Abstract
Purpose
Epidemiological and biological evidence suggest a preventative effect of selenium and vitamin E on bladder cancer. We assessed the effect of selenium and/or vitamin E on bladder cancer development.
Materials and Methods
This was a secondary analysis of the randomized, placebo-controlled SELECT (Selenium and Vitamin E Cancer Prevention Trial), which included 34,887 men randomly assigned to 4 groups (selenium, vitamin E, selenium plus vitamin E and placebo) in a double-blind fashion between August 22, 2001, and June 24, 2004. The primary end point was bladder cancer incidence, as determined by routine clinical management.
Results
During a median follow-up of 7.1 years (IQR 6.4 – 8.0 years), 224 bladder cancer cases were recorded. Patients with bladder cancer were older, and more likely to be white and have a smoking history than those without bladder cancer. Most cancers were urothelial and non-muscle invasive. There were no significant difference in the bladder cancer incidence between the 53 men in the placebo group and the 56 in the vitamin E group (HR 1.05, IRQ 0.64 – 1.73, p = 0.79), the 60 in the selenium group (HR 1.13, 0.70 – 1.84, p = 0.52) or the 55 in the vitamin E plus selenium group (HR 1.05, 0.63 – 1.70, p = 0.86).
Conclusions
This secondary analysis showed no preventive effect of selenium or vitamin E alone or combined on bladder cancer in this population of men. Further studies are needed to assess the effect in women, and at different doses and formulations.
INTRODUCTION
Bladder cancer represents an important cause of morbidity and mortality. In 2010 it was again the second most common genitourinary cancer in the United States with an established 70,530 new cases and 14,680 deaths.(1) Currently it is estimated that more than 500,000 men and women in the United States have a history of bladder cancer. The etiology of most bladder urothelial carcinoma is associated with tobacco exposure, occupational exposures to aromatic amines and exposure to the chemical and rubber industries.(2–4)Bladder cancer is the most expensive cancer in the United States, accounting for almost $3.7 billion (2001 value) in direct costs.(5)
There is substantial epidemiological and biological evidence that selenium and vitamin E may prevent bladder cancer. A recent meta-analysis of 7 published epidemiologic studies, including 3 case-control, 3 nested case-control and 1 case-cohort series, examined the association between selenium levels and bladder cancer.(6) The total number of cases and controls/cohort members in the identified studies was 1,910 and 17,339, respectively.(7–13) Selenium status was based on analysis of toenails and serum in 4 and 3 studies, respectively. This meta-analysis showed that the overall risk of bladder cancer was inversely associated with increased selenium according to a random-effects model (mortality OR 0.61, 95% CI 0.42–0.87). The mortality OR was 0.95 (95% CI 0.69–1.27) in men and 0.55 (95% CI 0.32–0.95) in women. In the analysis stratified by gender only women showed a significantly decreased risk associated with selenium (OR 0.55, 95% CI 0.32–0.95). An opposite gender pattern, with protective effects in men but not in women, was reported in a meta-analysis of selenium supplementation, primary cancer incidence and mortality.(14)
Evidence for the impact of vitamin E on the bladder cancer incidence is also contradictory. The association between individual vitamin C and vitamin E supplements, and bladder cancer mortality among 991,522 American adults in the Cancer Prevention Study II cohort showed that regular vitamin E supplement use for 10 years or greater was associated with a decreased risk of bladder cancer mortality (RR 0.60, 95% CI 0.37–0.96) while regular use of shorter duration was not (RR 1.04, 95% CI 0.77 – 1.40). (15) On the other hand, the Danish Diet, Cancer and Health Study, comprising 55,557 men and women 50 to 64 years old at study inclusion with no previous cancer diagnosis, used a detailed food frequency questionnaire with information on the consumption of vitamins C and E, folate and β-carotene from diet and supplements.(16) During a median of 10.6 years of followup bladder cancer was diagnosed in 322 participants, and vitamins C and E, and folate showed no association with bladder cancer regardless of source. A case-control study of plasma α-tocopherol demonstrated that mean plasma α-tocopherol was significantly lower in cases than in controls (23.93 vs. 27.48 µg/ml, p < 0.001). (17) A significant decrease in bladder cancer risk was associated with increasing plasma α-tocopherol (adjusted OR 0.91, 95% CI 0.85–0.97).
