Background: Growth arrest is a hallmark of cellular senescence.
Results: Loss of cell proliferation in senescent cells was associated with impaired ERK1/2 activation, which was caused by p53-mediated elevation of MKP-3.
Conclusion: The p53/MKP-3/ERK1/2 cascade contributed to the establishment of the senescent phenotype.
Significance: Our study provides novel insights into the actions and mechanisms of p53 on cellular senescence.
Keywords: Extracellular Signal-regulated Kinase (ERK), Oxidative Stress, p53, Proliferation, Senescence, MAP Kinase Phosphatase 3 (MKP-3)
Abstract
Growth arrest is one of the essential features of cellular senescence. At present, the precise mechanisms responsible for the establishment of the senescence-associated arrested phenotype are still incompletely understood. Given that ERK1/2 is one of the major kinases controlling cell growth and proliferation, we examined the possible implication of ERK1/2. Exposure of normal rat epithelial cells to etoposide caused cellular senescence, as manifested by enlarged cell size, a flattened cell body, reduced cell proliferation, enhanced β-galactosidase activity, and elevated p53 and p21. Senescent cells displayed a blunted response to growth factor-induced cell proliferation, which was preceded by impaired ERK1/2 activation. Further analysis revealed that senescent cells expressed a significantly higher level of mitogen-activated protein phosphatase 3 (MKP-3, a cytosolic ERK1/2-targeted phosphatase), which was suppressed by blocking the transcriptional activity of the tumor suppressor p53 with pifithrin-α. Inhibition of MKP-3 activity with a specific inhibitor or siRNA enhanced basal ERK1/2 phosphorylation and promoted cell proliferation. Apart from its role in growth arrest, impairment of ERK1/2 also contributed to the resistance of senescent cells to oxidant-elicited cell injury. These results therefore indicate that p53-mediated up-regulation of MKP-3 contributes to the establishment of the senescent cellular phenotype through dephosphorylating ERK1/2. Impairment of ERK1/2 activation could be an important mechanism by which p53 controls cellular senescence.
Introduction
Senescence was first described by Hayflick around 40 years ago (1). The term comes from Latin and means “growing old.” In general, senescence refers to a process that limits the proliferation of normal cells in culture. Most division-competent cells undergo senescence in response to various stress stimuli. Cellular senescence has been implicated in various pathophysiological situations, including tissue repair, tumor progression, and aging-related diseases (2).
Cellular senescence is classified as replicative senescence, stress-induced premature senescence, and oncogene-induced senescence. Replicative senescence refers to senescence resulting from the gradual loss of DNA at the ends of chromosomes, also called telomeres, following repeated cell replication (3). The eroded telomeres cause a persistent DNA damage response, thereby resulting in cellular senescence (3–5). Stress-induced premature senescence is defined as early senescence resulting from repeated exposure to various stresses at subcytotoxic concentrations, e.g. H2O2, chemotherapeutic agents, and ultraviolet and ionizing radiation (6). The third type of senescence is oncogene-induced senescence. It refers to senescence caused by oncogenic mutations. Many mutated oncogenes, such as Ras, Raf, MEK, and c-Myc, have been shown to induce senescence (7, 8). All of these types of senescence have several similar characteristic morphological and biochemical features, including loss of cell division, resistance to apoptosis, and an altered secretory profile (9).
The fundamental feature of cell senescence is the loss of cell proliferation as in its definition. The cell cycle of senescent cells is generally believed to arrest in G0 and G1 phase (10). This has been considered to be the result of the increased braking mechanisms that blocks the progression of the cell cycle. Normally cell proliferation is regulated by the cell cycle, the progression of which is driven by the activation and inactivation of cyclin-dependent kinases (CDKs)2 through interaction with the cyclin subunit. Activated CDKs phosphorylate retinoblastoma protein and prevent the formation of the E2F complex, therefore promoting progression of the cell cycle from G1 phase to S phase (10). In senescent cells, the cell cycle inhibitors p53/p21Waf1/Cif1 and p16INK4a are activated, which interact with CDKs and prevent retinoblastoma protein from phosphorylation, therefore maintaining it in the E2F-DP1-retinoblastoma protein complex and growth-inhibitory state (11).
One of the important molecules that regulate cell growth and proliferation is ERK1/2. Cell proliferation is associated with an early activation of ERK1/2, the inhibition of which abolishes growth factor-induced cell proliferation (12). ERK1/2 regulates cell proliferation via multiple mechanisms (13, 14). ERK1/2 induces the expression of immediate-early genes such as c-Fos through phosphorylation and activation of the transcriptional factor Elk-1. ERK1/2 also stabilizes c-Fos through direct phosphorylation and promotes its association with c-Jun. The formation of transcriptionally active AP-1 complexes leads to the expression of cyclin D1, a protein that interacts with CDKs and permits G1/S transition and cell cycle progression (13, 14). Apart from its role in cell proliferation, ERK1/2 also regulates many other cell behaviors that are closely related to cell senescence, such as cell apoptosis and secretion (9). In this context, a critical involvement of ERK1/2 in the establishment of senescent phenotype is highly probable. The purpose of this study was to test this hypothesis.
Here we present our data showing that impaired ERK1/2 activation is a key molecular event implicated in the establishment of cellular senescence. Furthermore, we characterize p53-mediated up-regulation of MKP-3 as the mechanism behind the defect in ERK1/2 activation in senescent cells.
EXPERIMENTAL PROCEDURES
Reagents
PDGF-BB, hepatocyte growth factor, and FGF were purchased from R&D Systems (Minneapolis, MN). Etoposide (ETO), doxorubicin (Dox), and hydrogen peroxide (H2O2) were obtained from Wako Pure Chemicals (Tokyo, Japan). PD98059 and SB203580 were from Calbiochem. SP600125, U0126, FR180204, pifithrin-α (2-benzylidene-3-(cyclohexylamino)-1-indanone hydrochloride), menadione, and anti-β-actin antibody were purchased from Sigma-Aldrich Japan (Tokyo, Japan). The phosphoPlus-44/42 MAPK (ERK1/2) (Thr-202/Tyr-204) antibody kit, phospho-MEK1/2 antibody (Beverly, MA). MKP-3 (F-12) and MKP-1 (C-19) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Culture
NRK-52E rat renal tubular epithelial cells and the WI-38 human diploid cell line (WI-38) were purchased from the ATCC. Cells were cultured in Dulbecco's modified Eagle's medium/Ham's F-12 medium (Invitrogen) supplemented with 5 to10% FBS.
BrdU ELISA assay
Proliferation was measured by cell proliferation ELISA BrdU kit (Roche Applied Science) following the manual of the manufacturer. Briefly, cells were incubated with BrdU labeling solution for 3 h at 37 °C and then fixed and denatured by FixDenat solution for 30 min. After 90 min of incubation with anti-BrdU-peroxidase, substrate solution was added until the color development was sufficient for photometric detection. H2SO4 was applied to stop the reaction. Absorbance was measured using an ELISA reader at 450 nm.
Transfection
NRK-52E cells were transiently transfected with pTRE-MKP-1–631Luc (provided by Dr. Fumio Tashiro, Tokyo University of Science, Japan), pGL3B/DUSP6 promoter-Luc (provided by Dr. Stephen M. Keyse, Ninewells Hospital and Medical School, UK), or pGL3B/p21Waf1/Cif1-Luc (provided by Dr. Naoko Ohtani, Japanese Foundation for Cancer Research, Japan) by electroporation. Transient transfection of NRK-52E cells with pcDNA3.1-DUSP-6-V5/His vector (provided by Dr. Toru Furukawa, Tokyo Woman's Medical University, Japan) and GFP-tagged human DUSP6 (catalog no. RG216245, Origene) were done with GeneJuice transfection reagent (Novagen). After 24 or 48 h, cells were seeded into 96- or 12-well plates and subjected to various stimulations.
siRNA Treatment
NRK cells were transfected with siRNA specifically targeting MKP-3 (Thermo Fisher Scientific) or negative control siRNA (AllStars negative control siRNA) at a final concentration of 20 nm using Hyperfect transfection reagent for 48 h. Then cell proliferation was evaluated by BrdU assay. The cell lysate was used for analysis of MKP-3 and p-ERK levels.
