Abstract
Background
Functional defects in mitochondria are involved in the induction of cell death in cancer cells. The process of programmed cell death may occur through the mechanisms of apoptosis. Several potential lead molecules such as Camptothecin (CPT) and its analogues have been isolated from plants with anticancer effect. The aim of the present study was to understand the necrotic effect versus apoptotic nature of CPT in HeLa cancer cells.
Methods
The anti-proliferative activity of CPT was estimated through 3-(4, 5- Dimethyl Thiazol-2-yl)-2, 5-diphenyl Tetrazolium bromide (MTT) assay and DNA fragmentation analysis using gel electrophoresis. Lactate Dehydrogenase (LDH) activity and cell morphology were assessed under control and CPT exposed conditions to evaluate the necrotic effect of CPT.
Results
The results showed that CPT inhibited the proliferation of HeLa cells in a dose-dependent manner with an Inhibitory Concentration 50% (IC50) of 0.08±0.012 µg/ml. However the significant (p<0.05) increase happens in LDH activity at concentrations 1×10-1µg/ml and above. Morphological changes showed that CPT in low concentrations induced an apoptotic mechanism of cell death, such as cell shrinkage and characteristic rounding of dying cells, while at high concentrations showed necrosis changes. The characteristic DNA ladder formation of CPT-treated cells in agarose gel electrophoresis confirmed the results obtained by light microscopy and LDH assay.
Conclusion
Camptothecin as an anticancer drug may have anti-proliferative effect on HeLa cancer cells in low concentrations, through its nature of induction of apoptosis. The border line between necrotic effect and apoptotic nature of CPT in HeLa cancer cells has been found to be at concentration of 1×10-1 µg/ml.
Keywords: Cell death, Camptothecin, HeLa cells, Necrosis, Apoptosis
Introduction
Apoptosis and necrosis represent two fundamental types of cell death [1-4]. Apoptosis is an important and active regulatory pathway of cell growth and proliferation in which cells respond to specific induction signals by initiating intracellular processes that result in characteristic physiological changes occurring over hours or days [5-10]. In contrast, necrosis occurs suddenly with mitochondrial and cellular swelling and ensuing plasma membrane disruption [11]. However, cell death, through necrosis or apoptosis mechanisms, is currently the subject of a considerable number of investigations [12-14].
Some natural and chemical compounds are said to be cytotoxic to cell that cause its death [3, 4]. In the early sixties, the discovery of Camptothecin (CPT) by Wall and Wani, as an anticancer drug with a unique mode of action, which is inhibition of DNA topoisomerase I, was an entirely new dimension in chemotherapy [15-17]. This natural alkaloid was first extracted from the stem wood of the Chinese ornamental tree Camptotheca acuminate [17-20]. There are many studies that confirmed anti-proliferative and cytotoxic effect of CPT in different cancer cells [17, 21-25].
Although CPT showed strong antitumor activity among cancer patients, it also caused unpredictable and severe adverse effects including myelosuppression, vomiting, diarrhoea, and severe haemorrhagic cystitis [17]. However, at present, CPT is not used as a drug of choice due to its severe toxicity effect in deferent cells. Thus, several groups have been tried to synthesize derivatives with a lower toxicity [26-29]. The determination of border line concentration between necrotic effect and apoptotic nature of CPT in cancer cells is not clear so we attempt in the present study to determine it.
Materials and Methods
Materials
Streptomycin, Penicillin, Ambistyrin, Tris- Acetate- Ethylenediaminetetraacetic acid (TAE) buffer, Trypsin, Phosphate Buffered Saline (PBS) and Sodium Dodecyl Sulphate were purchased from Himedia Laboratories Pvt. Ltd. (India). Dulbecco’s Modified Eagle Medium (DMEM) and 3-(4, 5- Dimethyl Thiazol-2-yl)-2, 5-diphenyl Tetrazolium bromide (MTT) were purchased from Sigma chemical company (St. Louis, MO. USA). The Fetal Bovine Serum (FBS) was also purchased from Gibco BRL (Life Technologies, Paisley, Scotland). Human cervical carcinoma HeLa cells were obtained from cell bank (Razi Vaccine and Serum Research Institute, Karaj, Iran). Camptothecin and Lactate Dehydrogenase (LDH) assay Kit were purchased from Sigma-Aldrich (USA).
