxious mechanical, thermal, and chemical stimuli to the muscle, indicating that they transmit nociceptive information from the muscle (Mense 1977; Kniffki et al. 1978; Pickar et al. 1994). The endings of group III and IV afferents are distributed throughout the muscle, terminating in extrafusal fibers, intrafusal fibers, connective tissue, fat, and the adventitia of both venules and arterioles. The majority of these nerves terminate as free endings in the adventitia of blood vessels, an ideal location for sampling blood for metabolites released as by-products of muscle contraction (Stacey 1969).
Upon leaving the muscle, nociceptive and proprioceptive muscle afferents project to different layers of the spinal cord. Group I and II afferents project to deep layers of the dorsal horn (layers III-IV), while group III and IV afferents project to superficial layers (I, II, and the edge of III) (Mense and Craig 1988; Ling et al. 2003). Nociceptive muscle afferents then project to the brain via the spinothalamic tract (Foreman et al. 1979), where they terminate in the nucleus submedius, paraventricular nucleus anterior (PVA)(Kawakita et al. 1993; Min et al. 2011), and ventral posterolateral nucleus of the thalamus (Kniffki and Mizumura 1983). Muscle pain, like cutaneous pain, activates multiple regions of the brain —including the anterior cingulate cortex, primary and secondary somatosensory cortex, dorsolateral prefrontal cortex, and the insula (Peyron et al. 2000); however, muscle pain more strongly activates regions of the brain associated with emotional processing than skin pain: bilateral amygdala, caudate, orbitofrontal cortex, hippocampus, parahippocampus, and the superior temporal pole (Cheng et al. 2011; Takahashi et al. 2011). Further, muscle pain is associated with activation of the periaqueductal grey and rostral ventromedial medulla, which have been implicated in modifying descending inhibitory pain pathways (Keay et al. 2000; Da Silva et al. 2010a).
Sensory Transduction
Muscle afferents are well suited for detecting the condition of muscle tissue. While skin afferents provide detailed information about the external environment and transmit pain-related information in response to external sources of injury, muscle afferents are sensitive to the capacity of the muscle to function as a force-generating organ with intermittent periods of high metabolic demand. Muscle afferents have poor spatial resolution as compared to skin, with single mechanically sensitive fibers innervating multiple receptive fields as far as 2 cm apart (Kumazawa and Mizumura 1977). This fits with the clinical description of muscle pain as diffuse and hard to localize. Yet, muscles are equipped with mechanically sensitive fibers specialized to detect innocuous pressure, noxious pressure, and graded forces of contraction (Mense and Meyer 1985). Patients often report mild muscle pain at rest that is acutely worsened by either pressure or use of injured muscle. In addition to mechanical forces, muscle afferents appear to be attuned to by-products released during muscle contraction or accumulated under ischemic conditions, as ischemia itself is sufficient to elicit pain (Sacchetti et al. 1980). Thermal sensitive fibers, activated by cool or warm temperatures, are also present in muscle (Hertel et al. 1976; Kumazawa and Mizumura 1977), but muscles do not reach noxious levels of heat under physiological conditions (Saltin et al. 1968; Brooks et al. 1971).
Substantial changes occur in muscle during fatigue. The fibers of the muscle themselves are placed under significant strain, which correlates with the release of interstitial ATP (Li et al. 2003; Dessem et al. 2010). As the metabolic demands of muscle contraction increase, elevated oxygen uptake for aerobic respiration stimulates the production of reactive oxygen species (Delliaux et al. 2009) until the capacity for aerobic respiration is exceeded, at which point muscle begins accumulating lactic acid (Bangsbo et al. 1993). Norepinephrine concentration is also elevated in the interstitial fluid of the muscle during exercise (Li et al. 2005). Damage to muscle tissue during use plays an important role in muscle pain, as muscle activity that results in greater tissue damage produces significantly more pain than non-damaging activity (Faulkner et al. 1993; Proske and Morgan 2001; Gibson et al. 2009; Martin et al. 2009). This damage results in recruitment of neutrophils and macrophages(Malm et al. 2000; Tidball 2005) and the release of a wide range of factors including prostaglandin E2 (Karamouzis et al. 2001; Uchida et al. 2009), bradykinin (Murase et al. 2010), serotonin (Ernberg et al. 1999; Shah et al. 2005), and nerve growth factor (NGF) (Murase et al. 2010; Urai et al. 2013). Multiple targets for these molecules have been studied both in isolated cells and whole animals.
Acid Sensing Ion Channels
Acid sensing ion channels (ASICs) are non-selective cation channels activated by increases in extracellular proton concentrations (Waldmann et al. 1997b). Four genes code for six subunit types (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, ASIC4) with differential distributions across the central and peripheral nervous systems depending on the subunit (Waldmann et al. 1997a; Lingueglia et al. 1997; Waldmann 1998; Sherwood et al. 2012). It is particularly interesting that ASICs are found on fibers terminating in the tunica adventitia of muscular arteries, an ideal location for detecting protons released during muscle contraction (Molliver et al. 2005). A functional channel is composed of three subunits. In the case of muscle afferents, ASICs form as heterotrimers of the ASIC1a, ASIC2, and ASIC3 subunits. In DRG innervating muscle, ASICs are activated by pH 6.8–7.0 and demonstrate a rapidly desensitizing peak current followed by a smaller sustained current (Gautam and Benson 2013). Application of acidic solutions activates group IV afferents (Hoheisel et al. 2004) and produces enhanced pain behavior in animals (Sluka et al. 2001) and pain in humans (Frey Law et al. 2008). In animal models, ASIC3−/− mice do not develop hyperalgesia after repeated intramuscular acidic saline injection and show reduced secondary hyperalgesia after injection with an inflammatory compound (Sluka et al. 2003; Walder et al. 2011). ASIC1a−/− mice, on the other hand, do develop hyperalgesia with intramuscular acidic saline injections but show reduced primary hyperalgesia in animals with muscle inflammation (Sluka et al. 2003; Walder et al. 2010). However downregulation of ASIC3 in adult mice prevents the development of both primary and secondary hyperalgesia after muscle inflammation (Walder et al. 2011).
A number of factors can alter the function of ASICs. Expression of ASIC3 is enhanced under ischemic conditions and inflammation (Ikeuchi et al. 2008; Deval et al. 2008) (Xing et al. 2011). Further, fatiguing muscle prior to treatment with acid or inflammatory compounds results in enhanced pain behavior in animals (Yokoyama et al. 2007; Sluka and Rasmussen 2010). Electrophysiological studies have shown lactate, independent of protons, increases the sensitivity and magnitude of currents in ASIC1a and ASIC3 (Immke and McCleskey 2001). Finally, release of ATP can enhance ASIC currents through an interaction between ASIC3 and P2X5 (Birdsong et al. 2010). Thus, ASICs critically contribute to the development of muscle hyperalgesia, as they can be activated under a number of conditions that are related to inflammation and fatigue, and their selective knockdown or antagonism significantly reduces mechanical sensitivity in conditions known to produce muscle hyperalgesia.
Ionotropic Purine Receptors
The P2X family of ligand-gated ion channels is large and plays a role in diverse functions. Notably, P2X channels have been implicated in detecting stretch in the bladder (Sun and Chai 2004; Zagorodnyuk et al. 2007) and muscle afferents (Li and Sinoway 2002), as well as detection of hypoxia in the carotid bodies (Prasad et al. 2001; Campanucci et al. 2006) and lungs (Fu et al. 2004; De Proost et al. 2009; Syed et al. 2010). P2X receptors also contribute to a wide range of pain conditions including cardiac (Zhang et al. 2008; Wang et al. 2009), visceral(Xu et al. 2008), inflammatory (Oliveira et al. 2009; Teixeira et al. 2010; Prado et al. 2013), neuropathic(Honore et al. 2002; Wang et al. 2003), and cancer (Kaan et al. 2010; Wu et al. 2012) pain. Application of P2X receptor agonists in the gastrocnemius (Reinöhl et al. 2003; Hanna and Kaufman 2004) and masseter (Connor et al. 2005) muscles activates group III and IV muscle afferents; similar treatments in animals (Shinoda et al. 2008) and humans(Mørk et al. 2003) are painful. The specific subtype of P2X receptor contributing to these effects is unclear. P2X3 is well studied and localizes to the DRG (Chen et al. 1995) as well as to the terminals of nerve fibers innervating the tunica adventitia of muscle afferents (Molliver et al. 2005). Eccentric muscle contractions, which are associated with muscle pain, increase P2X3 expression in muscle afferents (Dessem et al. 2010). Similarly, P2X4 and P2X5 are also found in the DRG (Xiang et al. 1998; Kobayashi et al. 2005) and studies of calcium imaging in cultured DRGs show that cells responding to physiologically relevant combinations of lactate, protons, and ATP are inhibited by an array of P2X antagonists in a pattern suggesting that both P2X4 and P2X5 contribute to activation of DRG (Light et al. 2008). Further, P2X5 is capable of physically interacting with and enhancing the proton-gated current through ASIC3, which is expressed in muscle afferents (Molliver et al. 2005; Birdsong et al. 2010). The contribution of these specific P2X subtypes to muscle pain has not yet been studied in behavioral experiments. In summary, purinergic receptors are sensitive to the ATP released during muscle activit,y and may contribute to fatigue-related muscle pain either directly, by generating an inward current through P2X3 or P2X4, and/or by enhancing the proton-gated current via P2X5-ASIC3 interactions.
