Background: Endoglycoceramidase-related protein 1 (EGCrP1) is a glucocerebrosidase in Cryptococcus neoformans; however, the functions of its paralogue, EGCrP2, remain unknown.
Results: EGCrP2, but not EGCrP1, hydrolyzed steryl-β-glucosides. Disruption of egcrp2 accumulated ergosteryl-3β-glucoside in C. neoformans.
Conclusion: EGCrP2 degraded steryl-β-glucosides both in vivo and in vitro.
Significance: EGCrP2 is a missing link in steryl-β-glucoside metabolism in fungi. In addition, EGCrP2 is a potential target for antifungal drugs.
Keywords: Fungi, Glycolipid, Glycosidase, Metabolism, Sterol
Abstract
Cryptococcosis is an infectious disease caused by pathogenic fungi, such as Cryptococcus neoformans and Cryptococcus gattii. The ceramide structure (methyl-d18:2/h18:0) of C. neoformans glucosylceramide (GlcCer) is characteristic and strongly related to its pathogenicity. We recently identified endoglycoceramidase-related protein 1 (EGCrP1) as a glucocerebrosidase in C. neoformans and showed that it was involved in the quality control of GlcCer by eliminating immature GlcCer during the synthesis of GlcCer (Ishibashi, Y., Ikeda, K., Sakaguchi, K., Okino, N., Taguchi, R., and Ito, M. (2012) Quality control of fungus-specific glucosylceramide in Cryptococcus neoformans by endoglycoceramidase-related protein 1 (EGCrP1). J. Biol. Chem. 287, 368–381). We herein identified and characterized EGCrP2, a homologue of EGCrP1, as the enzyme responsible for sterylglucoside catabolism in C. neoformans. In contrast to EGCrP1, which is specific to GlcCer, EGCrP2 hydrolyzed various β-glucosides, including GlcCer, cholesteryl-β-glucoside, ergosteryl-β-glucoside, sitosteryl-β-glucoside, and para-nitrophenyl-β-glucoside, but not α-glucosides or β-galactosides, under acidic conditions. Disruption of the EGCrP2 gene (egcrp2) resulted in the accumulation of a glycolipid, the structure of which was determined following purification to ergosteryl-3β-glucoside, a major sterylglucoside in fungi, by mass spectrometric and two-dimensional nuclear magnetic resonance analyses. This glycolipid accumulated in vacuoles and EGCrP2 was detected in vacuole-enriched fraction. These results indicated that EGCrP2 was involved in the catabolism of ergosteryl-β-glucoside in the vacuoles of C. neoformans. Distinct growth arrest, a dysfunction in cell budding, and an abnormal vacuole morphology were detected in the egcrp2-disrupted mutants, suggesting that EGCrP2 may be a promising target for anti-cryptococcal drugs. EGCrP2, classified into glycohydrolase family 5, is the first steryl-β-glucosidase identified as well as a missing link in sterylglucoside metabolism in fungi.
Introduction
Cryptococcosis is an infectious disease caused by pathogenic fungi, such as Cryptococcus neoformans and Cryptococcus gattii. The prevalence of cryptococcosis has increased over the past 20 years because of the increase in AIDS patients and expanded use of immunosuppressive drugs. More than 600,000 patients with immune deficiencies were reported to have died within 3 months of being infected with C. neoformans (1). The highly virulent C. gattii, a primary pathogen in healthy individuals and animals, was recently detected in the United States and Canada (2). Thus, the development of new drugs against cryptococcosis is urgently needed.
C. neoformans synthesizes a glucosylceramide (GlcCer)3 composed of β-linked glucose and ceramide that possesses a characteristic sphingoid base, which has two double bonds at C4/C8 in the trans conformation and a methyl substitution at C9 (3). Previous studies reported that this fungus-specific GlcCer may be strongly associated with the pathogenicity of C. neoformans; thus, the enzymes involved in the synthesis of GlcCer (e.g. UDP-glucose:ceramide glucosyltransferase (4, 5), sphingoid base C4/C8 desaturase (6, 7), and C9 methyltransferase (8, 9)) have been intensively studied, and the genes responsible for these enzyme activities have also been identified. However, how GlcCer is catabolized in fungi remains unclear because the enzyme(s) responsible for degrading GlcCer in fungi have not yet been identified.
Endoglycoceramidase-related protein 1 (EGCrP1) is a homologue of endoglycoceramidase (EGCase, ceramide glycanase; EC.3.2.1.123), which is an endo-type glycosidase capable of cleaving the β-glycosidic linkage between the ceramide (Cer) and oligosaccharide of various glycosphingolipids (GSLs) to release an intact oligosaccharide and Cer (10, 11). EGCase very weakly hydrolyzes GlcCer, whereas EGCrP1 specifically hydrolyzes GlcCer but not oligosaccharide-linked GSLs, such as LacCer, GM1a, and Gb3Cer, which are favorite substrates for EGCase. Thus, EGCrP1 was the first identified GlcCer-degrading enzyme (glucocerebrosidase) in fungi (12). Although the disruption of egcrp1 in C. neoformans reduced glucocerebrosidase activity under neutral conditions, the activity remained almost unchanged under acidic conditions, suggesting the presence of other glucocerebrosidase(s) that function in C. neoformans under acidic conditions.
Fungi have two major glycolipids: GlcCer and sterylglucoside. The former is related to pathogenicity of fungi (6), and the latter is involved in stress-mediated signal transduction (13). Sterylglucoside synthase (UGT51) was identified in budding yeast (14). However, the enzyme(s) involved in sterylglucoside catabolism have not yet been identified in fungi or yeasts.
We herein report the molecular cloning, enzymatic characterization, and physiological relevance of EGCrP2, a homologue of EGCrP1, in C. neoformans. The specificity of EGCrP2 for aglycone moiety differed completely from that of EGCrP1; the former hydrolyzed not only GlcCer but also various β-glucosides, including steryl-β-glucosides, para-nitrophenyl (pNP)-β-glucoside, and 4-methylumberifellyl (4MU)-β-glucoside, whereas the latter specifically hydrolyzed GlcCer but not the other β-glucosides tested. Neither EGCrP1 nor EGCrP2 hydrolyzed β-galactosides or α-glucosides, indicating that both enzymes were β-glucosidases with different aglycone specificities. The disruption of egcrp2, but not egrcp1, resulted in the accumulation of an unknown glycolipid, which was subsequently identified as an ergosteryl-3β-glucoside (EG) after purification. EG is a major molecular species of sterylglucoside in fungi and yeasts. These results indicated that EGCrP2 functioned in vivo as a steryl-β-glucosidase, which is a missing link in sterylglucoside metabolism in fungi. This study also provided evidence to show that EGCrP2 may be a promising target for the development of anti-fungus drugs for C. neoformans.
EXPERIMENTAL PROCEDURES
Materials
C6–7-nitro-2,1,3-benzoxadiazole (NBD)-Cer, C6-NBD-GlcCer, and C12-NBD-Gb3Cer were purchased from Matreya, and C6-NBD-LacCer, C6-NBD-GalCer, pNP glycosides, 4MU glycosides, and resorufin-β-d-glucopyranoside were from Sigma-Aldrich. C12-NBD-GM1 and C12-NBD-sphingomyelin were prepared using the sphingolipid Cer N-deacylase by the method described previously (15).
Strain and Culture
C. neoformans var. grubii serotype A strain H99 (ATCC 208821) was purchased from the American Type Culture Collection. C. neoformans was cultured at 30 °C in YPD medium (2% Glc, 2% peptone, 1% yeast extract).
Construction of the Expression Vector
Total RNA was obtained from fungus cells using Sepasol-RNA I Super G (Nacalai Tesque). First strand cDNA was synthesized from 1 μg of total RNA using PrimeScript reverse transcriptase (Takara Bio Inc.). To insert the restriction sites, PCR was carried out using first strand cDNA as a template and the expression primers listed in Table 1. Amplification was performed using PrimeSTAR GXL DNA polymerase (Takara Bio Inc.). The amplified product was digested with appropriate restriction enzymes and inserted into the corresponding sites of pET23a (Novagen).
TABLE 1.
Oligonucleotide primers used in this study
Restriction enzyme sites are underlined.
| Oligonucleotide | Sequence (5′–3′) | Aim |
|---|---|---|
| EGCrP-05607S-NotI | ATGGCGGCCGCATGCCTCCTCCACCAGAAGTCTCTCCTGTCA | Expression |
| EGCrP-05607AS-XhoI | ATGCTCGCGAGCAATAACGCATTCAGGACATC | |
| 13CN005607seq-U | GACGGCAAGAACATTCAAGACT | Sequencing |
| 14CN005607seq-D | CGTGAGGGTAGCTGAGGAGTT | |
| CN05607N-U | ATGCCTCCTCCACCAGAAGTCT | Gene disruption |
| CN05607N-AP-D | AATAGCGAGTCCATCGTCGAGATTCAAAGGT | |
| ActinP-U | ACTCGCTATTGTCCAGGCTGC | |
| Act-Nat-Down | CTTCTTCTTCCATAGACATGTTGGGCGAG | |
| Actin-HygroR-D | CTCGACAGACGTCGCGGTGAGCATAGACATGTTGGGCGAGT | |
| Nat-Up | ATGGAAGAAGAAGTCACTCTTGACGACACGGCTTACC | |
| Hygro-U | CTCACCGCGACGTCTGTCGAG | |
| NSL-2 | AAGGTGTTCCCCGACGACGAATCG | |
| NSR-2 | AACTCCGTCGCGAGCCCCATCAAC | |
| HSL | GGATGCCTCCGCTCGAAGTA | |
| HSR | CGTTGCAAGACCTGCCTGAA | |
| Nat-Ttrp-Down | TAACCCCTTACCGCCTTGGGGCAGGGCATGCTCA | |
| Hygro-TrpT-D | GTATATATACACCCTCTAAGGAAAACTATTCCTTTGCCCTCGGACG | |
| Ttrp-Up | AAGGCGGTAAGGGGTTAATTTTCCTTAGAGGGTG | |
| Trp-U(hyg) | TTTTCCTTAGAGGGTGTATATATAC | |
| TrpT-CN05607C-D | GGATCGATACAGATGAGGGGTGCGACAGAAGAGA | |
| CN05607C-U | ACCCCTCATCTGTATCGATCC | |
| CN05607C-D | CTAAGCAATAACGCATTCAGG | |
| E2-5SENSE | CGGTGACTTAATTAGAACGCTG | Southern blot |
| E2-5ANTISENSE | CCATCGTCGAGATTCAAAGGT | |
| 3Probe-S | TCCGAAAAGAGTCCACAGAG | |
| 3Probe-A | TTGTTCCTGCCCTGGTTG |
Expression of Recombinant EGCrP2
The EGCrP2 gene (egcrp2) of C. neoformans (CNAG_05607) was expressed in Escherichia coli BL21 (DE3) by inserting a pET23a vector (Novagen) containing egcrp2. After incubating the transformants at 37 °C in Luria-Bertani (LB) medium containing 100 μg/ml ampicillin until the A600 nm reached ∼0.6, isopropyl β-d-thiogalactopyranoside was then added to the culture at a final concentration of 1 mm. After cultivation for 24 h, the cells were harvested by centrifugation (8,000 × g for 15 min) and suspended in 50 mm Tris-HCl buffer, pH 7.5, containing 150 mm NaCl and 20 mm imidazole. The suspension was kept in a sonic bath for 30 s, this procedure was repeated four times to crush the cells, and cell debris was removed by centrifugation (18,000 × g for 15 min). The supernatant was applied to Ni Sepharose 6 Fast Flow resin (GE Healthcare) packed in a Muromac mini column M (Muromachi Technos), and the column was then washed with 50 mm Tris-HCl, pH 7.5, containing 150 mm NaCl and 40 mm imidazole. Recombinant EGCrP2 was eluted with 50 mm Tris-HCl, pH 7.5, containing 150 mm NaCl and 200 mm imidazole. The purified enzyme was dialyzed against 50 mm Tris-HCl, pH 7.5, containing 150 mm NaCl using an Amicon Ultra-4 30k device (Merck Millipore) and subjected to gel filtration chromatography on a Superdex 200 10/300 GL (GE Healthcare) column equilibrated with 25 mm MES, pH 6.0, containing 100 mm NaCl. EGCrP2 was eluted from the column with the same buffer at a flow rate of 0.5 ml/min, and each 0.5-ml fraction was collected using a fraction collector (GE Healthcare).