We determined whether selenium and/or vitamin E would prevent bladder cancer. Towards this goal we used data from the randomized, placebo-controlled SELECT on 34,887 men from a total of 427 participating sites in the United States, Canada, and Puerto Rico (18) to determine if there were differences in the bladder cancer incidence. Study participants were randomly assigned to 4 groups (selenium, vitamin E, selenium plus vitamin E, and placebo) in a double-blind fashion. Participants received oral selenium (200 µg daily from L-selenomethionine) and matched vitamin E placebo, vitamin E (400 IU daily of all- rac-α-tocopheryl acetate) and matched selenium placebo, selenium plus vitamin E or placebo plus placebo for a planned followup of a minimum of 7 years and a maximum of 12.
The SELECT design is suitable for this secondary analysis due to randomized, controlled nature of the study, which included a large number of older men, and former (48%) and current (8%) smokers. This is also a unique opportunity to evaluate the impact of selenium and vitamin E on the bladder cancer incidence since a bladder cancer trial may not be feasible due to the obstacles involved with performing a prospective, randomized trial in a large number of subjects.
METHODS
SELECT was a phase 3 randomized, placebo-controlled trial of selenium (200 µg daily from L-selenomethionine) and/or vitamin E (400 IU daily of all-rac-α-tocopherylacetate) for prostate cancer prevention with a planned minimum and maximum followup of 7and 12 years, respectively.(18) Major study eligibility requirements were age 50 years or greater for black men and 55 years or greater for all other men, no prior prostate cancer diagnosis, serum prostate specific antigen 4 ng/ml or less and digital rectal examination not suspicious for cancer. Also required was no current anticoagulant therapy other than daily 175 mg or less of acetylsalicylic acid or 81 mg or less of acetylsalicylic acid with clopidogrel bisulfate, no history of hemorrhagic stroke and normal blood pressure due to the antiplatelet effects of vitamin E and the related findings of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (http://atbcstudy.cancer.gov/).
Participant characteristics were based on self-report, including self-identification of race and ethnicity, which were defined according to the United States Census Bureau. All potentially eligible men were required to provide written informed consent before being allowed to participate in the trial. The local institutional review board at each study site approved the study for activation and reviewed its progress annually. The trial was activated in July 2001 and followup blinded to trial results ended on October 23, 2008. This report includes data as of July 5, 2011.
Participants without prostate cancer visited the clinic once every 6 months throughout the trial while those with prostate cancer visited it annually. Adherence and adverse events were monitored every 6 months. Limited physical examination, including assessments of blood pressure, weight and smoking status, was done annually. A formal pre-randomization period (28–90 days with no placebo run-in capsules allowed potential participants time to decide whether they would agree to stop any disallowed over-the-counter selenium or vitamin E supplements throughout the study and show willingness to adhere the trial by returning for randomization. Adherence during the trial was facilitated by the availability of a multivitamin containing no vitamin E or selenium.
Bladder Cancer Ascertainment
At each 6-month and annual visit, participants were asked by the local study site staff about new cancer diagnoses. Source documentation to verify the diagnosis was requested and submitted to the statistical center. Only patients with pathology reports confirming a bladder cancer diagnosis were included in analysis. Source documents were reviewed by two of us (YL and RFY) and relevant data were abstracted. There was no central pathology confirmation of the diagnosis and grade.
Analysis
The end point of analysis was bladder cancer incidence, as determined by routine clinical management. SELECT was designed as a 4 group trial with 5 prespecified comparisons, including selenium vs placebo, vitamin E vs placebo, selenium plus vitamin E vs placebo, selenium vs selenium plus vitamin E and vitamin E vs selenium plus vitamin E. This was a secondary analysis of the overall trial, in which prostate cancer prevention was the primary analysis. While overall cancer rate was a preplanned analysis, the bladder cancer incidence was not. However, the medical records to support this endpoint were collected in a prospective manner.
The diagnosis of any bladder cancer regardless of stage or grade was the outcome of interest. Bladder cancer stage and grade were based on data abstraction from primary source documentation. The baseline characteristics evaluated were age, race (white, black, Hispanic or other), education (less than a high school graduate, high school graduate, some vocational school or some college, college graduate or post-college education) and smoking status (never, former, current). Body mass index was categorized as normal—less than 25, overweight—25.1 to 30, obese—30.1 to 40 and morbidly obese—greater than 40 kg/m2.