Transient Receptor Potential Channels
The Transient Receptor Potential (TRP) family of ligand-gated ion channels contributes to many sensory modalities including cool (M8), noxious cold (A1), warm (TRPV1), noxious heat (TRPV2), acidic (TRPV1), inflammatory (TRPV1), and mechanical (TRPV4) stimuli. Among the TRP channels, TRPV1 is particularly well studied and has been implicated in detection of tissue ischemia (Wang et al. 2008; Zhong and Wang 2008; Xing et al. 2008; Xing et al. 2009) and contributes to the response of mechanically sensitive fibers, though TRPV1 itself does not appear to mediate mechanical transduction (Spencer et al. 2008; Inoue et al. 2009). Outside the muscle, TRPV1 also plays a role in a wide range of pain conditions, including neuropathic (Nakao et al. 2012; Wu et al. 2013), visceral (Sakurai et al. 2008; Matsumoto et al. 2012), skin (Barabas and Stucky 2013), and orofacial (Shinoda et al. 2011) pain. Within muscle, TRPV1 is important for mediating the exercise pressor reflex (Li et al. 2004; Kindig et al. 2005; Gao et al. 2007). TRPV1 is expressed on muscle cells themselves and contributes to adaptive responses to exercise, such has muscle hypertrophy (Ito et al. 2013) and improved endurance (Luo et al. 2012). Skeletal muscle afferents also express TRPV1 (Connor et al. 2005; Molliver et al. 2005; Tsukagoshi et al. 2006) and are excited by TRPV1 activation (Light et al. 2008; Jankowski et al. 2013). TRPV1 is activated by many diverse ligands including naturally occurring vanilloids (eg, capsaicin, capisate, piperine), endogenous lipids (e.g., oxidized lineoleic acid metabolites, anandamide, N-oleoyldopamine, and N-arachidonoyldopamine) (Calixto et al. 2005; Green et al. 2013), inflammatory mediators(Messeguer et al. 2006), protons (Tominaga and Tominaga 2005), and purine triphosphates (Lishko et al. 2007). TRP channels, however, have not been well studied in muscle pain. Administration of either TRPV1 or TRPA1 agonists produces mechanical hyperalgesia in animals (Ro et al. 2009). In animal models of muscle pain, antagonism of TRPV1 prevents mechanical hyperalgesia caused by eccentric contractions, but not by intramuscular injection of the inflammatory agent carrageenan (Fujii et al. 2008). Studies in knockout animals confirm the contribution of TRPV1 to the mechanical hyperalgesia caused by lengthening muscle contractions and also suggest that TRPV4 plays an important role in the generation of hyperalgesia (Ota et al. 2013). TRPV1 knockout mice also show decreased thermal hyperalgesia, but not mechanical hyperalgesia, in the carrageen muscle inflammation model (Walder et al. 2012). Thus, several members of the TRP family contribute to muscle hyperalgesia, likely by responding to distinct stimuli produced by tissue damage, inflammation, and muscle activity.
Models of Muscle Pain
Muscle pain has been studied using multiple approaches, both in terms of measuring and inducing pain. Muscle pain is commonly studied in the gastrocnemius (Hoheisel et al. 1993; Sluka et al. 2001) and masseter (Cairns et al. 2001; Dessem et al. 2010) muscles. Assessment of muscle pain is typically done by applying force-sensitive instruments to the muscle belly and observing the threshold at which a nocifensive behavior is triggered (Tillu et al. 2007; Khasar et al. 2009; Nasu et al. 2010).
Induction of muscle pain can be grouped into three broad categories–methods that rely on application of noxious compounds to muscle tissue, methods that use muscle activity, and methods that use stressful conditions to produce pain. Noxious substances including, but not limited to, acidic saline (Sluka et al. 2001), hypertonic saline (Ro et al. 2007), carrageenan (Diehl et al. 1988; Radhakrishnan et al. 2003), mustard oil(Han et al. 2008), complete freund’s adjuvant(CFA) (Ambalavanar et al. 2007; Chacur et al. 2009), and PGE2 combined with carrageenan (Dina et al. 2008; Dina et al. 2011) are commonly used to produce muscle hypersensitivity. Of these, repeated acidic saline (pH 4 in 0.9% saline) (Sluka et al. 2001), CFA (Ambalavanar et al. 2007; Chacur et al. 2009), carrageenan (3% in 0.9% saline)(Diehl et al. 1988; Radhakrishnan et al. 2003), and PGE2 (Dina et al. 2008; Dina et al. 2011) injections produce long-lasting enhanced pain behavior in animals. Notably, in each of these paradigms, the enhanced pain behaviors last substantially longer than inflammatory or histological changes in the affected muscle. Further, both the repeated acidic saline injections and single injection of carrageenan produced widespread muscle hypersensitivity in the untreated contralateral muscle, indicating that these methods of pain induction may recruit central mechanisms of sensitization (Coderre and Melzack 1985; Coderre and Melzack 1987).
Fatiguing exercise has good face validity for the induction of muscle pain as many painful muscle conditions either result from damage during use (Gibson et al. 2009) or demonstrate exacerbation of pain with activity (Staud 2007). Eccentric contractions, in which a muscle lengthens during contraction, produce measurable tissue damage and inflammation and are associated with delayed onset muscle soreness (DOMS) (Proske and Morgan 2001). Animal models consist of stimulating muscle contractions with electrical pulses while applying a stronger mechanical force to the limb in the opposite direction, thus forcing the muscle to lengthen (Taguchi et al. 2005). This reflects the real-life condition of being handed an object and, finding that it is too heavy, easing the object to the ground. In this sense, eccentric contractions may serve as a model for muscle over-use or high intensity effort, which often results in temporary soreness. Another approach uses muscle activity to enhance muscle hypersensitivity to low-threshold insults. In this method, a sub-threshold stimulus like 0.03% carrageenan or pH 5 saline is injected into the muscle in combination with an exercise task. No changes in pain behavior are observed when these are given alone, but when combined with muscle activity, animals show robust, long-lasting and wide-spread mechanical hypersensitivity (Yokoyama et al. 2007; Sluka and Rasmussen 2010; Gregory et al. 2013). Further, a prior inflammatory stimulus, such as carrageenan, can prime the muscle to respond in an exaggerated way to a subsequent stimulus, such as PGE2 (Aley et al. 2000; Parada et al. 2003; Dina et al. 2008). Thus, multiple muscle insults can be combined to produce a long-lasting and widespread hyperalgesia that persists despite minimal tissue damage.
Finally, stress has been used to induce widespread muscle pain. Diseases of chronic muscle pain, including FM and MPS, are associated with anxiety and disturbances in stress response (Staud 2002; Martinez-Lavin 2007). Common methods for inducing stress are exposure to water (Green et al. 2011), unexpected blasts of noise (Strausbaugh et al. 2003; Khasar et al. 2005; Khasar et al. 2009), and cooling of ambient temperature (Nasu et al. 2010). These approaches produce long lasting mechanical hypersensitivity in the muscle and exaggerated responses to subsequent mild muscle insults (Alvarez et al. 2013).