Cell Culture and Drug Treatment
The HeLa cells (density=1×106 cells/ml) were cultured in DMEM, supplemented with 10% FBS, 50 U/ml penicillin and 5 mg/ml streptomycin, at an incubator setting of 5% CO2 and 37°C. The cultured cells were exposed to various concentrations of CPT (7×10-4 to 8×10-1 µg/ml) for 48 hrs. The medium of all wells of plate were exchanged with fresh medium and then MTT and Dimethyl Sulfoxide (DMSO) were added and absorbance of each well was read using ELISA plate reader [30, 31].
Assessment of Cytotoxicity
The effect of CPT on HeLa cells was determined with MTT (3-(4, 5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide) assay. Anticancer drugs/cytotoxic compounds damage the cancerous cells and change the mitochondrial activity. When tetrazolium salts come in contact with the cancerous cells, salts are taken up into the mitochondria due to its net positive charge and plasma membrane potential.
In mitochondria, these coloured tetrazolium salts get reduced to formazan dye by NADH+ dependent reaction catalysed by the Mitochondrial Succinate Dehydrogenase Enzymes (MSDE). As this conversion takes place only in the living cell mitochondria, the amount of formazan formed is proportional to the number of living cells. Quantification of viable cells is made possible by quantification of formazan at 570 nm by a spectrophotometer [32-35]. Cells were seeded in a 96-well flat-bottom plate (10,000 cells/well) and left to adhere for 24 hrs at 37°C with 5% CO2. Various concentrations of CPT were added and incubated for further 48 hrs and then MTT (5mg/ml) was added. The absorbance of each well was determined at 570 nm using an ELISA plate reader. The percentage of growth inhibition was calculated using the following formula [36, 37].
Growth inhibition (%) =100 - [OD of individual test group/ OD of control group] ×100
LDH Determination
LDH is a cytoplasmic enzyme retained by viable cells with intact plasma membranes, but it released from necrotic cells with damaged membranes. Cytotoxicity induced by CPT was also assessed by LDH leakage into the culture medium. Following exposure to CPT, the cells were harvested and LDH activity was assayed spectrophotometrically at 340 nm by LDH assay kit [38, 39].
Light Microscopy
HeLa cells (1×106 cells/ml) were cultured in the absence (control cells), and presence of CPT at concentrations 5×10-2 and 4×10-1 µg/ml for 48 hrs in DMEM medium supplemented with 10% FBS at 37°C. Morphological changes of treated cells were observed by invert microscopy and compared with control cells [40].
DNA Fragmentation Assay
For DNA fragmentation assay, 2×106 HeLa cells, 2μl trypsin and CPT at concentrations of 5×10-2 and 4×10-1µg/ml were added to the medium and final volume was adjusted to 2ml. The cells incubated at 37°C for 48 hrs, and they were then centrifuged at 10,000 rpm for 10mins. After discarding the medium, the cells were washed twice in NaCl-Tris- Ethylenediaminetetraacetic acid (NTE) buffer. The cells suspended in 2ml NTE buffer and 2% trypsin (100μg/ml), 20% Sodium Dodecyl Sulfate (SDS) (25μl/ml) and proteinase K (100μg/ml) were added. After overnight incubation at 37°C, saturated phenol and chloroform were added and after shaking the vials, they were centrifuged at 10,000 rpm for 10 min. Having transferred the upper portion to another vial and adding 1ml chloroform, it was repeated for 4 times. RNase was added and incubated at 35°C for 2 hrs. Finally it was centrifuged at 10,000 rpm for 10 min and decanted the solvent. The pellet (DNA) was dissolved in Tris-Acetate- Ethylenediaminetetraacetic acid (TAE) buffer and subjected to horizontal electrophoresis [41, 42].
