Background: GALNT, the initial enzyme in mucin-type O-glycosylation, plays critical roles in cancer etiology.
Results: GALNT10-induced cellular proliferation was associated with EGFR activation mediated by down-regulation of miR-122 in HBV-associated HCC.
Conclusion: A regulatory pathway of Hnf4α/miR-122/GALNT10/EGFR may represent a possible mechanism underlying HBV-associated hepatocarcinogenesis.
Significance: This finding provides a novel role for O-glycosylation in HCC pathogenesis.
Keywords: Glycobiology, Glycosylation, Glycosyltransferase, Hepatitis Virus, Liver Cancer, GALNT10, Hnf4α, Hepatitis B Virus, miR-122
Abstract
MicroRNA-122 (miR-122), a mammalian liver-specific miRNA, has been reported to play crucial roles in the control of diverse aspects of hepatic function and dysfunction, including viral infection and hepatocarcinogenesis. In this study, we explored the clinical significance, transcriptional regulation, and direct target of miR-122 in hepatitis B virus (HBV)-associated hepatocellular carcinoma. Reduced expression of miR-122 in patients with HBV-associated hepatocellular carcinoma was correlated with venous invasion and poor prognosis. Furthermore, UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase-10 (GALNT10) was identified as a bona fide target of miR-122 in hepatoma cells. Ectopic expression and knockdown studies showed that GALNT10 indeed promotes proliferation and apoptosis resistance of hepatoma cells in a glycosyltransferase-dependent manner. Critically, adverse correlation between miR-122 and GALNT10, a poor prognosticator of clinical outcome, was demonstrated in hepatoma patients. Hepatocyte nuclear factor 4α (Hnf4α), a liver-enriched transcription factor that activates miR-122 gene transcription, was suppressed in HBV-infected hepatoma cells. Chromatin immunoprecipitation assay showed significantly reduced association of Hnf4α with the miR-122 promoter in HBV-infected hepatoma cells. Moreover, GALNT10 was found to intensify O-glycosylation following signal activation of the epidermal growth factor receptor. In addition, in a therapeutic perspective, we proved that GALNT10 silencing increases sensitivity to sorafenib and doxorubicin challenge. In summary, our results reveal a novel Hnf4α/miR-122/GALNT10 regulatory pathway that facilitates EGF miR-122 activation and hepatoma growth in HBV-associated hepatocarcinogenesis.
Introduction
Hepatocellular carcinoma (HCC)4 is the second leading cause of cancer-related mortality worldwide, and the burden of this devastating malignancy is expected to increase further in the coming years (1, 2). Epidemiological studies have provided overwhelming evidence for a causal role of chronic hepatitis B virus (HBV) infection in the development of HCC (3), but the molecular mechanisms underlying the incidence of this disease remain far from being fully understood. Although comprehensive genomic and proteomic analyses have identified many key drivers of HBV-associated HCC, the post-translational modifications remain poorly understood (4, 5).
Glycosylation, the most common post-translational modification of secreted and membrane proteins, can be found in the peripheral region of carbohydrates in glycoproteins, in addition to the structural variation in the core regions of carbohydrate chains bound to proteins through N- or O-glycosides (6). Glycosylation of glycoproteins and glycolipids is one of many molecular changes that accompany malignant transformation, reflecting tumor-specific changes in glycan biosynthesis caused by glycosyltransferases and glycosidases (7–10). A well characterized example is that elevated activities of N-acetylglucosaminyltransferase-III and acetylglucosaminyltransferase-V in HCC were positively correlated with the malignant and metastatic potential of tumor cells (11–13). In addition, the α-1,6-fucosylation of α-fetoprotein provides a more accurate diagnosis of HCC from chronic liver disease and a good monitoring and prognosis marker for the early diagnosis of HCC (14). Our previous study revealed that hepatitis B virus x protein (HBx) promotes cancer behaviors by up-regulating β-1,4-galactosyltransferase-I in hepatocellular carcinoma cells (15). However, the role of O-linked glycosylation in hepatocarcinogenesis has been overlooked because of the lack of consensus with the amino acid sequence of O-glycosylation and effective releasing enzymes for O-glycans (16).
O-Linked glycans encompass diverse classes of glycoproteins. In mammals, the most common types are mucin-type O-linked glycans, which constitute up to 80% of the total amount of cell-surface carbohydrate antigens (17, 18). Mucin-type linkage (GalNAc-O-Ser/Thr) synthesis is initiated by a family of uridine diphosphate (UDP)-N-acetyl-α-d-galactosamine (GalNAc) polypeptide:N-acetylgalactosaminyltransferases, which catalyze the transfer of GalNAc from the sugar donor UDP-GalNAc to the hydroxyl group of a serine and threonine residue. A total of at least 20 isoforms of this family has been reported and shown to have unique acceptor substrate specificities as well as tissue-specific expression patterns (19–21). Several lines of evidence indicate that changes in O-linked glycans allow neoplastic cells to differentiate, invade, and spread throughout the organism (20, 21). The alteration of O-glycans attached to the epidermal growth factor receptor (EGFR) mediated by the down-regulation of GALNT2 plays a critical role in the malignant phenotype of HCC cells (22). Moreover, overexpression of core 1β-1,3-galactosyltransferase activates hepatocyte growth factor signaling in hepatocellular carcinoma (23). Therefore, understanding the roles of O-glycosylation in hepatocellular carcinoma may provide novel insights into the pathogenesis of hepatocellular carcinoma.
A glycan determinant is produced by the concerted action of several related genes, making it difficult to elucidate the genetic regulatory mechanism for expression of some cell-surface glycans (24). Accumulating evidence suggests that alterations of microRNA (miRNA) expression participate in diverse biological functions and in carcinogenesis (25). miR-30d has been shown to negatively regulate GALNT7, and it exerts both pro-invasive and immunomodulatory effects, which indicate a direct association between glycan alteration and the miRNA for malignancies (26).
In this study, we identify the mucin-type O-glycosylation enzyme GALNT10 as a direct target of miR-122. Overexpression of GALNT10 correlates with HBV infection and a poor prognosis in HCC patients. Moreover, GALNT10 confers the malignant phenotype of HCC cells through modifying EGFR O-glycosylation and activating the PI3K/AKT signaling pathway. These data suggest that GALNT10 regulates the malignant growth of hepatocellular carcinoma cells and contributes to the pathogenesis of HBV-associated hepatocellular carcinoma.
MATERIALS AND METHODS
Cell Lines and Patient Samples
Human hepatoma cell lines Hep3B, PLC/PRF/5, HepG2, Huh7, SK-Hep1, BEL-7402, and BEL-7404 were obtained directly from Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS at 37 °C in a humidified 5% CO2 incubator. The cell lines have been characterized at the Shanghai Cell Bank by DNA fingerprinting analysis using short tandem repeat markers. All cell lines were placed under cryostage after they were obtained from the Shanghai Cell Bank and used within 6 months of thawing fresh vials. HBV producing HepG2.2.15 cells containing four copies of 1.1-fold HBV genome (genotype A) were derived from HepG2 cells as described previously (27). 142 pairs of HCC and adjacent nontumor tissues, and an additional 20 pairs of HBV-associated HCC specimens and corresponding nontumor tissues were collected from HCC patients undergoing partial hepatectomy in Nantong Tumor Hospital (Nantong University, Jiangsu, China) from 2003 to 2005. Overall survival (OS) was defined as the interval between the date of surgery and the date of death or the last observation time. The last follow-up was May, 2012, with a median follow-up period of 33.6 months (range 1–80 months). Use of human tissues with informed consent was approved by the Institutional Review Board of Fudan University.
Plasmid Construction
The HBV replicon plasmid pHBV1.3 harboring a 1.3-fold HBV genome (subtype adw), the plasmids encoding HBx amplified from the HBV genome, and the HBV replicon plasmid ΔHBx containing HBV genome mutant incompetent to express HBx protein were generated as described previously (27, 28). Expression plasmids encoding wild-type GALNT10 with a Myc tag was kindly provided by Dr. Hisashi Narimatsu (National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan). An inactive GALNT10 mutant (GALNT10-DN) incompetent to localize to Golgi was constructed using the QuikChange kit (Invitrogen). Luciferase reporter plasmids containing the 5′-flanking region of miR-122 (−636/+141) and the 3′-untranslated region of galnt10 (+561/+2445) were constructed into pGL3-basic and pGL3-control plasmid, respectively. Human Hnf4α expression plasmid was obtained from Addgene (Addgene plasmid 31100) (29). All plasmid constructs were confirmed by DNA sequencing. Primers used are presented in Table 1.
TABLE 1.
Primer sequences used for plasmids construction
The abbreviations used are as follows: GALNT10, UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 10; WT, wide type; Mut, mutant.
| Primer name | Sequence (5′ to 3′) | |
|---|---|---|
| galnt10 3′-UTR-WT | Sense | GGCACTAGTCAGGTGATTTGGTCCGGTCA |
| Antisense | GGCGGCCGGCCGCACCCCTTAACCCAATGGT | |
| galnt10 3′-UTR-Mut | Sense | GTCAGATTTATTTCAGTCAGTGCAA |
| Antisense | CCCAGCCTTTAGTTTGGAGTGTGAC | |
| miR-122 promoter | Sense | CGCGGTACCCCTCCTCTGCTGTCCGTGCG |
| Antisense | GCGCTCGAGGCCACCCCAAAGAACGGCCT | |
| GALNT10-DNa | Sense | GGGCCCGAATTCATGCGCGAGCGGCAGCCCGACGG |
| Antisense | GCCCCTCGAGGACTCGAGCGGCCGCCA | |
a This is an inactive form of GALNT10.