Luciferase Assay
Luciferase activity was evaluated by a luciferase assay system (Promega, Madison, WI) according to the protocol of the manufacturer. Assays were performed in quadruplicate.
Western Blot Analysis
Equal amounts of cell lysates were separated with 12% SDS-polyacrylamide gels and electrotransferred onto 0.4 μm polyvinylidene difluoride membranes. After being blocked with 5% nonfat milk in PBS, the membranes were incubated with the first antibodies (1:1000 dilution). Then the membranes were washed with PBS containing 0.05% Tween 20 and probed with horseradish peroxidase-conjugated sheep anti-rabbit IgG or horse anti-mouse IgG (1:2000 dilution, Cell Signaling Technology). Immunoreactivity was detected using ECL (Amersham Biosciences). The chemiluminescent signal was captured with a Fujifilm luminescent image FAS-4000 analyzer (Fujifilm, Tokyo, Japan). Densitometric analysis of individual bands was done using NIH ImageJ software (http://rsb.info.nih.gov/ij/).
Northern Blot Analysis
Cells were treated with various stimulants. Total RNA was extracted by a single-step method. Equal amounts of RNA (5 μg) were separated with electrophoresis and transferred onto nylon membranes (Hybond N+, Amersham Biosciences). The levels of MKP-3, c-Fos, Ho-1, and MKP-1 were examined as described previously (15). cDNAs for c-Fos (provided by Dr. Rolf Müller, Institut für Molekularbiologie und Tumorforschung, Germany), HO-1 (provided by Dr. Silvia Cardoso, Instituto Gulbenkian de Ciência, Portugal), MKP-3 (provided by Dr. Toru Furukawa, Tokyo Women's Medical University, Japan) were used to prepare radiolabeled probes. GAPDH was used as a loading control.
SA-β-gal Staining
SA-β-gal activity was evaluated as described by Dimri et al. (16). In brief, cells were fixed with 3% formaldehyde for 4 min and incubated at 37 °C overnight in X-gal solution containing 1 mg/ml X-gal (Sigma-Aldrich Japan), 40 mm citric acid/sodium phosphate (pH 6.0), 5 mm potassium ferricyanide, 5 mm potassium ferrocyanide, and 150 mm NaCl, and the experiment was performed in quadruplicate.
Formazan Assay
The number of viable cells was assessed by formazan assay using cell counting kit 8 (Dojindo Laboratory, Kumamoto, Japan) according to the protocol of the manufacturer. Assays were performed in quadruplicate.
Statistic Analysis
Data were expressed as mean ± S.E. Statistical analysis was performed using the non-parametric Mann-Whitney U test to compare data in different groups. p < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Loss of Cell Proliferation in Senescent Cells Is Associated with Impaired ERK1/2 Activation
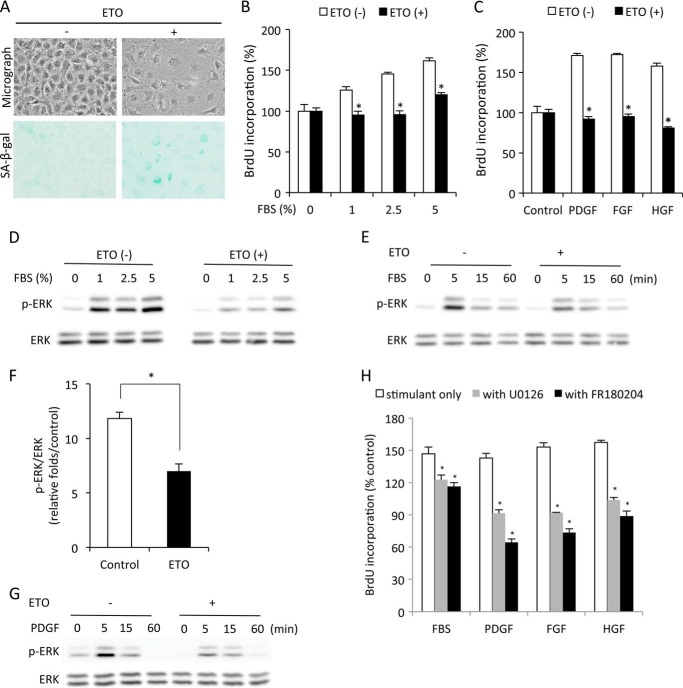
To investigate the potential implication of ERK1/2 in cell senescence, we used a premature senescent model induced by ETO (17). Incubation of NRK cells with subtoxic concentrations of ETO resulted in an enlargement of cell size and elevation of SA-β-gal activity, indicative of cellular senescence (Fig. 1A).
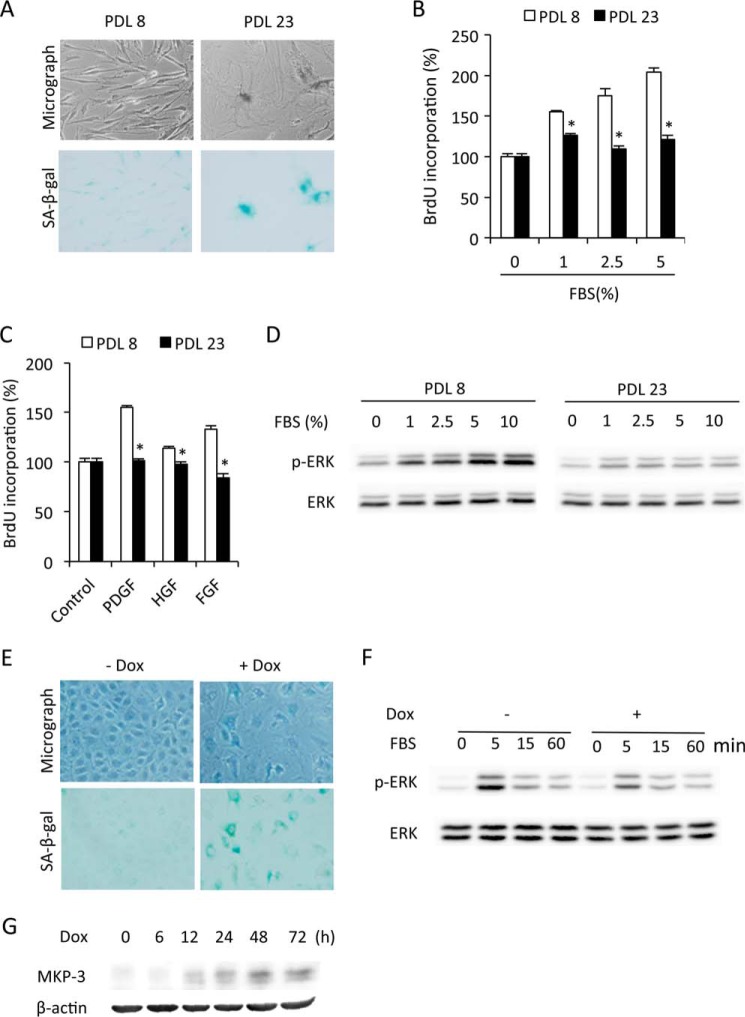
FIGURE 1.
ERK1/2 phosphorylation is impaired in senescent cells. A, induction of senescence by ETO. NRK cells were treated with 1 μg/ml ETO for 3 days and subjected to phase-contrast microscopy and SA-β-gal staining. B and C, reduced cell proliferation in senescent cells. NRK cells were treated with or without 1 μg/ml ETO for 3 days. Then cells were quiescent for 48 h and exposed to the indicated concentrations of FBS (B) or various growth factors (10 ng/ml, C) for 24 h. Cell proliferation was evaluated through BrdU incorporation. Data are mean ± S.E. (n = 4). *, p < 0.05 versus respective untreated control. HGF, hepatocyte-derived growth factor. D–G, impaired ERK1/2 phosphorylation in senescent cells. Cells were pretreated with or without ETP for 3 day and then exposed to the indicated concentration of FBS for 5 min or 5% FBS or 10 ng/ml PDGF for the indicated time intervals. Cellular lysates were extracted and subjected to Western blot analysis of p-ERK1/2 and ERK1/2 (D, E, and G). The densitometric data of p-ERK1/2 protein 5 min after exposure to 5% FBS was normalized to total ERK1/2 and control (F, data are mean ± S.E., n = 3). *, p < 0.05 versus untreated control. H, inhibition of ERK1/2 on cell proliferation. NRK cells were treated with 2.5% FBS, 10 ng/ml PDGF, FGF, or hepatocyte growth factor in the presence or absence of 20 μm U0126 or 100 μm FR180204 for 24 h and subjected to BrdU incorporation. Data are mean ± S.E. (n = 4). *, p < 0.05 versus respective untreated control.