Since this was a time-to-event analysis, statistical significance of the differences in the baseline characteristics between men who were and were not subsequently diagnosed with bladder cancer versus those who were not were calculated based on the Cox proportional hazard model. The model was parameterized using age (continuous), race (white vs other), education (high school or less vs some vocational/college vs college graduate vs post-college education), smoking (never vs former vs current) and BMI (continuous). Missing values were excluded from Cox models.
For the cumulative incidence curve and Cox models men were censored at the last follow-up or death. Recent results showed an unexpected increase in prostate cancer. Thus, we will report 2-sided p values since the comparison of risk vs benefit is a 2-sided question and we used 99% CI due to multiple comparisons. Using the CI allowed us to rule out the benefit or risk of a specific magnitude.
RESULTS
A total of 235 participants had a diagnosis of bladder cancer that was reported to the statistical center. Source documentation was obtained on 229 cases. Upon review 3 patients were excluded from analysis because they were misreported as bladder cancer, and 2 were excluded due to insufficient source documentation to confirm the diagnosis, leaving 224 bladder cancer among the 34,887 eligible, evaluable SELECT participants.(18) Of the 35,533 men who were randomized 646 were excluded from all analyses, including 10 due to prior diagnosis of prostate cancer (in another the diagnosis was discovered after primary publication) and 15 who never received proper informed consent, while all 621 participants from 2 clinical sites were excluded due to severe data and participant management problems.
Table 1 lists the characteristics of the 224 men with and the 34,663 without bladder cancer. Median age in the men with and without cancer, and in evaluable, eligible men in SELECT was 67 (IQR 62–72), 67 (58–72) and 62 years (IQR 58–67), respectively (p < 0.001). Mean ± SD body mass index was 28.6 ± 4.5 kg/m2.
Table 1.
Baseline characteristics of SELECT patients with and without bladder cancer
|
No. SELECT (%) |
No. No Bladder Ca (%) |
No. Bladder Ca (%) |
p Value | |
|---|---|---|---|---|
| Age: | <0.001 | |||
| 50 – 54 | 1,480 ( 4.2) | 1,476 (4.3) | 4 ( 1.8) | |
| 55 – 59 | 11,172 (32.0) | 1,144 (32.2) | 28 (12.5) | |
| 60 – 64 | 9,174 (26.3) | 9,124 (26.3) | 50 (22.3) | |
| 65 – 69 | 6,859 (19.7) | 6,799 (19.3) | 60 (26.8) | |
| 70 – 74 | 3,950 (11.3) | 3,903 (11.3) | 47 (21.0) | |
| 75 + | 2,252 ( 6.5) | 2,217 (6.4) | 35 (15.6) | |
| Race: | <0.001 | |||
| White | 27,571 (79.0) | 27,367 (79.0) | 204 (91.0) | |
| Black, nonHispanic | 4,318 (12.4) | 4,304 (12.4) | 14 (6.3) | |
| Hispanic, nonblack | 1,940 (5.6) | 1,939 (5.6) | 1 (0.5) | |
| Hispanic, black | 361 (1.0) | 359 (1.0) | 2 (1.0) | |
| Other | 697 (2.0) | 694 (2.0) | 3 (1.5) | |
| Education: | <0.001 | |||
| < High school graduate | 2,339 (6.7) | 2,323 (6.7) | 16 (7.1) | |
| High school graduate | 5,343 (15.3) | 5,297 (15.3) | 46 (20.5) | |
| Vocational/some college | 9,355 (26.8) | 9,281 (26.8) | 74 (33.0) | |
| College graduate | 6,605 (18.9) | 6,575 (19.0) | 30 (13.4) | |
| Post-college | 10,910 (31.3) | 10,853 (31.1) | 57 (25.5) | |
| Missing | 335 (1.0) | 334 (1.0) | 1 (0.5) | |
| Smoking: | <0.001 | |||
| Never | 15,110 (43.3) | 15,056 (43.4) | 54 (24.1) | |
| Current | 2,700 (7.6) | 16,899 (48.8) | 16 (7.1) | |
| Former | 17,053 (49.0) | 2,684 (7.7) | 154 (68.8) | |
| Missing | 25 (0.1) | 24 (0.1) | 0 | 0.87 |
| Body mass index: | ||||
| Normal | 7,104 (20.4) | 7,058 (20.4) | 46 (20.5) | |
| Overweight | 16,718 (47.9) | 16,614 (47.9) | 104 (46.4) | |
| Obese | 10,066 (28.9) | 9,999 (28.9) | 67 (29.9) | |
| Morbidly obese | 810 (2.3) | 804 (2.3) | 6 (2.7) | |
| Missing | 189 (0.5) | 188 (0.5) | 1 (0.5) |
Median followup for men in this analysis was 7.1 years, including a median of 6.0 years under active intervention and an additional 1.1 year of followup. In the placebo group median followup was 7.0 years (IQR 6.1, 8.0) while in the vitamin E, selenium and combination groups it was 7.0 years (IQR 6.0, 8.0).