Chronic Pain Mechanisms
Chronic pain has been defined in two ways–the duration of the pain and the underlying mechanisms for the pain. In the first case, any pain that exceeds some duration can be chronic pain and no assertions about the mechanisms of that pain are made. Levine and others suggest, however, that long lasting (chronic) pain can be broken down into two types. The first type of is the result of a repeated or persistent acute injury. When the source of acute pain is removed, the pain rapidly subsides. The second type may be initiated by some acute injury, but the pain continues even after the injury is resolved because of plastic changes in the nociceptive system (Reichling et al. 2013). This second type of long lasting pain includes diseases like fibromyalgia and is the most difficult to treat.
Peripheral Mechanisms
Peripheral sensitization involves changes in the function of the afferent terminals of nociceptive neurons. These changes can occur at the level of the receptor, intracellular signaling, or excitability of the cell. Likely, peripheral sensitization involves a synergistic combination of all three. Peripheral sensitization can occur after either inflammatory or non-inflammatory events.
Prostaglandins are released in settings of tissue damage and can result in long-lasting muscle pain. In naive mice, application of PGE2 results in a short-lasting, acute pain; however, after treatment with carrageenan, PGE2 triggers a long lasting mechanical hypersensitivity. The shorter-duration pain in naive animals is mediated by activation of protein kinase A by adenylyl cyclase and subsequent cAMP production (Reichling and Levine 2009). The longer-duration pain in carrageenan-treated animals, on the other hand, appears to be mediated by a change from Gs to Gi/o signaling downstream of PGE2 that requires protein kinase C epsilon (PKCε) (Khasar et al. 2008). In a process that requires the EP1 receptor, recruitment of PKCε leads to the sensitization of TRPV1 by activating phospholipase C and subsequently reducing concentrations of phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid that normally inhibits TRPV1 function (Moriyama et al. 2005). PGE2 binding to another receptor, EP3, results in activation of protein kinase A (PKA) which in turn phosphorylates members of the P2X receptor family, among other targets. This enhances their response to ATP (Wang et al. 2007). Further, PGE2 application can enhance the response to acidic solutions (Rukwied et al. 2007).
Bradykinin is also released during inflammation and results in mechanical hypersensitivity (Murase et al. 2010). Bradykinin activates intracellular signaling pathways by binding to the B1 and B2 receptors. Several mitogen activated protein kinases (MAPKs) are activated by bradykinin binding to either of these receptors, including p38 and JNK. Further, B1 signaling can recruit PKC-ε, which may result in a similar activation of TRPV1, though this has not been tested directly (Meotti et al. 2012).
NGF is also released in the muscle during exercise (Murase et al. 2010; Urai et al. 2013) and after muscle incision(Wu et al. 2009). NGF is painful in both humans (Svensson et al. 2003; Deising et al. 2012) and animals (Woolf et al. 1994; Hathway and Fitzgerald 2006). NGF binds two receptors–TrkA and p75NTR (Eibl et al. 2012). NGF binding to TrkA activates the tyrosine kinase domain of TrkA, which triggers pathways involving ERK1/2, Akt, and PLCγ (Chao et al. 2006). NGF-enhanced pain in eccentric muscle contractions and ischemia is dependent on TRPV1, consistent with NGF’s recruitment of PLC (Xing et al. 2009; Ota et al. 2013). Further, application of NGF to muscle can strengthen the synapses of primary afferent projections to the dorsal horn, though it is unclear if this is independent from or a consequence of enhanced sensitivity of receptors at the terminal (Lewin et al. 1992).
Non-inflammatory mechanisms of peripheral sensitization are less well understood. Repeated acidic saline injections result in long-lasting muscle pain and are associated with lymphoplasmacytic infiltrates (Sluka et al. 2001), though these infiltrates do not appear to be necessary for mechanical hypersensitivity (Gregory et al. 2013). ASIC3 is necessary for long-lasting mechanical hypersensitivity in muscle (Sluka et al. 2003), but the role of ASIC3 in mediating this effect is unclear. Repeated acidic saline injections reduce substance P (SP) release from peripheral terminals. SP is typically considered an algesic substance, but signaling through the NK1 receptor at the terminal activates M-type potassium channels that reduce primary afferent excitability. Thus, by inhibiting SP, repeated acidic saline injections may enhance neuronal excitability through ASIC3 (Lin et al. 2011).
In the sound stress-induced muscle pain model, epinephrine is critical for maintaining the long-lasting mechanical hyperalgesia in rats (Khasar et al. 2009). Sustained exposure to epinephrine, either from stress or exogenous sources, switches intracellular signaling via EP receptors from Gs to Gi/o, resulting in activation of PCKε (Khasar et al. 2008). Prolonged exposure to epinephrine also triggers elevated expression of IL-6 and TNFR1 (Alvarez et al. 2013).
Peripheral sensitization is an important component of chronic muscle pain. Enhanced sensitivity of peripheral receptors may account for pain sensation in response to normally non-painful stimuli. Further, the enhanced excitability may translate into more frequent action potential firing, which may set the stage for alterations in the central nervous system that can continue to enhance muscle pain and spread it beyond the affected tissue (Staud et al. 2009).
Central Mechanisms
Central sensitization refers to changes in the brain and spinal cord that result in enhanced nociceptive processing. The processes underlying central sensitization are not well understood, but central changes may begin with enhanced peripheral input (Staud et al. 2009). Manipulation of muscle with inflammatory substances or eccentric contractions not only produces peripheral sensitization, but also results in changes in the dorsal horn that correlate with nocifensive behavior (Mebane et al. 2003; Sluka et al. 2003; Taguchi et al. 2005; Chacur et al. 2009). Noxious stimulation of the muscle results in expansion of the receptive field for dorsal horn neurons (Hoheisel and Mense 1989; Hoheisel et al. 1993; Sluka et al. 2003; Chacur et al. 2009). Further, repeated injection of acidic saline into the muscle increases concentration of glutamate and aspartate in the spinal cord(Skyba et al. 2005) and phosphorylation of the NR1 subunit of the NMDA receptor(Bement and Sluka 2007). Antagonism of either NMDA or non-NMDA type glutamate receptors reverses mechanical hyperalgesia after acidic saline injection(Skyba et al. 2002). Microglial activation in the spinal cord has been shown in an inflammatory model of muscle pain and inhibition of microglia with minocycline reverses behavioral measures of pain (Chacur et al. 2009).
In the acid-saline model, removal of afferent input does not alter the widespread hyperalgesia once fully developed; supporting the idea that hyperalgesia in this model is maintained by changes in the central nervous system(Sluka et al. 2001). However, in other models like the hyperalgesic priming model, blockade of PCKε in primary afferent fibers reversed the hyperalgesia. Thus, while nociceptive input is clearly necessary for initiation of muscle pain, changes in peripheral nociceptors and/or changes in central pathways could maintain the hyperalgesia. Whether plasticity in peripheral or central pathways plays a predominant role may depend on the animal model and likely mimics the diversity observed in clinical populations.
Several subcortical structures have been implicated in chronic muscle pain. Descending input from the vagus nucleus modifies baseline nociceptive thresholds and the effects of bradykinin on muscle pain (Khasar et al. 2003b; Khasar et al. 2003c). The RVM shows increased staining of phosphorylated NR1 subunit of the NMDA receptor after repeated acidic saline injection (Da Silva et al. 2010a). The hyperalgesia caused by repeated acidic saline injections is absent in animals lacking the NR1 subunit and is reversed by injection of NMDA receptor antagonists or ropivicaine into the RVM (Tillu et al. 2007; Da Silva et al. 2010a; Da Silva et al. 2010b; Sluka et al. 2012). Further, acidic saline injection into the muscle increased staining of phosphorylated ERK in the central nucleus of the amygdala, piriform cortex, paraventricular hypothalamic nucleus, and anterior nucleus of the paraventricular thalamus, and the intensity of the pERK signal correlated with muscle pain. Interestingly, inhibition of ERK in the anterior nucleus of the paraventricular thalamus was sufficient to prevent long lasting muscle pain (Chen et al. 2010; Min et al. 2011).
The contribution of cortical structures to chronic muscle pain is less well understood. A functional MRI study found that when female fibromyalgia patients were given an intramuscular solution of protons and PGE2, more regions of the cortex – specifically, the left anterior insula ipsilateral to treatment – were activated as compared to healthy controls (Diers et al. 2011). A structural analysis of the brains of female fibromyalgia patients found a greater striatal grey matter volume in fibromyalgia patients as compared to healthy controls(Schmidt-Wilcke et al. 2007). The significance of these patterns is unclear. More work must be done to understand potential contributions of cortical structures to chronic muscle pain.