The ETO-treated cells displayed a significantly reduced proliferation in response to FBS and growth factors (PDGF, FGF, and hepatocyte growth factor) (Fig. 1, B and C). Because ERK1/2 mediates mitogen-triggered cell proliferation (12), we examined ERK1/2 activation in senescent cells. As shown in Fig. 1, D–G, activation of ERK1/2 triggered by FBS and PDGF was reduced markedly in ETO-pretreated senescent cells compared with control cells.
To confirm the role of ERK1/2 in cell proliferation, we treated cells with inhibitors that target ERK1/2 or its upstream kinase MEK-1. Both ERK1/2 inhibitor FR180204 and MEK-1 inhibitor U0126 significantly prevented BrdU uptake induced by various mitogens (Fig. 1G). These results therefore indicate that the loss of cell proliferation in senescent cells is associated with the impaired activation of ERK1/2.
Elevated MKP-3 in Senescent Cells Underlies the Defect in ERK Activation
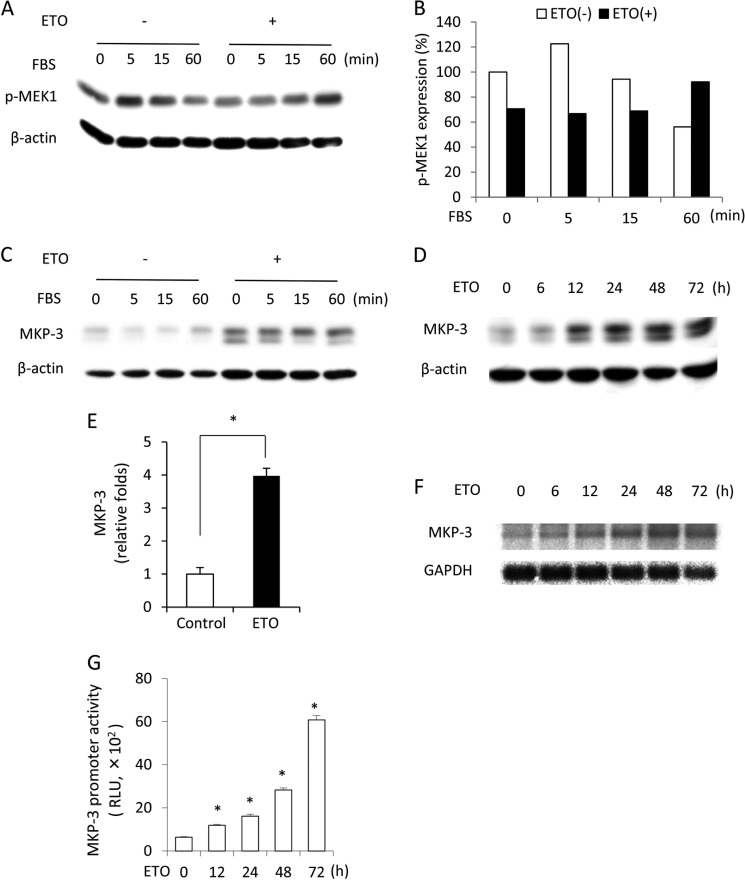
ERK1/2 is phosphorylated by its upstream kinase MEK-1 and dephosphorylated by phosphatases (18). We therefore examined the possible implication of these molecules in impaired ERK1/2 activation. First, we compared the phosphorylation level of MEK-1 between mitogen-stimulated control and senescent cells. It appeared that ETO-treated cells had a lower basal level of phosphorylated MEK-1 in comparison with control cells. In addition, they displayed a blunted response to FBS-induced MEK-1 phosphorylation. In control cells, FBS induced a peak activation of MEK-1 at 5 min and declined thereafter to below the basal level at 60 min. In ETO-treated cells, however, the activation of MEK-1 by FBS reached a maximum at 60 min. These results indicate that ETO treatment might affect MEK-1 activation (Fig. 2, A and B). We then proceeded to test the role of ERK1/2 phosphatases. We focused on MKP-3 because it specially dephosphorylates cytosolic ERK1/2 (19). Fig. 2C shows that ETO-pretreated cells expressed higher level of MKP-3 in comparison with control cells. Indeed, induction of senescence with ETO caused a time-dependent elevation of MKP-3 protein (Fig. 2, D and E). The level of MKP-3 mRNA and transcriptional activities were also enhanced by ETO, as revealed by Northern blot analysis and a promoter activity assay (Fig. 2, F and G).
FIGURE 2.
Influence of the senescence-inducing agent ETO on MEK-1 activation and MKP-3 protein level. A–C, levels of phosphorylated MEK-1 and MKP-3 in control and senescent cells. NRK cells were treated with 1 μg/ml ETO for 3 days or left untreated. Then cells were exposed to 5% FBS for the indicated time. Cellular lysates were subjected to Western blot analysis of p-MEK1 (A) and MKP-3 (B). Densitometric quantitation of the level of the phosphorylated MEK-1 in A is shown in C. The result was normalized to zero point control. D–G, changes in MKP-3 during induction of senescence with ETO. Cells were treated with 1 μg/ml ETO for the indicated time and subjected to Western blot analysis of MKP-3. β-Actin was used as a loading control (D). Densitometric quantitation of the level of MKP-3 at the 48-h point following exposure to 1 μg/ml ETO is shown in E. The result was normalized to the control. Data are mean ± S.E. (n = 3). *, p < 0.05 versus control. F, cells were treated with 1 μg/ml ETO for the indicated time and subjected to Northern blot analysis of MKP-3. GAPDH was used as a loading control. G, cells transfected with pGL3B/DUSP6-luc were exposed to 1 μg/ml ETO for the indicated time. Cell lysates were subjected to a luciferase assay. Data are mean ± S.E. (n = 4). RLU, relative light unit. *, p < 0.05 versus zero point control.