Plasmid Transfection and RNA Interference
Transient and stable transfections with various plasmids were performed as described previously (28). Short hairpin RNA (shRNA) against galnt10 shRNA (human) lentiviral particles, and corresponding control shRNA lentiviral particles (Santa Cruz Biotechnology, Santa Cruz, CA) were used for RNA interference as described previously (30). Gene silencing effect was confirmed by Western blot and quantitative real time (RT)-PCR.
Bioinformatics
Three software programs, TargetScan 5.2, DINAN, and miRanda, were used to predict the potential target genes of miR-122-5p.
Enforcing or Reducing Expression of miR-122-5p in HCC Cells
To force expression of miR-122-5p in HCC cells, cells were transfected with precursor molecules mimicking miR-122-5p or scrambled sequence (Invitrogen), and to reduce expression of miR-122-5p, an inhibitor of miR-122-5p or a negative inhibitor control (Invitrogen) was transfected into hepatoma cells by using FuGENE HD transfection reagent (Roche Applied Science) according to the manufacturer's instructions. Experiments were repeated at least three times.
RNA Extraction and qRT-PCR
Total RNA from cultured cells and frozen tissue specimens were extracted using TRIzol (Invitrogen) according to the manufacturer's instructions. qRT-PCR assays were performed as described in our previous study (28). GAPDH was used as an internal control. Furthermore, miR-122-5p expression was measured by using TaqMan miRNA assays (Applied Biosystems, Foster City, CA). U6 small nuclear RNA was used as an internal control. Experiments were repeated at least three times. The PCR primer sets used are shown in Table 2.
TABLE 2.
Primer sequences used for qRT-PCR
| Primer name | Sequence (5′ to 3′) | |
|---|---|---|
| GAPDH | Sense | AAGGTCGGAGTCAACGGATTTG |
| Antisense | CCATGGGTGGAATCATATTGGAA | |
| GALNT10 | Sense | ACAGCCAGGTAATGGGTGAG |
| Antisense | GAAGATGGGATGGCTTTTCA | |
| Hnf4α | Sense | TGTCCCGACAGATCACCTC |
| Antisense | CACTCAACGAGAACCAGCAG | |
| GALNT1 | Sense | ATGGCCCAGTTACAATGCTC |
| Antisense | ATATTTCTGGCAGGGTGACG | |
| GALNT3 | Sense | AAAGCGTTGGTCAGCCTCTA |
| Antisense | AACGAGACCTTGAGCAGCAT | |
| GALNT8 | Sense | GAGCTTAGCCTGAGGGTGTG |
| Antisense | CCAGGCCAAGTAGACCATGT | |
| GALNT12 | Sense | CATCTTGCAGGAGGATGGAT |
| Antisense | CTGGCTCCACAGTCTCCTTC | |
| GALNT13 | Sense | CATCTATCCGGACTCCCAGA |
| Antisense | TCGGTTCGGATTTCTTTGTC | |
| GALNTL1 | Sense | GAGCTCTCCTTCAGGGTGTG |
| Antisense | CACTTCTGCAGTGCGCTTAG | |
| B4GALT1 | Sense | CAATGGATAAGTTTGGATTCAGCCT |
| Antisense | GGTGTCCCGATGTCCACTGTGATTT | |
| FUT8 | Sense | CCCGTCCTCCATATTTACCCTTG |
| Antisense | ACTGAGACACCCACCACACTG | |
| ST6GALNAC4 | Sense | GCACCCTGCGTGTCGTCTCA |
| Antisense | CGGTTCTTGCCCGTCTCGTC | |
Western Blot, Immunoprecipitation, and Lectin Blot Analysis
Western blot was performed as described previously (28). Primary antibodies used included those against Hnf4α, GAPDH (Santa Cruz Biotechnology), GALNT10 (Sigma), AKT, p-Akt (Ser-473), Mcl-1, Bcl-2, and Bcl-xl (Cell Signaling Technology, Beverly, MA). For lectin blot, the membranes were detected with biotinylated vicia villosa agglutinin (VVA), peanut agglutinin (PNA), and Jacalin. The immunoreactive bands were visualized using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA). For immunoprecipitation, the supernatant (2 mg of protein) was incubated for 1 h at 4 °C with anti-EGFR monoclonal antibody (3 μg/ml) (Santa Cruz Biotechnology). Protein G beads (30 μl in 50% slurry) were then added, followed by incubation overnight at 4 °C with a rotator. For lectin immunoprecipitation, the cell lysate was incubated with 50 μl of VVA-agarose (Vector Laboratories, Burlingame, CA) for 4 h at 4 °C, followed by washing three times with lysis buffer. Neuraminidase (New England Biolabs, Ipswich, MA) was used to remove sialic acid. The pulldown protein was then subjected to Western blot analysis.
Cell Surface Labeling and Immunoprecipitation
Cells were washed twice with ice-cold PBS and incubated with 1 mg/ml NHS-LC-biotin in PBS for 30 min at 4 °C on a rocking platform. After washing with ice-cold PBS, cells were lysed, and cell-surface proteins were precipitated using 50 μl of streptavidin-agarose at 4 °C overnight and detected by Western blotting.
Cell Proliferation Assay
Cell proliferation was measured using the Cell Counting kit-8 (Dojindo, Kamimashiki-gun Kumamoto, Japan) according to the manufacturer's instructions. Before detection, cells were incubated with CCK-8 for 1 h. Cell proliferation rate was assessed by measuring the absorbance at 450 nm with the Universal Microplate Reader (Bio-Tek Instruments, Minneapolis, MN).
5-Bromo-2′-deoxyuridine Incorporation Assay
Cells cultured in a 35-mm dish were added 10 μm BrdU (Sigma) in the medium 30 min before harvesting. Cells were trypsinized and fixed with ice-cold 70% ethanol. For BrdU incorporation analysis, cells were incubated with 10 μl of anti-BrdU FITC, (347583, BD Biosciences) in the presence of 100 μg/ml RNase for 30 min at room temperature in the dark. BrdU incorporation was determined in the FACSCaliburTM (BD Biosciences). The percentage of BrdU-positive cells relative to total cells was determined by using the Cell Fit Program (BD Biosciences).
Caspase 3/7 Activity Assay and Annexin V Apoptosis Assay
Caspase 3/7 activity assay and annexin V/propidium iodide staining were performed with caspase-Glo 3/7 assay (Promega, Madison, WI) and annexin V-FITC apoptosis detection kit (BD Biosciences) as described previously (31).
Luciferase Activity Assay and Flow Cytometry
Luciferase activity assay was performed with Dual-LuciferaseTM reporter assay system (Promega, Madison, WI) according to the manufacturer's instructions. The co-expressed Renilla luciferase activity was used for the normalization of transfection efficiency. Flow cytometry was performed as described previously (32–34). Briefly, single cells were incubated with FcR blocking reagent (Miltenyi Biotec, Auburn, CA) for 15 min at 4 °C and stained with phycoerythrin-conjugated anti-EGFR antibody (Abcam, Cambridge, MA) for 1 h at 4 °C. Samples were washed with PBS supplemented with 1% BSA, and data were acquired with FACSCalibur (BD Biosciences).
ChIP Assay
ChIP was performed using the EZ-ChIP chromatin immunoprecipitation kit (Millipore, Billerica, MA) following the manufacturer's protocol. Immunoprecipitation complexes were immunoprecipitated with an Hnf4α antibody (Santa Cruz Biotechnology) and mouse IgG antibodies (Millipore, Billerica, MA) overnight at 4 °C. The captured genomic DNA was obtained and used for qPCR analysis. Ten percent of total genomic DNA from the nuclear extract was used as input. The primers used for detection of the miR-122 promoter sequence were as follows: 5′-GACCTGTCTTCTCTGCCTCGG-3′ and 5′-GGGGGAGGGAGGGCACGAC-3′. Amplification efficiency was calculated, and the data were expressed as enrichment related to input.
In Situ Hybridization (ISH)
Paraffin-embedded liver tissues were deparaffinized and rehydrated through an ethanol series. Slides were washed in PBS containing 3% H2O2 for 30 min to quench endogenous peroxidase activity. After proteinase K digestion for 5 min, slides were fixed in 4% paraformaldehyde and rinsed in PBS. Slides were incubated in hybridization buffer for 2 h at 60 °C. Probes for miR-122-5p or scrambled miRNA control (50 nm; digoxigenin-labeled LNA probes) (Exiqon, Vedbaek, Denmark) were mixed with hybridization buffer, heated to 90 °C for 4 min, chilled on ice, and added to the sections, followed by incubation overnight at 60 °C and stringent washes in wash buffer (50% formamide in 2× saline/sodium citrate and PBS plus Tween 20 (PBST)). For the alkaline phosphatase reaction, alkaline phosphatase substrate (nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate) was used, and the slides were mounted in aqueous mounting medium. Signals in tumor cells were visually quantified using a scoring system from 0 to 300, multiple intensities of signal, and percentage of positive cells (signal: 0 = no signal; 1 = weak signal; 2 = intermediate signal; and 3 = strong signal; percentage: 0–100). Patients were dichotomized into high and low expression subgroup according to medium intensity score.
Immunohistochemistry (IHC)
IHC on formalin-fixed and paraffin-embedded sections was performed, as described previously (35), using rabbit polyclonal antibody against GALNT10 (Sigma). The staining intensity of each specimen was assessed by two pathologists blinded to the clinicopathological information. A semi-quantitative H-score ranging from 0 to 300 was calculated for each specimen by multiplying the distribution areas (0–100%) at each staining intensity level by the intensities (0, negative; 1, weak staining; 2, moderate staining; and 3, strong staining) (36). Patients were dichotomized into a high and low expression subgroup according to medium intensity score.