Statistical Analysis
Values are expressed as means±SD of three repeats in each group. Data were analysed using students' t-test with a statistical significance of p<0.05.
Results
CPT Toxicity Against HeLa Cells
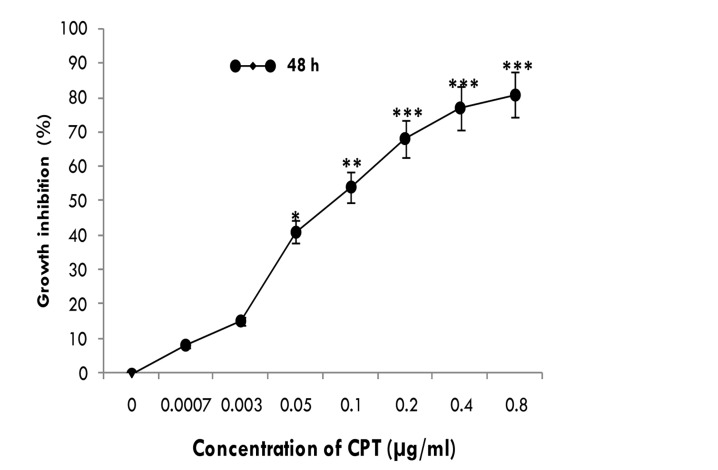
The inhibitory effects of CPT on the proliferation of HeLa cells were tested at different concentrations (7×10-4 to 8×10-1µg/ml) for 48 hrs using MTT assay and the IR% was determined (Figure1). Data analysis showed that the growth of HeLa cells was significantly (p<0.05) inhibited as compared to control cells (CPT-unexposed cells) in dose-dependent manner. The starting dose which inhibited the HeLa cells proliferation was 7×10-4 µg/ml, and nearly 80% of HeLa cells were inhibited by 8×10-1µg/ml CPT. The Inhibitory Concentration 50% (IC50) was found to be 0.08±0.012 µg/ml.
Figure 1.
<bold>Anti-proliferative effect of CPT on HeLa cells.</bold> Growth inhibition of HeLa cells exposed to CPT in concentrations ranging from 7×10-4 to 8×10-1 μg/ml at 48 hrs and measured by MTT assay. Data are mean ± SD from three independent determinations in triplicate. *p<0.05, **p<0.005 and ***p<0.001 were considered to be statistically significant, compared with values from cells incubated in the absence of CPT (control).
Lactate Dehydrogenase (LDH) Activity
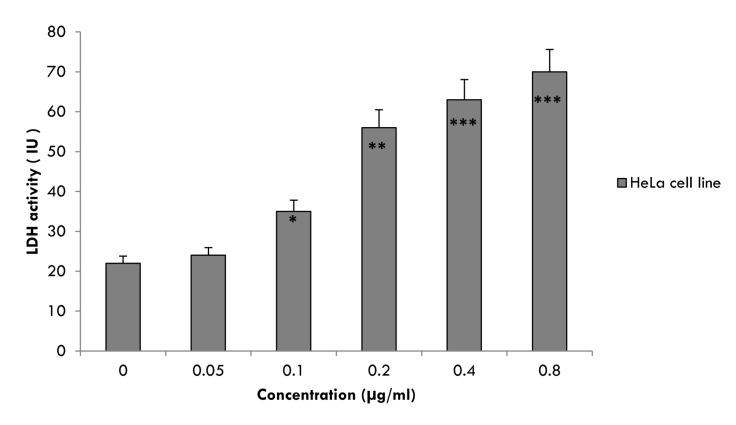
CPT-induced cell membrane damage was assessed by the LDH release assay. Cells were treated with CPT for 48 hrs resulted in a significant (p<0.05) increase in LDH release relative to the untreated cells (Figure 2). When CPT concentration increased to 1×10-1 µg/ml and above, the LDH activity in the cultured media increased significantly (p<0.05). However, CPT caused highly significant (p<0.005) increase in the activity of LDH enzyme at concentration of 2×10-1 µg/ml by 2.5 folds and at maximum concentration (8×10-1 µg/ml) by 3.2 folds as compared with unexposed cells. By contrast, treatment of HeLa cells with CPT at concentrations blow 1×10-1 µg/ml did not significantly increase LDH release (Figure 2).
