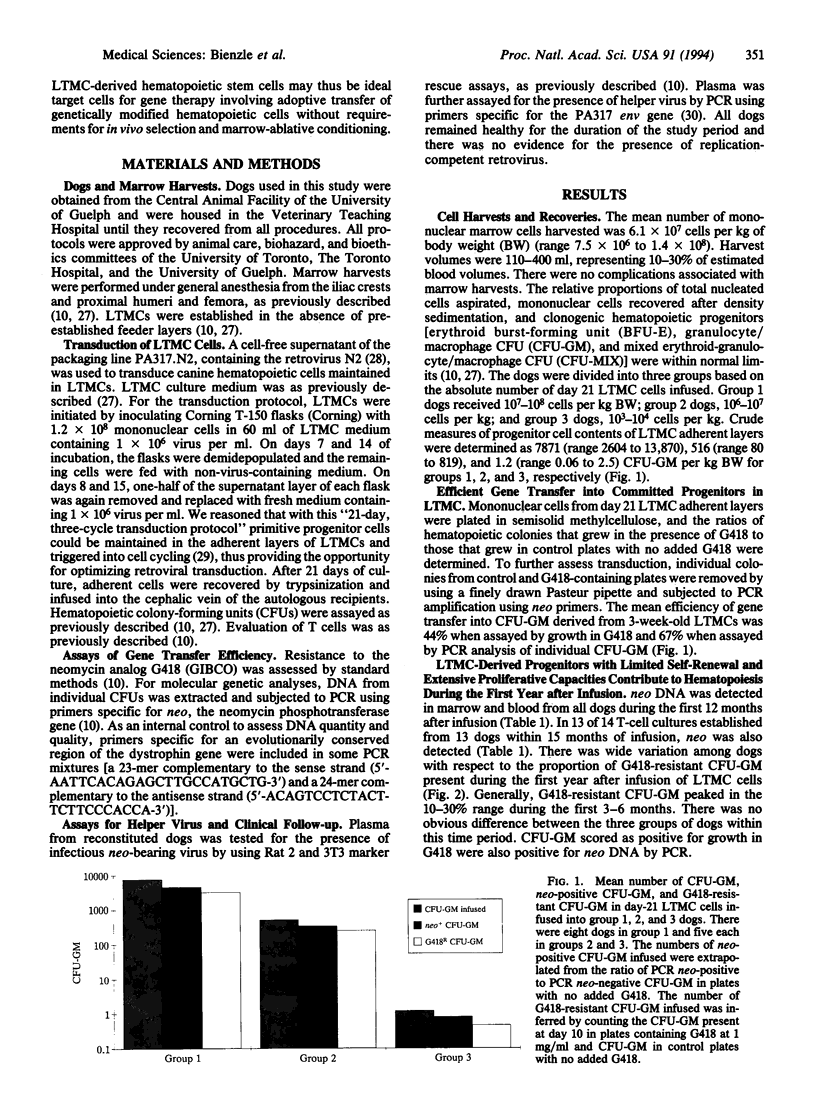
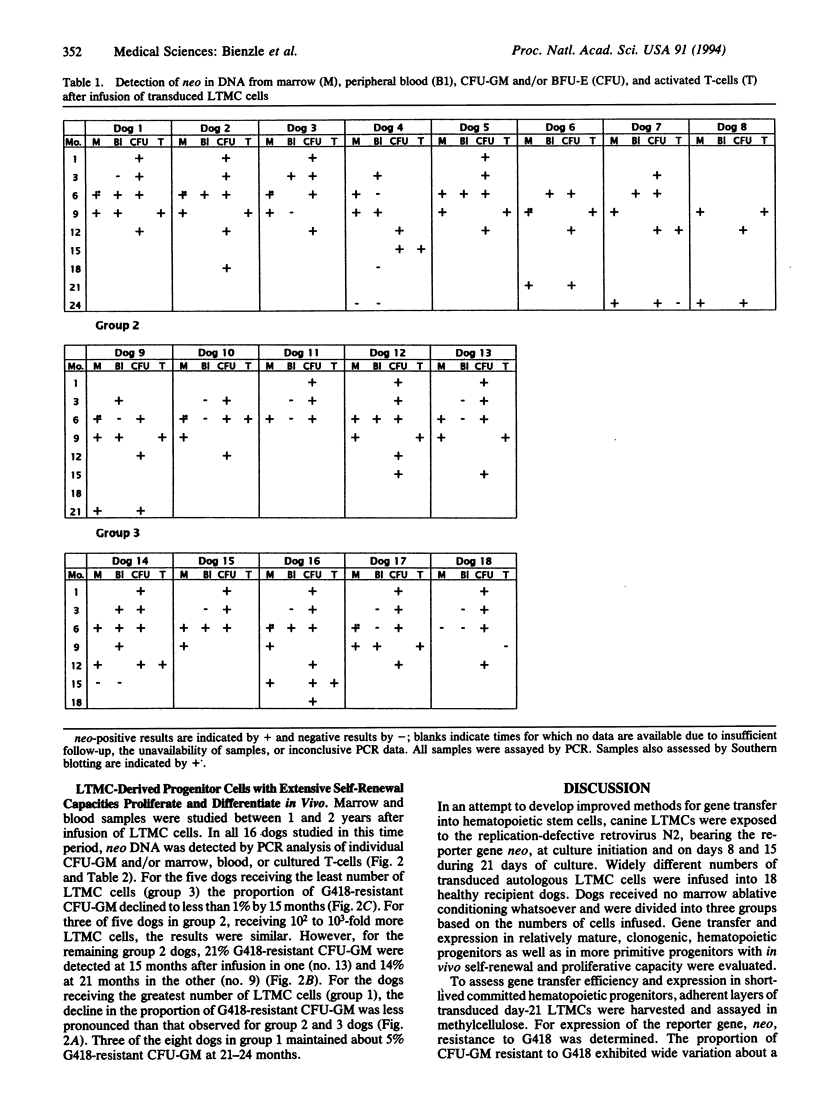
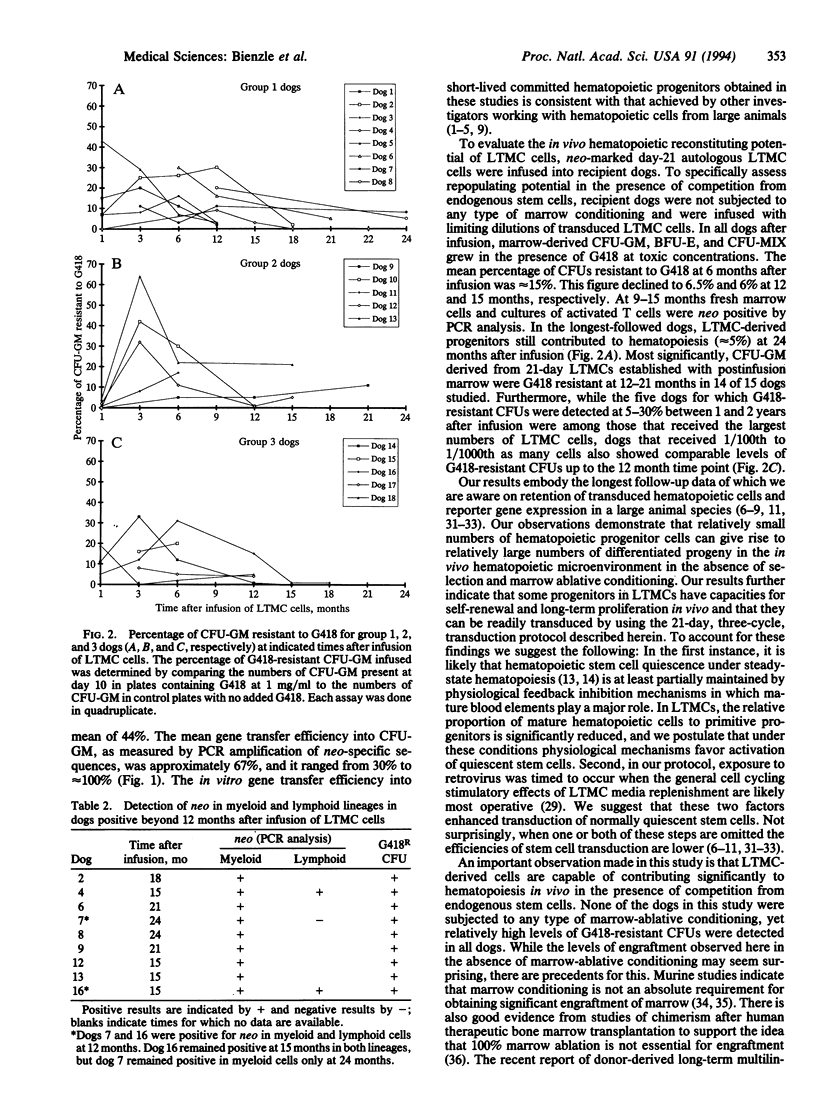
Abstract
Adoptive transfer of genetically modified somatic cells will play an increasingly important role in the management of a wide spectrum of human diseases. Among the most appealing somatic cells as potential gene transfer vehicles are hematopoietic cells, because of their wide distribution and their well-characterized capacities for proliferation, differentiation, and self-renewal. Genes can be readily transferred into short-lived and lineage-restricted hematopoietic cells, but there remains a need to develop reliable methods for gene transfer into hematopoietic stem cells in large animals. In this work, we used a gene transfer approach in which hematopoietic cells in long-term marrow cultures were exposed to the replication-defective retrovirus N2, bearing the reporter gene neo, on multiple occasions during 21 days of culture. Genetically marked cultured autologous cells were infused into 18 canine recipients in the absence of marrow-ablative conditioning. neo was detected by Southern blotting and/or the polymerase chain reaction in the marrow, blood, marrow-derived granulocyte/macrophage and erythroid progenitors, and cultured T cells in dogs after infusion. In most dogs, the proportion of long-term marrow culture cells contributing to hematopoiesis rose during the first 3 months after infusion and peaked within the first 6. The maximal levels attained were between 10% and 30% G418-resistant (neo-positive) granulocyte/macrophage progenitors. At 12 months, five dogs maintained greater than 10% G418-resistant progenitors, and for two of them this level exceeded 20%. Two dogs had greater than 5% G418-resistant hematopoietic progenitors at 24 months after infusion. Our data suggest that very primitive hematopoietic progenitors are maintained in long-term marrow cultures, where they can be triggered into entering the cell cycle. In vivo, these activated cells likely continue normal programs of proliferation, differentiation, and self-renewal. Their progeny can be maintained at clinically relevant levels for up to 2 years without the requirement that endogenous hematopoiesis be suppressed through chemo- or radiotherapy prior to adoptive transfer. Long-term marrow culture cells may thus be ideal targets for gene therapy involving adoptive transfer of transduced hematopoietic cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abkowitz J. L., Linenberger M. L., Newton M. A., Shelton G. H., Ott R. L., Guttorp P. Evidence for the maintenance of hematopoiesis in a large animal by the sequential activation of stem-cell clones. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9062–9066. doi: 10.1073/pnas.87.22.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., Karlsson S., Nienhuis A. W. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8897–8901. doi: 10.1073/pnas.86.22.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., McDonagh K. T., Brandt S. J., Ney P. A., Agricola B., Byrne E., Nienhuis A. W. Development of a high-titer retrovirus producer cell line capable of gene transfer into rhesus monkey hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3738–3742. doi: 10.1073/pnas.87.10.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., McDonagh K. T., Seidel N. E., Nienhuis A. W. Survival and retrovirus infection of murine hematopoietic stem cells in vitro: effects of 5-FU and method of infection. Exp Hematol. 1991 Mar;19(3):206–212. [PubMed] [Google Scholar]
- Bordignon C., Yu S. F., Smith C. A., Hantzopoulos P., Ungers G. E., Keever C. A., O'Reilly R. J., Gilboa E. Retroviral vector-mediated high-efficiency expression of adenosine deaminase (ADA) in hematopoietic long-term cultures of ADA-deficient marrow cells. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6748–6752. doi: 10.1073/pnas.86.17.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregni M., Magni M., Siena S., Di Nicola M., Bonadonna G., Gianni A. M. Human peripheral blood hematopoietic progenitors are optimal targets of retroviral-mediated gene transfer. Blood. 1992 Sep 15;80(6):1418–1422. [PubMed] [Google Scholar]
- Carter R. F., Abrams-Ogg A. C., Dick J. E., Kruth S. A., Valli V. E., Kamel-Reid S., Dubé I. D. Autologous transplantation of canine long-term marrow culture cells genetically marked by retroviral vectors. Blood. 1992 Jan 15;79(2):356–364. [PubMed] [Google Scholar]
- Carter R. F., Kruth S. A., Valli V. E., Dubé I. D. Long-term culture of canine marrow: cytogenetic evaluation of purging of lymphoma and leukemia. Exp Hematol. 1990 Oct;18(9):995–1001. [PubMed] [Google Scholar]