Statistical Analysis
Experimental data were presented as means ± S.E. of at least three independent replicates by analyzing with Medcalc software (version 12.7.0.0, Medcalc, Mariakerke, Belgium) and assessing comparisons between different groups by the Student's t test and one-way analysis of variance. Differences between scatter plots for evaluated scores of IHC staining or ISH in tumor tissue sections were determined by nonparametric Mann-Whitney test. Survival was calculated starting from the date of death or last follow-up. Survival curves were estimated using the Kaplan-Meier method, and the log-rank test was used to compute differences between the curves. The correlations between GALNT10 with miR-122 staining obtained by IHC or ISH were determined using Spearman correlation test. Differences were considered significant at values of p < 0.05.
RESULTS
Decreased miR-122 Correlates with Poor Prognosis in Patients with HBV-associated HCC
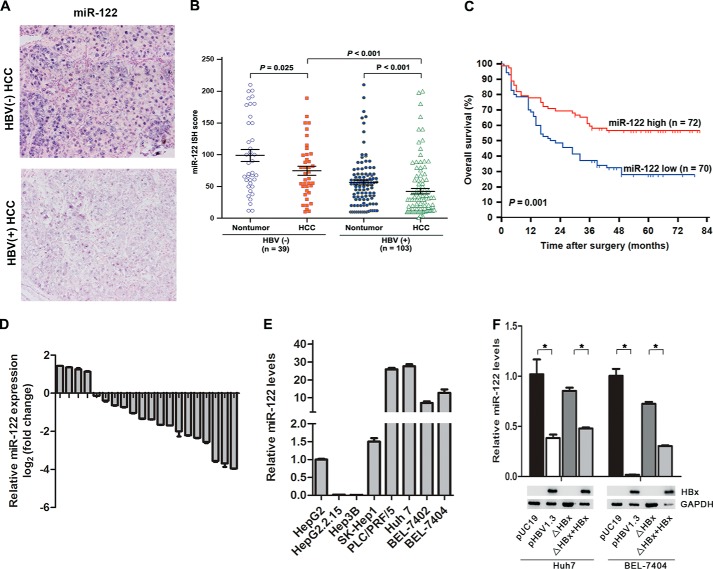
miR-122, a diagnostic and prognostic marker for HCC progression, was significantly down-regulated in patients with HBV infection. Therefore, we decided to investigate the clinical relevance of miR-122 in HBV-associated HCC patients. We first detected miR-122 expression in a panel of 142 primary liver tumors and matched nontumor surrounding liver tissues by ISH. We observed that miR-122 expression was significantly decreased in HBV-associated HCC tissues compared with those without HBV infection (p < 0.001). Furthermore, the expression levels of miR-122 were decreased in HCC samples compared with the corresponding nontumor tissues (p < 0.001) (Fig. 1, A and B). Validation by real time PCR on RNA from an additional 20 pairs of HBV-associated HCC specimens and corresponding nontumor tissues showed that the expression of miR-122 was down-regulated 16/20 (80%) of the HCC specimens (Fig. 1D). We next investigated the relationship between miR-122 expression and clinicopathologic features in patients with hepatocellular carcinoma. As shown in Table 3, the level of miR-122 closely correlated with HBV infection (p = 0.004), tumor size (p = 0.019), venous invasion (p = 0.035), and tumor differentiation (p = 0.037). A Kaplan-Meier survival analysis showed that the survival rates of HCC patients with low miR-122 expression were significantly lower than those with high miR-122 expression (p = 0.001, Fig. 1C). Collectively, these data suggest that miR-122 is frequently down-regulated in HBV-associated HCC and indicated a poor survival in HCC patients.
FIGURE 1.
Down-regulated miR-122 correlates with poor prognosis in patients with HBV-associated HCC. A, representative miRNA in situ hybridization staining for miR-122 in human HCC specimens (original magnification ×200). B, scatter plots for corresponding evaluated score in tumor and matched nontumor tissue sections from 142 HCC patients stratified with HBV infection status. p value was determined by a nonparametric Mann-Whitney test. C, Kaplan-Meier survival analysis of HCC patients stratified with miR-122 staining (n = 72 for high miR-122 group versus n = 70 for low miR-122 group). p value is determined by log-rank test. D, quantitative RT-PCR analysis of miR-122 levels for 20 pairs of matched HBV-infected HCC patient tissues. Values represent mean ± S.E. from three independent experiments. *, p < 0.05. E and F, quantitative RT-PCR analysis of miR-122 levels for eight HCC cell lines (HepG2, HepG2.2.15, Hep3B, PLC/PRF/5, Huh7, BEL-7402, BEL-7404, and SK-Hep1) (E), and for Huh7 and BEL-7404 cells transiently transfected with pUC19, pHBV1.3, ΔHBx, and ΔHBx + HBx, respectively (F, upper panel). Western blot analysis of indicated proteins (F, lower panel). Values represent mean ± S.E. from three independent experiments. *, p < 0.05.
TABLE 3.
Correlation between miR-122 expression and HCC patient characteristics
The abbreviations used are as follows: HBsAg, hepatitis B surface antigen, TNM, tumor node metastasis. Boldface values indicate p < 0.05 (t test for continuous variables and χ2 test for categorical variables). Microsatellite nodules are defined as tumors <2 cm located within 2 cm of the primary lesion.
| Characteristic | Patients (n = 142) |
miR-122 expression |
p valuea | ||
|---|---|---|---|---|---|
| No. | % | High (n = 72) | Low (n = 70) | ||
| Age (years)a | 142 | 100 | 53.42 ± 7.93 | 56.07 ± 8.93 | 0.063 |
| Gender | 0.947 | ||||
| Male | 123 | 86.6 | 63 | 60 | |
| Female | 19 | 13.4 | 9 | 10 | |
| HBsAg | 0.004 | ||||
| Negative | 39 | 27.5 | 28 | 11 | |
| Positive | 103 | 72.5 | 44 | 59 | |
| Liver cirrhosis | 0.979 | ||||
| Absent | 60 | 42.3 | 30 | 30 | |
| Present | 82 | 57.7 | 42 | 40 | |
| Maximal tumor size (cm)a | 142 | 100 | 6.30 ± 4.01 | 8.37 ± 6.25 | 0.019 |
| Tumor no. | 0.851 | ||||
| Single | 124 | 87.3 | 62 | 62 | |
| Multiple | 18 | 12.7 | 10 | 8 | |
| Microsatellite nodules | 0.315 | ||||
| Absent | 116 | 81.7 | 56 | 60 | |
| Present | 26 | 18.3 | 16 | 10 | |
| Tumor encapsulation | 0.771 | ||||
| Absent | 106 | 74.6 | 55 | 51 | |
| Present | 36 | 25.4 | 17 | 19 | |
| Venous invasion | 0.035 | ||||
| Absent | 126 | 88.7 | 68 | 58 | |
| Present | 16 | 11.3 | 4 | 12 | |
| Tumor differentiation | 0.037 | ||||
| I–II | 96 | 67.6 | 55 | 41 | |
| III–IV | 46 | 32.4 | 17 | 29 | |
| TNM stage | 0.971 | ||||
| I–II | 86 | 60.6 | 44 | 42 | |
| III–IV | 56 | 39.4 | 28 | 28 | |
a The results of continuous variables are expressed as mean ± S.D.
We detected miR-122 in eight human hepatoma cell lines, and HepG2.2.15 cells with endogenously driven HBV replication had lower miR-122 expression (Fig. 1E). Further studies revealed that HBV replication with HBx expression significantly inhibits miR-122 expression compared with that without HBx expression (Fig. 1F). These data suggest that HBV infection inhibits miR-122 expression mainly due to HBx expression.
GALNT10 mRNA Is a Bona Fide Target of miR-122
To identify the miR-122 target, we used miRNA target-predicting algorithms (TargetScan, DIANA, and miRanda) based on the presence of the binding site in 3′UTR. Among the predicted and validated genes, we took special interest in the 10 glycosyltransferases. In parallel, we found that GALNT10 expression was significantly down-regulated in HepG2 cells transfected with miR-122 mimics (Fig. 2A). Analysis of the 3′-UTR of galnt10 revealed one highly conserved miR-122-binding site (Fig. 2B). Treatment with the miR-122 mimic significantly reduced the activity of firefly luciferase with the wild type but not the miR-122-binding site mutant 3′-UTR of galnt10, although the anti-miR-122 increased the luciferase activity but not mutant 3′-UTR (Fig. 2C). Both gain-of-function and loss-of-function analyses verified a suppressive effect of miR-122 on the expression of endogenous GALNT10. HepG2 and SK-Hep1 cells were chosen for enforcing the expression of miR-122, because the cell lines exhibit lower levels of miR-122. Conversely, miR-122 silencing was performed in Huh7 and BEL-7404 cells because the cell lines exhibit miR-122 constitutive expression. miR-122 mimic-transfected HepG2 and SK-Hep1 cells decreased the mRNA and protein levels of GALNT10, whereas the depletion of endogenous miR-122 in Huh7 and BEL-7404 cells by its inhibitor led to an increase of GALNT10 level (Fig. 2, D and E). We then examined the effect of HBV infection on GALNT10 expression. Significant increases of GALNT10 expression were detected in Huh7 and BEL-7404 cells following ectopic expression of HBV plasmid pHBV1.3 (Fig. 2F). Collectively, these data show that GALNT10, a direct target of miR-122, is up-regulated following HBV infection.
FIGURE 2.