Protein Assay
Protein content was determined by Pierce 660 nm protein assay reagent (Thermo Fisher Scientific) with bovine serum albumin as a standard. SDS-PAGE was carried out according to the method of Laemmli (16) with prestained protein markers (Nacalai Tesque) as a standard. Proteins were stained with CBB Stain One (Nacalai Tesque).
Enzyme Assay
An aliquot of each substrate (NBD-labeled GSLs, pNP glycosides, 4MU-β-glucosides, and resorufin-β-d-glucoside) was incubated at 30 °C for an appropriate period with 100 ng of enzyme in 20 μl of 50 mm MES buffer, pH 6.0, containing 0.025% cholic acid. The reaction mixture, dried using a SpeedVac concentrator, was dissolved in 10 μl of chloroform/methanol (1:2, v/v) and applied to a Silica Gel 60 TLC plate (Merck Millipore), which was developed with chloroform/methanol/water (65:25:4 or 65:16:2, v/v/v). NBD-labeled GSLs were visualized using AE-6935B VISIRAYS-B and EZ-capture II (ATTO). The extent of the hydrolysis of NBD-labeled GSLs was calculated as follows: hydrolysis (%) = (peak area for the NBD-Cer generated) × 100/(peak area for the NBD-Cer generated + peak area for the remaining NBD-GSLs). GlcCer and glucose were visualized by spraying the TLC plate with orcinol-H2SO4 reagent. p-Nitrophenol released from pNP glycosides by the action of the enzyme was measured at 405 nm by Multiskan FC microplate reader (Thermo Fisher Scientific). 4-Methylumbelliferone and resorufin released from 4MU-β-glucoside and resorufin-β-d-glucoside, respectively, were measured at 355/460 nm and 544/590 nm of excitation/emission, respectively, by an ARVO MX 1420 fluorescence microplate reader (PerkinElmer Life Sciences).
Characterization of Recombinant EGCrP2
The pH dependence of EGCrP2 activity was determined in a pH range of 4–9 using the GTA buffer (3,3-dimethyl-glutaric acid, tris(hydroxymethyl) aminomethane, and 2-amino-2-methyl-1,3-ropanediol) at a final concentration of 150 mm. Temperature dependence was determined in the range between 15 and 40 °C. The effects of DMSO were examined in the range between 0 and 50%. The effects of detergents were examined using sodium cholate and Triton X-100 at the concentration indicated. The kinetic constants of EGCrP2 were measured using various glucosides (Table 2) at concentrations ranging between 0.08 and 10 μm. The reaction product C6-NBD-Cer was separated from C6-NBD-GlcCer on a normal phase HPLC column (Inertsil SIL 150A-5, GL Science) and quantified according to the method described by Hayashi et al. (17).
TABLE 2.
Kinetic parameters of recombinant EGCrP2 and EGCrP1
Values are the mean ± S.D. of three experiments. The values for EGCrP1 are from Ref. 12.
| Substrate | Enzyme | Km | Kcat | Kcat/Km |
|---|---|---|---|---|
| μm | s−1 | m−1 s−1 | ||
| C6-NBD-GlcCer | EGCrP2 | 38.4 ± 2.6 | 0.53 ± 0.03 | (13.8 ± 0.05) × 103 |
| 4MU-β-Glc | EGCrP2 | 340 ± 13.4 | 8.8 ± 0.18 | (25.7 ± 1.4) × 103 |
| Resorufin-β-Glc | EGCrP2 | 43.6 ± 2.4 | 21.9 ± 0.36 | (504 ± 24.2) × 103 |
| pNP-β-Glc | EGCrP2 | 817 ± 37.4 | 13.8 ± 0.15 | (16.9 ± 0.16) × 103 |
| C6-NBD-GlcCer | EGCrP1 | 5.80 ± 0.3 | (38.3 ± 0.20) × 10−3 | (6.6 ± 0.40) × 103 |
Generation of egcrp1-knock-out Mutant (1KO)
1KO was generated from C. neoformans var. grubii H99 by a method described previously (12).
Generation of egcrp2-knock-out Mutant (2KO) and egcrp1/egcrp2 Double Knock-out Mutant (DKO)
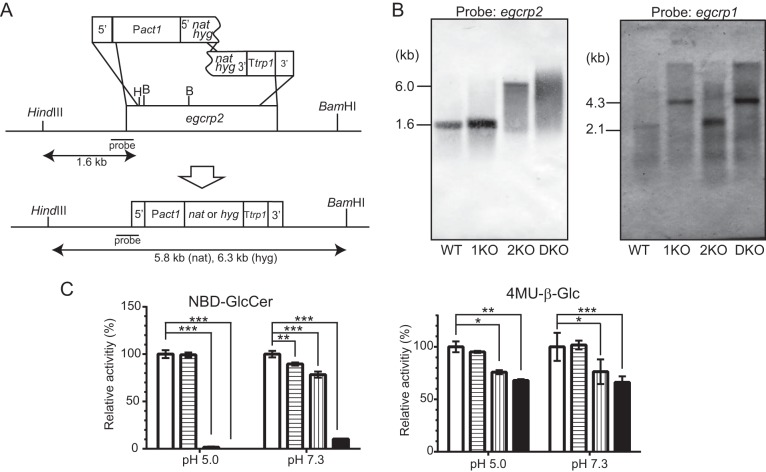
The C. neoformans EGCrP2 gene (egcrp2) (locus number CNAG_05607 in the C. neoformans var. grubii H99 database) was disrupted with the NAT split marker according to the method described previously (12, 18). A gene-specific disruption cassette contained ∼350 bp of the 5′- and 3′-flanking regions of egcrp2, an 860-bp fragment of the promoter sequence with the ATG start codon of the C. neoformans actin gene (19), a 310-bp fragment of the terminator sequence with the stop codon of C. neoformans TRP1 (20), and the selectable marker NAT gene (21) (Fig. 7). DNA fragments were amplified in the first round of PCR using the primers CN05607N-U and CN05607N-AP-D for the 5′-flanking region, CN05607C-U and CN05607C-D for the 3′-flanking region, ActinP-U and Act-Nat-Down for the actin promoter, and Ttrp-U and Ttrp-CN05607C-D for the TRP1 terminator with genome DNA as a template. Nat-Up and Nat-Ttrp-Down primers were used to amplify the NAT gene with pYL16 (WERNER BioAgents) as a template. The sequences of primers used in this study are summarized in Table 1. C. neoformans genome DNA was prepared using ISOPLANT II (NIPPON GENE). PCR products were separated on a 1% agarose gel and then extracted from the gel and used as a template in overlap PCR to combine DNA fragments. All PCR amplifications were performed using PrimeSTAR GXL DNA polymerase (Takara Bio Inc.). The combined overlap PCR product was then inserted into T-vector pGEM-T (Easy) to construct pGEM/EGCrP2–5′ and pGEM/EGCrP2–3′ after adding adenine overhang using 10× A-attachment Mix (TOYOBO) and 2× Ligation Mix (NIPPON GENE). A NAT split marker containing the 200-bp overlapping sequence was amplified by PCR using the primer sets of CN05607N-U and NSL-2 for the 5′-region of NAT and NSR-2 and CN05607C-D for the 3′-region of NAT with pGEM/EGCrP2–5′ and pGEM/EGCrP2–3′ as a template, respectively (18). The two PCR fragments were purified and then precipitated onto 500 μg of gold microcarrier beads (0.6 μm; Bio-Rad), and introduced into C. neoformans H99 by biolistic transformation, as described previously (22), using a model PDS-1000/He Biolistic particle delivery system (Bio-Rad). Stable transformants were selected on a YPD-agarose plate containing 100 μg/ml nourseothricin (WERNER BioAgents).
FIGURE 7.