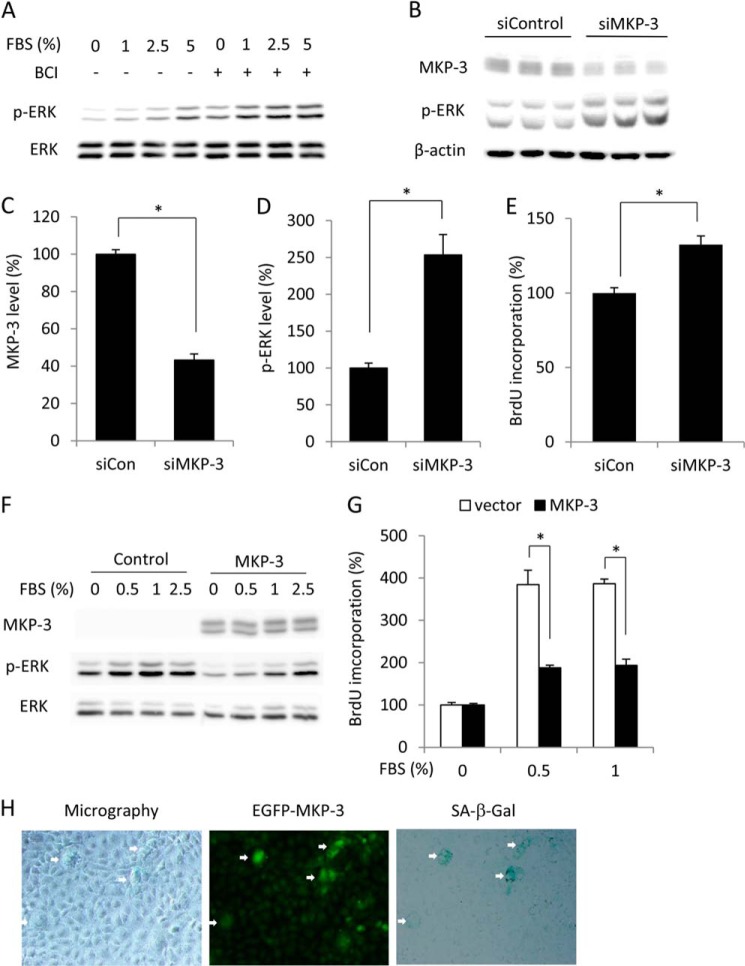
To evaluate the role of MKP-3 in impaired ERK1/2 activation, we examined the cell response to FBS in the presence of 2-benzylidene-3-(cyclohexylamino)-1-indanone hydrochloride, a novel MKP-3 inhibitor that inhibits the activity of MKP-3 through competitive binding to ERK1/2 (20). Inhibition of MKP-3 with 2-benzylidene-3-(cyclohexylamino)-1-indanone hydrochloride recovered ERK1/2 phosphorylation in response to FBS stimulation (Fig. 3A). Down-regulation of MKP-3 by siRNA enhanced the basal level of ERK1/2 phosphorylation and promoted cell proliferation (Fig. 3, B–E). On the contrary, overexpression of MKP-3 by transfection of cells with a MKP-3 expression plasmid increased MKP-3 and decreased FBS-induced ERK activation as well as cell proliferation (Fig. 3, F and G). To determine whether there was a correlation between MKP-3 and formation of a senescent phenotype, we transfected cells with GFP-tagged MKP-3 and analyzed cell morphology, β-gal activity, and fluorescence intensity of GFP. As shown in Fig. 3H, cells with a strong GFP signal were more likely to have a large cell size and higher activity of SA-β-gal. Similar results were achieved through simultaneous staining of the transfected cells with MKP-3 and SA-β-gal (data not shown). These results therefore implicate elevated MKP-3 in the induction of the senescent cellular phenotype.
FIGURE 3.
Effect of MKP-3 on ERK1/2 phosphorylation, cell proliferation, cell size, and SA-β-gal activity. A–E, down-regulation of MKP-3 on ERK1/2 phosphorylation and cell proliferation. A, cells were pretreated with 1 μg/ml ETO for 3 days. After that, they were treated with 1 μm 2-benzylidene-3-(cyclohexylamino)-1-indanone hydrochloride (BCI) for 15 min or left untreated before being exposed to the indicated concentrations of FBS for an additional 5 min. Cell lysates were subjected to Western blot analysis of p-ERK1/2 and ERK1/2. B–E, cells were transfected with control or MKP-3 siRNA. Cell lysates were subjected to Western blot analysis of MKP-3 and p-ERK1/2. β-Actin was used as a loading control (B). Densitometric quantitations of the levels of MKP-3 and p-ERK1/2 in B are shown in C and D. The result was normalized to siRNA control (siCon). Data are mean ± S.E. (n = 3). *, p < 0.05 versus control. E, cells transfected with control or MKP-3 siRNA were subjected to BrdU incorporation. Data are mean ± S.E. (n = 4). *, p < 0.05 versus siRNA control. F and G, overexpression of MKP3 on ERK1/2 and cell proliferation. Cells that were transiently transfected with a control or MKP-3 plasmid were exposed to the indicated concentration of FBS for 5 min. Cellular protein was extracted and subjected to Western blot analysis of MKP-3, p-ERK, and ERK. G, cells transfected with control or MKP-3 plasmid were subjected to BrdU incorporation. Data are mean ± S.E.; n = 4; *, p < 0.05. H, transfection of NRK cells with a GFP-tagged MKP-3 gene on cell morphology and SA-β-gal activity. Cells were transfected with a GFP-tagged MKP-3 gene. After 4 days, cells were subjected to phase-contrast/immunofluorescence microscopy and SA-β-gal staining. Note the cell size and SA-β-gal activity in the GFP-positive cells (arrows).
MKP-3 and ERK1/2 Are Downstream Targets of p53
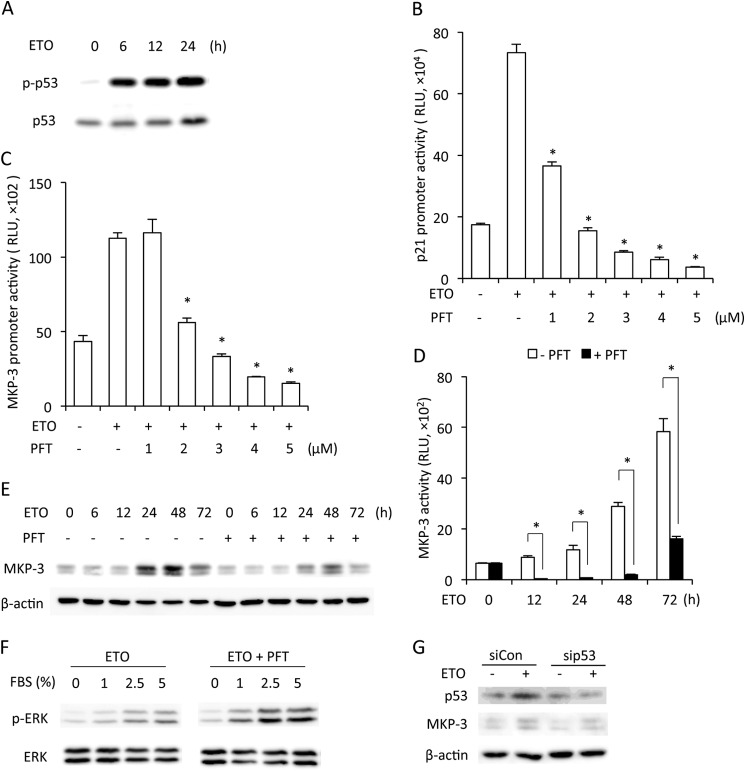
Given that p53 plays a pivotal role in the establishment and maintenance of cellular senescence (21) and that p53 was characterized recently as a transcriptional factor of MKP-3 (22), we investigated the role of p53. Induction of cellular senescence with ETO was associated with an increased level of total and phosphorylated p53 (Fig. 4A). The promoter activity of p21, a downstream target of p53, was also elevated, indicating increased p53 transcription activity (Fig. 4B). Incubation of cells with pifithrin-α (PFT), a chemical inhibitor that specifically suppresses the transcription activity of p53 (23), completely abolished the activation of p21 gene (Fig. 4B). It also inhibited the promoter activity of MKP-3 induced by ETO (Fig. 4, C and D), indirectly indicating a critical role of p53 in the transcriptional activation of MKP-3. Consistently, PFT also inhibited the up-regulation of the MKP-3 protein (Fig. 4E) and recovered FBS-triggered ERK1/2 phosphorylation in senescent cells (Fig. 4F). Further analysis using siRNA specifically against p53 revealed that down-regulation of p53 was associated with a decreased level of MKP-3 (Fig. 4G). Collectively, these results indicate that a p53-mediated enhancement of MKP-3 is responsible for the impairment of ERK1/2 activation in senescent cells.
FIGURE 4.