Men with bladder cancer were older than those without bladder cancer and more likely to be white and former smokers. Most cancers were urothelial and nonmuscle invasive (table 2).
Table 2.
Bladder cancers detected
| No. Placebo |
No. Vitamin E |
No. Selenium |
No. Combination |
|
|---|---|---|---|---|
| Stage: | ||||
| CIS | 3 | 4 | 4 | 3 |
| Ta | 32 | 26 | 30 | 36 |
| T1 | 10 | 18 | 19 | 7 |
| Greater than T1 | 6 | 6 | 6 | 7 |
| Unknown | 2 | 2 | 1 | 2 |
| Grade: | ||||
| Well differentiated | 21 | 18 | 23 | 29 |
| Moderately differentiated | 9 | 7 | 10 | 5 |
| Poorly differentiated | 22 | 29 | 26 | 19 |
| Unknown | 1 | 2 | 1 | 2 |
| N-stage: | ||||
| N0 | 3 | 2 | 3 | 5 |
| N1 | 3 | 2 | 1 | 0 |
| NX | 47 | 52 | 56 | 50 |
| M-stage: | ||||
| M0 | 2 | 0 | 3 | 3 |
| M1 | 3 | 3 | 0 | 1 |
| MX | 48 | 53 | 57 | 51 |
| Histology: | ||||
| Urothelial cell | 51 | 53 | 59 | 50 |
| Squamous cell | 1 | 0 | 1 | 0 |
| Adenoca | 0 | 0 | 0 | 1 |
| Small cell | 0 | 1 | 0 | 0 |
| Other/unknown | 1 | 2 | 0 | 4 |
| Death cause: | ||||
| Bladder Ca | 6 | 6 | 3 | 3 |
| Other | 5 | 3 | 4 | 5 |
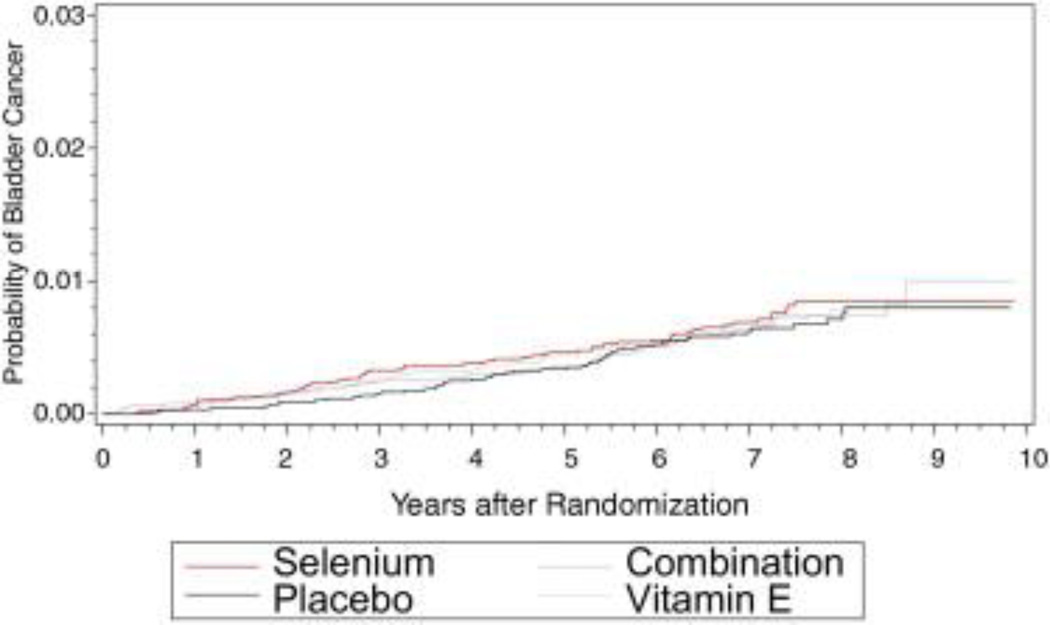
Table 2 lists cancers by treatment arm. There were 53, 56, 60 and 55 bladder cancers in the placebo, vitamin E, selenium and combination arms, respectively. Bladder cancer developed in a similar distribution of smokers and nonsmokers in the different study arms. The bladder cancer rate was higher in the 3 treatment arms than in the placebo arm but no