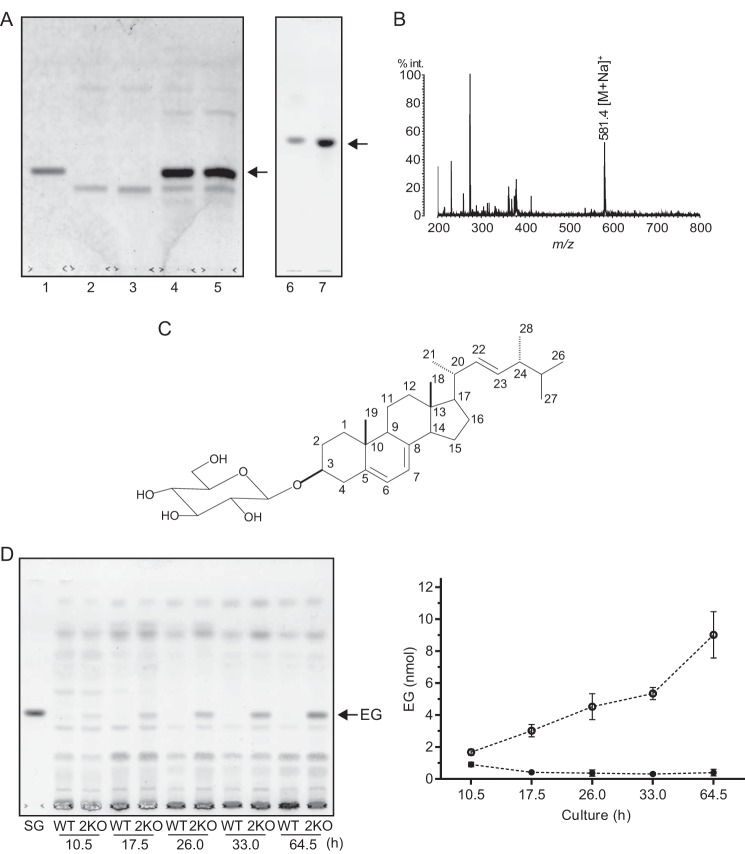
Identification of the lipid that accumulated in egcrp2-disrupted mutant (2KO) of C. neoformans. A, TLC showing the glycolipid that accumulated in the mutants. Glycolipids were extracted by chloroform/methanol (1:2, v/v) from the cells after cultivation at 25 °C for 3 days and purified using a Sep-Pak plus silica column. Lipids corresponding to 4 mg of dry cells were loaded onto a TLC plate, which was developed with chloroform/methanol/water (65:16:2, v/v/v) for lanes 1–5 (left) and chloroform/methanol/water (65:25:4, v/v/v) for lanes 6 and 7 (right). Glycolipids were visualized by orcinol sulfate reagent. Lane 1, sitosteryl-β-glucoside standard (5 nmol); lane 2, WT; lane 3, 1KO; lane 4, 2KO; lane 5, DKO; lane 6, sitosteryl-β-glucoside standard; lane 7, purified glycolipid from 2KO. B, MALDI-TOF-MS of the glycolipid that accumulated in 2KO. C, the proposed structure of the glycolipid that accumulated in 2KO. D, TLC showing the accumulation of EG in 2KO during the course of cultivation. Total lipids were extracted by chloroform/methanol (1:2, v/v) from cells cultivated at 25 °C for the period indicated. SG, sitosteryl-β-glucoside standard (10 nmol). EG contents on the TLC were calculated by a TLC chromatoscanner (right column). Open circle, 2KO; closed circle, WT. Data represent the mean ± S.D. (error bars) of three experiments.
DKO was generated from 1KO using hygromycin-resistant split marker, according to the methods described previously (18). The gene-specific disruption cassette used was the same as that for the generation of 2KO except for the selectable maker. Hygromycin-resistant split marker was used instead of NAT split marker for the generation of DKO. The primers used are summarized in Table 1.
Southern Blot Analysis
It was conducted using 2 μg of genomic DNA digested with BamHI-HindIII for egcrp2 and HindIII for egcrp1. The gene-specific probes were amplified with primer sets of E2-5SENSE and E2-5ANTISENSE for egcrp2 and 3Probe-S and 3Probe-A for egcrp1 with genomic DNA as a template. The primers used are summarized in Table 1.
Growth Curve
The growth of C. neoformans was examined using a YPD liquid medium at 30 °C with shaking (150 rpm). C. neoformans (A600 nm, 0.02) precultured for 2 days was transferred into fresh YPD medium, and growth was evaluated by measuring A600 nm after the appropriate periods indicated.
Extraction of Glycolipids
Total lipids were extracted from C. neoformans by chloroform/methanol (1:2, v/v). Total lipids, dissolved in chloroform, were loaded onto a Sep-Pak plus silica cartridge (Waters) equilibrated with chloroform. The glycolipid fraction, which contained GlcCer and ergosterylglucoside, was eluted by acetone. The elution of the glycolipid fraction was monitored by TLC using chloroform/methanol/water (65:16:2, v/v/v) as a developing solution and stained with orcinol-sulfate reagent.
Purification of the Glycolipid That Accumulated in Egcrp2-disrupted Mutants
The glycolipid that accumulated in the egcrp2-disrupted mutants was extracted using the conventional Bligh and Dyer method (23). The lower phase was collected and dried using a SpeedVac concentrator. The dried lipid was dissolved in chloroform and loaded onto a Sep-Pak plus silica column cartridge equilibrated with chloroform. The glycolipid was eluted from the cartridge by a stepwise elution with chloroform/methanol (98:2, v/v), chloroform/methanol (95:5, v/v), and chloroform/methanol (2:1, v/v). The glycolipid was mainly recovered in the chloroform/methanol (95:5, v/v) fraction. The glycolipid was purified using an HPLC (EZChrom Elite, Hitachi, Japan) equipped with a Cosmosil 5SL-II column (4.6 × 150 mm; particle size, 5 μm), which was equilibrated and eluted with methanol at a flow rate of 1 ml/min and column temperature of 40 °C. The elution of glycolipid was monitored at 278 nm.
MS Spectrometry
An AXIMA-CFR instrument (Shimadzu) was used for the MALDI-TOF-MS analysis. Mass spectra were acquired in the positive mode, and 2,5-dihydroxybenzoic acid was used as the matrix.
NMR Spectroscopy
Spectra were recorded on a Varian INOVA600 spectrometer. The operating conditions were as follows: 1H: frequency, 600 MHz; sweep width, 8 kHz; sampling point, 44 K; accumulation, 128 pulses; temperature 30 °C. 13C: frequency, 150 MHz; sweep width, 32 kHz, sampling point 160 K: accumulation, 12,000 pulses; temperature 300 K. Chemical shifts were referenced to tetramethylsilane (δH, δC 0) in CD3OD and CDCl3 (1:1, v/v). Conventional pulse sequences were used in the MQ-COSY, TOCSY, NOESY, HSQC, and HMBC experiments.
Flow Cytometric Analysis
Cells harvested from 3-day cultures were fixed with cold 70% EtOH for 3 h, washed with PBS, and then incubated for 5 min in Hoechst 33342 solution (50 μg/ml in distilled water) at room temperature. The samples were placed on ice before the analysis and were then analyzed using an EC800 flow cytometer (Sony). Cell volumes were estimated by the flow cytometer according to the manufacturer's instructions. To eliminate the signals for aggregated cells, Hoechst 33342-based gating was performed in the analysis.
Vacuole Analysis
The vacuole-enriched fraction was isolated from C. neoformans cells by density gradient centrifugation in accordance with Cabrera and Ungermann (24). Typically, a 300-ml culture was subjected to centrifugation at 4,400 × g for 5 min at room temperature and washed twice with PBS. The pellet was resuspended with spheroplasting buffer containing with 14 ml of Mcllvain buffer, 6 ml of 1 m sodium tartrate, and 250 mg of Westase (Takara Bio.) and incubated at 30 °C for 2 h with shaking at 60 rpm. After centrifuging at 5,300 × g, for 5 min at 4 °C, the supernatants were discarded. The pellet was dissolved in 2.5 ml of 15% Ficoll in PS buffer (15% (w/v) Ficoll 400, 20 mm PIPES/KOH, pH 6.8, 200 mm sorbitol), added to 200 μl of 0.4 mg/ml DEAE-dextran in PS buffer, and incubated for 5 min on ice and at 30 °C for 90 s. Each 3 ml of the supernatant was transferred into centrifuge tubes, and 800 μl of 8% Ficoll in PS buffer (8% (w/v) Ficoll 400, 20 mm PIPES/KOH, pH 6.8, 200 mm sorbitol) was carefully layered, followed by 800 μl of 4% Ficoll in PS buffer (4% (w/v) Ficoll 400, 20 mm PIPES/KOH, pH 6.8, 200 mm sorbitol). Finally, 300 μl of PS buffer without Ficoll (20 mm PIPES/KOH, pH 6.8, 200 mm sorbitol) was layered on the top. The tubes were centrifuged using a RPS65T Swing rotor (Hitachi) at 110,000 × g for 90 min at 4 °C. The vacuole and lipid droplet fractions were collected from the top to 0–4% interface using a 1-ml tip. Vacuoles and lipid droplets were separated according to the method described by Zinser et al. (25). Lipids were extracted from the lysate and vacuole vesicles (50 μg of protein) by chloroform/methanol (1:2, v/v) and dried under a stream of N2. The lipid was analyzed by TLC using chloroform/methanol/water (65:16:2, v/v/v) as a developing solvent. Vacuoles in cells were visualized with the incorporation of 5-(and 6)-carboxy-2′,7′-dichlorofluorescein diacetate (carboxy-DCFDA; Molecular Probes) under microscopy (26).