Role of p53 in activation of MKP-3 transcription. A, change of p53 during senescence. NRK cells were treated with 1 μg/ml ETO for the indicated time. Cell lysate were extracted and subjected to Western blot analysis of p-p53 and p53. B–E, involvement of p53 in MKP-3 expression. Cells transfected with the pGL3B/DUSP6-luc (B) or pGL3B/p21WAF1/CIF1-Luc plasmids (C) were treated with 1 μg/ml ETO in the absence or presence of the indicated concentration of PFT for 24 h. Cell lysates were extracted and assayed for luciferase activity. Data are mean ± S.E. (n = 4). *, p < 0.05 versus ETO alone. RLU, relative light units. D, cells transfected with the pGL3B/DUSP6-luc plasmid were treated with 1 μg/ml ETO with or without 5 μm PFT for the indicated time. Cell lysates were extracted and assayed for luciferase activity. Data are mean ± S.E. (n = 4). *, p < 0.05 versus the respective time point control. E, cells were treated with 1 μg/ml ETO with or without 5 μm PFT for the indicated time. Cell lysates were detected for expression of MKP-3. β-Actin was used as a loading control. F, regulation of ERK1/2 by p53. Cells were treated with 1 μg/ml ETO with or without 5 μm PFT for 3 days. Then cells were stimulated with the indicated concentrations of FBS for 5 min. Cell lysates were analyzed for the level of p-ERK1/2 and total ERK1/2. G, down-regulation of p53 with siRNA on MKP-3 expression. Cells were transfected with control or p53 siRNA. Cell lysates were subjected to Western blot analysis of p53 and MKP-3. β-Actin was used as a loading control. siCon, control siRNA.
Loss of Cell Proliferation in Other Models of Cellular Senescence Is Also Associated with Impaired ERK1/2 Activation
To determine whether our findings could also be applicable to other models of cellular senescence, we compared ERK1/2 phosphorylation between passages 8 and 23 of WI-38 cells, which is known to undergo replicative senescence with extended passage (1). Indeed, WI38 cells at passage 23 exhibited a senescent phenotype, as manifested by declined growth ability, enlarged cell size, and increased SA-β-gal activity (Fig. 5A). Consistent with the results obtained from ETO-induced premature senescence, WI38 cells at passage 23 displayed an obvious reduction in proliferation and ERK1/2 activation in response to FBS and growth factors in comparison with the juvenile counterpart at passage 8 (Fig. 5, B–D).
FIGURE 5.
Impaired ERK1/2 activation in other models of cellular senescence. A, morphological changes in replicative senescent cells. WI-38 cells at passages 8 and 23 (PDL8 and PDL23) were subjected to SA-β-gal staining. B–D, impaired cell proliferation and ERK1/2 phosphorylation in replicative senescent cells. PDL8 and PDL23 cells were quiescent for 48 h. Then cells were exposed to the indicated concentrations of FBS (B) or 10 ng/ml growth factors (C) for 24 h. Cell proliferation was evaluated through BrdU incorporation. Data are mean ± S.E. (n = 4). *, p < 0.05 versus the respective control. HGF, hepatocyte-derived growth factor. D, PDL8 and PDL23 cells were stimulated with the indicated concentrations of FBS for 5 min and subjected to Western blot analysis of p-ERK1/2 and ERK1/2. E, induction of senescence by Dox. NRK cells were treated with 100 μg/ml Dox for 3 days and then subjected to phase-contrast microscopy and β-gal staining. F, impaired ERK1/2 activation in Dox-pretreated cells. NRK cells were treated with 100 μg/ml Dox for 3 days. Then cells were exposed to 5% FBS for the indicated time. The cellular lysates were subjected to Western blot analysis of p-ERK and ERK. G, effect of Dox on MKP-3 expression. NRK cells were treated with 100 μg/ml Dox for the indicated time and subjected to Western blot analysis of MKP-3. β-Actin was used as a loading control.
We also looked at ERK1/2 activation and the MKP-3 level in a premature model of cellular senescence induced by Dox. As shown in Fig. 5, E and F, induction of senescence by Dox was also associated with impaired ERK activation and an increased level of MKP-3. Collectively, these observations indicate that impaired ERK1/2 activation might also contribute to the declined proliferative ability in other models of cellular senescence.
Impaired ERK1/2 Activation Contributes to Senescence-associated Cellular Resistance to Oxidative Injury
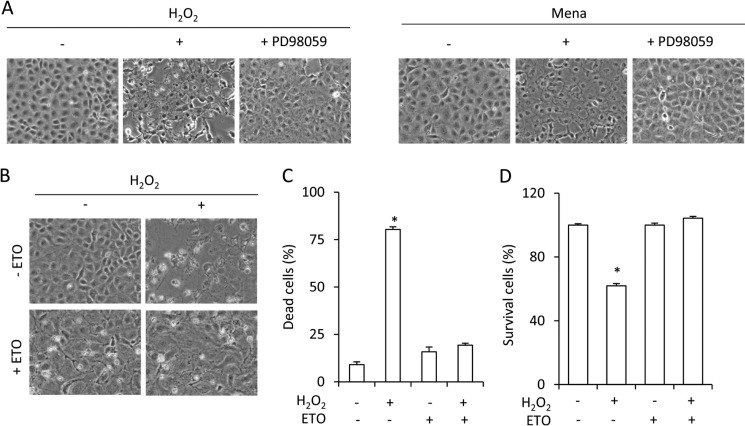
Apart from its critical role in cell proliferation, ERK1/2 also mediates cell response to the cytotoxic effects of oxidative stress (24, 25). As shown in Fig. 6A, H2O2 and superoxide donor menadione-elicited loss of cell viability was mediated by ERK1/2. In the presence of the MEK-1 inhibitor PD98059, the cell death induced by these chemicals was greatly inhibited. In comparison with control cells, senescent cells displayed a dramatic resistance to H2O2-induced cell injury (Fig. 6B). There were few round and detached cells in senescent cells after exposure to H2O2 (Fig. 6C). Most of them survived after the stimulation (Fig. 6D).
FIGURE 6.
Resistance to oxidative cell injury in senescent cells. A, role of ERK1/2 in oxidative stress-induced cell injury. NRK cells were exposed to 100 μm H2O2 (left panel) or 50 μm menadione (Mena, right panel) in the presence or absence of 50 μm PD98059 for 12 h. The cell morphology under phase-contrast microscopy was photographed (magnification, ×200). B–D, resistance to oxidative injury in senescent cells. Cells were treated with or without 1 μg/ml ETO for 3 days, and then cells were exposed to 100 μm H2O2 for 12 h and subjected to phase-contrast microscopy (B) or formazan assay (D). The quantitative result of B is shown in C. Results are expressed as mean ± S.E. (n = 4). *, p < 0.05 versus untreated control.
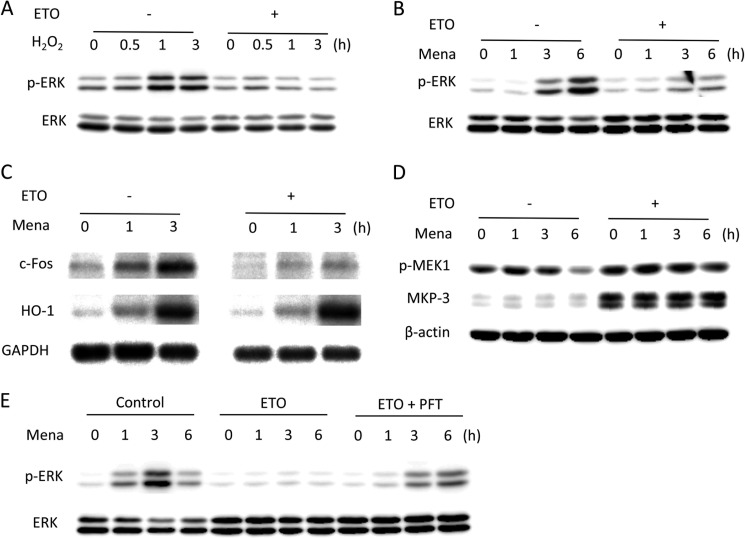
To examine the role of ERK1/2, we compared the oxidant-induced activation of ERK1/2 between control and senescent cells. Both H2O2 and menadione failed to activate ERK1/2 in ETO-pretreated cells (Fig. 7, A and B). Accordingly, the induction of c-Fos, a downstream substrate of ERK1/2, was also defective in senescent cells (Fig. 7C). Interestingly, the defect was not associated with the altered level of HO-1 (Fig. 7C), a parameter of oxidative stress (26), suggesting that, under equal reactive oxygen species stimulation, senescent cells displayed a preferential response to reactive oxygen species-induced activation of ERK1/2.