GALNT10 is a bona fide target of miR-122. A, heatmap representation of predicted gene mRNA levels assessed by qRT-PCR in HepG2 cells transfected with miR-control (miR-con) and miR-122 mimics (miR-122). B, sequences of WT and mutant (Mut) miR-122 target sites in the GALNT10 3′UTR. C, luciferase activity assay for GALNT10–3′UTR-WT or GALNT10–3′UTR-Mut relative to pGL3-control for SK-Hep1 cells harboring miR-con or miR-122 transfection (left panel), and Huh7 cells harboring anti-miR-control or anti-miR-122 transfection (right panel). Firefly luciferase activity was normalized to Renilla luciferase activity. D, quantitative RT-PCR analysis of GALNT10 levels for HepG2 and SK-Hep1 cell-transfected miR-122 mimics (left panel) and Huh7 and BEL-7404 cells with anti-miR-122 (right panel) or their negative control. Values represent mean ± S.E. from three independent experiments; *, p < 0.05. E, Western blot analysis of GALNT10 and GAPDH for HepG2 and SK-Hep1 cells transfected with miR-con or miR-122 (upper panel) and Huh7 and BEL-7404 cells transfected with anti-miR-control or anti-miR-122 (lower panel). F, Western blot analysis of GALNT10 and GAPDH for Huh7 and BEL-7404 cells transfected with pUC19 or pHBV1.3.
GALNT10 Promotes Proliferation and Apoptosis Resistance of Hepatoma Cells Dependent on Glycosyltransferase Activity
To investigate the role of GALNT10 in the hepatocarcinogenesis, we first analyzed GALNT10 expression in eight hepatoma cell lines by Western blotting. Consequently, SK-Hep1 expressed higher levels of GALNT10, whereas Huh7 expressed lower levels of GALNT10 in these cell lines (Fig. 3A). Therefore, SK-Hep1 and Huh7 were chosen to knock down and overexpress the GALNT10 expression, respectively. Stable GALNT10 knockdown in clones 1 and 2 compared with control SK-Hep1 cells was confirmed by Western blot analysis, and SK-Hep1-shGALNT10 clone 1 was selected for further investigation because of a better efficiency (Fig. 3B). Mock, GALNT10-WT, and GALNT10-DN stable transfectants were obtained from the pooled colonies of Huh7 cells transfected with empty vector, GALNT10-WT, and GALNT10-DN plasmids, respectively (Fig. 3B). GALNT10 knockdown was found to inhibit cellular proliferation of SK-Hep1 cells, whereas overexpression of GALNT10-WT but not GALNT10-DN promoted proliferation of Huh7 cells (Fig. 3, C and D). To further characterize the potential role of GALNT10 in survival, GALNT10 knockdown increased the apoptosis rate in SK-Hep1 cells under serum starvation, whereas overexpression of GALNT10-WT but not GALNT10-DN reduced apoptosis rate in Huh7 cells (Fig. 3, E and F).
FIGURE 3.
GALNT10 promotes proliferation and apoptosis resistance of hepatoma cells dependent on glycosyltransferase activity. A, Western blot analysis of GALNT10 and GAPDH protein levels in eight HCC cell lines. B, Western blot analysis of GALNT10 protein levels in SK-Hep1 cells stably transfected with control shRNA (sh-Con) or GALNT10 shRNA-1,2 (sh-GALNT10–1,2) (left panel), and in Huh7 cells stably transfected with empty vector, wild-type GALNT10 (GALNT10-WT) or inactive form GALNT10 (GALNT10-DN) (right panel). C and D, cell proliferation assay. BrdU incorporation assay and subsequent flow cytometry analysis (C) and CCK-8 assay (D) for SK-Hep1 cells stably transfected with sh-GALNT10 and Huh7 cells stably transfected with empty vector, GALNT10-WT, or -DN. All values represent the mean ± S.E. of three independent experiments; *, p < 0.05. E and F, caspase 3/7 activity assay (E) and annexin V apoptosis assay (F) for SK-Hep1 cells stably transfected with sh-GALNT10 and Huh7 cells stably transfected with empty vector, GALNT10-WT, or -DN after 48 h of serum starvation. All values represent the mean ± S.E. of three independent experiments; *, p < 0.05.
A previous study has proved that miR-122 overexpression attenuated cellular proliferation (37). We hypothesized that miR-122 might inhibit hepatocyte proliferation through modulation of GALNT10. As shown in Fig. 4A, miR-122 mimics significantly reduced proliferation of SK-Hep1 cells but not in GALNT10 knockdown cells. Moreover, depletion of miR-122 by its inhibitor led to a significant increase in cell proliferation in Huh7 cells but not in GALNT10 shRNA-transfected cells (Fig. 4B). These data indicate that miR-122 might suppress cell proliferation through down-regulation of GALNT10.
FIGURE 4.
Effects of restoration of GALNT10 expression on HBV/miR-122-mediated cell proliferation and survival. A–D, cell proliferation assay. BrdU incorporation assay and subsequent flow cytometry analysis for SK-Hep1 cells transiently transfected with miR-con, miR-122, sh-GALNT10, or sh-GALNT10+miR-122 (A); for Huh7 cells transiently transfected with anti-miR-con, anti-miR-122, sh-GALNT10, or sh-GALNT10+anti-miR-122 (B); for SK-Hep1 cells transiently transfected with empty vector (pUC19), pHBV1.3, miR-122 mimic, and pHBV1.3+miR-122 mimic (C); and for SK-Hep1 cells transiently transfected with empty vector (pUC19), pHBV1.3, sh-GALNT10, and pHBV1.3+sh-GALNT10 (D). All values represent the mean ± S.E. of three independent experiments; *, p < 0.05. E and F, caspase 3/7 activity assay for SK-Hep1 cells transiently transfected with empty vector (pUC19), pHBV1.3, miR-122 mimic, and pHBV1.3+miR-122 mimic (E) and for SK-Hep1 cells transiently transfected with empty vector (pUC19), pHBV1.3, sh-GALNT10, and pHBV1.3+ sh-GALNT10 (F). All values represent the mean ± S.E. of three independent experiments; *, p < 0.05.
Furthermore, we investigated the role of GALNT10 in the hepatitis B virus-induced tumorigenesis. pHBV1.3 transfection promoted SK-Hep1 cell proliferation, which was almost abolished by co-transfection of miR-122 or GALNT10 shRNA (Fig. 4, C and D). Furthermore, transfection with pHBV1.3 in SK-Hep1 cells increased survival under serum deprivation, which was largely abolished by simultaneous transfection of miR-122 or GALNT10 shRNA (Fig. 4, E and F), indicating that the miR-122/GALNT10 pathway participates in HBV-induced tumorigenesis.
Clinical Correlation of miR-122 with GALNT10 and Patient Outcome
The pathological significance of GALNT10 in HCC is still unknown. Analysis of the GALNT10 protein expression in HCC tissues using immunohistochemical staining revealed an inverse relationship between miR-122 and GALNT10 expression (r = −0.417, p < 0.001, Fig. 5, A and B). Patients with high GALNT10 expression had significantly reduced OS (p < 0.001, Fig. 5C). In an analysis of the prognostic indicator, patients with concomitantly lower miR-122 and higher GALNT10 were found to have the poorest OS (Fig. 5D). These results indicate that GALNT10 expression is inversely correlated with miR-122 expression and the overall survival of HCC patients.
FIGURE 5.
Adverse correlation between miR-122 and GALNT10 in patients with HBV-associated HCC. A, representative ISH and IHC staining with miR-122 and GALNT10 (original magnification ×200) in tumor tissue sections from HCC patients. B, correlation analysis between GALNT10 and miR-122 staining scores in tumor tissue sections from 142 HCC patients (r = −0.417, p < 0.001). C, overall survival was calculated using Kaplan-Meier analysis according to low and high GALNT10. The p value was determined by log-rank test. D, Kaplan-Meier plots for overall survival were stratified by combined miR-122 and GALNT10 expression. The p value is determined by log-rank test.
Hnf4α Orchestrates miR-122 Down-regulation in HBV-infected Hepatoma Cells
To further investigate the mechanism of HBV-mediated miR-122 down-regulation, luciferase activity assay was employed. HBV infection has inhibitory effects on miR-122 5′-UTR luciferase activity, which was partly reversed by ΔHBx (Fig. 6A). We then analyzed the sequence of the promoter region of miR-122. Consequently, several potent binding sites upstream of the miR-122 promoter were defined as described previously (38). There exists a predicted Hnf4α-binding site on the upstream region of the miR-122 promoter (Fig. 6B). As Hnf4α is a positive regulator of miR-122, we wondered whether HBx inhibited miR-122 expression in an Hnf4α-dependent manner. Further studies revealed a significant reduction of the recruitment of Hnf4α at the promoter of miR-122 in HepG2.2.15 cells compared with HepG2 cells, as well as in Huh7 cells stably transfected with HBx compared with control vector-transfected cells (Fig. 6C). Consistent with the aforementioned phenomenon, HBV infection repressed Hnf4α mRNA and protein expression in an HBx-dependent manner (Fig. 6, D and E). Furthermore, HBV infection and HBx expression-induced reduction of miR-122 promoter activity could be reversed by Hnf4α overexpression (Fig. 6F). These data suggest that Hnf4α participates in the HBV-induced miR-122 down-regulation.
FIGURE 6.