RESULTS
Molecular Cloning and Characterization of EGCrP2
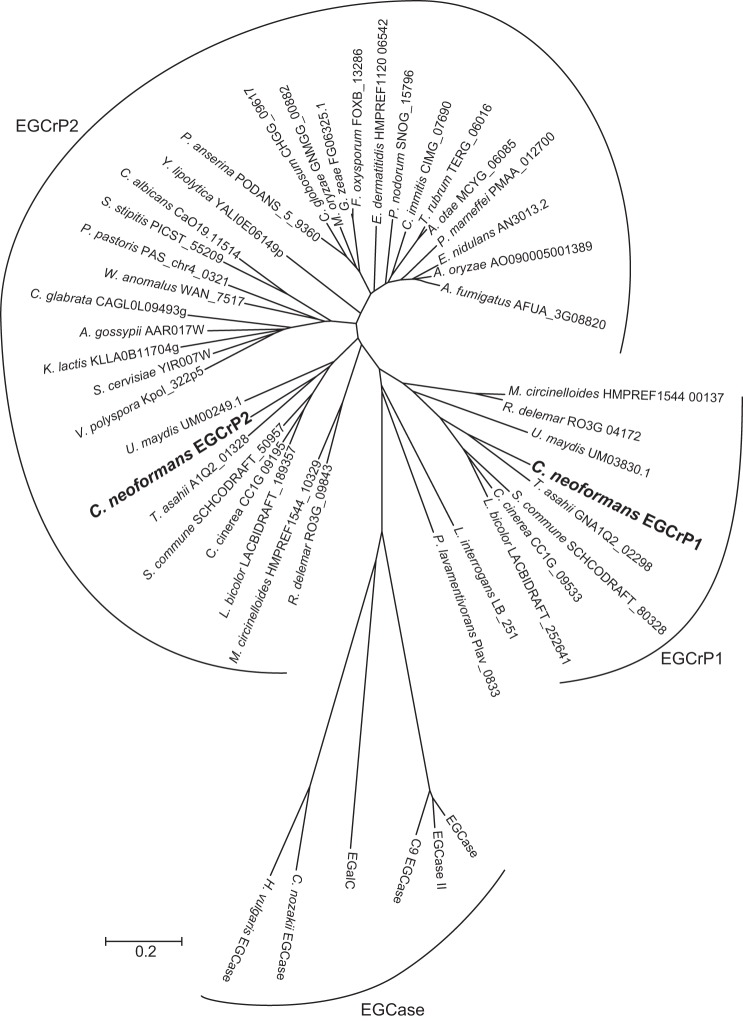
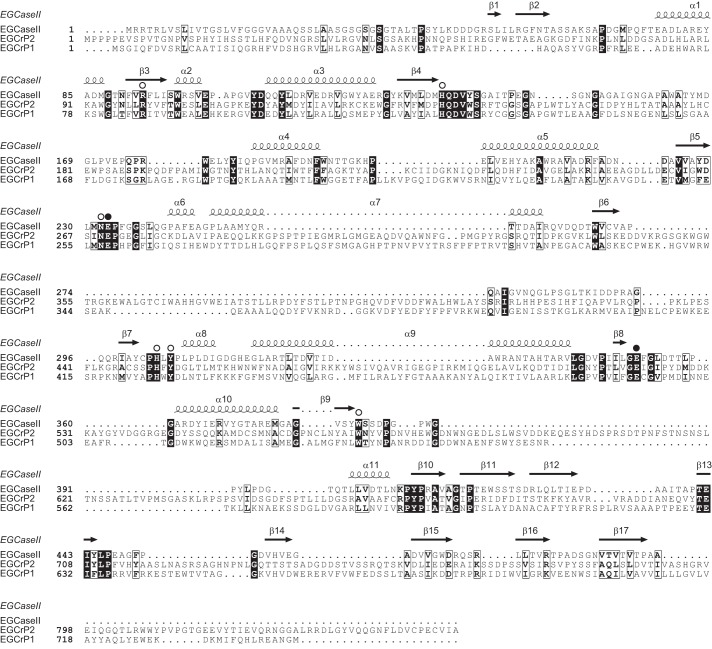
EGCase, an enzyme capable of cleaving the β-glycosidic linkage between the oligosaccharide and Cer of various GSLs, is distributed in bacteria, actinomycetes, and some invertebrates, such as jellyfish and hydra (Fig. 1). We previously reported that the EGCase homologue, EGCrP1, was a glucocerebrosidase of C. neoformans, involved in the quality control of GlcCer, possibly associated with the GlcCer synthesis pathway (12). In the present study, we identified another homologue of EGCase in C. neoformans and designated it as EGCrP2. It showed 28% identity to EGCrP1 and rhodococcal EGCase II. The alignment of the deduced amino acid sequence of EGCrP2 with those of EGCrP1 and EGCase II revealed that 8 residues, essential for the catalytic activity of glycoside hydrolase family 5 glycosidases (27), were completely conserved in these enzymes (Fig. 2, open circles). Of the 8 residues of EGCrP2, two catalytic glutamates, Glu-270 and Glu-520, at the end of β-strands 5 and 8, respectively, were thought to be an acid/base catalyst and nucleophile, respectively (Fig. 1, closed circles). EGCrP2-like homologues were widely distributed across the phyla/genera of fungi and formed a gene family, which was independent of the EGCrP1 and EGCase families (Fig. 1).
FIGURE 1.
Phylogenetic tree of EGCrP1, EGCrP2, and EGCase. The amino acid sequences of EGCrPs and EGCases were reconstructed using the neighbor-joining method. The scale bar represents 0.2 amino acid substitutions/site.
FIGURE 2.
Alignments of EGCrP1 and EGCrP2 with EGCase II. The amino acid sequences of Rhodococcus EGCase (accession number AAB67050.1), C. neoformans EGCrP1 (accession number BAL46040.1), and C. neoformans EGCrP2 were aligned using ClustalW (42) and ESPript (43). White letters on a black background and black letters in an open box show identical and similar residues, respectively. Open circles indicate amino acid residues conserved in glycoside hydrolase family 5 glycosidase. Two glutamates, the Glu-258 and Glu-492 of EGCrP1 (12) and Glu-270 and Glc-520 of EGCrP2, are indicated by closed circles as possible acid/base catalyst and nucleophile, respectively. The secondary structural elements are shown above the amino acid sequence of EGCase II (44).
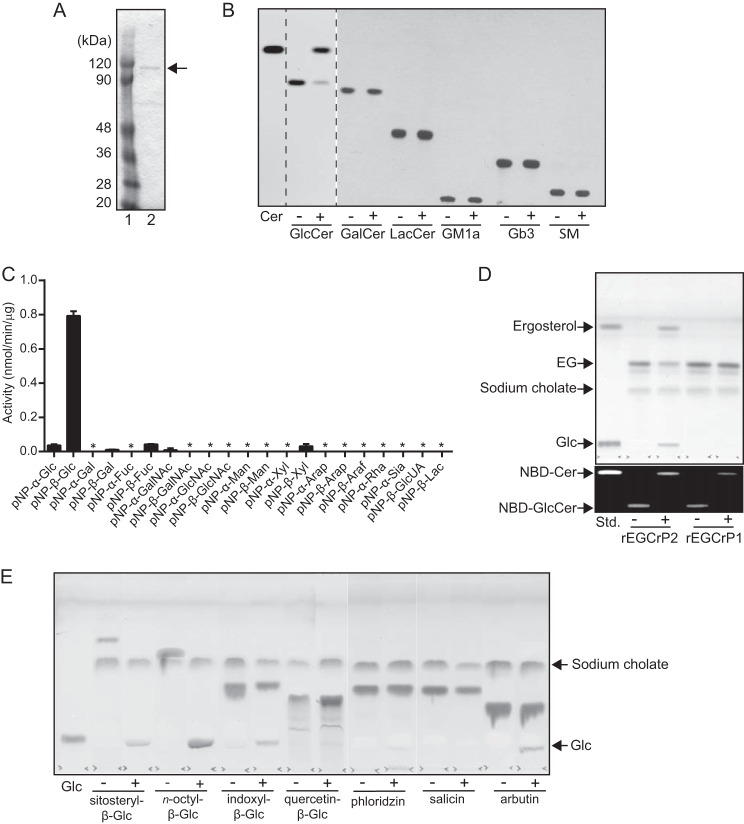
To characterize EGCrP2, the egcrp2 open reading frame encoding 851 amino acid residues was cloned from the complementary DNA of C. neoformans and expressed in E. coli BL21 (DE3) as a His tag-fused protein. Recombinant EGCrP2 (rEGCrP2) was purified by affinity chromatography using a nickel-conjugated Sepharose column and gel filtration using a Superdex 200 10/300 GL column. The purified enzyme showed a single protein band possessing a molecular mass of ∼120 kDa on SDS-PAGE after staining with Coomassie Brilliant Blue (CBB Stain One) (Fig. 3A). Purified rEGCrP2 hydrolyzed C6-NBD-GlcCer to generate C6-NBD-Cer; however, it did not hydrolyze any of the other NBD-GSLs tested (Fig. 3B). rEGCrP2 was also found to hydrolyze native GlcCer, which was not labeled with NBD; however, it did not degrade any other native GSLs (data not shown). The specificity of rEGCrP2 toward GSLs appeared to be identical to that of EGCrP1 but was completely different from that of EGCase, which preferred oligosaccharide-linked GSLs such as LacCer and GM1a (10). Both EGCrP1 and EGCrP2 were β-glucosidases; however, the specificity of EGCrP2 was very broad for aglycone moieties (i.e. EGCrP2-hydrolyzed pNP-β-glucoside (Fig. 3C) and 4MU-β-glucoside (Table 2)), whereas these β-glucosides were completely resistant to hydrolysis by EGCrP1. Neither EGCrP1 nor EGCrP2 hydrolyzed other pNP-glycosides, including pNP-β-galactoside and pNP-α-glucoside (Fig. 3C). Differences in the aglycone specificities of the two EGCrPs were also shown with various β-glycosides (i.e. EGCrP2 hydrolyzed EG to generate ergosterol and glucose, but this glycolipid was not degraded by EGCrP1) (Fig. 3D). Furthermore, various β-glucosides, such as sitosteryl-β-glucoside, n-octyl-β-glucoside, indoxyl-β-glucoside, and arbutin, were hydrolyzed by EGCrP2 under the conditions used (Fig. 3E). Salicin and phloridzin were hydrolyzed by EGCrP2 when the amount of the enzyme was increased 10-fold; however, quercetin-β-glucoside was still resistant to hydrolysis by EGCrP2 under the same condition (data not shown). The kinetic parameters of EGCrP2 for various β-glucosides and their structures are summarized in Table 2 and Fig. 4, respectively.
FIGURE 3.
Purification and characterization of the recombinant EGCrP2. A, final preparation of rEGCrP2 on 10% SDS-PAGE. The protein eluted from the nickel-Sepharose column was purified using Superdex 200 10/300 GL. Lane 1, protein marker; lane 2, final preparation. B, TLC showing the specificity of EGCrP2 toward various NBD-GSLs. Each NBD-GSL (100 pmol) was incubated in 20 μl of 50 mm MES buffer, pH 6.0, with 100 ng of rEGCrP2 (+) or heat-inactivated EGCrP2 (−) at 30 °C for 16 h, except for C6-NBD-GlcCer, which was incubated at 30 °C for 1 h. Samples were loaded onto a TLC plate, which was developed with chloroform/methanol/water (65:25:4, v/v/v). C, hydrolysis of pNP substrates by rEGCrP2. Error bars, S.D. of three experiments. An asterisk indicates no hydrolysis of pNP substrates. D, top TLC shows hydrolysis of EG by rEGCrP2. Fungal EG, purified from 2-mg dry cells of 2KO, was incubated at 30 °C for 18 h with 40 μg of EGCrP1 or 20 ng of EGCrP2. TLC was developed with chloroform/methanol/water (65:16:2, v/v/v) and stained with orcinol sulfate reagent. The bottom TLC shows C6-NBD-Cer released from C6-NBD-GlcCer by rEGCrP1 and -2. 50 pmol of C6-NBD-GlcCer was incubated at 30 °C with 40 μg of rEGCrP1 and 20 ng of rEGCrP2 for 18 h. +, with EGCrP2; −, without EGCrP2. E, TLC showing the hydrolysis of various β-glucosides by rEGCrP2. Each 100 nmol of substrate was incubated at 30 °C with 100 μg of enzyme for 18 h. Samples were loaded onto a TLC plate, which was developed with chloroform/methanol/water (65:25:4, v/v/v) and visualized by orcinol sulfate reagent. Glc, glucose released from various β-glucosides by rEGCrP2. +, with EGCrP2; −, without EGCrP2.