Sex Differences in Chronic Muscle Pain
While substantial sex differences have been observed in diseases of chronic muscle pain, pain mechanism research has primarily focused on males. Most chronic muscle pain conditions are more common in women, with fibromyalgia affecting up to ten times more women than men, depending on the diagnostic criteria. In muscle, a study of eccentric exercise in humans found no significant sex differences in the levels of myoglobin, TNFα, IL1β, and nitric oxide, suggesting that the muscles of males and females are equally prone to damage under strenuous conditions (Dannecker et al. 2012). Instead of at the muscle, sex differences in muscle pain appear to be mediated by variations in the sensitivity of peripheral terminals and processing in central structures. Peripheral injection of NMDA into the masseter muscle evoked greater and longer lasting pain in human females(Cairns et al. 2001; Castrillon et al. 2012). In animals, this same treatment evoked more intense nociceptor firing (Cairns et al. 2001). This NMDA effect is modulated by peripheral treatment with estrogen, suggesting that estrogen modifies NMDA receptors, either directly or indirectly. A similar effect has been found in the epinephrine-induced muscle pain model, with greater mechanical sensitivity observed in female rats. Estrogen contributes to sex differences by enhancing signaling through PKCε and PKA in nociceptive neurons (Dina et al. 2001; Hucho et al. 2006). Thus, circulating or local estrogen may contribute to sex differences in pain by directly modifying the sensitivity of nociceptors through multiple mechanisms.
Sexual dimorphism in the central processing of pain has not been well-studied. However, central processing may be an important link in the development of chronic, widespread muscle pain. Specifically, low-intensity muscle insults (local fatigue and muscle insult), separated by time or distance, produce hyperalgesia that is greater, longer-lasting, and more widespread in female mice when compared to male mice. These data suggest sex differences in the way stimuli are processed centrally may increase the risk for developing widespread pain in female mice. In this model, sex differences are explained neither by changes in the RVM, an area of the brainstem implicated in exercise-enhanced pain, nor by circulating estrogen (Tillu et al. 2007; Da Silva et al. 2010a; Gregory et al. 2013). In contrast, whole-body-fatigue-induced pain results in enhanced hyperalgesia in female mice that is dependent on circulating estrogen (Sluka and Rasmussen 2010). Thus different models may show different mechanisms underlying sexual dimorphism. Greater excitability of dorsal horn neurons in female rats may contribute to such sex differences (Ji et al. 2012). Further, sexual dimorphism in the autonomic system may also contribute, as females differ substantially in their response to vagotomy and medullectomy in both bradykinin- and epinephrine-evoked muscle pain (Khasar et al. 2003c). Although the source of this dimorphism remains unclear, gonadectomy of juvenile animals does not reverse this effect (Khasar et al. 2003a). Whether sex differences in the exercise- or epinephrine-enhanced pain are due to organizational effects of sex hormones during development, or some other mechanism, requires further examination (Greenspan et al. 2007).
Conclusion
Chronic muscle pain remains an important, but poorly understood clinical issue. Muscle pain is mediated by nerve endings specialized for the detection of ischemia, mechanical forces, and tissue damage within muscle tissue. These peripheral nerves transmit nociceptive information to anatomically distinct regions of the spinal cord and brain, which may contribute to the substantial differences in the experience of muscle pain as compared to skin pain. Further, activation of these distinct regions may contribute to the affective symptoms seen in some diseases associated with chronic muscle pain. The mechanisms underlying the development and maintenance of chronic muscle pain are still poorly understood. Figure 1 shows a schematic diagram underlying the multiple factors that can contribute to the development of chronic muscle pain. Development of chronic muscle pain is influenced not only by muscle insults, but also by activity level, stress, fatigue, and sex. These factors can result in molecular and cellular changes in both nociceptors and in central neurons that result in sensitization to maintain the pain. Furthermore, peripheral and central pathways can interact to further enhance the observed molecular and cellular changes and enhance pain. Thus, chronic muscle pain involves peripheral modification of receptors as well as alterations of nociceptive neurons and central processing. Sex differences are similarly not well understood. While sex hormones in the adult may contribute to some of the difference, they clearly are not the only factor. Further studies are needed to better understand both the transition to chronic pain and the sex differences in chronic muscle pain.
Figure 1.
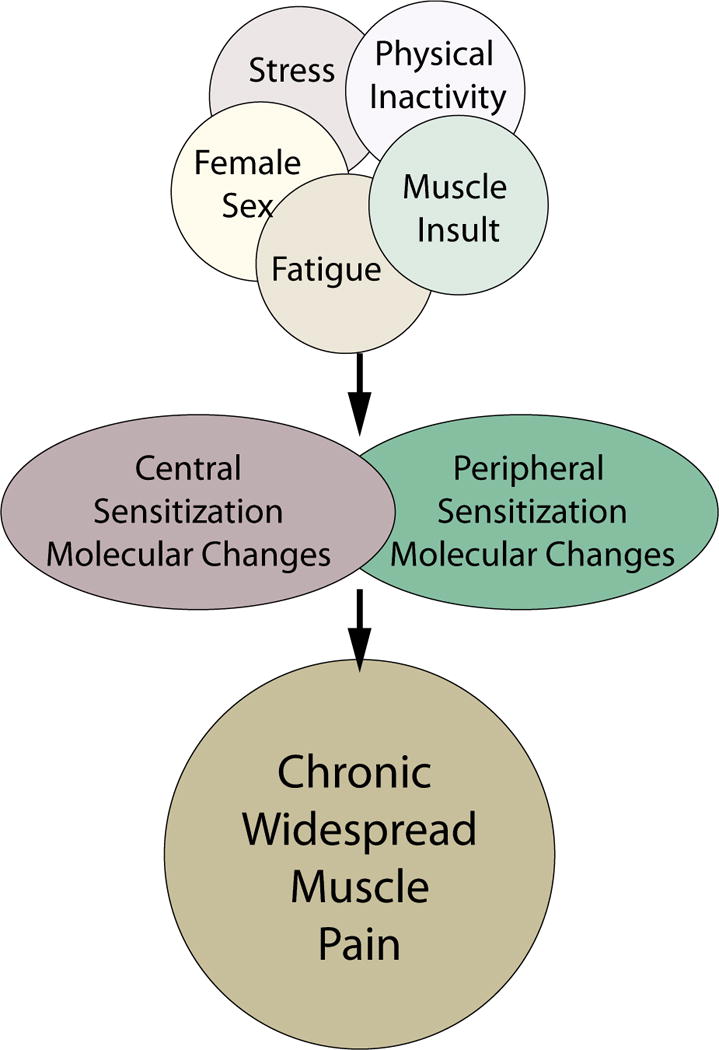
Schematic diagram outline factors that contribute to the development of widespread and long-lasting muscle pain. We propose that combination of multiple stressors result in peripheral and/or central sensitization to produce widespread and long lasting muscle pain. These stressors can include multiple muscle insults separated in time and space, or they include physical inactivity combine with muscle insult, fatigue combined with muscle insult, or stress combined with muscle insult. Further sex influences the development of chronic muscle pain with females more likely to develop chronic muscle pain. Molecular and cellular changes can occur anywhere along the nociceptive pathways from the nociceptor to the cortical neuron and are likely involved in driving the chronic muscle pain and hyperalgesia. Lastly, the peripheral and central pathways can interact with each other to result in enhanced chronic pain.