Figure 2.
<bold>Lactate Dehydrogenase (LDH) activity.</bold> Activity of cytosolic enzyme Lactate Dehydrogenase (LDH) after treatment of HeLa cells in the presence and in the absence (control cells) of CPT at various concentrations of 5×10-2 to 8×10-1μg/ml. The cells were cultured in DMEM medium supplemented with 10% FBS for 48 hrs at 37°C. Data are mean±SD from three independent determinations in triplicate. *p<0.05, **p<0.005 and ***p<0.001 were considered to be statistically significant, compared with values from cells incubated in the absence of CPT (control).
Cell Morphology
To assess alterations of cell morphology, HeLa cells treated with CPT for 48 hrs and morphology of cells observed by invert microscopy and compared with untreated cells (control cells, Figure 3-A). Figure 3-B shows that the HeLa cells treated with CPT at 5×10-2 µg/ml were changed into round shapes as compared to untreated HeLa cells (Figure 3-A). The untreated cells also showed a high confluency of monolayer cells (Figure 3-A) compared to CPT-treated cells (Figure 3-B and C), which Figure 3-B showed a reduction in cell volume and cell shrinkage that finally resulted in generation of apoptotic bodies. As shown in Figure 3-C, significant morphological changes were observed at concentration of 4×10-1µg/ml after CPT exposure, making features of necrosis such as loss of membrane integrity, no vesicle formation and complete lysis as compared to control cells (Figure 3-A).
Figure 3.
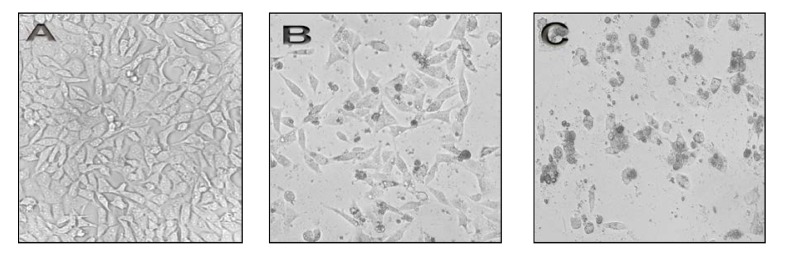
<bold>Effect of CPT on morphology of HeLa cells.</bold> HeLa cells (1×106 cells/ml) were cultured in DMEM medium supplemented with 10% FBS and treated in the absence (control cells), or in the presence of CPT at 5×10-2 and 4×10-1 μg/ml for 48 hrs at 37°C. Morphological changes of treated cells were observed by invert microscopy and compared with control cells. (A) Control of HeLa cells. (B) HeLa cells treated with 5×10-2 μg/ml of CPT. (C) HeLa cells treated with 4×10-1μg/ml of CPT. CPT= Camptothecin.
Effect of CPT on DNA Fragmentation
DNA fragmentation was observed from the HeLa cells cultured in the presence of CPT at various concentrations for 48 hrs as to compare with untreated cells (Figure 4, lane 2, 3, 4). Data analysis showed that CPT at 5×10-2 µg/ml concentration clearly produced characteristic DNA laddering (Figure 4, lane 3). However, as shown in lane 4 no DNA laddering is observed when HeLa cells exposed to CPT at concentration of 4×10-1µg/ml.