- Cashman J., Eaves A. C., Eaves C. J. Regulated proliferation of primitive hematopoietic progenitor cells in long-term human marrow cultures. Blood. 1985 Oct;66(4):1002–1005. [PubMed] [Google Scholar]
- Collins R. H., Jr, Anastasi J., Terstappen L. W., Nikaein A., Feng J., Fay J. W., Klintmalm G., Stone M. J. Brief report: donor-derived long-term multilineage hematopoiesis in a liver-transplant recipient. N Engl J Med. 1993 Mar 18;328(11):762–765. doi: 10.1056/NEJM199303183281104. [DOI] [PubMed] [Google Scholar]
- Cornetta K., Wieder R., Anderson W. F. Gene transfer into primates and prospects for gene therapy in humans. Prog Nucleic Acid Res Mol Biol. 1989;36:311–322. doi: 10.1016/s0079-6603(08)60179-8. [DOI] [PubMed] [Google Scholar]
- Cournoyer D., Scarpa M., Mitani K., Moore K. A., Markowitz D., Bank A., Belmont J. W., Caskey C. T. Gene transfer of adenosine deaminase into primitive human hematopoietic progenitor cells. Hum Gene Ther. 1991 Fall;2(3):203–213. doi: 10.1089/hum.1991.2.3-203. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Dick J. E., Kamel-Reid S., Murdoch B., Doedens M. Gene transfer into normal human hematopoietic cells using in vitro and in vivo assays. Blood. 1991 Aug 1;78(3):624–634. [PubMed] [Google Scholar]
- Fraser C. C., Szilvassy S. J., Eaves C. J., Humphries R. K. Proliferation of totipotent hematopoietic stem cells in vitro with retention of long-term competitive in vivo reconstituting ability. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1968–1972. doi: 10.1073/pnas.89.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E. Competitive repopulation in unirradiated normal recipients. Blood. 1993 May 15;81(10):2473–2474. [PubMed] [Google Scholar]
- Harrison D. E., Stone M., Astle C. M. Effects of transplantation on the primitive immunohematopoietic stem cell. J Exp Med. 1990 Aug 1;172(2):431–437. doi: 10.1084/jem.172.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P. F., Thacker J. D., Hogge D., Sutherland H. J., Thomas T. E., Lansdorp P. M., Eaves C. J., Humphries R. K. Retroviral gene transfer to primitive normal and leukemic hematopoietic cells using clinically applicable procedures. J Clin Invest. 1992 Jun;89(6):1817–1824. doi: 10.1172/JCI115786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff P. W., Flake A. W., Eglitis M. A., Scharf S., Bond S., Gilboa E., Erlich H., Harrison M. R., Zanjani E. D., Anderson W. F. In utero gene transfer and expression: a sheep transplantation model. Blood. 1989 Mar;73(4):1066–1073. [PubMed] [Google Scholar]