Footnotes
Contributing Author: Nicholas Gregory, Neuroscience Graduate Program, 3144 Med Labs, University of Iowa, Iowa City, IA, 52246, 319 384-4442, Nicholas-s-gregory@uiowa.edu
References
- Ahacic K, Kåreholt I. Prevalence of musculoskeletal pain in the general Swedish population from 1968 to 2002: age, period, and cohort patterns. PAIN. 2010;151:206–214. doi: 10.1016/j.pain.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Green PG, Levine JD. Stress in the Adult Rat Exacerbates Muscle Pain Induced by Early-Life Stress. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambalavanar R, Yallampalli C, Yallampalli U, Dessem D. Injection of adjuvant but not acidic saline into craniofacial muscle evokes nociceptive behaviors and neuropeptide expression. Neuroscience. 2007;149:650–659. doi: 10.1016/j.neuroscience.2007.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo LF, Costa-Pereira A, Mendonça L, et al. Epidemiology of chronic pain: a population-based nationwide study on its prevalence, characteristics and associated disability in Portugal. J Pain. 2012;13:773–783. doi: 10.1016/j.jpain.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Johansen L, Graham T, Saltin B. Lactate and H+ effluxes from human skeletal muscles during intense, dynamic exercise. J Physiol (Lond) 1993;462:115–133. doi: 10.1113/jphysiol.1993.sp019546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabas ME, Stucky CL. TRPV1, but not TRPA1, in primary sensory neurons contributes to cutaneous incision-mediated hypersensitivity. Molecular pain. 2013;9:9. doi: 10.1186/1744-8069-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement MKH, Sluka KA. Co-localization of p-CREB and p-NR1 in spinothalamic neurons in a chronic muscle pain model. Neurosci Lett. 2007;418:22–27. doi: 10.1016/j.neulet.2007.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RM. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol. 2007;21:427–445. doi: 10.1016/j.berh.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Birdsong WT, Fierro L, Williams FG, et al. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–749. doi: 10.1016/j.neuron.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA, Hittelman KJ, Faulkner JA, Beyer RE. Tissue temperatures and whole-animal oxygen consumption after exercise. 1971 doi: 10.1152/ajplegacy.1971.221.2.427. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Hu JW, Arendt-Nielsen L, et al. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2001;86:782–791. doi: 10.1152/jn.2001.86.2.782. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Kassuya CAL, André E, Ferreira J. Contribution of natural products to the discovery of the transient receptor potential (TRP) channels family and their functions. Pharmacol Ther. 2005;106:179–208. doi: 10.1016/j.pharmthera.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Campanucci VA, Zhang M, Vollmer C, Nurse CA. Expression of multiple P2X receptors by glossopharyngeal neurons projecting to rat carotid body O2-chemoreceptors: role in nitric oxide-mediated efferent inhibition. J Neurosci. 2006;26:9482–9493. doi: 10.1523/JNEUROSCI.1672-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon EE, Cairns BE, Wang K, et al. Comparison of glutamate-evoked pain between the temporalis and masseter muscles in men and women. PAIN. 2012;153:823–829. doi: 10.1016/j.pain.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Chacur M, Lambertz D, Hoheisel U, Mense S. Role of spinal microglia in myositis-induced central sensitisation: an immunohistochemical and behavioural study in rats. Eur J Pain. 2009;13:915–923. doi: 10.1016/j.ejpain.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci. 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, et al. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Chen W-K, Liu IY, Chang Y-T, et al. Ca(v)3.2 T-type Ca2+ channel-dependent activation of ERK in paraventricular thalamus modulates acid-induced chronic muscle pain. J Neurosci. 2010;30:10360–10368. doi: 10.1523/JNEUROSCI.1041-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S-J, Chen C-C, Yang H-W, et al. Role of extracellular signal-regulated kinase in synaptic transmission and plasticity of a nociceptive input on capsular central amygdaloid neurons in normal and acid-induced muscle pain mice. J Neurosci. 2011;31:2258–2270. doi: 10.1523/JNEUROSCI.5564-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25:173–183. doi: 10.1016/j.berh.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. Cutaneous hyperalgesia: contributions of the peripheral and central nervous systems to the increase in pain sensitivity after injury. Brain Res. 1987;404:95–106. doi: 10.1016/0006-8993(87)91359-x. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. Increased pain sensitivity following heat injury involves a central mechanism. Behavioural brain research. 1985;15:259–262. doi: 10.1016/0166-4328(85)90181-0. [DOI] [PubMed] [Google Scholar]
- Connor M, Naves LA, McCleskey EW. Contrasting phenotypes of putative proprioceptive and nociceptive trigeminal neurons innervating jaw muscle in rat. Molecular pain. 2005;1:31. doi: 10.1186/1744-8069-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva LF, DeSantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. J Pain. 2010a;11:378–387. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva LFS, Walder RY, Davidson BL, et al. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulate pain behaviors. PAIN. 2010b;151:155–161. doi: 10.1016/j.pain.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannecker EA, Liu Y, Rector RS, et al. Sex Differences in Exercise-Induced Muscle Pain and Muscle Damage. The Journal of Pain. 2012;13:1242–1249. doi: 10.1016/j.jpain.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Proost I, Pintelon I, Wilkinson WJ, et al. Purinergic signaling in the pulmonary neuroepithelial body microenvironment unraveled by live cell imaging. FASEB J. 2009;23:1153–1160. doi: 10.1096/fj.08-109579. [DOI] [PubMed] [Google Scholar]
- Deising S, Weinkauf B, Blunk J, et al. NGF-evoked sensitization of muscle fascia nociceptors in humans. PAIN. 2012:1–7. doi: 10.1016/j.pain.2012.04.033. [DOI] [PubMed] [Google Scholar]
- Delliaux S, Brerro-Saby C, Steinberg JG, Jammes Y. Reactive oxygen species activate the group IV muscle afferents in resting and exercising muscle in rats. Pflugers Arch. 2009;459:143–150. doi: 10.1007/s00424-009-0713-8. [DOI] [PubMed] [Google Scholar]
- Dessem D, Ambalavanar R, Evancho M, et al. Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X(3) expression in a functionally relevant manner. PAIN. 2010;149:284–295. doi: 10.1016/j.pain.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E, Noël J, Lay N, et al. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;27:3047–3055. doi: 10.1038/emboj.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl B, Hoheisel U, Mense S. Histological and neurophysiological changes induced by carrageenan in skeletal muscle of cat and rat. Agents Actions. 1988;25:210–213. doi: 10.1007/BF01965013. [DOI] [PubMed] [Google Scholar]
- Diers M, Schley MT, Rance M, et al. Differential central pain processing following repetitive intramuscular proton/prostaglandin E2 injections in female fibromyalgia patients and healthy controls. Eur J Pain. 2011;15:716–723. doi: 10.1016/j.ejpain.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Dina OA, Aley KO, Isenberg W, et al. Sex hormones regulate the contribution of PKCε and PKA signalling in inflammatory pain in the rat. Eur J Neurosci. 2001;13:2227–2233. doi: 10.1046/j.0953-816x.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase Cepsilon-dependent chronic-latent muscle pain. The Journal of Pain. 2008;9:457–462. doi: 10.1016/j.jpain.2008.01.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Levine JD, Green PG. Enhanced cytokine-induced mechanical hyperalgesia in skeletal muscle produced by a novel mechanism in rats exposed to unpredictable sound stress. Eur J Pain. 2011;15:796–800. doi: 10.1016/j.ejpain.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibl JK, Strasser BC, Ross GM. Structural, biological, and pharmacological strategies for the inhibition of nerve growth factor. Neurochem Int. 2012;61:1266–1275. doi: 10.1016/j.neuint.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Ernberg M, Hedenberg-Magnusson B, Alstergren P, Kopp S. The level of serotonin in the superficial masseter muscle in relation to local pain and allodynia. Life Sci. 1999;65:313–325. doi: 10.1016/s0024-3205(99)00250-7. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Brooks SV, Opiteck JA. Injury to skeletal muscle fibers during contractions: conditions of occurrence and prevention. Phys Ther. 1993;73:911–921. doi: 10.1093/ptj/73.12.911. [DOI] [PubMed] [Google Scholar]