FIGURE 7.
Impaired ERK1/2 activation in oxidant-exposed senescent cells. A–C, ERK1/2 phosphorylation induced by oxidative stress in senescent cells. Control and senescent cells were exposed to 100 μm H2O2 (A) or 50 μm menadione (Mena, B) for the indicated times and subjected to Western blot analysis of p-ERK1/2 and ERK1/2. C, cells were exposed to 50 μm menadione for the indicated time and subjected to Northern blot analysis of c-Fos and Ho-1. GAPDH was used as a loading control. D and E, involvement of p53 in the regulation of ERK1/2 phosphorylation. D, control and senescent cells were treated with 50 μm menadione for the indicated time and subjected to Western blot analysis of p-MEK1 and MKP-3. β-Actin was used as a loading control. E, control cells were treated with 1 μg/ml ETO in the absence or presence of 5 μm PFT for 3 days, and then cells were exposed to 50 μm menadione for the indicated time. Cell lysates were subjected to Western blot analysis of p-ERK1/2 and ERK1/2.
Impaired phosphorylation of ERK1/2 was associated with an obvious elevation in MKP-3 level but without change in MEK-1 (Fig. 7D). In support of a role of MKP-3 in dephosphorylating ERK1/2, blockade of p53 transcription activity by PFT reversed ERK1/2 phosphorylation (Fig. 7E). These results indicate that the same regulatory mechanism might contribute to senescence-associated cell resistance to oxidative injury.
DISCUSSION
In this study, we demonstrated that p53-mediated up-regulation of MKP-3 contributed to the arrested phenotype of senescence through inactivation of ERK1/2. Our study therefore unraveled an unreported mechanism of cellular senescence. Furthermore, we characterized MKP-3/ERK1/2 as an important downstream target of p53 function.
We used a well documented premature senescence model induced by ETO to investigate the role of ERK1/2 in cellular senescence (27, 28). ETO-pretreated cells exhibited a markedly declined proliferative response to mitogens, a hallmark feature of senescence. Traditionally, the loss of cell proliferation in senescent cells is considered to be the result of the braking mechanisms that block progress through the cell cycle (29). Indeed, cell senescence is associated with a markedly elevated level of cell cycle-inhibitory proteins, such as p16, p21, and p53 (7, 27, 29–32). Besides the braking mechanisms, impairment of the mechanisms that drive the progression of the cell cycle is also a determining factor. Because it is positioned at the upstream of the braking mechanism, it should play an even greater role. However, information on this aspect is still limited. In this study, we focused on ERK1/2, a molecule that mediates extracellular stimuli-elicited cell proliferation. ERK1/2 promotes progression of the cell cycle through multiple mechanisms. It induces the expression of genes that are important for cell cycle progression through activation of the transcription factors Myc and AP-1 (33). It also promotes the formation of the complexes of cyclin E/CDK2 and cyclin B/CDK1 that are critically involved in the transition of G1/S and G2/M phase, respectively (34–36). Furthermore, ERK1/2 participates in the regulation of many cellular processes necessary for cell growth and division, including protein translation, chromatin remodeling, ribosome synthesis, etc. (14). Therefore, a defect in ERK1/2 activation in senescent cells, as shown in this study, might be an important mechanism behind the blunted cell response to mitogens.
What were the mechanisms underlying the defect in ERK1/2 activation in senescent cells? ERK1/2 is dynamically regulated through phosphorylation. Its full activation relies on dual phosphorylation at both sites of Tyr-204/187 and Thr-202/185 by its upstream kinase MEK-1 (18). Upon extracellular mitogen stimuli, receptor tyrosine kinases convert the inactive Ras-GDP to active Ras-GTP, which activates Raf kinase and, subsequently, leads to activation of MEK-1. The activated MEK-1 combines and phosphorylates ERK1/2 (13). In this study, we confirmed the pivotal role of MEK-1 in the phosphorylation of ERK1/2. Inhibition of MEK-1 with U0126 abolished ERK1/2 activation and cell proliferation. Furthermore, ETO-treated cells appeared to have a delayed response to FBS-induced activation of MEK-1. Therefore, MEK-1 might also contribute to the blunted ERK1/2 activation. However, considering that the late activation of MEK-1 in ETO-treated cells was not associated with an increased ERK1/2 phosphorylation, we speculated that the mechanisms of dephosphorylating ERK 1/2 might play an even greater role in this investigation.
Dephosphorylation of ERK1/2 by phosphatases also plays a critical role in the control of ERK1/2 activity. Among many phosphatases identified to dephosphorylate MAP kinases, dual specificity phosphatase 3 (MKP-3) has been shown to selectively inactivate cytosolic ERK1/2 through dephosphorylation of both threonine and tyrosine residues (37). Expression of MKP-3 completely blocked ERK1/2 activation induced by epidermal growth factor or by P21ras (37). In line with this report, our study provided additional evidence supporting a role of MKP-3 in dephosphorylating ERK1/2. Inhibition or down-regulation of MKP-3 with a specific inhibitor or siRNA enhanced basal ERK1/2 phosphorylation and promoted cell proliferation. On the contrary, overexpression of MKP-3 blunted ERK1/2 activation and cell proliferation. Therefore, the markedly elevated MKP-3 in senescent cells might contribute to the blunted cell response to mitogen-induced ERK1/2 phosphorylation. In addition, it also contributed to the establishment of other senescent features, including enlargement of cell size and enhanced activity of SA-β-gal. Cells with a high level of MKP-3 were found to be more likely to have a larger cell size and stronger intensity of SA-β-gal staining. These observations are consistent with a recent paper demonstrating a causative role of the increased MKP-3 (also known as dual-specific phosphatase 6) in defect in T cell receptor-induced ERK1/2 phosphorylation and proliferation in naive CD4+ T cells of elderly persons (38). Besides in rat renal epithelial cells, we also detected a defect in ERK1/2 activation at highly passaged WI-38 cells These cells undergoes replicative senescence upon the extended passages (1). Furthermore, doxorubicin-induced premature senescence was also associated with a decreased ERK1/2 activation and an increased MKP-3 protein level. It appears that inactivation of ERK1/2 by MKP-3 occurred at both replicative and premature senescence. Furthermore, it is cell type-, species-, and stimulant-independent.
Beside cytosolic phosphatase MKP-3, the nuclearly located MKP-1 and MKP-2 have also been reported to participate in dephosphorylating MAPKs in the nucleus (39). We also have data showing that MKP-1 was increased at both the mRNA and protein levels under our experimental settings (data not shown). Because MKP-1 mainly dephosphorylates JNK and p38 (40), which were only marginally altered in this investigation (data not shown), further study on this enzyme has not been done. There was one previous report demonstrating a key role of MKP-2 in impairment of nuclear ERK1/2 phosphorylation and function in replicative senescence (41). The question naturally occurs how two spatially differently located phosphatases exert similar effects on ERK1/2 activation and function. One explanation is that dephosphorylating cytosolic ERK1/2 by MKP-3 prevented its nuclear translocation. Indeed, ERK1/2 phosphorylation has been reported to be requisite for its nuclear entry (42). The multistep regulation of ERK1/2 activation by different phosphatases in senescent cells may imply that inactivation of ERK1/2 is an indispensable event for the establishment of the senescent phenotype.
Our study further identified p53 as the underlying mechanism for the elevation of MKP-3. p53 is a tumor suppressor and functions as a sequence-specific transcription factor. Similar to ERK1/2, it is elevated and activated in response to various stress signals (43). In this study, we observed that ETO-induced senescence was associated with an elevation in p53 protein and phosphorylation. Consistent with a recent report showing that p53 transcriptionally regulated MKP-3 expression, we observed that blocking p53 transcriptional activity with a specific inhibitor or down-regulation of p53 with siRNA attenuated the elevation of MKP-3 in senescent cells. Furthermore, we demonstrated that inhibition of p53 also led to improved ERK1/2 phosphorylation. Our observations therefore characterized MKP-3 and its substrate ERK1/2 as downstream targets of p53. p53 has been established as a pivotal mediator of cellular senescence. Its level and/or activity was increased in senescent cells (5, 7, 44). Overexpression of p53 induced premature senescence in p53-null cells (45–47), whereas inactivation of p53 extended the cell lifespan (5, 7, 44, 48, 49). Several p53-targeted molecules, such as p21, promyelocytic leukemia protein, PAI-1, and DEC1, have been shown to mediate senescence downstream of p53 (30–32, 50–54). Currently, the mechanisms by which p53-targeted genes regulate senescence are still poorly understood. Most of them act on blockade of cell cycle progression and/or promotion of p53 activity (21). Linking p53 to ERK1/2 provides a novel mechanistic insight into the regulatory role of p53 on cellular senescence.