FIGURE 4.
Structures of β-glucosides used to analyze the specificity of rEGCrP2. The specificity of EGCrP2 was examined using various β-glucosides, as indicated.
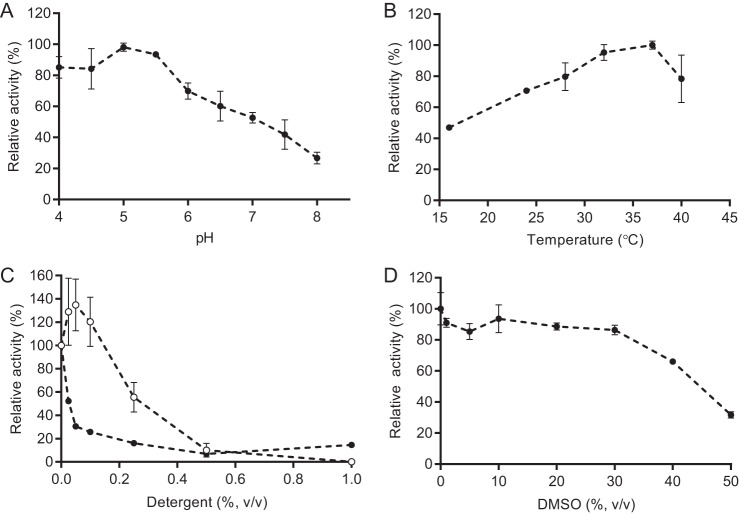
The maximal activity of EGCrP2 was observed at pH 5.0–5.5 when C6-NBD-GlcCer was used as a substrate, indicating that EGCrP2 was an acid β-glucosidase (Fig. 5A). In contrast, the pH optimum of EGCrP1 was previously shown to be ∼7.5 (12). The optimal temperature of EGCrP2 was found between 32 and 37 °C (Fig. 5B), which was a suitable temperature for the growth of C. neoformans. The activity of EGCrP2 was enhanced by the addition of sodium cholate at a concentration of 0.025% when C6-NBD-Cer was used as a substrate; however, higher concentrations of the detergent inhibited activity. Triton X-100 strongly inhibited the activity of EGCrP2 (Fig. 5C) and EGCrP1 (12). Although the addition of DMSO did not affect activity up to 30% of the reaction mixture, it inhibited activity at higher concentrations (Fig. 5D).
FIGURE 5.
General properties of rEGCrP2. A, pH dependence of C. neoformans rEGCrP2. 150 mm of GTA buffer was used for the assay. B, effects of temperature on EGCrP2 activity. C, effects of detergents on EGCrP2 activity. Closed circles, Triton X-100; open circles, sodium cholate. D, effects of DMSO on EGCrP2 activity. Data represent the mean ± S.D. (error bars) of three experiments. The assay was conducted using C6-NBD-ClcCer as the substrate.
Generation of 2KO and egcrp1/egcrp2 DKO
To determine whether EGCrP2 was involved in the catabolism of β-glucosides in vivo, 2KO and DKO were generated from C. neoformans var. grubii serotype A strain H99 (WT) and 1KO (12) by gene-targeting homologous recombination using NAT and hygromycin (hyg)-resistant gene as a marker, respectively (Fig. 6A). Southern blot analysis using the BamHI-HindIII-digested genome DNA revealed that the egcrp2 gene was disrupted by this method in 2KO and DKO, as expected (Fig. 6B, left), whereas the egcrp1 gene was present in WT and 2KO but not in 1KO or DKO (Fig. 6B, right). The β-glucocerebrosidase activity decreased in the cell lysate of 1KO at pH 7.3 but not at pH 5.0 when the activity was measured using C6-NBD-GlcCer, as shown previously (12). On the other hand, the activity markedly decreased in the cell lysates of 2KO and DKO when it was measured using C6-NBD-GlcCer at pH 5.0, indicating that EGCrP2 possesses an acid β-glucocerebrosidase activity (Fig. 6C, left). However, the apparent decrease of β-glucosidase activity of 2KO and DKO was much lower when the activity was measured using 4MU-β-Glc as a substrate at pH 5.0 (Fig. 6C, right), suggesting the presence of β-glucosidase(s) that may act on 4MU-β-Glc but not C6-NBD-GlcCer under acidic conditions.
FIGURE 6.
Disruption of the egcrp2 gene in C. neoformans. A, strategy for the disruption of C. neoformans egcrp2 using the split marker method. B, Southern blot analysis of BamHI-HindIII-digested genomic DNA for egcrp2 (left) and HindIII-digested genomic DNA for egcrp1 (right). The disruption of egcrp1 and Southern blot analysis of HindIII-digested genomic DNA were performed by the method described previously (12). C, the activity of β-glucosidase was measured using NBD-GlcCer (left) and 4MU-β-Glc (right). Activities were measured at 37 °C for 18 h using NBD-GlcCer and 2 μg of protein for pH 5.0 or 10 μg of protein for pH 7.3. When 4MU-β-Glc was the substrate, 1 μg of protein was incubated at 37 °C for 18 h for both pH values. The activity was expressed as a percentage of that of WT. *, **, and ***, p < 0.01, p < 0.001, and p < 0.0001, respectively. Error bars, S.D.
Identification of the Lipid That Accumulated in 2KO
TLC analysis of cell extracts showed that the accumulation of GlcCer was not significant in 2KO under the conditions used; alternatively, an unknown lipid, whose Rf corresponded to that of sitosteryl-β-glucoside (SG), highly accumulated in both 2KO and DKO mutants (Fig. 7A, lanes 4 and 5). To clarify its structure, the accumulated lipid was extracted from 2KO, purified as described under “Experimental Procedures” (Fig. 7B, lane 7) and then analyzed by MALDI-TOF-MS in positive mode using 2,5-dihydroxybenzoic acid as a matrix. The main molecular ion peak, [M + Na]+, was observed at m/z 581.4 for the lipid (Fig. 7B), which corresponded to the molecular mass of EG (C32H54O6, 558.39). The NMR spectra of the lipid accumulated in 2KO are summarized in Table 3. The 1H NMR spectrum of the purified lipid showed the characteristic signals of a glycoside structure composed by a sterol part and hexopyranose. The 1H NMR spectrum for the sterol moiety displayed signals for four secondary methyls as a doublet, two tertialy methyls as a singlet, four olefinic protons, and one oxygen-bearing methine proton. In the 13C NMR spectrum, 28 carbon signals (6 methyls, 7 methylenes, 6 methines, 4 quaternary carbons, 6 sp2 carbons, and 1 oxygen-bearing methine) suggested that the sterol moiety could be an ergosterol (Table 3). Furthermore, the deshielded signal at δC 78.2 (C-3) in comparison with the ergosterol suggested a glycosidic linkage at C-3. The structure of the sugar moiety was assignable to β-glucopyranoside because of its chemical shift values and the correlation from the H-1 (δH 4.43 (d, J = 7.8 Hz)) to H2-6 (δH 3.73 and 3.87) in the MQ-COSY and TOCSY spectra, and the glycosidic linkage at the C-3 of the ergosterol was also confirmed by the HMBC correlation between the H-1 of Glc (δH 4.43) and C-3 of the ergosterol (δC 78.2). Collectively, the structure of the accumulated lipid was determined to be EG, a major sterylglucoside in fungi, as shown in Fig. 7C. EG accumulated in the 2KO in a time-dependent manner; however, the content of this glycolipid was very low and not increased in wild type during the course of cultivation (Fig. 7D). These results indicated that EGCrP2 is involved in the degradation of EG in C. neoformans, and disruption of egcrp2 resulted in the accumulation of EG.
TABLE 3.
13C and 1H NMR spectral data of EG and ergosterol
NMR chemical shifts of standard ergosterol were assigned by MQ-COSY, TOCSY, HSQC, and HMBC experimental data, which measured at the same condition of EG.