- Foreman RD, Schmidt RF, Willis WD. Effects of mechanical and chemical stimulation of fine muscle afferents upon primate spinothalamic tract cells. J Physiol (Lond) 1979;286:215–231. doi: 10.1113/jphysiol.1979.sp012615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey Law LA, Sluka KA, McMullen T, et al. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. PAIN. 2008;140:254–264. doi: 10.1016/j.pain.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XW, Nurse CA, Cutz E. Expression of functional purinergic receptors in pulmonary neuroepithelial bodies and their role in hypoxia chemotransmission. Biol Chem. 2004;385:275–284. doi: 10.1515/BC.2004.022. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Ozaki N, Taguchi T, et al. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. PAIN. 2008;140:292–304. doi: 10.1016/j.pain.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Gao Z, Li JD, Sinoway LI, Li J. Effect of muscle interstitial pH on P2X and TRPV1 receptor-mediated pressor response. J Appl Physiol. 2007;102:2288–2293. doi: 10.1152/japplphysiol.00161.2007. [DOI] [PubMed] [Google Scholar]
- Gautam M, Benson CJ. Acid-sensing ion channels (ASICs) in mouse skeletal muscle afferents are heteromers composed of ASIC1a, ASIC2, and ASIC3 subunits. FASEB J. 2013;27:793–802. doi: 10.1096/fj.12-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W, Arendt-Nielsen L, Taguchi T, et al. Increased pain from muscle fascia following eccentric exercise: animal and human findings. Exp Brain Res. 2009;194:299–308. doi: 10.1007/s00221-008-1699-8. [DOI] [PubMed] [Google Scholar]
- Green DP, Ruparel S, Roman L, et al. Role of endogenous TRPV1 agonists in a postburn pain model of partial-thickness injury. PAIN. 2013 doi: 10.1016/j.pain.2013.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PG, Alvarez P, Gear RW, et al. Further Validation of a Model of Fibromyalgia Syndrome in the Rat. The Journal of Pain. 2011;12:811–818. doi: 10.1016/j.jpain.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007:S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory NS, Gibson-Corley K, Frey-Law L, Sluka KA. Fatigue-enhanced hyperalgesia in response to muscle insult: Induction and development occur in a sex-dependent manner. PAIN. 2013 doi: 10.1016/j.pain.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SR, Lee MK, Lim KH, et al. Intramuscular administration of morphine reduces mustard-oil-induced craniofacial-muscle pain behavior in lightly anesthetized rats. Eur J Pain. 2008;12:361–370. doi: 10.1016/j.ejpain.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol. 2004;96:1166–1169. doi: 10.1152/japplphysiol.01020.2003. [DOI] [PubMed] [Google Scholar]
- Hathway G, Fitzgerald M. Time Course and Dose-Dependence of Nerve Growth Factor–Induced Secondary Hyperalgesia in the Mouse. The Journal of Pain. 2006;7:57–61. doi: 10.1016/j.jpain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Hertel HC, Howaldt B, Mense S. Responses of group IV and group III muscle afferents to thermal stimuli. Brain Res. 1976;113:201–205. doi: 10.1016/0006-8993(76)90020-2. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Mense S. Long-term changes in discharge behaviour of cat dorsal horn neurones following noxious stimulation of deep tissues. PAIN. 1989;36:239–247. doi: 10.1016/0304-3959(89)90029-8. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Mense S, Simons DG, Yu XM. Appearance of new receptive fields in rat dorsal horn neurons following noxious stimulation of skeletal muscle: a model for referral of muscle pain? Neurosci Lett. 1993;153:9–12. doi: 10.1016/0304-3940(93)90064-r. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Reinöhl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. PAIN. 2004;110:149–157. doi: 10.1016/j.pain.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Honore P, Kage K, Mikusa J, et al. Analgesic profile of intrathecal P2X(3) antisense oligonucleotide treatment in chronic inflammatory and neuropathic pain states in rats. PAIN. 2002;99:11–19. doi: 10.1016/s0304-3959(02)00032-5. [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Kuhn J, Levine JD. Estrogen controls PKCepsilon-dependent mechanical hyperalgesia through direct action on nociceptive neurons. Eur J Neurosci. 2006;24:527–534. doi: 10.1111/j.1460-9568.2006.04913.x. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Kolker SJ, Burnes LA, et al. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. PAIN. 2008;137:662–669. doi: 10.1016/j.pain.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- Inoue R, Jian Z, Kawarabayashi Y. Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Ther. 2009;123:371–385. doi: 10.1016/j.pharmthera.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Ito N, Ruegg UT, Kudo A, et al. Capsaicin mimics mechanical load-induced intracellular signaling events: involvement of TRPV1-mediated calcium signaling in induction of skeletal muscle hypertrophy. Channels (Austin) 2013;7:221–224. doi: 10.4161/chan.24583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Ekmann KM, et al. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol. 2013;109:2374–2381. doi: 10.1152/jn.01067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Tang B, Cao D-Y, et al. Sex differences in spinal processing of transient and inflammatory colorectal stimuli in the rat. PAIN. 2012;153:1965–1973. doi: 10.1016/j.pain.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaan TKY, Yip PK, Patel S, et al. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain. 2010;133:2549–2564. doi: 10.1093/brain/awq194. [DOI] [PubMed] [Google Scholar]
- Karamouzis M, Karamouzis I, Vamvakoudis E, et al. The response of muscle interstitial prostaglandin E2(PGE2), prostacyclin I2(PGI2) and thromboxane A2(TXA2) levels during incremental dynamic exercise in humans determined by in vivo microdialysis. Prostaglandins Leukot Essent Fatty Acids. 2001;64:259–263. doi: 10.1054/plef.2001.0269. [DOI] [PubMed] [Google Scholar]
- Kawakita K, Dostrovsky JO, Tang JS, Chiang CY. Responses of neurons in the rat thalamic nucleus submedius to cutaneous, muscle and visceral nociceptive stimuli. PAIN. 1993;55:327–338. doi: 10.1016/0304-3959(93)90008-D. [DOI] [PubMed] [Google Scholar]
- Keay KA, Li QF, Bandler R. Muscle pain activates a direct projection from ventrolateral periaqueductal gray to rostral ventrolateral medulla in rats. Neurosci Lett. 2000;290:157–160. doi: 10.1016/s0304-3940(00)01329-x. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Burkham J, Dina OA, et al. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Dina OA, Green PG, Levine JD. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J Pain. 2009;10:1073–1077. doi: 10.1016/j.jpain.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Green PG, Gear RW, et al. Gonadal hormones do not account for sexual dimorphism in vagal modulation of nociception in the rat. The Journal of Pain. 2003a;4:190–196. doi: 10.1016/s1526-5900(03)00560-1. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. PAIN. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Green PG, Miao FJ-P, Levine JD. Vagal modulation of nociception is mediated by adrenomedullary epinephrine in the rat. Eur J Neurosci. 2003b;17:909–915. doi: 10.1046/j.1460-9568.2003.02503.x. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Miao FJ-P, Gear RW, et al. Vagal modulation of bradykinin-induced mechanical hyperalgesia in the female rat. The Journal of Pain. 2003c;4:278–283. doi: 10.1016/s1526-5900(03)00631-x. [DOI] [PubMed] [Google Scholar]
- Kindig AE, Heller TB, Kaufman MP. VR-1 receptor blockade attenuates the pressor response to capsaicin but has no effect on the pressor response to contraction in cats. Am J Physiol Heart Circ Physiol. 2005;288:H1867–73. doi: 10.1152/ajpheart.00735.2004. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Mense S, Schmidt RF. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res. 1978 doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Mizumura K. Responses of neurons in VPL and VPL-VL region of the cat to algesic stimulation of muscle and tendon. J Neurophysiol. 1983;49:649–661. doi: 10.1152/jn.1983.49.3.649. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, et al. Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J Comp Neurol. 2005;481:377–390. doi: 10.1002/cne.20393. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol (Lond) 1977;273:179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Winter J, McMahon SB. Regulation of Afferent Connectivity in the Adult Spinal Cord by Nerve Growth Factor. European Journal of Neuroscience. 1992;4:700–707. doi: 10.1111/j.1460-9568.1992.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol. 2003;95:577–583. doi: 10.1152/japplphysiol.00185.2003. [DOI] [PubMed] [Google Scholar]
- Li J, King NC, Sinoway LI. Interstitial ATP and norepinephrine concentrations in active muscle. Circulation. 2005;111:2748–2751. doi: 10.1161/CIRCULATIONAHA.104.510669. [DOI] [PubMed] [Google Scholar]
- Li J, Maile MD, Sinoway AN, Sinoway LI. Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J Appl Physiol. 2004;97:1709–1714. doi: 10.1152/japplphysiol.00389.2004. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol. 2002;283:H2636–43. doi: 10.1152/ajpheart.00395.2002. [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, et al. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-CJ, Chen W-N, Chen C-J, et al. An antinociceptive role for substance P in acid-induced chronic muscle pain. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1108903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L-J, Honda T, Shimada Y, et al. Central projection of unmyelinated (C) primary afferent fibers from gastrocnemius muscle in the guinea pig. J Comp Neurol. 2003;461:140–150. doi: 10.1002/cne.10619. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, De Weille JR, Bassilana F, et al. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Procko E, Jin X, et al. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Lloyd DPC, Chang HT. Afferent fibers in muscle nerves. J Neurophysiol. 1948;11:199–207. doi: 10.1152/jn.1948.11.3.199. [DOI] [PubMed] [Google Scholar]