difference was statistically significant (vitamin E HR 1.06, 99% CI 0.72 – 1.53, selenium HR 1.13, 99% CI 0.78–1.63, vitamin E plus selenium HR 1.05, 99% CI 0.71 – 1.51). While the number of bladder cancers was relatively small and differences were not statistically significant, we ruled out the potential for a 25% decrease in the HR of the 3 treatment arms compared to the placebo arm (each treatment arm p < 0.05). However, we could not rule out the potential of a 25% decrease at p < 0.01. Figures 1 to 4 show the cumulative incidence of bladder cancer in men in the placebo, selenium, vitamin E and combined arms.
Figure 1.
Bladder cancer cumulative incidence in placebo, selenium, vitamin E and combined arms.
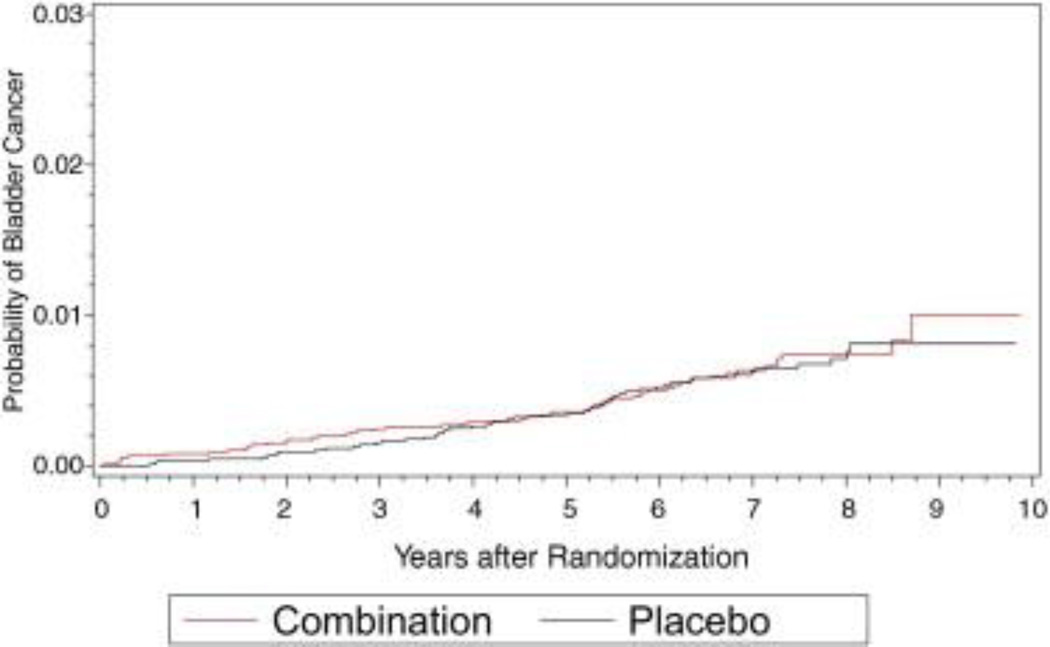
Figure 4.
Bladder cancer cumulative incidence in placebo and combined arms.
DISCUSSION
Significant cost and effort are needed to perform large, prospective, randomized trials. As such, it is important to gather as much information as possible from these trials. This secondary analysis of SELECT showed no benefit from selenium or vitamin E independently or combined to decrease the bladder cancer incidence. While a modest number of men were diagnosed with incident bladder cancer during the course of the trial, we could still rule out the possibility 25% or greater decrease in the bladder cancer incidence. That is, neither vitamin E nor selenium decreased the bladder cancer rate compared by 25% or more compared to placebo.