- Kantoff P. W., Gillio A. P., McLachlin J. R., Bordignon C., Eglitis M. A., Kernan N. A., Moen R. C., Kohn D. B., Yu S. F., Karson E. Expression of human adenosine deaminase in nonhuman primates after retrovirus-mediated gene transfer. J Exp Med. 1987 Jul 1;166(1):219–234. doi: 10.1084/jem.166.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Lothrop C. D., Jr, al-Lebban Z. S., Niemeyer G. P., Jones J. B., Peterson M. G., Smith J. R., Baker H. J., Morgan R. A., Eglitis M. A., Anderson W. F. Expression of a foreign gene in cats reconstituted with retroviral vector infected autologous bone marrow. Blood. 1991 Jul 1;78(1):237–245. [PubMed] [Google Scholar]
- Mitani K., Wakamiya M., Caskey C. T. Long-term expression of retroviral-transduced adenosine deaminase in human primitive hematopoietic progenitors. Hum Gene Ther. 1993 Feb;4(1):9–16. doi: 10.1089/hum.1993.4.1-9. [DOI] [PubMed] [Google Scholar]
- Moore K. A., Deisseroth A. B., Reading C. L., Williams D. E., Belmont J. W. Stromal support enhances cell-free retroviral vector transduction of human bone marrow long-term culture-initiating cells. Blood. 1992 Mar 15;79(6):1393–1399. [PubMed] [Google Scholar]
- Nolta J. A., Kohn D. B. Comparison of the effects of growth factors on retroviral vector-mediated gene transfer and the proliferative status of human hematopoietic progenitor cells. Hum Gene Ther. 1990 Fall;1(3):257–268. doi: 10.1089/hum.1990.1.3-257. [DOI] [PubMed] [Google Scholar]
- Petz L. D., Yam P., Wallace R. B., Stock A. D., de Lange G., Knowlton R. G., Brown V. A., Donis-Keller H., Hill L. R., Forman S. J. Mixed hematopoietic chimerism following bone marrow transplantation for hematologic malignancies. Blood. 1987 Nov;70(5):1331–1337. [PubMed] [Google Scholar]
- Scarpa M., Cournoyer D., Muzny D. M., Moore K. A., Belmont J. W., Caskey C. T. Characterization of recombinant helper retroviruses from Moloney-based vectors in ecotropic and amphotropic packaging cell lines. Virology. 1991 Feb;180(2):849–852. doi: 10.1016/0042-6822(91)90105-k. [DOI] [PubMed] [Google Scholar]