Reduced Hnf4α is responsible for miR-122 down-regulation in HBV-infected hepatoma cells. A, luciferase activity analysis of miR-122 promoter relative to pGL3-Basic for HepG2.2.15 versus HepG2 (left panel), and Huh7 and BEL-7404 cells transiently transfected with pUC19, pHBV1.3, and ΔHBx, respectively (right panel). Firefly luciferase activity was normalized to Renilla luciferase activity. B, schematic model of miR-122 promoter sequence analysis showing the predicted binding elements. TSS, transcription start site. C, ChIP-qPCR analysis of Hnf4α enrichment at upstream of miR-122 promoter in HepG2 and HepG2.2.15 cells (left panel) and in HepG2 cells stably transfected with HBx or its vehicle pcDNA3.1 (right panel). D, qRT-PCR analysis of Hnf4α mRNA levels for HepG2 and HepG2.2.15 cells (left panel), for Huh7 and BEL-7404 cells transiently transfected with empty vector or HBx, respectively (right panel). E, Western blot analysis of Hnf4α and GAPDH for HepG2 and HepG2.2.15 cells (upper panel) and for Huh7 and BEL-7404 cells transiently transfected with empty vector or HBx, respectively (lower panel). F, luciferase activity analysis of miR-122 promoter relative to pGL3-Basic for HepG2.2.15 cells transiently transfected with empty vector or Hnf4α, respectively (left panel) for Huh7 and BEL-7404 cells transiently transfected with empty vector, HBx, Hnf4α, or HBx together with Hnf4α (right panel). Firefly luciferase activity was normalized to Renilla luciferase activity. All values represent mean ± S.E. from three independent experiments; *, p < 0.05.
GALNT10 Intensifies Glycosylation and Activation of EGFR
To further determine the role of O-glycosyltransferase activity in the oncogenic role of GALNT10, lectin blot was employed to examine the short O-glycans. A decreased binding of VVA lectin to glycoproteins was observed in SK-Hep1 cells with GALNT10 stably knocked down, whereas increased binding of VVA lectin in Huh7 cells with exogenously expressed GALTN10 but not its inactive form was observed. Moreover, the binding of PNA and Jacalin lectin to glycoproteins in hepatoma cells with altered GALNT10 expression had no changed (Fig. 7A). Because a previous study indicated that EGFR carries O-glycans, we wondered whether EGFR could be modified by GALNT10. Our data showed that genetic inhibition of GALNT10 reduced EGFR expression on the cell membrane, whereas overexpression of wild-type GALNT10, not the inactive form of GALNT10, led to increased surface retention of EGFR (Fig. 7, B and C). Moreover, knockdown of GALNT10 diminished binding of VVA to neuraminidase-treated EGFR, whereas overexpression of GALNT10-WT but not GALNT10-DN increased binding of VVA to neuraminidase-treated EGFR, indicating that sialyl-Tn is presented on the EGFR (Fig. 7D).
FIGURE 7.
GALNT10 intensifies mucin-like O-glycosylation-mediated activation of EGFR. A and B, lectin blot analysis of VVA, PNA, and Jacalin (A), flow cytometry analysis of EGFR (B) for SK-Hep1 cells stably transfected with sh-GALNT10 (left), and Huh7 cells stably transfected with empty vector, GALNT10-WT, or -DN, respectively (right). C, cell lysates were immunoprecipitated (IP) with streptavidin beads and then immunoblotted (IB) with anti-EGFR antibody for membrane biotin-labeled SK-Hep1 cells stably transfected with sh-GALNT10 (left panel), and Huh7 cells stably transfected with empty vector, GALNT10-WT or -DN, respectively (right panel). D, cell lysates treated with neuraminidase were pulled down (PD) with VVA and then immunoblotted (IB) with anti-EGFR antibody (upper panel), and the cell lysates treated with neuraminidase were immunoprecipitated with anti-EGFR antibody and then immunoblotted with VVA, PNA, or anti-EGFR antibody (lower panel) for SK-Hep1 cells stably transfected with sh-GALNT10, and Huh7 cells stably transfected with empty vector, GALNT10-WT or -DN, respectively. E, Western blot analysis of GALNT10, AKT, p-Akt(Ser-473), Mcl-1, Bcl-xl, Bcl-2, and GAPDH for SK-Hep1 cells stably transfected with sh-GALNT10 (left panel), and Huh7 cells stably transfected with empty vector, GALNT10-WT, or -DN, respectively (right panel).
Because activation of the PI3K/AKT pathway has been demonstrated to be a downstream event of EGFR signal, we then investigated the effects of GALNT10 on the activation of the PI3K/AKT pathway. The levels of phosphorylated AKT, Mcl-1, and Bcl-2 were diminished in GALNT10 knockdown cells and increased in GALNT10-WT but not in GALNT10-DN overexpression cells (Fig. 7E). These results suggest that GALNT10 enhances EGFR membrane retention via O-glycosylation, thus activates PI3K/AKT signaling.
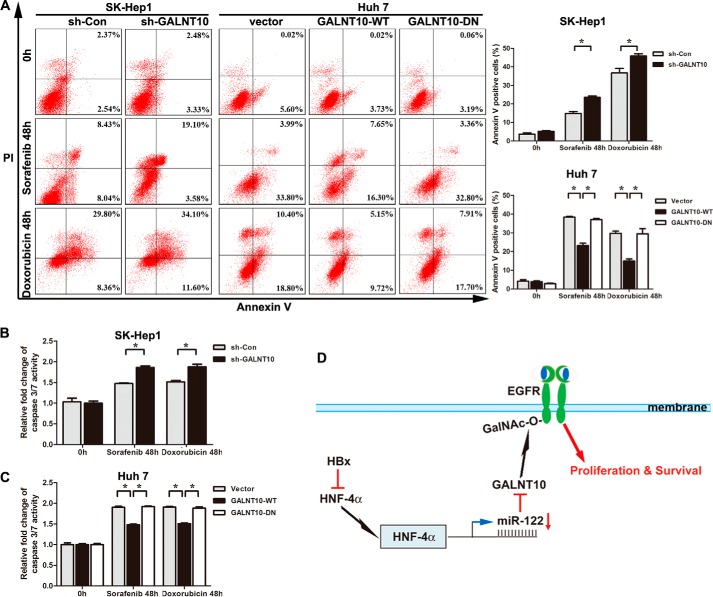
GALNT10 Silencing Increases Sorafenib and Doxorubicin Sensitivity of Hepatoma Cells
Sorafenib, an oral multiple tyrosine kinase inhibitor, has been approved for use in advanced HCC. EGFR activation is a potential determinant of primary resistance of hepatocellular carcinoma cells to sorafenib (39). Therefore, we have further confirmed the effects of GALNT10 on the sensitivity of sorafenib. Control short hairpin RNA-transfected and short hairpin GALNT10-transfected SK-Hep1 cells were treated with sorafenib for 48 h. The apoptotic index was calculated using flow cytometry. The results showed that the apoptotic index was significantly increased in stably GALNT10 knocked down SK-Hep1 cells (Fig. 8, A, left panel, and B). Overexpression of GALNT10 decreased the apoptosis index following sorafenib treatment in Huh7 cells, which was reversed by GALNT10-DN (Fig. 8A, right panel, and C). A previous study reported that restored miR-122 expression increases sensitivity to doxorubicin challenge (37). Thus, we investigate the effect of GALNT10 on the sensitivity of doxorubicin in hepatoma cells. Consistent with aforementioned results, the apoptotic index was significantly increased in GALNT10-knockdown SK-Hep1 cells and reduced in Huh7 cells stably transfected with GALNT10-WT but not GALNT10-DN during doxorubicin treatment (Fig. 8, A–C).
FIGURE 8.
GALNT10 silencing increases sorafenib and doxorubicin sensitivity of hepatoma cells. A, GALNT10 knockdown SK-Hep1 cells (left panel) and stably transfected with wild-type or inactive form GALNT10 Huh7 cells (right) were treated with sorafenib or doxorubicin for 48 h. Sorafenib concentration for both cells was 5 μm, whereas 5 and 0.5 μg/ml doxorubicin were applied on SK-Hep1 cells and Huh7 cells, respectively. The cells were harvested, and the apoptotic rates were measured by annexin V apoptosis assay. The summarized results are expressed as the mean ± S.E. of three independent experiments; *, p < 0.05. B and C, caspase 3/7 activity assay for sorafenib- and doxorubicin-treated SK-Hep1 (B) and Huh7 (C) cells. All values represent mean ± S.E. from three independent experiments; *, p < 0.05. D, schematic model of Hnf4α/miR-122/GALNT10/EGFR regulatory pathway in HBV-infected HCC cells.
DISCUSSION
Accumulating evidence suggests a linked deregulation of miRNA expression with liver cancers, but studies related to HBV-related HCC are limited. In this study, we confirmed miR-122 abundance is significantly repressed in HBV-associated HCC, and we further elucidated the transcriptional inhibition of miR-122 by down-regulation of Hnf4α. This suppression of miR-122 levels correlates with etiology, tumor size, venous invasion, differentiation grade, and poor prognosis. More importantly, we demonstrated that down-regulation of miR-122 leads to increased expression of its target galnt10, which thus promoted HCC cell growth and survival.
miR-122 has been identified as a critical regulator of hepatic gene expression and is implicated in several important aspects of liver pathobiology, including lipid metabolism, hepatocarcinogenesis, and HCV replication (40, 41). The level of miR-122 is decreased in patients with HBV infection (42), but the mechanism underlying is not fully elucidated. Song et al. (43, 44) reported that HBx conferred epigenetic down-regulation of miR-122 by peroxisome proliferator-activated receptor-γ·retinoid X receptor α complex through interaction with the co-repressors and H3K9 histone methyltransferase (SUV39H1) in HCC cells. In addition, Li et al. (45) reported that HBV mRNAs act as sponges to bind and sequester endogenous miR-122 to facilitate HBV replication. The promoter region of the miR-122 gene contains specific binding sites for liver-enriched transcription factors, such as hepatocyte nuclear factor-1α (Hnf1α), Hnf3β, and Hnf4α, which regulate miR-122 transcription (38, 46, 47). Here, we indicate that HBV infection inhibited the endogenous level of Hnf4α due to HBx protein, thus leading to the transcriptional repression of the miR-122 promoter and inhibition of miR-122 expression. In support of this notion, recent work showed that HBx activates interleukin-6 (IL-6) production, whereas signaling to Stat3 leads to production of more miR-24 and miR-629, which keeps Hnf4α inactivation (48, 49).