Figure 4.
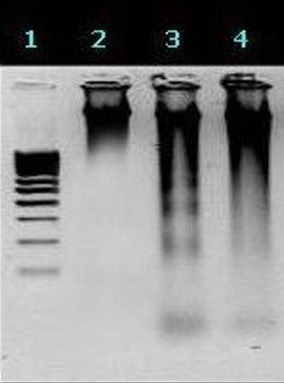
<bold>Shows the DNA fragmentation analysis.</bold> Lane 1: Molecular weight marker, Lane 2: Untreated HeLa cells, Lane 3: CPT (5×10-2μg/ml) treated HeLa cells, Lane 4: CPT (4×10-1μg/ml) treated HeLa cells. CPT= Camptothecin.
Discussion
Many studies showed that CPT and its analogues are used clinically against many forms of cancers including lung, breast, ovarian, and colorectal cancer, because they cause cell death and inhibition mechanism of DNA topoisomerase I [43-45]. Although, CPT was considered as a well anti-proliferative agent in cancer cells but at present, it is not used as a drug of choice due to its severe toxicity effect on cells [46-49]. Recently, many CPT analogues are under investigation as cell cancer inhibitors [50, 51]. Finding the border line between necrotic effect and apoptotic nature of CPT may be useful to find the cause of high toxicity of this agent in cancer patients. This study was designed to investigate the border line between necrotic effects versus apoptotic nature of CPT in human cervical cancer cells (HeLa).
In the present study, HeLa monolayer cell line was used. The use of monolayer cells, instead of floating cells, has some advantages as they are much easier to maintain and handle [52]. Various concentrations of CPT are proved to be cytotoxic in MTT assay (Figure 1). The MTT assay has been used in many experiments for assessment of cytotoxic effects to test agents. In this method the MTT dye, is reduced by living cells and this reaction is used as the end point in a rapid drug-screening assay [36, 53]. The efficacy of this method has been extensively demonstrated [54-57]. In our study, the MTT assay showed that CPT has anti-proliferative effect on HeLa cells in a dose-dependent manner with an IC50 of 0.08±0.012 µg/ml. IC50 is a useful parameter for quantification of drug effect on cell survival [58-60]. Also, the MTT clearly demonstrated that the growth inhibition of HeLa cells by CPT is through induction of apoptosis, at concentrations below 1×10-1µg/ml. Recent studies have shown that CPT and its analogues can strongly induce apoptosis in human leukemic cells [27, 50], prostate [26, 50], colon [29] and breast [28, 51] cancer cells as well as glioma cells [61].
LDH is a cytoplasmic enzyme retained by viable cells with intact plasma membranes, but it is released from necrotic cells with damaged membranes [38, 39]. We also studied released Lactate Dehydrogenase (LDH) activity in HeLa cells. Our results showed an increase in LDH activity in culture media of HeLa cells exposed to CPT at concentration above 1×10-1 µg/ml, while no significant rise was observed in LDH release below concentration of 5×10-2 µg/ml. It has been well documented that lactate dehydrogenase levels, as a marker of necrosis in the cell medium, elevate when cells are exposed to high concentration of anticancer agents [62, 63]. Also, it is a well known phenomenon that cytotoxic drugs, which can induce apoptosis, promote necrosis when they are administered at higher concentrations which can induce toxicity to normal cells [64-67].
In this study, treatment of the HeLa cells with CPT induced morphological changes and apoptosis, including rounding of cells, reduction in cell volume and cell shrinkage at concentration of 5×10-2 µg/ml. In contrast, morphological studies on cells treated with CPT at concentration of 4×10-1µg/ml, revealed loss of membrane integrity, no vesicle formation and complete lysis of cells. Cell death has been characterized as either apoptotic or necrotic, based on morphological features [4, 68, 69]. Two prominent morphological changes which distinguish apoptosis from necrosis are the formation of apoptotic bodies, and the maintenance of cell membrane integrity [70-74]. However, these morphological features described and confirmed necrotic effect of CPT at high concentrations.