- Luo Z, Ma L, Zhao Z, et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1α upregulation in mice. Cell Res. 2012;22:551–564. doi: 10.1038/cr.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm C, Nyberg P, Engstrom M, et al. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol (Lond) 529 Pt. 2000;1:243–262. doi: 10.1111/j.1469-7793.2000.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin V, Dousset E, Laurin J, et al. Group III and IV muscle afferent discharge patterns after repeated lengthening and shortening actions. Muscle Nerve. 2009;40:827–837. doi: 10.1002/mus.21368. [DOI] [PubMed] [Google Scholar]
- Martinez-Lavin M. Stress, the stress response system, and fibromyalgia. Arthritis Res Ther. 2007 doi: 10.1186/ar2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Lo MW, Hosoya T, et al. Experimental colitis alters expression of 5-HT receptors and transient receptor potential vanilloid 1 leading to visceral hypersensitivity in mice. Lab Invest. 2012;92:769–782. doi: 10.1038/labinvest.2012.14. [DOI] [PubMed] [Google Scholar]
- Mease P. Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. J Rheumatol Suppl. 2005;75:6–21. [PubMed] [Google Scholar]
- Mebane H, Turnbach ME, Randich A. Spinal EP receptors mediating prostaglandin E2-induced mechanical hyperalgesia, thermal hyperalgesia, and touch-evoked allodynia in rats. J Pain. 2003;4:392–399. doi: 10.1016/s1526-5900(03)00715-6. [DOI] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol (Lond) 1977;267:75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, Craig AD. Spinal and supraspinal terminations of primary afferent fibers from the gastrocnemius-soleus muscle in the cat. Neuroscience. 1988;26:1023–1035. doi: 10.1016/0306-4522(88)90117-0. [DOI] [PubMed] [Google Scholar]
- Mense S, Meyer H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. J Physiol (Lond) 1985;363:403–417. doi: 10.1113/jphysiol.1985.sp015718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meotti FC, Campos R, da Silva K, et al. Inflammatory muscle pain is dependent on the activation of kinin B1 and B2 receptors and intracellular kinase pathways. Br J Pharmacol. 2012;166:1127–1139. doi: 10.1111/j.1476-5381.2012.01830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messeguer A, Planells-Cases R, Ferrer-Montiel A. Physiology and pharmacology of the vanilloid receptor. Curr Neuropharmacol. 2006;4:1–15. doi: 10.2174/157015906775202995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min M-Y, Yang H-W, Yen C-T, et al. ERK, synaptic plasticity and acid-induced muscle pain. Commun Integr Biol. 2011;4:394–396. doi: 10.4161/cib.4.4.15694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda H, Kaila-Kangas L, Heliövaara M, et al. Musculoskeletal pain at multiple sites and its effects on work ability in a general working population. Occup Environ Med. 2010;67:449–455. doi: 10.1136/oem.2009.048249. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Immke DC, Fierro L, et al. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Molecular pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Higashi T, Togashi K, et al. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Molecular pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, Terazawa E, Queme F, et al. Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness) J Neurosci. 2010;30:3752–3761. doi: 10.1523/JNEUROSCI.3803-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mørk H, Ashina M, Bendtsen L, et al. Experimental muscle pain and tenderness following infusion of endogenous substances in humans. Eur J Pain. 2003;7:145–153. doi: 10.1016/S1090-3801(02)00096-4. [DOI] [PubMed] [Google Scholar]
- Nakao A, Takahashi Y, Nagase M, et al. Role of capsaicin-sensitive C-fiber afferents in neuropathic pain-induced synaptic potentiation in the nociceptive amygdala. Molecular pain. 2012;8:51. doi: 10.1186/1744-8069-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasu T, Taguchi T, Mizumura K. Persistent deep mechanical hyperalgesia induced by repeated cold stress in rats. Eur J Pain. 2010;14:236–244. doi: 10.1016/j.ejpain.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Oliveira MCG, Pelegrini-da-Silva A, Tambeli CH, Parada CA. Peripheral mechanisms underlying the essential role of P2X3,2/3 receptors in the development of inflammatory hyperalgesia. PAIN. 2009;141:127–134. doi: 10.1016/j.pain.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Ota H, Katanosaka K, Murase S, et al. TRPV1 and TRPV4 play pivotal roles in delayed onset muscle soreness. PLoS ONE. 2013;8:e65751. doi: 10.1371/journal.pone.0065751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Pickar JG, Hill JM, Kaufman MP. Dynamic exercise stimulates group III muscle afferents. J Neurophysiol. 1994;71:753–760. doi: 10.1152/jn.1994.71.2.753. [DOI] [PubMed] [Google Scholar]
- Prado FC, Araldi D, Vieira AS, et al. Neuronal P2X3 receptor activation is essential to the hyperalgesia induced by prostaglandins and sympathomimetic amines released during inflammation. Neuropharmacology. 2013;67:252–258. doi: 10.1016/j.neuropharm.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Prasad M, Fearon IM, Zhang M, et al. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: role in chemosensory signalling. J Physiol (Lond) 2001;537:667–677. doi: 10.1111/j.1469-7793.2001.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol (Lond) 2001;537:333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. PAIN. 2003;104:567–577. doi: 10.1016/s0304-3959(03)00114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Green PG, Levine JD. The fundamental unit of pain is the cell. PAIN. 2013 doi: 10.1016/j.pain.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinöhl J, Hoheisel U, Unger T, Mense S. Adenosine triphosphate as a stimulant for nociceptive and non-nociceptive muscle group IV receptors in the rat. Neurosci Lett. 2003;338:25–28. doi: 10.1016/s0304-3940(02)01360-5. [DOI] [PubMed] [Google Scholar]
- Ro JY, Capra NF, Lee J-S, et al. Hypertonic saline-induced muscle nociception and c-fos activation are partially mediated by peripheral NMDA receptors. Eur J Pain. 2007;11:398–405. doi: 10.1016/j.ejpain.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Ro JY, Lee J-S, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. PAIN. 2009;144:270–277. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukwied R, Chizh BA, Lorenz U, et al. Potentiation of nociceptive responses to low pH injections in humans by prostaglandin E2. The Journal of Pain. 2007;8:443–451. doi: 10.1016/j.jpain.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Sacchetti G, Lampugnani R, Battistini C, Mandelli V. Response of pathological ischaemic muscle pain to analgesics. Br J Clin Pharmacol. 1980;9:165–169. doi: 10.1111/j.1365-2125.1980.tb05828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai J, Obata K, Ozaki N, et al. Activation of extracellular signal-regulated protein kinase in sensory neurons after noxious gastric distention and its involvement in acute visceral pain in rats. Gastroenterology. 2008;134:1094–1103. doi: 10.1053/j.gastro.2008.01.031. [DOI] [PubMed] [Google Scholar]
- Saltin B, Gagge AP, Stolwijk JA. Muscle temperature during submaximal exercise in man. 1968. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Luerding R, Weigand T, et al. Striatal grey matter increase in patients suffering from fibromyalgia–a voxel-based morphometry study. PAIN. 2007;132(Suppl 1):S109–16. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99:1977–1984. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- Sherwood TW, Frey EN, Askwith CC. Structure and activity of the acid-sensing ion channels. Am J Physiol, Cell Physiol. 2012;303:C699–710. doi: 10.1152/ajpcell.00188.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda M, Asano M, Omagari D, et al. Nerve Growth Factor Contribution via Transient Receptor Potential Vanilloid 1 to Ectopic Orofacial Pain. J Neurosci. 2011;31:7145–7155. doi: 10.1523/JNEUROSCI.0481-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda M, Ozaki N, Sugiura Y. Involvement of ATP and its receptors on nociception in rat model of masseter muscle pain. PAIN. 2008;134:148–157. doi: 10.1016/j.pain.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. PAIN. 2002;98:69–78. doi: 10.1016/s0304-3959(01)00471-7. [DOI] [PubMed] [Google Scholar]