|
13C NMR data |
1H NMR data |
|||||
|---|---|---|---|---|---|---|
| Carbon | EG | Ergosterol | Multiplicity | Proton | EG | Ergosterol |
| 1 | 39.0 | 39.0 | CH2 | 1α | 1.32 | 1.30 |
| 1β | 1.93 | 1.90 | ||||
| 2 | 30.5 | 32.0 | CH2 | 2α | 2.00 | 1.86 |
| 2β | 1.67 | 1.50 | ||||
| 3 | 78.2 | 70.4 | CH | 3 | 3.72 (m) | 3.54 (m) |
| 4 | 37.8 | 40.9 | CH2 | 4α | 2.60 (brd, 14.7) | 2.44 |
| 4β | 2.33 (brt, 13.0) | 2.27 | ||||
| 5 | 140.1 | 140.6 | C= | |||
| 6 | 120.5 | 120.1 | CH= | 6 | 5.57 (brs) | 5.55 |
| 7 | 116.9 | 116.9 | CH= | 7 | 5.38 (brs) | 5.38 |
| 8 | 141.8 | 141.6 | C= | |||
| 9 | 46.9 | 46.9 | CH | 9 | 1.99 | 1.97 |
| 10 | 37.8 | 37.6 | C | |||
| 11 | 21.6 | 21.7 | CH2 | 11α | 1.74 | 1.74 |
| 11β | 1.62 | 1.62 | ||||
| 12 | 39.7 | 39.7 | CH2 | 12α | 1.30 | 1.28 |
| 12β | 2.09 | 2.09 | ||||
| 13 | 43.4 | 43.4 | C | |||
| 14 | 55.1 | 55.1 | CH | 14 | 1.92 | 1.90 |
| 15 | 23.5 | 23.5 | CH2 | 15α | 1.69 | 1.67 |
| 15β | 1.32 | 1.38 | ||||
| 16 | 28.8 | 28.8 | CH2 | 16α | 1.79 | 1.78 |
| 16β | 1.36 | 1.34 | ||||
| 17 | 56.4 | 56.4 | CH | 17 | 1.30 | 1.28 |
| 18 | 12.4 | 12.4 | CH3 | 18 | 0.65 (3H, s) | 0.65 |
| 19 | 16.5 | 16.5 | CH3 | 19 | 0.96 (3H, s) | 0.95 |
| 20 | 41.1 | 41.0 | CH | 20 | 2.06 | 2.06 |
| 21 | 21.4 | 21.4 | CH3 | 21 | 1.05 (3H, d, 6.7) | 1.05 |
| 22 | 136.3 | 136.2 | CH= | 22 | 5.20 (dd, 7.8, 15.3) | 5.19 |
| 23 | 132.6 | 132.6 | CH= | 23 | 5.24 (dd, 7.3, 15.3) | 5.24 |
| 24 | 43.6 | 43.5 | CH | 24 | 1.87 | 1.86 |
| 25 | 33.7 | 33.7 | CH | 25 | 1.48 | 1.48 |
| 26 | 19.9 | 19.9 | CH3 | 26 | 0.84 (3H, d, 6.9) | 0.84 |
| 27 | 20.2 | 20.2 | CH3 | 27 | 0.85 (3H, d, 6.9) | 0.85 |
| 28 | 17.9 | 18.0 | CH3 | 28 | 0.93 (3H, d, 6.7) | 0.93 |
| β-Glc | ||||||
| C-1′ | 101.9 | CH | H-1′ | 4.43 (d, 7.8) | ||
| C-2′ | 74.3 | CH | H-2′ | 3.22 (t, 8.4 | ||
| C-3′ | 77.3 | CH | H-3′ | 3.41 (t, 8.8) | ||
| C-4′ | 80.0 | CH | H-4′ | 3.38 (t, 9.0) | ||
| C-5′ | 76.9 | CH | H-5′ | 3.30 (m) | ||
| C-6′ | 62.3 | CH2 | H-6a′ | 3.73 (dd, 5.2, 11.9) | ||
| H-6b′ | 3.87 (dd, 2.7, 11.9) | |||||
Phenotype Analysis of 2KO and DKO
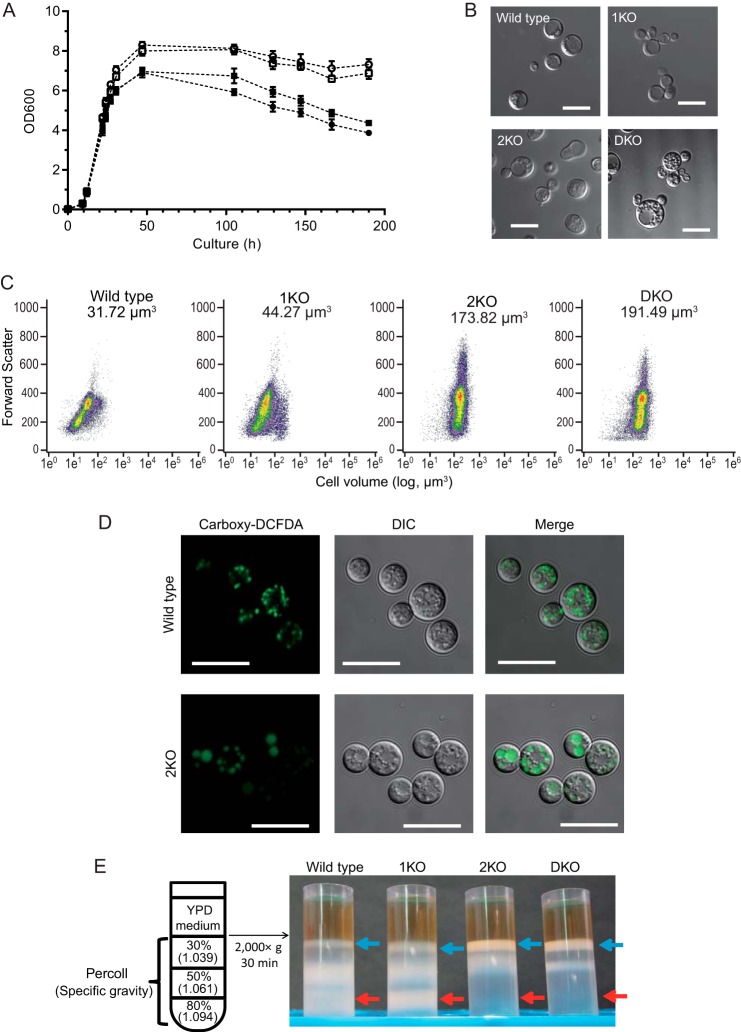
To assess the physiological effects of the disruption of the egcrp2 gene, we compared the cell growth of 2KO and DKO with that of WT and 1KO. The disruption of egcrp2, but not egcrp1, resulted in the arrest of cell growth from the middle log phase to the early stationary phase (Fig. 8A). Observations with differential interference-contrast microscopy revealed that 2KO and DKO cells were larger than WT and 1KO cells, possibly due to dysfunction of the budding process (Fig. 8B). In support of this observation, the flow cytometric analysis revealed that the average cell volumes of 2KO (173.82 μm3) and DKO (191.49 μm3) were 5–6-fold larger than those of WT (31.72 μm3) and 1KO (44.27 μm3). Small cells (cell volume, ∼10 μm3) were found in WT and 1KO populations but not in 2KO and DKO populations (Fig. 8C). The vacuoles of 2KO were larger than those of WT and 1KO when carboxy-DCFDA was incorporated into these cells as an indicator to visualize vacuoles (Fig. 8D). The cell densities of 2KO and DKO were compared with those of WT and 1KO using Percoll cell gradient centrifugation. The distribution of cells in the Percoll gradient was markedly different (i.e. 2KO and DKO cells were mainly recovered in the 30% Percoll fraction (the specific gravity was estimated to be 1.039; blue arrows), whereas WT and 1KO cells were recovered in the 50% (specific gravity, 1.061; yellow arrows) and 80% Percoll fractions (specific gravity, 1.094; red arrows)). Almost no 2KO and DKO cells were detected in the 80% Percoll fraction (Fig. 8E). These results indicated that the specific gravities of 2KO and DKO cells were markedly lower than those of 1KO and WT cells.
FIGURE 8.
Analysis of the phenotype of egcrp2-disrupted mutants (2KO) of C. neoformans. A, growth curves of WT (open circles), 1KO (open squares), 2KO (closed circles), and DKO (closed squares). Data represent the mean ± S.D. (error bars) of three experiments. B, cells observed under a differential interference-contrast microscope. 2KO and DKO cells exhibited cell-budding dysfunction, resulted in enlarged cells. Scale bars = 10 μm. C, flow cytometric analysis. The average cell volumes of WT, 1KO, 2KO, and DKO are presented in each panel. D, vacuoles stained with carboxy-DCFDA of WT and 2KO. Scale bars = 10 μm. Cells, cultured at 25 °C for 1 day, were incubated with 100 μm carboxy-DCFDA at 25 °C for 20 min and observed with confocal microscopy. E, density gradient centrifugation of WT, 1KO, 2KO, and DKO cells by Percoll. Blue and red arrows indicate the layers, including cells with the lightest and heaviest specific gravities, respectively.
Vacuole Analysis of WT and 2KO
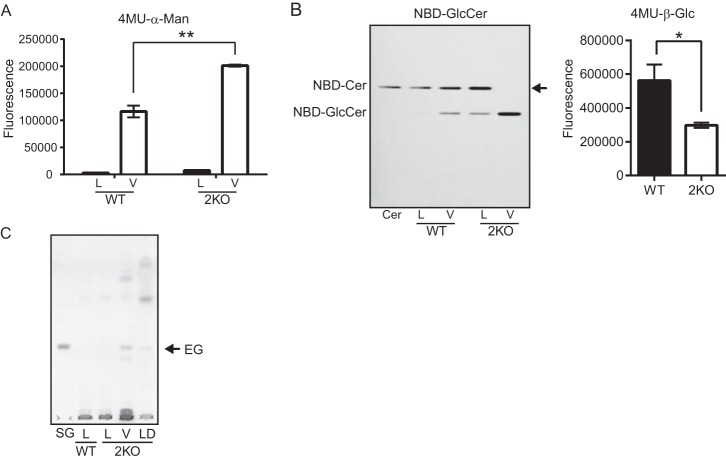
The disruption of egcrp2, but not egcrp1, led to the accumulation of EG (Fig. 7, A and D) and hypertrophy of cell body (Fig. 8, B and C) and vacuoles (Fig. 8D). EGCrP2 degraded EG most efficiently at an acidic pH (Fig. 5A), and, thus, it was hypothesized that EG may be catabolized by EGCrP2 in acidic compartments in the cell, such as the vacuoles. Vacuoles were thus isolated from WT and 2KO cells using Ficoll gradient centrifugation as described under “Experimental Procedures.” The final fractions obtained from both WT and 2KO cells possessed high specific activity for the acid α-mannosidase, which is a marker enzyme localized in the vacuole, indicating that vacuoles were enriched in these fractions as expected (Fig. 9A). The specific activity of the acid α-mannosidase in the vacuole-enriched fraction of 2KO was markedly higher than that of WT (Fig. 9A); however, the specific activity of β-glucosidase was significantly lower in the same fraction of 2KO than that of WT when its activity was measured using C6-NBD-GlcCer (Fig. 9B, left) and 4MU-β-glucoside (Fig. 9B, right) as substrates, indicating that EGCrP2 was present in the vacuole-enriched fraction. To determine whether EG accumulated in vacuoles, total lipids in the vacuole-enriched fraction were extracted and analyzed by TLC. As shown in Fig. 9C, EG was detected in the vacuole-enriched fraction of 2KO but not in that of WT. These results strongly suggested that EGCrP2 catabolizes EG in the vacuoles of C. neoformans.
FIGURE 9.
Cellular localization of EG that accumulated in egcrp2-disrupted mutants (2KO) of C. neoformans. A, the α-mannosidase activity of the cell lysate and vacuole fractions. Activity was measured at 37 °C for 1 h using 0.5 μg of protein from each fraction and 20 nmol of 4MU-α-mannoside as a substrate in 100 μl of 50 mm phosphate buffer, pH 6.5. L, lysate; V, vacuole fraction. B, the β-glucosidase activity of the vacuole fraction of WT and 2KO. Activity was measured at 30 °C for 18 h by using 0.25 μg of protein from vacuole fraction and 50 nmol of C6-NBD-GlcCer in 20 μl or 30 nmol of 4MU-β-glucoside as a substrate in 100 μl of 50 mm sodium acetate buffer, pH 5.0. C, TLC showing EG that accumulated in 2KO. Glycolipids were extracted from the lysate and vacuole fractions of WT and 2KO (each 50 μg as protein) and analyzed by TLC using the method described in the legend to Fig. 5A. L, lysate; V, vacuole fraction; LD, lipid droplet fraction. * and **, p < 0.05 and p < 0.0001, respectively. Error bars, S.E.