Also DNA fragmentation was analysed in HeLa cells treated with CPT. The results suggest that CPT induces apoptosis in HeLa cells at downstairs concentration (5×10-2 µg/ml). However, no DNA ladder was observed when the cells were exposed to high concentrations of 4×10-1 µg/ml CPT. Recent studies have suggested that during apoptosis, a specific nuclease cuts the genomic DNA in nucleosomes to generate DNA fragments. The presence of this ladder has been extensively used as a marker for apoptotic cell death [75, 76].
Conclusion
In conclusion, CPT has an anti-proliferative effect on HeLa cells through apoptosis at a low concentration which is very close to the concentration that cause necrosis to cells. The narrow border line between necrotic and apoptotic effects of this drug was the main reason for its high toxicity in patients. Therefore, a close monitoring of both modes of cell death is vital to evaluate accurately the cytotoxic effects of antitumor agents.
Acknowledgments
This research was supported by Razi Vaccine and Serum Research Institute of Iran.
Footnotes
Conflicts of Interest
There is no conflict of interest in this article.
Authors' Contribution
Abbas Zare-Mirakabadi reviewed the manuscript. Ali Sarzaeem designed the study and wrote the manuscript. Saeed Moredhaseli reported the results. Aida Sayad and Masoud Negahdary analyzed the data.
REFERENCES
- 1.Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: different modes of dying. Biochemistry (Mosc). 2005;70:231–9. doi: 10.1007/s10541-005-0105-4. [DOI] [PubMed] [Google Scholar]
- 2.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death . Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartzman RA, Cidlowski JA. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocrine Rev. 1993;14:133. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- 4.Vermes I, Haanan C. Apoptosis and programmed cell death in health and disease. Adv Clin Chem. 1994;31:177. doi: 10.1016/s0065-2423(08)60336-4. [DOI] [PubMed] [Google Scholar]
- 5.Sang WH, Yun JK, Wonyong K, Chung SL. Antitumor Effects of Camptothecin Combined with Conventional Anticancer Drugs on the Cervical and Uterine Squamous Cell Carcinoma Cell Line SiHa. Korean J Physiol Pharmacol. 2009;13:115–21. doi: 10.4196/kjpp.2009.13.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 8.Wyllie AH. Apoptosis (the1992 Frank Rose Memorial Lecture). Br J Cancer. . 1993;67:205–8. doi: 10.1038/bjc.1993.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3. [PMC free article] [PubMed] [Google Scholar]
- 10.Rudin CM, Thompson CB. Apoptosis and disease: regulation and clinical relevance of programmed cell death. Ann Rev Med. 1997;48:267–81. doi: 10.1146/annurev.med.48.1.267. [DOI] [PubMed] [Google Scholar]
- 11.Fiers W, Beyaert R, Declercq W, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–30. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 12.Silva RJ, Silva MG, Vilela LC, Fecchio D. Antitumor effect of Bothrops jararaca venom. Mediators Inflamm. 2002;11:99–104. doi: 10.1080/09629350220131953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Son DJ, Park MH, Chae SJ, Moon SO, Lee JW, Song HS, et al. Inhibitory effect of snake venom toxin from Vipera lebetina turanica on hormone-refractory human prostate cancer cell growth: induction of apoptosis through inactivation of nuclear factor B. Mol Cancer Therapeutics. 2007;6:675–83. doi: 10.1158/1535-7163.MCT-06-0328. [DOI] [PubMed] [Google Scholar]