Of note, MKP-3 is also known to be transcriptionally regulated by the transcription factors ETS1 and ETS2 (55). However, the participation of these transcription factors in the induction of MKP-3 under our experimental settings is less likely. Previous studies have indicated that the transactivation of these transcription factors requires functional ERK1/2 (56, 57), which was defective in senescent cells.
In addition to proliferation, ERK1/2 also controls cell survival (58, 59). ERK1/2 mediates the extracellular stimulus-elicited activation of cellular intrinsic and extrinsic apoptotic signaling pathways (60). Functional ERK1/2 has been reported to be requisite for activation of caspase-8, alteration of the Bax/BCL2 ratio, release of cytochrome c, and cleavage of caspase-3 (24). In this context, it is not surprising to see that the impairment of ERK1/2 contributed to the resistance of senescent cells to oxidative stress-elicited cell injury.
Our finding could have significant implications. First, we identified an unrecognized mechanism implicated in the establishment of the senescent phenotypes. As an essential molecule in the control of cell proliferation and survival, impaired ERK1/2 activation provided a novel explanation for the loss of cell proliferation and cellular resistance to oxidative injury. Second, our data indicate that MKP-3/ERK1/2 serves as a downstream target of p53. Because both of them are activated in response to stress stimuli and participate in the control of many similar cellular functions, such as proliferation, apoptosis, and senescence, it is conceivable that some of the actions of p53 are through MKP-3-mediated inactivation of ERK1/2. As for senescence, p53 might inhibit cell proliferation through its action on both the “driving force” and “braking mechanism” of cell cycle progression. Our findings provide another good example for the complicated and integrated cellular events in control of cell proliferation by p53.
Taken together, our results indicate that inactivation of ERK1/2 by p53-mediated up-regulation of MKP-3 is a key molecular event implicated in the establishment of cellular senescence. Although cellular arrest can also be imposed through other mechanisms, given the role and position of ERK1/2 in cell proliferation, regulation of ERK1/2 by p53 might be an important mechanisms by which p53 exert its regulatory actions on the senescent phenotype.
Acknowledgment
We thank Dr. Masanori Kitamura for advice and support during the study period.
This work was supported by Ministry of Education, Culture, Sports, Science, and Technology, Japan Grant-in-Aid for Scientific Research 26461219 (to J. Y.).
- CDK
- cyclin-dependent kinase
- ETO
- etoposide
- Dox
- doxorubicin
- SA-β-gal
- senescence-associated β-galactosidase
- PFT
- pifithrin-α.
REFERENCES
- 1. Hayflick L. (1965) The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 [DOI] [PubMed] [Google Scholar]
- 2. Shay J. W., Roninson I. B. (2004) Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene 23, 2919–2933 [DOI] [PubMed] [Google Scholar]
- 3. Shay J. W., Wright W. E. (2004) Telomeres are double-strand DNA breaks hidden from DNA damage responses. Mol. Cell 14, 420–421 [DOI] [PubMed] [Google Scholar]
- 4. d'Adda di Fagagna F., Reaper P. M., Clay-Farrace L., Fiegler H., Carr P., Von Zglinicki T., Saretzki G., Carter N. P., Jackson S. P. (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 [DOI] [PubMed] [Google Scholar]
- 5. Herbig U., Jobling W. A., Chen B. P., Chen D. J., Sedivy J. M. (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol. Cell 14, 501–513 [DOI] [PubMed] [Google Scholar]
- 6. Mallette F. A., Ferbeyre G. (2007) The DNA damage signaling pathway connects oncogenic stress to cellular senescence. Cell Cycle 6, 1831–1836 [DOI] [PubMed] [Google Scholar]
- 7. Serrano M., Lin A. W., McCurrach M. E., Beach D., Lowe S. W. (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 [DOI] [PubMed] [Google Scholar]
- 8. Serrano M., Blasco M. A. (2007) Cancer and ageing: convergent and divergent mechanisms. Nat. Rev. Mol. Cell Biol. 8, 715–722 [DOI] [PubMed] [Google Scholar]
- 9. Kuilman T., Michaloglou C., Mooi W. J., Peeper D. S. (2010) The essence of senescence. Genes Dev. 24, 2463–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blagosklonny M. V. (2006) Cell senescence: hypertrophic arrest beyond the restriction point. J. Cell. Physiol. 209, 592–597 [DOI] [PubMed] [Google Scholar]
- 11. Murray A. W. (2004) Recycling the cell cycle: cyclins revisited. Cell 116, 221–234 [DOI] [PubMed] [Google Scholar]
- 12. Pagès G., Lenormand P., L'Allemain G., Chambard J. C., Meloche S., Pouysségur J. (1993) Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. U.S.A. 90, 8319–8323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roskoski R., Jr. (2012) ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol. Res. 66, 105–143 [DOI] [PubMed] [Google Scholar]
- 14. Chambard J. C., Lefloch R., Pouysségur J., Lenormand P. (2007) ERK implication in cell cycle regulation. Biochim. Biophys. Acta 1773, 1299–1310 [DOI] [PubMed] [Google Scholar]
- 15. Yao J., Kitamura M., Zhu Y., Meng Y., Kasai A., Hiramatsu N., Morioka T., Takeda M., Oite T. (2006) Synergistic effects of PDGF-BB and cAMP-elevating agents on expression of connexin43 in mesangial cells. Am. J. Physiol. Renal Physiol. 290, F1083–F1093 [DOI] [PubMed] [Google Scholar]
- 16. Dimri G. P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E. E., Linskens M., Rubelj I., Pereira-Smith O. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. U.S.A. 92, 9363–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schonn I., Hennesen J., Dartsch D. C. (2010) Cellular responses to etoposide: cell death despite cell cycle arrest and repair of DNA damage. Apoptosis 15, 162–172 [DOI] [PubMed] [Google Scholar]
- 18. Shaul Y. D., Seger R. (2007) The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim. Biophys. Acta 1773, 1213–1226 [DOI] [PubMed] [Google Scholar]
- 19. Muda M., Boschert U., Dickinson R., Martinou J. C., Martinou I., Camps M., Schlegel W., Arkinstall S. (1996) MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J. Biol. Chem. 271, 4319–4326 [DOI] [PubMed] [Google Scholar]
- 20. Molina G., Vogt A., Bakan A., Dai W., Queiroz de Oliveira P., Znosko W., Smithgall T. E., Bahar I., Lazo J. S., Day B. W., Tsang M. (2009) Zebrafish chemical screening reveals an inhibitor of Dusp6 that expands cardiac cell lineages. Nat. Chem. Biol. 5, 680–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rufini A., Tucci P., Celardo I., Melino G. (2013) Senescence and aging: the critical roles of p53. Oncogene 32, 5129–5143 [DOI] [PubMed] [Google Scholar]
- 22. Piya S., Kim J. Y., Bae J., Seol D. W., Moon A. R., Kim T. H. (2012) DUSP6 is a novel transcriptional target of p53 and regulates p53-mediated apoptosis by modulating expression levels of Bcl-2 family proteins. FEBS Lett. 586, 4233–4240 [DOI] [PubMed] [Google Scholar]
- 23. Komarova E. A., Gudkov A. V. (2000) Suppression of p53: a new approach to overcome side effects of antitumor therapy. Biochemistry 65, 41–48 [PubMed] [Google Scholar]
- 24. Cagnol S., Chambard J. C. (2010) ERK and cell death: mechanisms of ERK-induced cell death: apoptosis, autophagy and senescence. FEBS J. 277, 2–21 [DOI] [PubMed] [Google Scholar]