DISCUSSION
Because the activity of the glucocerebrosidase did not decrease under acidic conditions after the disruption of egcrp1 in C. neoformans (12), we searched for glucocerebrosidase(s) capable of working under acidic conditions in this study. EGCrP2, a homologue of EGCrP1, was found to hydrolyze GlcCer in vitro, and the disruption of egcrp2 greatly reduced glucocerebrosidase activity when C6-NBD-GlcCer was used as a substrate under acidic conditions (Fig. 6C, left). However, we found that DKO still exhibited glucocerebrosidase activity (Fig. 6C, left), suggesting that C. neoformans may possess glucocerebrosidase(s) other than EGCrP1 and EGCrP2.
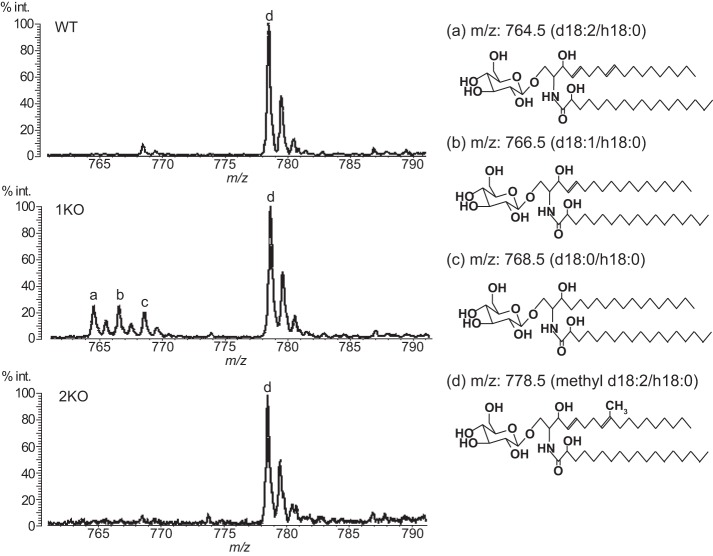
The GlcCer of C. neoformans (WT) shows homogeneity in the sphingoid base, which possesses two double bonds at C4/C8 and a methyl substitution at C9 (methyl d18:2) (9, 12). However, the EGCrP1-knock-out mutant (1KO) accumulated GlcCer possessing several sphingoid bases without methyl substitution (d18:2, d18:1, and d18:0) that are intermediates generated from the pathway of GlcCer synthesis in C. neoformans (12). This indicates that EGCrP1 is involved in the quality control of GlcCer in C. neoformans. On the other hand, the sphingoid base of GlcCer in 2KO was exclusively methyl d18:2, as in WT (Fig. 10), indicating that EGCrP2 is unlikely to participate in the elimination of aberrant GlcCer found in 1KO. This discrepancy in the physiological role of EGCrPs may stem from the different localization of each EGCrP. The different pH optimum for each enzyme may support this hypothesis; however, the precise localization of EGCrPs in C. neoformans remains to be elucidated.
FIGURE 10.
MALDI-TOF-MS analysis of GlcCer from WT, 1KO, and 2KO. GlcCer, purified from WT, 1KO, and 2KO, was analyzed by MALDI-TOF-MS in a positive ion mode using 2,5-dihydroxybenzoic acid as a matrix. The major ion peak at m/z 778.5 (d), which corresponds to the molecular mass of mature GlcCer possessing Cer composed of methyl d18:2/h18:0, was detected in GlcCer from WT, 1KO, and 2KO. However, ion peaks at m/z 764.5 (a), 766.5 (b), and 768.5 (c), which correspond to the molecular mass of immature GlcCer possessing Cer composed of d18:2/h18:0, d18:1/h18:0, and d18:0/h18:0, respectively, were detected in GlcCer from 1KO but not WT or 2KO.
EGCrP2 seems to be a major EG-degrading enzyme in C. neoformans because egcrp2-disrupted mutants led to the significant accumulation of EG (Fig. 7, A and D), a major sterylglucoside in fungi and yeast (28). Sterylglucosides, sterol-containing glucosides, are a major class of glycolipids in fungi; however, they are also found in algae, plants, and animals (28). Sterol 3β-glucosyltransferase (EC 2.4.1.173), an enzyme that catalyzes the transfer of glucose from UDP-glucose to sterols, has been found in Candida albicans (14), Colletotrichum gloeosporioides (29), Fusarium graminearum (28), Leptosphaeria maculans (30), Saccharomyces cerevisiae (14), and Pichia pastoris (14). The sterol 3β-glucosyltransferase-deficient mutants of P. pastoris were found to lack pexophagy, which is a process for the degradation of peroxisomes in vacuoles, whereas sterol 3β-glucosyltransferase did not appear to be essential for pexophagy in S. cerevisiae and Yarrowia lipolytica (31–33). It remains unclear whether the accumulation of EG affects pexophagy in C. neoformans. In the present study, the accumulation of EG due to a dysfunction in egcrp2 may have led to the abnormal cell proliferation (Fig. 8, A–C) and vacuole morphology (Fig. 8D) in C. neoformans; however, the mechanism responsible remains unknown.
Although both EGCrP1 and EGCrP2 are β-glucohydrolases belonging to glycoside hydrolase family 5, the specificity of both enzymes completely differs for the aglycone moiety; EGCrP1 only cleaved the β-glucosidic linkage of GlcCer among the substrates tested (12), whereas EGCrP2 hydrolyzed various β-glucosides, including GlcCer, steryl-β-glucosides, and artificial substrates, such as pNP-β-glucoside and 4MU-β-glucoside (Fig. 3 and Table 2). A comparison of the primary structure of EGCrP2 with EGCrP1 revealed the presence of several inserts in EGCrP2 between possible β-strands 6 and 7, β-strand 9, and α-helix 11, and the carboxyl-terminal region (Fig. 2). Further studies are required to elucidate the relationship between the structures and aglycone specificities of EGCrPs. X-ray crystal analysis of EGCrP1 and EGCrP2 could provide valuable information on mutual relationships, and experiments are ongoing.
In the present study, we identified EGCrP2 as the first steryl-β-glusosidase in fungi. Similar activity was found in Sinapis alba (34) and Sulfolobus solfataricus (35); however, these enzymes show no sequence homology with EGCrP1/EGCrP2. In addition, it remains unclear whether these plant and archaea enzymes are actually involved in sterylglucoside metabolism in vivo because knock-out of the corresponding genes in plants and archaea has not been reported.
EGCrP2 appeared to localize to the vacuole because β-glucosidase activity was detected in the vacuole-enriched fraction, and its activity was decreased in the fraction after the disruption of egcrp2 in C. neoformans (Fig. 9B). The optimum pH of EGCrP2 was found to be 5.0–5.5 (Fig. 5A), which approximately corresponded to the vacuole pH determined in C. neoformans strain H99 (36). The expression of GFP-tagged EGCrP2 could help to estimate the intracellular localization of EGCrP2; however, the expression of GFP-EGCrP2 has yet to be successfully achieved in C. neoformans.
The abnormal morphology of vacuoles in C. albicans mutants led to a decrease in pathogenicity in a mouse model (37–40); thus, the vacuole proteins of this fungi are now being considered as targets for the development of antifungal drugs (41). In this context, EGCrP2 could be a promising candidate for the development of anti-Cryptococcus drugs because a dysfunction in EGCrP2 resulted in an abnormal morphology in the vacuoles (Fig. 8D). One of the reasons for this abnormality could stem from the accumulation of EG in the vacuoles; however, the molecular mechanism responsible remains unknown. Taken together, we herein uncovered the missing link in sterylglucoside metabolism in C. neoformans by identifying the enzyme responsible for degrading EG in vivo and in vitro.
Acknowledgments
We thank Dr. Kaoru Takegawa and Dr. Ken Matsuoka (Kyushu University) for valuable suggestions regarding vacuole morphology and physiology. Thanks also due to Emiko Matsunaga (Kyushu University) for MS analysis of EG.
This work was supported in part by Basic Research B Grant 24380058 from the Japanese Ministry of Education, Culture, Science, and Technology and the Japan Foundation for Applied Enzymology (to M. I.).
- GlcCer
- glucosylceramide
- Cer
- ceramide
- EG
- ergosteryl-3β-glucoside
- EGCase
- endoglycoceramidase
- EGCrP
- endoglycoceramidase-related protein
- rEGCrP
- recombinant EGCrP
- Glc
- d-glucose
- GSL
- glycosphingolipid
- LacCer
- lactosylceramide
- NBD
- 7-nitro-2,1,3-benzoxadiazole
- pNP
- para-nitrophenyl
- 4MU
- 4-methylumberifellyl
- 1KO
- egcrp1-knock-out mutant
- 2KO
- egcrp2-knock-out mutant
- DKO
- egcrp1/egcrp2 double knock-out mutant
- TOCSY
- total correlation spectroscopy
- HSQC
- heteronuclear single quantum correlation
- HMBC
- heteronuclear multiple-bond correlation
- carboxy-DCFDA
- 5-(and 6)-carboxy-2′,7′-dichlorofluorescein diacetate.