- Skyba DA, Lisi TL, Sluka KA. Excitatory amino acid concentrations increase in the spinal cord dorsal horn after repeated intramuscular injection of acidic saline. PAIN. 2005;119:142–149. doi: 10.1016/j.pain.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Danielson J, Rasmussen L, DaSilva LF. Exercise-induced pain requires NMDA receptor activation in the medullary raphe nuclei. Med Sci Sports Exerc. 2012;44:420–427. doi: 10.1249/MSS.0b013e31822f490e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, et al. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. PAIN. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Rasmussen LA. Fatiguing exercise enhances hyperalgesia to muscle inflammation. PAIN. 2010;148:188–197. doi: 10.1016/j.pain.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Kerrin A, Singer CA, et al. Identification of capsaicin-sensitive rectal mechanoreceptors activated by rectal distension in mice. Neuroscience. 2008;153:518–534. doi: 10.1016/j.neuroscience.2008.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey MJ. Free nerve endings in skeletal muscle of the cat. J Anat. 1969;105:231–254. [PMC free article] [PubMed] [Google Scholar]
- Staud R. Evidence of involvement of central neural mechanisms in generating fibromyalgia pain – Springer. Curr Rheumatol Rep. 2002 doi: 10.1007/s11926-002-0038-5. [DOI] [PubMed] [Google Scholar]
- Staud R. Treatment of fibromyalgia and its symptoms. Expert Opin Pharmacother. 2007;8:1629–1642. doi: 10.1517/14656566.8.11.1629. [DOI] [PubMed] [Google Scholar]
- Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. PAIN. 2009;145:96–104. doi: 10.1016/j.pain.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausbaugh HJ, Green PG, Dallman MF, Levine JD. Repeated, non-habituating stress suppresses inflammatory plasma extravasation by a novel, sympathoadrenal dependent mechanism. Eur J Neurosci. 2003;17:805–812. doi: 10.1046/j.1460-9568.2003.02493.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chai TC. Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J Urol. 2004;171:448–452. doi: 10.1097/01.ju.0000099660.46774.3c. [DOI] [PubMed] [Google Scholar]
- Svensson P, Cairns BE, Wang K, Arendt-Nielsen L. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. PAIN. 2003;104:241–247. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Syed N-I-H, Tengah A, Paul A, Kennedy C. Characterisation of P2X receptors expressed in rat pulmonary arteries. Eur J Pharmacol. 2010;649:342–348. doi: 10.1016/j.ejphar.2010.09.041. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Matsuda T, Tamura R, et al. Muscular mechanical hyperalgesia revealed by behavioural pain test and c-Fos expression in the spinal dorsal horn after eccentric contraction in rats. J Physiol (Lond) 2005;564:259–268. doi: 10.1113/jphysiol.2004.079483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Taguchi T, Tanaka S, et al. Painful muscle stimulation preferentially activates emotion-related brain regions compared to painful skin stimulation. Neurosci Res. 2011:1–9. doi: 10.1016/j.neures.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Teixeira JM, Oliveira MCG, Nociti FH, et al. Involvement of temporomandibular joint P2X3 and P2X2/3 receptors in carrageenan-induced inflammatory hyperalgesia in rats. Eur J Pharmacol. 2010;645:79–85. doi: 10.1016/j.ejphar.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Thompson JM. Exercise in muscle pain disorders. PM R. 2012;4:889–893. doi: 10.1016/j.pmrj.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–53. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- Tillu D, Gebhart G, Sluka K. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. PAIN. 2007 doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch. 2005;451:143–150. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi M, Goris RC, Funakoshi K. Differential distribution of vanilloid receptors in the primary sensory neurons projecting to the dorsal skin and muscles. Histochem Cell Biol. 2006;126:343–352. doi: 10.1007/s00418-006-0167-4. [DOI] [PubMed] [Google Scholar]
- Uchida MC, Nosaka K, Ugrinowitsch C, et al. Effect of bench press exercise intensity on muscle soreness and inflammatory mediators. J Sports Sci. 2009;27:499–507. doi: 10.1080/02640410802632144. [DOI] [PubMed] [Google Scholar]
- Urai H, Murase S, Mizumura K. Decreased nerve growth factor upregulation is a mechanism for reduced mechanical hyperalgesia after the second bout of exercise in rats. Scand J Med Sci Sports. 2013;23:e96–101. doi: 10.1111/sms.12013. [DOI] [PubMed] [Google Scholar]
- Valkeinen H, Häkkinen A, Alen M, et al. Physical fitness in postmenopausal women with fibromyalgia. Int J Sports Med. 2008;29:408–413. doi: 10.1055/s-2007-965818. [DOI] [PubMed] [Google Scholar]
- Vázquez-Delgado E, Cascos-Romero J, Gay-Escoda C. Myofascial pain syndrome associated with trigger points: a literature review. (I): Epidemiology, clinical treatment and etiopathogeny. Med Oral Patol Oral Cir Bucal. 2009;14:e494–8. doi: 10.4317/medoral.14.e494. [DOI] [PubMed] [Google Scholar]
- Verbunt JA, Pernot DHFM, Smeets RJEM. Disability and quality of life in patients with fibromyalgia. Health Qual Life Outcomes. 2008;6:8. doi: 10.1186/1477-7525-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder RY, Gautam M, Wilson SP, et al. Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. PAIN. 2011;152:2348–2356. doi: 10.1016/j.pain.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder RY, Radhakrishnan R, Loo L, et al. TRPV1 is important for mechanical and heat sensitivity in uninjured animals and development of heat hypersensitivity after muscle inflammation. PAIN. 2012;153:1664–1672. doi: 10.1016/j.pain.2012.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder RY, Rasmussen LA, Rainier JD, et al. ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury. J Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/S0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, et al. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997a;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, et al. A proton-gated cation channel involved in acid-sensing. Nature. 1997b;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Wang C, Li G-W, Huang L-YM. Prostaglandin E2 potentiation of P2X3 receptor mediated currents in dorsal root ganglion neurons. Molecular pain. 2007;3:22. doi: 10.1186/1744-8069-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Guo W, Ossipov MH, et al. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003;121:815–824. doi: 10.1016/s0306-4522(03)00491-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li G, Yu K, et al. Expressions of P2X2 and P2X3 receptors in rat nodose neurons after myocardial ischemia injury. Auton Neurosci. 2009;145:71–75. doi: 10.1016/j.autneu.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Wang YX, Wang J, Wang C, et al. Functional expression of transient receptor potential vanilloid-related channels in chronically hypoxic human pulmonary arterial smooth muscle cells. J Membr Biol. 2008;223:151–159. doi: 10.1007/s00232-008-9121-9. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Safieh-Garabedian B, Ma QP, et al. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Wu C, Erickson MA, Xu J, et al. Expression Profile of Nerve Growth Factor after Muscle Incision in the Rat. Anesthesiology. 2009;110:140–149. doi: 10.1097/ALN.0b013e318190bc84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JX, Xu MY, Miao XR, et al. Functional up-regulation of P2X3 receptors in dorsal root ganglion in a rat model of bone cancer pain. Eur J Pain. 2012;16:1378–1388. doi: 10.1002/j.1532-2149.2012.00149.x. [DOI] [PubMed] [Google Scholar]
- Wu Z, Yang Q, Crook RJ, et al. TRPV1 channels make major contributions to behavioral hypersensitivity and spontaneous activity in nociceptors after spinal cord injury. PAIN. 2013 doi: 10.1016/j.pain.2013.06.040. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Bo X, Burnstock G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci Lett. 1998;256:105–108. doi: 10.1016/s0304-3940(98)00774-5. [DOI] [PubMed] [Google Scholar]
- Xing J, Gao Z, Lu J, et al. Femoral artery occlusion augments TRPV1-mediated sympathetic responsiveness. Am J Physiol Heart Circ Physiol. 2008;295:H1262–H1269. doi: 10.1152/ajpheart.00271.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Lu J, Li J. J Physiol (Lond) 2011. ASIC3 function and immunolabeling increases in skeletal muscle sensory neurons following femoral artery occlusion. no–no. [DOI] [Google Scholar]
- Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2009;296:H1380–7. doi: 10.1152/ajpheart.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G-Y, Shenoy M, Winston JH, et al. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230–1237. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle fatigue increases the probability of developing hyperalgesia in mice. The Journal of Pain. 2007;8:692–699. doi: 10.1016/j.jpain.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Gibbins IL, Costa M, et al. Properties of the major classes of mechanoreceptors in the guinea pig bladder. J Physiol (Lond) 2007;585:147–163. doi: 10.1113/jphysiol.2007.140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li G, Liang S, et al. Myocardial ischemic nociceptive signaling mediated by P2X3 receptor in rat stellate ganglion neurons. Brain Res Bull. 2008;75:77–82. doi: 10.1016/j.brainresbull.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Zhong B, Wang DH. N-oleoyldopamine, a novel endogenous capsaicin-like lipid, protects the heart against ischemia-reperfusion injury via activation of TRPV1. Am J Physiol Heart Circ Physiol. 2008;295:H728–35. doi: 10.1152/ajpheart.00022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