- 14.Yang RS, Tang CH, Chuang WJ, Huang TH, Peng HC, Huang TF, et al. Inhibition of tumor formation by snake venom disintegrin. Toxicon. 2005;45:661–9. doi: 10.1016/j.toxicon.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Tsao YP, Russo A, Nyamuswa G, Silber R, Liu LF. Interaction between replication forks and topoisomerase-I-DNA cleavable complexes: studies in a cell-free SV40 DNA replication system. Cancer Res. 1993;53:5908–14. [PubMed] [Google Scholar]
- 16.Laco GS, Collins JR, Luke BT, Kroth H, Sayer JM, Jerina DM, et al. Human topoisomerase I inhibition: docking camptothecin and derivatives into a structure-based active site model. Biochemistry. 2002;41:1428–35. doi: 10.1021/bi011774a. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava V, Arvind SN, Kumar JK, Gupta MM, Suman PS. Plant-based anticancer molecules: A chemical and biological profile of some important leads. Bioorganic & Medicinal Chemistry. 2005;13:5892–908. doi: 10.1016/j.bmc.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 18.Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA. Plant antitumour agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumour inhibitor from Camptotheca acuminata. J Am Chem Soc. 1966;88:3888–90. [Google Scholar]
- 19.Wall ME. Camptothecin and taxol: discovery to clinic. Med Res Rev. 1998;18:299–314. doi: 10.1002/(sici)1098-1128(199809)18:5<299::aid-med2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz SB, Chang C, Grollman AP. Antiviral Action of Camptothecin. Antimicrobial Agents and Chemotherapy. 1972;2:395–401. doi: 10.1128/aac.2.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redinbo MR, Stewart L, Kuhn P, Champoux JJ, Hol WGJ. Crystal structures of human topoisomerase I in covalent and non-covalent complexes with DNA. Science. 1998;279:1504–13. doi: 10.1126/science.279.5356.1504. [DOI] [PubMed] [Google Scholar]
- 22.Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Stewart LP. The mechanism of topoisomerase I poisoning by a camptothecin analog. Natl Acad Sci U.S.A. 2002;99:15387–92. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz SB, Chang CK, Grollman AP. Studies on camptothecin: effects on nucleic acid and protein synthesis. Mol Pharmacol. 1971;7:632–44. [PubMed] [Google Scholar]
- 24.Kessel D. Effects of camptothecin on RNA synthesis in leukemia L1210 cells. Biochim Biophys Acta. 1971;246:225–32. doi: 10.1016/0005-2787(71)90131-6. [DOI] [PubMed] [Google Scholar]
- 25.Kassel D. Some determinants of camptothecin responsiveness in leukemia L1210 cells. Cancer Res. 1971;31:1883–7. [PubMed] [Google Scholar]
- 26.Li CJ, Wang C, Pardee AB. Induction of apoptosis by β-lapachone in human prostate cancer cells. Cancer Res. 1995;55:3712–5. [PubMed] [Google Scholar]
- 27.Nard U. The proapoptotic drug camptothecin stimulates phospholipase D activit y and diacylglycerol production in the nucleus of HL-60 human promyelocytic le ukemia cells. Cancer Res. 1999;59:3961–7. [PubMed] [Google Scholar]
- 28.Nieves NW, Pommie Y. Apoptotic response to camptothecin and 7-hydr oxystaurosporine (UCN-01) in the 8 human breast cancer cell lines of the NCI a nticancer Drug Screen: multi factorial relationships with topoisomerase I, pro tein kinase C, Bcl-2, p53, MDM-2 and caspase pathways. Int J Cancer. 1999;82:396–404. doi: 10.1002/(sici)1097-0215(19990730)82:3<396::aid-ijc13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 29.Ohyama T, Li Y, Utsugi T, Irie S, Yamada Y, Sato T. A dual topoisomerase inhibitor, TAS-103, induces apoptosis in human cancer cells. Jpn J Cancer Res. 1999;90:691–8. doi: 10.1111/j.1349-7006.1999.tb00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youngnam C, Riyi S, Richard BB, Albena I. Functionalized mesoporous silica nanoparticle-based drug delivery system to rescue acrolein-mediated cell death. Nanomedicine. 2008;3:1–13. doi: 10.2217/17435889.3.4.507. [DOI] [PubMed] [Google Scholar]