In an attempt to identify the underlying molecular mechanisms responsible for the impact of miR-122 on oncogenesis, we used microRNA target prediction algorithms on genes negatively correlated with miR-122. Consequently, an evolutionarily conserved binding site for miR-122 was detected in the 3′-UTR of GALNT10 mRNA. Interestingly, GALNT10 is not the only target of miR-122 relevant to HBV-associated HCC. Fan et al. (50) reported that miR-122 inhibits viral replication and cell proliferation in hepatitis B virus-related hepatocellular carcinoma by targeting NDRG3, a member of the N-myc downstream-regulated gene family. Li et al. (45) found that hepatitis B virus mRNA-mediated miR-122 inhibition up-regulates pituitary tumor-transforming gene 1 (PTTG1) binding factor, which promotes HCC cells growth and invasion. In addition, Wang et al. (42) also found that the miR-122 target cyclin G1 is overexpressed in chronic hepatitis B. Nevertheless, in this study the promoting effect of HBV on cellular proliferation and apoptosis resistance was shown to be largely dependent on the regulation of miR-122 and GALNT10.
Accumulating evidence suggests that miRNA maybe a principal regulator in the regulation of specific glycan biosynthetic enzymes. Mahal and co-workers (51) revealed critical nodes in the global glycosylation network accessible to microRNA regulation, providing an miRNA regulatory map for glycogenes both in the N-linked and O-linked glycosylation pathways, among which galnt1, galnt7, fut4, fut8, and manea are predicted to be highly regulated genes. Changes in microRNA may underlie the altered glycosylation observed in dynamic processes in cancer phenotypes (52). Bernardi et al. (53) reported that fucosyltransferase 8 (FUT8), catalyzing core fucosylation, was regulated by miR-122 and miR-34a during hepatocarcinogenesis. Galnt7 was revealed to be regulated by miR-378 (54), miR-30b/d (26), miR-214 (55), miR-17 (56), and miR-34a/c (57). This study identified galnt10 as a direct target of miR-122.
Aberrantly glycosylated mucins, carrying Tn among others, often alter the function of glycoproteins, such as antigenic property, and the potential of cancer cells to invade and metastasize (58). The expression of Tn and sialyl-Tn antigens has been detected in human hepatocellular carcinoma (59, 60). EGFR O-glycosylation was shown to be modified by GALNT10 with overexpression in HCC, and previous finding showed that EGFR O-glycosylation could be regulated by GALNT2 (22). The possible reason for this inconsistency is that many GALNTs appear to have a hierarchical relationship with one another, such that one enzyme cannot attach an N-acetylgalactosamine until an adjacent serine or threonine is glycosylated by a different GALNT. Thus, co-expression in the same cell of GALNTs with complementary and partly overlapping acceptor substrate specificities probably ensures efficient O-GalNAc glycosylation (24).
GALNT10 was first cloned as a homolog of human GALNT7 and found in the restricted expression (small intestine, stomach, pancreas, ovary, thyroid gland, and spleen) (61). The repertoire of GALNTs expressed in cells varies with cell type, differentiation, and maturation and is associated with substantial changes in expression of individual GALNT during malignant transformation (62, 63). Disregarding the relatively lower assembly of GALNT10 in liver, GALNT10 was up-regulated by HBV-induced down-regulation of miR-122 and exerted an oncogenic function during HCC progression. Moreover, previous studies suggested that glycosylation on HBV structural proteins, including both N- and O-glycosylation, may exert a crucial role in the assembly, secretion, and infection of HBV (64–66). Whether GALNT10 is involved in the O-glycosylation of HBV structural proteins or not needs to be investigated in the future.
HBV replication is inhibited by miR-122 through the inhibitory effect of p53 on HBV transcription (42). Conversely, miR-122 is down-regulated by HBV and leads to the increase of GALNT10 expression in our study. p53 stability was coordinately regulated by O-linked N-acetylglucosamine (O-GlcNAc) and O-phosphate modifications (67). Both O-GlcNAc and O-GalNAc modification happen at Ser/Thr residues. O-GalNAc synthesis is a hierarchical process, whereas O-GlcNAc is a highly dynamic modification. Whether GALNT10 participates in the regulation of p53 stability needs to be further investigated.
Increasing EGFR signaling has been reported to be associated with the development of HBV-associated HCC (68). Despite the high frequency of EGFR expression in HCC, activation mutations in the EGFR kinase domain are not detected (69). These results indicate that a different mechanism for the regulation of EGFR function may operate during HCC pathogenesis and progression. Glycosylation plays an important role in EGF binding and hence in kinase activation (70). Here, we showed that GALNT10 modifies O-glycans on EGFR and subsequent phosphorylation of AKT in HCC. It is noteworthy that EGFR may not be the only acceptor substrate of GALNT10. It is likely that, in addition to EGFR, other glycoproteins, including surface and secreted molecules, may be modified by GALNT10 to cooperatively mediate phenotypic changes in HCC. Muc1 (Mucin-1), one of several high molecular weight glycoprotein families that are often heavily O-glycosylated, is often overexpressed in HCC and associated with migration and invasion (71). Glycoproteomic technology will be helpful to further understand the precise mechanisms.
The oncogenic role of EGFR is well documented, and its activation has been implicated in limiting sorafenib efficiency in HCC (39). In this work, we found out that miR-122 down-regulation and its target galnt10 overexpression lead to increased tumor growth and survival by modifying EGFR activity. In line with this, a phase III randomized controlled trial of sorafenib conducted in the Asia-Pacific region confirmed that HBV-negative HCC patients had a more significant survival benefit than HBV-positive patients (prolonged median OS 3.7 months versus 1.8 months) (72). Thus, many clinical trials evaluate the efficacy and safety of sorafenib in combination with anti-EGFR pharmacologic agents for patients with HCC (73). Consistently, we found that knockdown of GALNT10 cooperates with sorafenib to increase apoptosis rates. Furthermore, Fornari et al. (37) reported that miR-122 increases sensitivity to doxorubicin challenge through a p53-dependent and p53-independent manner in hepatoma cells. p53 may regulate sensitivity to the EGFR inhibitor by modulating EGFR downstream signaling functionality and apoptosis induction (74). We found out that altered GALNT10 influenced the O-glycosylation and activity of EGFR. Therefore, we are motivated to elucidate the potential role of GALNT10 on the doxorubicin. Interestingly, knockdown of GALNT10 expression potentiates the sensitivity of doxorubicin in hepatoma cells. Consistently, the combination of tyrosine kinase inhibitor and doxorubicin was found to be superior to either alone in preclinical models (75).
On the basis of these results, we propose a schematic model illustrating a possible molecular mechanism and functional basis for HBV-associated hepatocarcinogenesis (Fig. 8D). In conclusion, our results reveal a novel association between HBV infection and the Hnf4α/miR-122/GALNT10 pathway in the development of HBV-associated HCC, thus revealing a putative molecular mechanism for the development and progression of HBV-associated HCC. These results shed new light for potential therapeutic intervention to prevent hepatocarcinogenesis in the high risk group of chronic hepatitis B patients with chemically modified oligonucleotides or artificial decoys that may depress the endogenous GALNT10 levels.
This work was supported by National Basic Research Program of China Grant 2012CB822104, National Key Projects for Infectious Diseases of China Grant 2012ZX10002-012, National Natural Science Foundation of China Grants 31100629, 31270863, 81471621, and 81472227, Program for New Century Excellent Talents in University Grant NCET-13-0146, and Shanghai Rising-Star Program Grant 13QA1400300.
- HCC
- hepatocellular carcinoma
- HBV
- hepatitis B virus
- HBx
- hepatitis B virus X protein
- EGFR
- epidermal growth factor receptor
- VVA
- vicia villosa
- PNA
- peanut agglutinin
- ISH
- in situ hybridization
- Hnf4α
- hepatocyte nuclear factor-4α
- RXRα
- retinoid X receptor α
- miRNA
- microRNA
- qRT-PCR
- quantitative real time PCR
- IHC
- immunohistochemistry
- GALNT
- UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase
- OS
- overall survival.