The results of this analysis are disappointing, considering previous evidence that increased selenium and vitamin E were inversely associated with the bladder cancer risk.(6) Notably a limitation of this analysis is that we did not analyze baseline plasma or toenail selenium or vitamin E in men with and without bladder cancer. To our knowledge, it is unknown whether or not supplementation with these agents would be effective in those with low baseline levels. As such, we could not determine definitively that selenium and vitamin E cannot prevent bladder cancer.
A future study of baseline plasma selenium and vitamin E, and bladder cancer is being discussed and planned using the samples from the SELECT biorepository. At this time one could argue that a large-scale prevention study should not be done without determination whether the intervention would have a high likelihood of success.
Another consideration is that SELECT was limited to men. In a recent meta-analysis of the association of selenium and bladder cancer outcome were stratified by gender and only women showed a significantly decreased risk associated with selenium.(6) If this meta-analysis is accurate, an association with selenium may not have not found because SELECT was limited to men. On the other hand, a separate meta-analysis of selenium supplementation indicated an opposite gender pattern with protective effects in men and not in women.(14)
In the current analysis men with bladder cancer were older and more likely to be white and smokers. This is consistent with prior epidemiologic studies. The etiology of most urothelial carcinoma of the bladder is associated with tobacco exposure and tobacco is the largest contributor to disease.(2,3) At this time, smoking cessation is the most reliable strategy to prevent bladder cancer.
Also, supplements can lead to detrimental side effects. A recent update of SELECT showed an increased risk of prostate cancer in the vitamin E group.(19) Another recent relevant series was a multicenter study in which patients with nonmuscle invasive bladder cancer were prospectively randomized to bacillus Calmette-Guérin alone vs bacillus Calmette-Guérin plus interferon α-2b and megadose vitamins, including vitamin E.(20) This study showed no benefit to nutritional supplements, including vitamin E, for reducing bladder cancer recurrence.
Our study has various limitations. This was a secondary analysis of a trial that did not focus on men at high risk for bladder cancer. Only 18% of the patients were in the peak age range for bladder cancer and the study was limited to men. The protective benefit of selenium in the meta-analysis of Amaral et al was seen only in women, (6) which was hypothesized to be due to gender specific differences in selenium metabolism.
Other potential limitations resulted from this secondary analysis. There was no way to control for all risk factors for bladder cancer, smoking duration and extent, or environmental or occupational exposure. It is also unclear whether the 1 year off supplements had an impact on the study outcome.
CONCLUSIONS
This secondary analysis of the SELECT showed no benefit from selenium or vitamin E independently or combined for decreasing the bladder cancer incidence. Since selenium was not measured, there may possibly be a relationship between selenium and the bladder cancer incidence. Furthermore, this secondary analysis was limited to men.
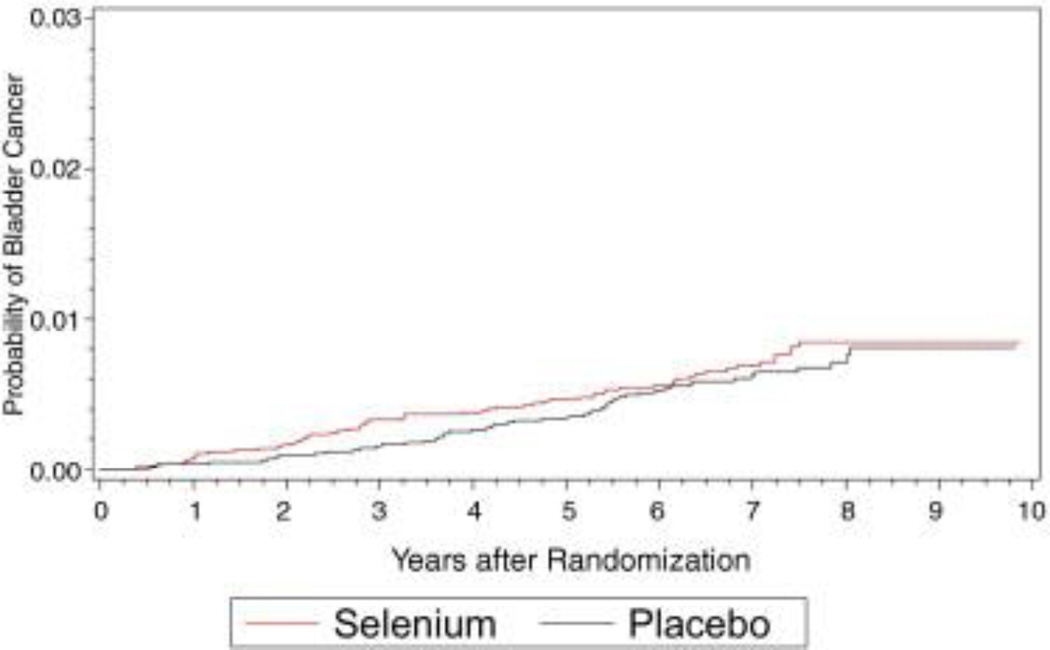
Figure 2.