- Schuening F. G., Kawahara K., Miller A. D., To R., Goehle S., Stewart D., Mullally K., Fisher L., Graham T. C., Appelbaum F. R. Retrovirus-mediated gene transduction into long-term repopulating marrow cells of dogs. Blood. 1991 Nov 15;78(10):2568–2576. [PubMed] [Google Scholar]
- Smith L. G., Weissman I. L., Heimfeld S. Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain L. M., Mulligan R. C. Purification and characterization of retrovirally transduced hematopoietic stem cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3790–3794. doi: 10.1073/pnas.89.9.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead R. B., Kwok W. W., Storb R., Miller A. D. Canine model for gene therapy: inefficient gene expression in dogs reconstituted with autologous marrow infected with retroviral vectors. Blood. 1988 Mar;71(3):742–747. [PubMed] [Google Scholar]
- Stewart F. M., Crittenden R. B., Lowry P. A., Pearson-White S., Quesenberry P. J. Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood. 1993 May 15;81(10):2566–2571. [PubMed] [Google Scholar]
- Stoeckert C. J., Jr, Nicolaides N. C., Haines K. M., Surrey S., Bayever E. Retroviral transfer of genes into erythroid progenitors derived from human peripheral blood. Exp Hematol. 1990 Dec;18(11):1164–1170. [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Eaves A. C., Dragowska W., Lansdorp P. M. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989 Oct;74(5):1563–1570. [PubMed] [Google Scholar]
- Varmus H. E., Padgett T., Heasley S., Simon G., Bishop J. M. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977 Jun;11(2):307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]
- Wieder R., Cornetta K., Kessler S. W., Anderson W. F. Increased efficiency of retroviral-mediated gene transfer and expression in primate bone marrow progenitors after 5-fluorouracil-induced hematopoietic suppression and recovery. Blood. 1991 Feb 1;77(3):448–455. [PubMed] [Google Scholar]
- Wu D. D., Keating A. Hematopoietic stem cells engraft in untreated transplant recipients. Exp Hematol. 1993 Feb;21(2):251–256. [PubMed] [Google Scholar]
- van Beusechem V. W., Kukler A., Heidt P. J., Valerio D. Long-term expression of human adenosine deaminase in rhesus monkeys transplanted with retrovirus-infected bone-marrow cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7640–7644. doi: 10.1073/pnas.89.16.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]