REFERENCES
- 1. Maluccio M., Covey A. (2012) Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 62, 394–399 [DOI] [PubMed] [Google Scholar]
- 2. El-Serag H. B., Mason A. C. (1999) Rising incidence of hepatocellular carcinoma in the United States. N. Engl. J. Med. 340, 745–750 [DOI] [PubMed] [Google Scholar]
- 3. Farazi P. A., DePinho R. A. (2006) Hepatocellular carcinoma pathogenesis: from genes to environment. Nat. Rev. Cancer 6, 674–687 [DOI] [PubMed] [Google Scholar]
- 4. Li C., Tan Y. X., Zhou H., Ding S. J., Li S. J., Ma D. J., Man X. B., Hong Y., Zhang L., Li L., Xia Q. C., Wu J. R., Wang H. Y., Zeng R. (2005) Proteomic analysis of hepatitis B virus-associated hepatocellular carcinoma: identification of potential tumor markers. Proteomics 5, 1125–1139 [DOI] [PubMed] [Google Scholar]
- 5. Okabe H., Satoh S., Kato T., Kitahara O., Yanagawa R., Yamaoka Y., Tsunoda T., Furukawa Y., Nakamura Y. (2001) Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res. 61, 2129–2137 [PubMed] [Google Scholar]
- 6. Hakomori S. (1985) Aberrant glycosylation in cancer cell membranes as focused on glycolipids: overview and perspectives. Cancer Res. 45, 2405–2414 [PubMed] [Google Scholar]
- 7. Blomme B., Van Steenkiste C., Callewaert N., Van Vlierberghe H. (2009) Alteration of protein glycosylation in liver diseases. J. Hepatol. 50, 592–603 [DOI] [PubMed] [Google Scholar]
- 8. Dennis J. W., Granovsky M., Warren C. E. (1999) Glycoprotein glycosylation and cancer progression. Biochim. Biophys. Acta 1473, 21–34 [DOI] [PubMed] [Google Scholar]
- 9. Fuster M. M., Esko J. D. (2005) The sweet and sour of cancer: glycans as novel therapeutic targets. Nat. Rev. Cancer 5, 526–542 [DOI] [PubMed] [Google Scholar]
- 10. Fukuda M. (1996) Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 56, 2237–2244 [PubMed] [Google Scholar]
- 11. Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. (1987) Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science 236, 582–585 [DOI] [PubMed] [Google Scholar]
- 12. Ito Y., Miyoshi E., Sakon M., Takeda T., Noda K., Tsujimoto M., Ito S., Honda H., Takemura F., Wakasa K., Monden M., Matsuura N., Taniguchi N. (2001) Elevated expression of UDP-N-acetylglucosamine: α-mannoside β1,6-N-acetylglucosaminyltransferase is an early event in hepatocarcinogenesis. Int. J. Cancer 91, 631–637 [PubMed] [Google Scholar]
- 13. Yanagi M., Aoyagi Y., Suda T., Mita Y., Asakura H. (2001) N-Acetylglucosaminyltransferase V as a possible aid for the evaluation of tumor invasiveness in patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 16, 1282–1289 [DOI] [PubMed] [Google Scholar]
- 14. Noda K., Miyoshi E., Uozumi N., Yanagidani S., Ikeda Y., Gao C., Suzuki K., Yoshihara H., Yoshikawa K., Kawano K., Hayashi N., Hori M., Taniguchi N. (1998) Gene expression of α1–6 fucosyltransferase in human hepatoma tissues: a possible implication for increased fucosylation of α-fetoprotein. Hepatology 28, 944–952 [DOI] [PubMed] [Google Scholar]
- 15. Wei Y., Liu D., Zhou F., Ge Y., Xu J., Yun X., Gu J., Jiang J. (2008) Identification of β-1,4-galactosyltransferase I as a target gene of HBx-induced cell cycle progression of hepatoma cell. J. Hepatol. 49, 1029–1037 [DOI] [PubMed] [Google Scholar]
- 16. Jensen P. H., Kolarich D., Packer N. H. (2010) Mucin-type O-glycosylation–putting the pieces together. FEBS J. 277, 81–94 [DOI] [PubMed] [Google Scholar]
- 17. Brockhausen I. (1999) Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta 1473, 67–95 [DOI] [PubMed] [Google Scholar]
- 18. Hollingsworth M. A., Swanson B. J. (2004) Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer 4, 45–60 [DOI] [PubMed] [Google Scholar]
- 19. Gerken T. A., Jamison O., Perrine C. L., Collette J. C., Moinova H., Ravi L., Markowitz S. D., Shen W., Patel H., Tabak L. A. (2011) Emerging paradigms for the initiation of mucin-type protein O-glycosylation by the polypeptide GalNAc transferase family of glycosyltransferases. J. Biol. Chem. 286, 14493–14507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwientek T., Bennett E. P., Flores C., Thacker J., Hollmann M., Reis C. A., Behrens J., Mandel U., Keck B., Schäfer M. A., Haselmann K., Zubarev R., Roepstorff P., Burchell J. M., Taylor-Papadimitriou J., Hollingsworth M. A., Clausen H. (2002) Functional conservation of subfamilies of putative UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosaminyltransferases in Drosophila, Caenorhabditis elegans, and mammals. One subfamily composed of l(2)35Aa is essential in Drosophila. J. Biol. Chem. 277, 22623–22638 [DOI] [PubMed] [Google Scholar]
- 21. Ten Hagen K. G., Fritz T. A., Tabak L. A. (2003) All in the family: the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology 13, 1R–16R [DOI] [PubMed] [Google Scholar]
- 22. Wu Y. M., Liu C. H., Hu R. H., Huang M. J., Lee J. J., Chen C. H., Huang J., Lai H. S., Lee P. H., Hsu W. M., Huang H. C., Huang M. C. (2011) Mucin glycosylating enzyme GALNT2 regulates the malignant character of hepatocellular carcinoma by modifying the EGF receptor. Cancer Res. 71, 7270–7279 [DOI] [PubMed] [Google Scholar]
- 23. Wu Y. M., Liu C. H., Huang M. J., Lai H. S., Lee P. H., Hu R. H., Huang M. C. (2013) C1GALT1 enhances proliferation of hepatocellular carcinoma cells via modulating MET glycosylation and dimerization. Cancer Res. 73, 5580–5590 [DOI] [PubMed] [Google Scholar]
- 24. Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E. (2009) Essentials of Glycobiology, 2nd Ed., p. 120, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 25. Croce C. M., Calin G. A. (2005) miRNAs, cancer, and stem cell division. Cell 122, 6–7 [DOI] [PubMed] [Google Scholar]
- 26. Gaziel-Sovran A., Segura M. F., Di Micco R., Collins M. K., Hanniford D., Vega-Saenz de Miera E., Rakus J. F., Dankert J. F., Shang S., Kerbel R. S., Bhardwaj N., Shao Y., Darvishian F., Zavadil J., Erlebacher A., Mahal L. K., Osman I., Hernando E. (2011) miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell 20, 104–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tian X., Zhao C., Zhu H., She W., Zhang J., Liu J., Li L., Zheng S., Wen Y. M., Xie Y. (2010) Hepatitis B virus (HBV) surface antigen interacts with and promotes cyclophilin a secretion: possible link to pathogenesis of HBV infection. J. Virol. 84, 3373–3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu J., Yun X., Jiang J., Wei Y., Wu Y., Zhang W., Liu Y., Wang W., Wen Y., Gu J. (2010) Hepatitis B virus X protein blunts senescence-like growth arrest of human hepatocellular carcinoma by reducing Notch1 cleavage. Hepatology 52, 142–154 [DOI] [PubMed] [Google Scholar]
- 29. Thomas H., Senkel S., Erdmann S., Arndt T., Turan G., Klein-Hitpass L., Ryffel G. U. (2004) Pattern of genes influenced by conditional expression of the transcription factors HNF6, HNF4α and HNF1β in a pancreatic beta-cell line. Nucleic Acids Res. 32, e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu H., Xu J., Zhou L., Yun X., Chen L., Wang S., Sun L., Wen Y., Gu J. (2011) Hepatitis B virus large surface antigen promotes liver carcinogenesis by activating the Src/PI3K/Akt pathway. Cancer Res. 71, 7547–7557 [DOI] [PubMed] [Google Scholar]
- 31. Xu J., Liu H., Chen L., Wang S., Zhou L., Yun X., Sun L., Wen Y., Gu J. (2012) Hepatitis B virus X protein confers resistance of hepatoma cells to anoikis by up-regulating and activating p21-activated kinase 1. Gastroenterology 143, 199–212.e194 [DOI] [PubMed] [Google Scholar]
- 32. Zhang W., Zhou Q., Xu W., Cai Y., Yin Z., Gao X., Xiong S. (2013) DNA-dependent activator of interferon-regulatory factors (DAI) promotes lupus nephritis by activating the calcium pathway. J. Biol. Chem. 288, 13534–13550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang W., Xu W., Xiong S. (2010) Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. J. Immunol. 184, 6465–6478 [DOI] [PubMed] [Google Scholar]
- 34. Zhang W., Xu W., Xiong S. (2011) Macrophage differentiation and polarization via phosphatidylinositol 3-kinase/Akt-ERK signaling pathway conferred by serum amyloid P component. J. Immunol. 187, 1764–1777 [DOI] [PubMed] [Google Scholar]
- 35. Ruan Y., Sun L., Hao Y., Wang L., Xu J., Zhang W., Xie J., Guo L., Zhou L., Yun X., Zhu H., Shen A., Gu J. (2012) Ribosomal RACK1 promotes chemoresistance and growth in human hepatocellular carcinoma. J. Clin. Invest. 122, 2554–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Detre S., Saclani Jotti G., Dowsett M. (1995) A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J. Clin. Pathol. 48, 876–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fornari F., Gramantieri L., Giovannini C., Veronese A., Ferracin M., Sabbioni S., Calin G. A., Grazi G. L., Croce C. M., Tavolari S., Chieco P., Negrini M., Bolondi L. (2009) miR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 69, 5761–5767 [DOI] [PubMed] [Google Scholar]