- 25. Lee W. C., Choi C. H., Cha S. H., Oh H. L., Kim Y. K. (2005) Role of ERK in hydrogen peroxide-induced cell death of human glioma cells. Neurochem. Res. 30, 263–270 [DOI] [PubMed] [Google Scholar]
- 26. Ryter S. W., Choi A. M. (2002) Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid. Redox Signal. 4, 625–632 [DOI] [PubMed] [Google Scholar]
- 27. Gu L., Kitamura M. (2012) Sensitive detection and monitoring of senescence-associated secretory phenotype by SASP-RAP assay. PloS ONE 7, e52305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leontieva O. V., Blagosklonny M. V. (2010) DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging 2, 924–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Becker T., Haferkamp S. (2013) Molecular mechanisms of cellular senescence. In Senescence and Senescence-Related Disorders (Wang Z., Inuzuka H., eds), pp. 25–50, InTech [Google Scholar]
- 30. el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 [DOI] [PubMed] [Google Scholar]
- 31. Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75, 805–816 [DOI] [PubMed] [Google Scholar]
- 32. Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. (1993) p21 is a universal inhibitor of cyclin kinases. Nature 366, 701–704 [DOI] [PubMed] [Google Scholar]
- 33. Burch P. M., Yuan Z., Loonen A., Heintz N. H. (2004) An extracellular signal-regulated kinase 1- and 2-dependent program of chromatin trafficking of c-Fos and Fra-1 is required for cyclin D1 expression during cell cycle reentry. Mol. Cell. Biol. 24, 4696–4709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lents N. H., Keenan S. M., Bellone C., Baldassare J. J. (2002) Stimulation of the Raf/MEK/ERK cascade is necessary and sufficient for activation and Thr-160 phosphorylation of a nuclear-targeted CDK2. J. Biol. Chem. 277, 47469–47475 [DOI] [PubMed] [Google Scholar]
- 35. Walsh S., Margolis S. S., Kornbluth S. (2003) Phosphorylation of the cyclin b1 cytoplasmic retention sequence by mitogen-activated protein kinase and Plx. Mol. Cancer Res. 1, 280–289 [PubMed] [Google Scholar]
- 36. Palmer A., Gavin A. C., Nebreda A. R. (1998) A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 17, 5037–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muda M., Theodosiou A., Rodrigues N., Boschert U., Camps M., Gillieron C., Davies K., Ashworth A., Arkinstall S. (1996) The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J. Biol. Chem. 271, 27205–27208 [DOI] [PubMed] [Google Scholar]
- 38. Li G., Yu M., Lee W. W., Tsang M., Krishnan E., Weyand C. M., Goronzy J. J. (2012) Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat. Med. 18, 1518–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kondoh K., Nishida E. (2007) Regulation of MAP kinases by MAP kinase phosphatases. Biochim. Biophys. Acta 1773, 1227–1237 [DOI] [PubMed] [Google Scholar]
- 40. Caunt C. J., Keyse S. M. (2013) Dual-specificity MAP kinase phosphatases (MKPs): shaping the outcome of MAP kinase signalling. FEBS J. 280, 489–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tresini M., Lorenzini A., Torres C., Cristofalo V. J. (2007) Modulation of replicative senescence of diploid human cells by nuclear ERK signaling. J. Biol. Chem. 282, 4136–4151 [DOI] [PubMed] [Google Scholar]
- 42. Pouysségur J., Lenormand P. (2003) Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Eur. J. Biochem. 270, 3291–3299 [DOI] [PubMed] [Google Scholar]
- 43. Menendez D., Inga A., Resnick M. A. (2010) Potentiating the p53 network. Discov. Med. 10, 94–100 [PubMed] [Google Scholar]
- 44. Vaziri H., Benchimol S. (1996) From telomere loss to p53 induction and activation of a DNA-damage pathway at senescence: the telomere loss/DNA damage model of cell aging. Exp. Gerontol. 31, 295–301 [DOI] [PubMed] [Google Scholar]
- 45. Sugrue M. M., Shin D. Y., Lee S. W., Aaronson S. A. (1997) Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc. Natl. Acad. Sci. U.S.A. 94, 9648–9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y., Blandino G., Oren M., Givol D. (1998) Induced p53 expression in lung cancer cell line promotes cell senescence and differentially modifies the cytotoxicity of anti-cancer drugs. Oncogene 17, 1923–1930 [DOI] [PubMed] [Google Scholar]
- 47. Qian Y., Chen X. (2010) Tumor suppression by p53: making cells senescent. Histol. Histopathol. 25, 515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hara E., Tsurui H., Shinozaki A., Nakada S., Oda K. (1991) Cooperative effect of antisense-Rb and antisense-p53 oligomers on the extension of life span in human diploid fibroblasts, TIG-1. Biochem. Biophys. Res. Commun. 179, 528–534 [DOI] [PubMed] [Google Scholar]
- 49. Bond J. A., Wyllie F. S., Wynford-Thomas D. (1994) Escape from senescence in human diploid fibroblasts induced directly by mutant p53. Oncogene 9, 1885–1889 [PubMed] [Google Scholar]
- 50. Pearson M., Carbone R., Sebastiani C., Cioce M., Fagioli M., Saito S., Higashimoto Y., Appella E., Minucci S., Pandolfi P. P., Pelicci P. G. (2000) PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature 406, 207–210 [DOI] [PubMed] [Google Scholar]
- 51. de Stanchina E., Querido E., Narita M., Davuluri R. V., Pandolfi P. P., Ferbeyre G., Lowe S. W. (2004) PML is a direct p53 target that modulates p53 effector functions. Mol. Cell 13, 523–535 [DOI] [PubMed] [Google Scholar]
- 52. Kortlever R. M., Higgins P. J., Bernards R. (2006) Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 8, 877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qian Y., Zhang J., Yan B., Chen X. (2008) DEC1, a basic helix-loop-helix transcription factor and a novel target gene of the p53 family, mediates p53-dependent premature senescence. J. Biol. Chem. 283, 2896–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhu Y., Xu L., Zhang J., Hu X., Liu Y., Yin H., Lv T., Zhang H., Liu L., An H., Liu H., Xu J., Lin Z. (2013) Sunitinib induces cellular senescence via p53/Dec1 activation in renal cell carcinoma cells. Cancer Sci. 104, 1052–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang Z., Kobayashi S., Borczuk A. C., Leidner R. S., Laframboise T., Levine A. D., Halmos B. (2010) Dual specificity phosphatase 6 (DUSP6) is an ETS-regulated negative feedback mediator of oncogenic ERK signaling in lung cancer cells. Carcinogenesis 31, 577–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ekerot M., Stavridis M. P., Delavaine L., Mitchell M. P., Staples C., Owens D. M., Keenan I. D., Dickinson R. J., Storey K. G., Keyse S. M. (2008) Negative-feedback regulation of FGF signalling by DUSP6/MKP-3 is driven by ERK1/2 and mediated by Ets factor binding to a conserved site within the DUSP6/MKP-3 gene promoter. Biochem. J. 412, 287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Furukawa T., Tanji E., Xu S., Horii A. (2008) Feedback regulation of DUSP6 transcription responding to MAPK1 via ETS2 in human cells. Biochem. Biophys. Res. Commun. 377, 317–320 [DOI] [PubMed] [Google Scholar]
- 58. Zhuang S., Schnellmann R. G. (2006) A death-promoting role for extracellular signal-regulated kinase. J. Pharmacol. Exp. Ther. 319, 991–997 [DOI] [PubMed] [Google Scholar]
- 59. Martin P., Pognonec P. (2010) ERK and cell death: cadmium toxicity, sustained ERK activation and cell death. FEBS J. 277, 39–46 [DOI] [PubMed] [Google Scholar]
- 60. Pognonec P. (2010) ERK and cell death: overview. FEBS J. 277, 1. [DOI] [PubMed] [Google Scholar]