Bladder cancer cumulative incidence in placebo and selenium arms.
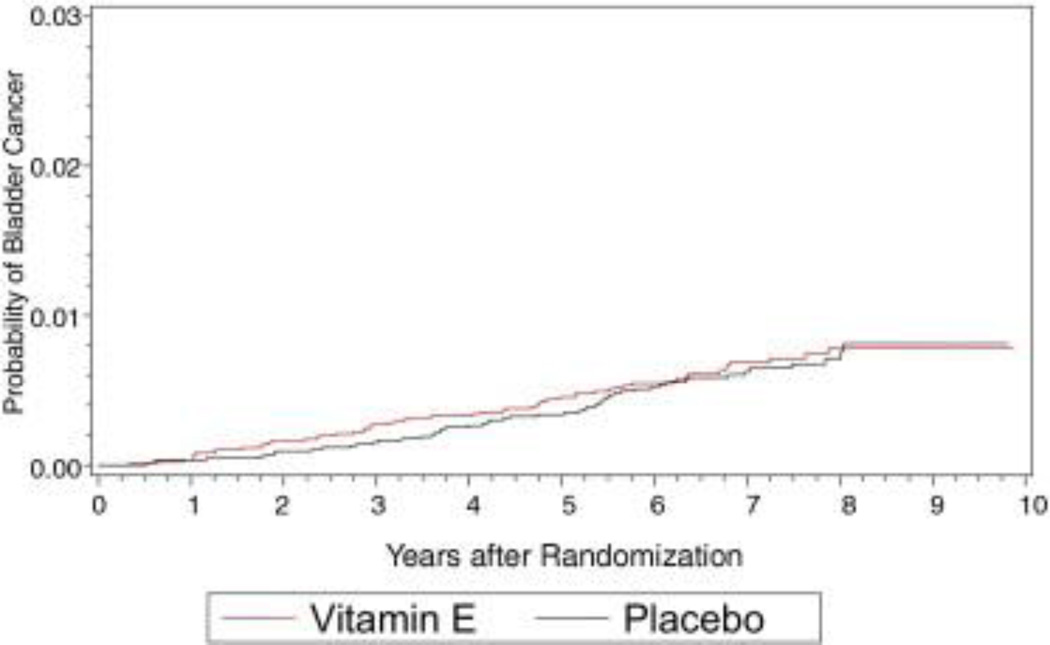
Figure 3.
Bladder cancer cumulative incidence in placebo and vitamin E arms.
ACKNOWLEDGMENTS
Study agents and packaging were provided by Perrigo Co., Allegan, Michigan; Sabinsa Corp., Piscataway and DSM Nutritional Products, Parsippany, New Jersey; and Tishcon Corp., Westbury, New York.