REFERENCES
- 1. Park B. J., Wannemuehler K. A., Marston B. J., Govender N., Pappas P. G., Chiller T. M. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23, 525–530 [DOI] [PubMed] [Google Scholar]
- 2. Byrnes E. J., 3rd, Bildfell R. J., Frank S. A., Mitchell T. G., Marr K. A., Heitman J. (2009) Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. J. Infect. Dis. 199, 1081–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rhome R., McQuiston T., Kechichian T., Bielawska A., Hennig M., Drago M., Morace G., Luberto C., Del Poeta M. (2007) Biosynthesis and immunogenicity of glucosylceramide in Cryptococcus neoformans and other human pathogens. Eukaryot. Cell 6, 1715–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leipelt M., Warnecke D., Zähringer U., Ott C., Müller F., Hube B., Heinz E. (2001) Glucosylceramide synthases, a gene family responsible for the biosynthesis of glucosphingolipids in animals, plants, and fungi. J. Biol. Chem. 276, 33621–33629 [DOI] [PubMed] [Google Scholar]
- 5. Rittershaus P. C., Kechichian T. B., Allegood J. C., Merrill A. H., Jr., Hennig M., Luberto C., Del Poeta M. (2006) Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Invest. 116, 1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Michaelson L. V., Zäuner S., Markham J. E., Haslam R. P., Desikan R., Mugford S., Albrecht S., Warnecke D., Sperling P., Heinz E., Napier J. A. (2009) Functional characterization of a higher plant sphingolipid Delta4-desaturase: defining the role of sphingosine and sphingosine-1-phosphate in Arabidopsis. Plant Physiol. 149, 487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oura T., Kajiwara S. (2008) Disruption of the sphingolipid Δ8-desaturase gene causes a delay in morphological changes in Candida albicans. Microbiology 154, 3795–3803 [DOI] [PubMed] [Google Scholar]
- 8. Ternes P., Sperling P., Albrecht S., Franke S., Cregg J. M., Warnecke D., Heinz E. (2006) Identification of fungal sphingolipid C9-methyltransferases by phylogenetic profiling. J. Biol. Chem. 281, 5582–5592 [DOI] [PubMed] [Google Scholar]
- 9. Singh A., Wang H., Silva L. C., Na C., Prieto M., Futerman A. H., Luberto C., Del Poeta M. (2012) Methylation of glycosylated sphingolipid modulates membrane lipid topography and pathogenicity of Cryptococcus neoformans. Cell. Microbiol. 14, 500–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ito M., Yamagata T. (1986) A novel glycosphingolipid-degrading enzyme cleaves of the linkage between the oligosaccharide and ceramide of neutral and acidic glycosphingolipids. J. Biol. Chem. 261, 14278–14282 [PubMed] [Google Scholar]
- 11. Izu H., Izumi Y., Kurome Y., Sano M., Kondo A., Kato I., Ito M. (1997) Molecular cloning, expression of the endoglycoceramidase II gene from Rhodococcus species strain M-777. J. Biol. Chem. 272, 19846–19850 [DOI] [PubMed] [Google Scholar]
- 12. Ishibashi Y., Ikeda K., Sakaguchi K., Okino N., Taguchi R., Ito M. (2012) Quality control of fungus-specific glucosylceramide in Cryptococcus neoformans by endoglycoceramidase-related protein 1 (EGCrP1). J. Biol. Chem. 287, 368–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kunimoto S., Murofushi W., Kai H., Ishida Y., Uchiyama A., Kobayashi T., Kobayashi S., Murofushi H., Murakami-Murofushi K. (2002) Steryl glucoside is a lipid mediator in stress-responsive signal transduction. Cell Struct. Funct. 27, 157–162 [DOI] [PubMed] [Google Scholar]
- 14. Warnecke D., Erdmann R., Fahl A., Hube B., Müller F., Zank T., Zähringer U., Heinz E. (1999) Cloning and functional expression of UGT genes encoding sterol glucosyltransferases from Saccharomyces cerevisiae, Candida albicans, Pichia pastoris, and Dictyostelium discoideum. J. Biol. Chem. 274, 13048–13059 [DOI] [PubMed] [Google Scholar]
- 15. Nakagawa T., Tani M., Kita K., Ito M. (1999) Preparation of fluorescence-labeled GM1 and sphingomyelin by the reverse hydrolysis reaction of sphingolipid ceramide N-deacylase as substrates for assay of sphingolipid-degrading enzymes and for detection of sphingolipid-binding proteins. J. Biochem. 126, 604–611 [DOI] [PubMed] [Google Scholar]
- 16. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 17. Hayashi Y., Zama K., Abe E., Okino N., Inoue T., Ohno K., Ito M. (2008) A sensitive and reproducible fluorescent-based HPLC assay to measure the activity of acid as well as neutral β-glucocerebrosidases. Anal. Biochem. 383, 122–129 [DOI] [PubMed] [Google Scholar]
- 18. Kim M. S., Kim S. Y., Yoon J. K., Lee Y. W., Bahn Y. S. (2009) An efficient gene-disruption method in Cryptococcus neoformans by double-joint PCR with NAT-split markers. Biochem. Biophys. Res. Commun. 390, 983–988 [DOI] [PubMed] [Google Scholar]
- 19. Cox G. M., Rude T. H., Dykstra C. C., Perfect J. R. (1995) The actin gene from Cryptococcus neoformans: structure and phylogenetic analysis. J. Med. Vet. Mycol. 33, 261–266 [DOI] [PubMed] [Google Scholar]
- 20. Perfect J. R., Rude T. H., Penning L. M., Johnson S. A. (1992) Cloning the Cryptococcus neoformans TRP1 gene by complementation in Saccharomyces cerevisiae. Gene 122, 213–217 [DOI] [PubMed] [Google Scholar]
- 21. McDade H. C., Cox G. M. (2001) A new dominant selectable marker for use in Cryptococcus neoformans. Med. Mycol. 39, 151–154 [DOI] [PubMed] [Google Scholar]
- 22. Davidson R. C., Cruz M. C., Sia R. A. L., Allen B., Alspaugh J. A., Heitman J. (2000) Gene disruption by biolistic transformation in serotype D strains of Cryptococcus neoformans. Fungal Genet. Biol. 29, 38–48 [DOI] [PubMed] [Google Scholar]
- 23. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 24. Cabrera M., Ungermann C. (2008) Purification and in vitro analysis of yeast vacuoles. Methods Enzymol. 451, 177–196 [DOI] [PubMed] [Google Scholar]
- 25. Zinser E., Paltauf F., Daum G. (1993) Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J. Bacteriol. 175, 2853–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richards A., Gow N. A. R., Veses V. (2012) Identification of vacuole defects in fungi. J. Microbiol. Methods 91, 155–163 [DOI] [PubMed] [Google Scholar]
- 27. Sakon J., Adney W. S., Himmel M. E., Thomas S. R., Karplus P. A. (1996) Crystal structure of thermostable family 5 endocellulase E1 from Acidothermus cellulolyticus in complex with cellotetraose. Biochemistry 35, 10648–10660 [DOI] [PubMed] [Google Scholar]
- 28. Grille S., Zaslawski A., Thiele S., Plat J., Warnecke D. (2010) The functions of steryl glycosides come to those who wait: recent advances in plants, fungi, bacteria and animals. Prog. Lipid Res. 49, 262–288 [DOI] [PubMed] [Google Scholar]
- 29. Kim Y. K., Wang Y., Liu Z. M., Kolattukudy P. E. (2002) Identification of a hard surface contact-induced gene in Colletotrichum gloeosporioides conidia as a sterol glycosyl transferase, a novel fungal virulence factor. Plant J. 30, 177–187 [DOI] [PubMed] [Google Scholar]
- 30. Idnurm A., Warnecke D. C., Heinz E., Howlett B. J. (2003) Characterisation of neutral trehalase and UDP-glucose: sterol glucosyltransferase genes from the plant pathogenic fungus Leptosphaeria maculans. Physiol. Mol. Plant Pathol. 62, 305–313 [Google Scholar]
- 31. Oku M., Warnecke D., Noda T., Müller F., Heinz E., Mukaiyama H., Kato N., Sakai Y. (2003) Peroxisome degradation requires catalytically active sterol glucosyltransferase with a GRAM domain. EMBO J. 22, 3231–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stasyk O. V., Nazarko T. Y., Stasyk O. G., Krasovska O. S., Warnecke D., Nicaud J. M., Cregg J. M., Sibirny A. A. (2003) Sterol glucosyltransferases have different functional roles in Pichia pastoris and Yarrowia lipolytica. Cell Biol. Int. 27, 947–952 [DOI] [PubMed] [Google Scholar]
- 33. Cao Y., Klionsky D. J. (2007) Atg26 is not involved in autophagy-related pathways in Saccharomyces cerevisiae. Autophagy 3, 17–20 [DOI] [PubMed] [Google Scholar]
- 34. Kalinowska M., Wojciechowski Z. A. (1978) Purification and some properties of steryl β-d-glucoside hydrolase from Sinapis alba seedlings. Phytochemistry 17, 1533–1537 [Google Scholar]
- 35. Aguirre A., Peiru S., Eberhardt F., Vetcher L., Cabrera R., Menzella H. G. (2014) Enzymatic hydrolysis of steryl glucosides, major contaminants of vegetable oil-derived biodiesel. Appl. Microbiol. Biotechnol. 98, 4033–4040 [DOI] [PubMed] [Google Scholar]
- 36. Harrison T. S., Chen J., Simons E., Levitz S. M. (2002) Determination of the pH of the Cryptococcus neoformans vacuole. Med. Mycol. 40, 329–332 [DOI] [PubMed] [Google Scholar]
- 37. Cornet M., Bidard F., Schwarz P., Da Costa G., Blanchin-Roland S., Dromer F., Gaillardin C. (2005) Deletions of endocytic components VPS28 and VPS32 affect growth at alkaline pH and virulence through both RIM101-dependent and RIM101-independent pathways in Candida albicans. Infect. Immun. 73, 7977–7987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Poltermann S., Nguyen M., Günther J., Wendland J., Härtl A., Künkel W., Zipfel P. F., Eck R. (2005) The putative vacuolar ATPase subunit Vma7p of Candida albicans is involved in vacuole acidification, hyphal development and virulence. Microbiology 151, 1645–1655 [DOI] [PubMed] [Google Scholar]
- 39. Franke K., Nguyen M., Härtl A., Dahse H. M., Vogl G., Würzner R., Zipfel P. F., Künkel W., Eck R. (2006) The vesicle transport protein Vac1p is required for virulence of Candida albicans. Microbiology 152, 3111–3121 [DOI] [PubMed] [Google Scholar]
- 40. Johnston D. A., Eberle K. E., Sturtevant J. E., Palmer G. E. (2009) Role for endosomal and vacuolar GTPases in Candida albicans pathogenesis. Infect. Immun. 77, 2343–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y., Rao R. (2012) The V-ATPase as a target for antifungal drugs. Curr. Protein Pept. Sci. 13, 134–140 [DOI] [PubMed] [Google Scholar]
- 42. Thompson J. D., Higgins D. G., Gibson T. J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gouet P., Robert X., Courcelle E. (2003) ESPript/ENDscript: extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 31, 3320–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Caines M. E. C., Vaughan M. D., Tarling C. A., Hancock S. M., Warren R. A. J., Withers S. G., Strynadka N. C. J. (2007) Structural and mechanistic analyses of endo-glycoceramidase II, a membrane-associated family 5 glycosidase in the Apo and GM3 ganglioside-bound forms. J. Biol. Chem. 282, 14300–14308 [DOI] [PubMed] [Google Scholar]