- 38. Li Z. Y., Xi Y., Zhu W. N., Zeng C., Zhang Z. Q., Guo Z. C., Hao D. L., Liu G., Feng L., Chen H. Z., Chen F., Lv X., Liu D. P., Liang C. C. (2011) Positive regulation of hepatic miR-122 expression by HNF4α. J. Hepatol. 55, 602–611 [DOI] [PubMed] [Google Scholar]
- 39. Blivet-Van Eggelpoël M. J., Chettouh H., Fartoux L., Aoudjehane L., Barbu V., Rey C., Priam S., Housset C., Rosmorduc O., Desbois-Mouthon C. (2012) Epidermal growth factor receptor and HER-3 restrict cell response to sorafenib in hepatocellular carcinoma cells. J. Hepatol. 57, 108–115 [DOI] [PubMed] [Google Scholar]
- 40. Jopling C. L., Yi M., Lancaster A. M., Lemon S. M., Sarnow P. (2005) Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science 309, 1577–1581 [DOI] [PubMed] [Google Scholar]
- 41. Lanford R. E., Hildebrandt-Eriksen E. S., Petri A., Persson R., Lindow M., Munk M. E., Kauppinen S., Ørum H. (2010) Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327, 198–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang S., Qiu L., Yan X., Jin W., Wang Y., Chen L., Wu E., Ye X., Gao G. F., Wang F., Chen Y., Duan Z., Meng S. (2012) Loss of microRNA 122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G1-modulated p53 activity. Hepatology 55, 730–741 [DOI] [PubMed] [Google Scholar]
- 43. Song K., Han C., Zhang J., Lu D., Dash S., Feitelson M., Lim K., Wu T. (2013) Epigenetic regulation of microRNA-122 by peroxisome proliferator activated receptor-gamma and hepatitis b virus X protein in hepatocellular carcinoma cells. Hepatology 58, 1681–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Song K. S., Han C., Wu T. (2013) Epigenetic regulation of miR-122 by PPARγR and hepatitis B virus X protein in hepatocellular carcinoma cells. FASEB J. 27, 872.10 [Google Scholar]
- 45. Li C., Wang Y., Wang S., Wu B., Hao J., Fan H., Ju Y., Ding Y., Chen L., Chu X., Liu W., Ye X., Meng S. (2013) Hepatitis B virus mRNA-mediated miR-122 inhibition upregulates PTTG1-binding protein, which promotes hepatocellular carcinoma tumor growth and cell invasion. J. Virol. 87, 2193–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu H., He J. H., Xiao Z. D., Zhang Q. Q., Chen Y. Q., Zhou H., Qu L. H. (2010) Liver-enriched transcription factors regulate microRNA-122 that targets CUTL1 during liver development. Hepatology 52, 1431–1442 [DOI] [PubMed] [Google Scholar]
- 47. Coulouarn C., Factor V. M., Andersen J. B., Durkin M. E., Thorgeirsson S. S. (2009) Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene 28, 3526–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hatziapostolou M., Polytarchou C., Aggelidou E., Drakaki A., Poultsides G. A., Jaeger S. A., Ogata H., Karin M., Struhl K., Hadzopoulou-Cladaras M., Iliopoulos D. (2011) An HNF4α-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell 147, 1233–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xiang W. Q., Feng W. F., Ke W., Sun Z., Chen Z., Liu W. (2011) Hepatitis B virus X protein stimulates IL-6 expression in hepatocytes via a MyD88-dependent pathway. J. Hepatol. 54, 26–33 [DOI] [PubMed] [Google Scholar]
- 50. Fan C. G., Wang C. M., Tian C., Wang Y., Li L., Sun W. S., Li R. F., Liu Y. G. (2011) miR-122 inhibits viral replication and cell proliferation in hepatitis B virus-related hepatocellular carcinoma and targets NDRG3. Oncol. Rep. 26, 1281–1286 [DOI] [PubMed] [Google Scholar]
- 51. Agrawal P., Kurcon T., Pilobello K. T., Rakus J. F., Koppolu S., Liu Z., Batista B. S., Eng W. S., Hsu K. L., Liang Y., Mahal L. K. (2014) Mapping posttranscriptional regulation of the human glycome uncovers microRNA defining the glycocode. Proc. Natl. Acad. Sci. U.S.A. 111, 4338–4343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kasper B. T., Koppolu S., Mahal L. K. (2014) Insights into miRNA regulation of the human glycome. Biochem. Biophys. Res. Commun. 445, 774–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bernardi C., Soffientini U., Piacente F., Tonetti M. G. (2013) Effects of microRNAs on fucosyltransferase 8 (FUT8) expression in hepatocarcinoma cells. PLoS One 8, e76540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kahai S., Lee S. C., Lee D. Y., Yang J., Li M., Wang C. H., Jiang Z., Zhang Y., Peng C., Yang B. B. (2009) MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7. PLoS One 4, e7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peng R. Q., Wan H. Y., Li H. F., Liu M., Li X., Tang H. (2012) MicroRNA-214 suppresses growth and invasiveness of cervical cancer cells by targeting UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 7. J. Biol. Chem. 287, 14301–14309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shan S. W., Fang L., Shatseva T., Rutnam Z. J., Yang X., Du W., Lu W. Y., Xuan J. W., Deng Z., Yang B. B. (2013) Mature miR-17–5p and passenger miR-17–3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways. J. Cell Sci. 126, 1517–1530 [DOI] [PubMed] [Google Scholar]
- 57. Li W., Ma H., Sun J. (2014) MicroRNA34a/c function as tumor suppressors in Hep2 laryngeal carcinoma cells and may reduce GALNT7 expression. Mol. Med. Rep. 9, 1293–1298 [DOI] [PubMed] [Google Scholar]
- 58. Brockhausen I. (2006) Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 7, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sasaki M., Yamato T., Nakanuma Y. (1999) Expression of sialyl-Tn, Tn and T antigens in primary liver cancer. Pathol. Int. 49, 325–331 [DOI] [PubMed] [Google Scholar]
- 60. Cao Y., Karsten U., Otto G., Bannasch P. (1999) Expression of MUC1, Thomsen-Friedenreich antigen, Tn, sialosyl-Tn, and α2,6-linked sialic acid in hepatocellular carcinomas and preneoplastic hepatocellular lesions. Virchows Arch. 434, 503–509 [DOI] [PubMed] [Google Scholar]
- 61. Cheng L., Tachibana K., Zhang Y., Guo Jm, Kahori Tachibana K., Kameyama A., Wang H., Hiruma T., Iwasaki H., Togayachi A., Kudo T., Narimatsu H. (2002) Characterization of a novel human UDP-GalNAc transferase, pp-GalNAc-T10. FEBS Lett. 531, 115–121 [DOI] [PubMed] [Google Scholar]
- 62. Bennett E. P., Mandel U., Clausen H., Gerken T. A., Fritz T. A., Tabak L. A. (2012) Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schjoldager K. T., Clausen H. (2012) Site-specific protein O-glycosylation modulates proprotein processing–deciphering specific functions of the large polypeptide GalNAc-transferase gene family. Biochim. Biophys. Acta 1820, 2079–2094 [DOI] [PubMed] [Google Scholar]
- 64. Schmitt S., Glebe D., Tolle T. K., Lochnit G., Linder D., Geyer R., Gerlich W. H. (2004) Structure of pre-S2 N- and O-linked glycans in surface proteins from different genotypes of hepatitis B virus. J. Gen. Virol. 85, 2045–2053 [DOI] [PubMed] [Google Scholar]
- 65. Werr M., Prange R. (1998) Role for calnexin and N-linked glycosylation in the assembly and secretion of hepatitis B virus middle envelope protein particles. J. Virol. 72, 778–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tolle T. K., Glebe D., Linder M., Linder D., Schmitt S., Geyer R., Gerlich W. H. (1998) Structure and glycosylation patterns of surface proteins from woodchuck hepatitis virus. J. Virol. 72, 9978–9985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang W. H., Kim J. E., Nam H. W., Ju J. W., Kim H. S., Kim Y. S., Cho J. W. (2006) Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat. Cell Biol. 8, 1074–1083 [DOI] [PubMed] [Google Scholar]
- 68. Schiffer E., Housset C., Cacheux W., Wendum D., Desbois-Mouthon C., Rey C., Clergue F., Poupon R., Barbu V., Rosmorduc O. (2005) Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology 41, 307–314 [DOI] [PubMed] [Google Scholar]
- 69. Su M. C., Lien H. C., Jeng Y. M. (2005) Absence of epidermal growth factor receptor exon 18–21 mutation in hepatocellular carcinoma. Cancer Lett. 224, 117–121 [DOI] [PubMed] [Google Scholar]
- 70. Bishayee S. (2000) Role of conformational alteration in the epidermal growth factor receptor (EGFR) function. Biochem. Pharmacol. 60, 1217–1223 [DOI] [PubMed] [Google Scholar]
- 71. Bozkaya G., Korhan P., Cokaklı M., Erdal E., Sağol O., Karademir S., Korch C., Atabey N. (2012) Cooperative interaction of MUC1 with the HGF/c-Met pathway during hepatocarcinogenesis. Mol. Cancer 11, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cheng A. L., Guan Z., Chen Z., Tsao C. J., Qin S., Kim J. S., Yang T. S., Tak W. Y., Pan H., Yu S., Xu J., Fang F., Zou J., Lentini G., Voliotis D., Kang Y. K. (2012) Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur. J. Cancer 48, 1452–1465 [DOI] [PubMed] [Google Scholar]
- 73. Villanueva A., Llovet J. M. (2011) Targeted therapies for hepatocellular carcinoma. Gastroenterology 140, 1410–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huang S., Benavente S., Armstrong E. A., Li C., Wheeler D. L., Harari P. M. (2011) p53 modulates acquired resistance to EGFR inhibitors and radiation. Cancer Res. 71, 7071–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Asghar U., Meyer T. (2012) Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J. Hepatol. 56, 686–695 [DOI] [PubMed] [Google Scholar]