Supported by Public Health Service Cooperative Agreement Grant CA37429 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and the National Center for Complementary and Alternative Medicine, National Institutes of Health.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010 Sep–2010 Oct 31. 60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Silverman DT, Hartge P, Morrison AS, Devesa SS. Epidemiology of bladder cancer. Hematol Oncol Clin North Am. 1992 Feb;6(1):1–30. [PubMed] [Google Scholar]
- 3.Zeegers MP, Tan FE, Dorant E, van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000 Aug 1;89(3):630–639. doi: 10.1002/1097-0142(20000801)89:3<630::aid-cncr19>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Samanic C, Kogevinas M, Dosemeci M, Malats N, Real FX, Garcia-Closas M, Serra C, Carrato A, Garcia-Closas R, Sala M, et al. Smoking and bladder cancer in Spain: effects of tobacco type, timing, environmental tobacco smoke, and gender. Cancer Epidemiol Biomarkers Prev. 2006 Jul;15(7):1348–1354. doi: 10.1158/1055-9965.EPI-06-0021. [DOI] [PubMed] [Google Scholar]
- 5.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21(18):1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 6.Amaral AF, Cantor KP, Silverman DT, Malats N. Selenium and bladder cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010 Sep;19(9):2407–2415. doi: 10.1158/1055-9965.EPI-10-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nomura A, Heilbrun LK, Morris JS, Stemmermann GN. Serum selenium and the risk of cancer, by specific sites: case-control analysis of prospective data. J Natl Cancer Inst. 1987 Jul;79(1):103–108. [PubMed] [Google Scholar]
- 8.Helzlsouer KJ, Comstock GW, Morris JS. Selenium, lycopene, alpha-tocopherol, beta-carotene, retinol, and subsequent bladder cancer. Cancer Res. 1989 Nov 1;49(21):6144–6148. [PubMed] [Google Scholar]
- 9.Kellen E, Zeegers M, Buntinx F. Selenium is inversely associated with bladder cancer risk: a report from the Belgian case-control study on bladder cancer. Int J Urol. 2006 Sep;13(9):1180–1184. doi: 10.1111/j.1442-2042.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 10.Michaud DS, De Vivo I, Morris JS, Giovannucci E. Toenail selenium concentrations and bladder cancer risk in women and men. Br J Cancer. 2005 Oct 3;93(7):804–806. doi: 10.1038/sj.bjc.6602788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michaud DS, Hartman TJ, Taylor PR, Pietinen P, Alfthan G, Virtamo J, Albanes D. No Association between toenail selenium levels and bladder cancer risk. Cancer Epidemiol Biomarkers Prev. 2002 Nov;11(11):1505–1506. [PubMed] [Google Scholar]
- 12.Zeegers MP, Goldbohm RA, Bode P, van den Brandt PA. Prediagnostic toenail selenium and risk of bladder cancer. Cancer Epidemiol Biomarkers Prev. 2002 Nov;11(11):1292–1297. [PubMed] [Google Scholar]
- 13.Wallace K, Kelsey KT, Schned A, Morris JS, Andrew AS, Karagas MR. Selenium and risk of bladder cancer: a population-based case-control study. Cancer Prev Res (Phila) 2009 Jan;2(1):70–73. doi: 10.1158/1940-6207.CAPR-08-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardia A, Tleyjeh IM, Cerhan JR, Sood AK, Limburg PJ, Erwin PJ, Montori VM. Efficacy of antioxidant supplementation in reducing primary cancer incidence and mortality: systematic review and meta-analysis. Mayo Clin Proc. 2008 Jan;83(1):23–34. doi: 10.4065/83.1.23. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs EJ, Henion AK, Briggs PJ, Connell CJ, McCullough ML, Jonas CR, Rodriguez C, Calle EE, Thun MJ. Vitamin C and vitamin E supplement use and bladder cancer mortality in a large cohort of US men and women. Am J Epidemiol. 2002 Dec 1;156(11):1002–1010. doi: 10.1093/aje/kwf147. [DOI] [PubMed] [Google Scholar]
- 16.Roswall N, Olsen A, Christensen J, Dragsted LO, Overvad K, Tjonneland A. Micronutrient intake and risk of urothelial carcinoma in a prospective Danish cohort. Eur Urol. 2009 Nov;56(5):764–770. doi: 10.1016/j.eururo.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Liang D, Lin J, Grossman HB, Ma J, Wei B, Dinney CP, Wu X. Plasma vitamins E and A and risk of bladder cancer: a case-control analysis. Cancer Causes Control. 2008 Nov;19(9):981–992. doi: 10.1007/s10552-008-9165-2. [DOI] [PubMed] [Google Scholar]
- 18.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009 Jan 7;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011 Oct 12;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nepple KG, Lightfoot AJ, Rosevear HM, O'Donnell MA, Lamm DL Bladder Cancer Genitourinary Oncology Study Group. Bacillus Calmette-Guérin with or without interferon α-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. J Urol. 2010 Nov;184(5):1915–1919. doi: 10.1016/j.juro.2010.06.147. Epub 2010 Sep 17. [DOI] [PubMed] [Google Scholar]