- 31.Pourhassan M, Zarghami N, Rahmati M, Alibakhshi A, Ranjbari J. The inhibitory effect of Curcuma longa extract on telomerase activity in A549 lung cancer cell line. African Journal of Biotechnolog. 2010;9:912–19. [Google Scholar]
- 32.Fiona MY, Wichaya P, Barbara JSS. Modification of MTT assay conditions to examine the cytotoxic effects of amitraz on the human lymphoblastoid cell line, WIL2NS. Toxicol In vitro. 2005;19:1051–9. doi: 10.1016/j.tiv.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Harbell JW, Koontz SW, Lewis RW, Lovell D. Acosta Cell cytotoxicity assays. Food Chem Toxicol. 1997;35:79126. doi: 10.1016/s0278-6915(96)00101-9. [DOI] [PubMed] [Google Scholar]
- 34.Husoy T, Syversen T, Jenssen J. Comparisons of four in vitro cytotoxicity tests: the MTT assay, NR assay, uridine incorporation and protein measurements. Toxicol In vitro. 1993;7:149–54. doi: 10.1016/0887-2333(93)90125-o. [DOI] [PubMed] [Google Scholar]
- 35.James FDO, William CB, Martin G, Kent C, Ulla C, Steven PM, et al. Comparison of cisplatin and carboplatin cytotoxicity in human ovarian cancer cell lines using the MTT assay. Gynecol Oncol. 1990;39:119–22. doi: 10.1016/0090-8258(90)90416-i. [DOI] [PubMed] [Google Scholar]
- 36.Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 37.Subhadradevi V, kalathil K, Kuppusamy A, Muthuswamy UAS, Puliyath J. Induction of Apoptosis and Cytotoxic Activities of Apium graveolens Linn. Using in vitro Models. Middle-East Journal of Scientific Research. 2011;9:90–94. [Google Scholar]
- 38.Chengbin X, Wei L, Jianhong W, Xiangliang Y, Huibi X. Chemoprotective effect of N-acetylcysteine (NAC) on cellular oxidative damages and apoptosis induced by nano titanium dioxide under UVA irradiation. Toxicology in Vitro. 2011;25:110–16. doi: 10.1016/j.tiv.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 39.José MC, Judit CM, Elena P, Gerardo AC, Maria AP, Manuel AP. The antimicrobial peptide cecropin A induces caspase-independent cell death in human promyelocytic leukemia cells. Peptides. 2010;31:1494–503. doi: 10.1016/j.peptides.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Saeed S, Mohammad HB, Saideh D. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharmacogn Mag. 2010;6:309–314. doi: 10.4103/0973-1296.71799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fehsel K, Kolb BV, Kolb H. Analysis of TNF alpha-induced DNA strand breaks at the single cell level. Am J Pathol. 1999;139:251–4. [PMC free article] [PubMed] [Google Scholar]
- 42.Negoescu A, Lorimier P, Labat-Moleur F, Drouet C, Robert C, Guillermet CC, et al. In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J Histochem Cytochem. 1996;44:959–68. doi: 10.1177/44.9.8773561. [DOI] [PubMed] [Google Scholar]
- 43.Pommier Y, Pourquier P, Urasaki Y, Wu J, Laco G. Topoisomerase I inhibitors: selectivity and cellular resistance. Drug Resistance Update. 1999;2:307–18. doi: 10.1054/drup.1999.0102. [DOI] [PubMed] [Google Scholar]
- 44.Chen AY, Liu LF. DNA Topoisomerases: essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol. 1994;94:194–218. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- 45.Beck WT, Khelifa T, Kusumoto H, Mo YY, Rodgers Q, Wolverton JS, et al. Novel mechanisms of resistance to inhibitors of DNA topoisomerases. Adv Enzyme Regul. 1997;37:17–26. doi: 10.1016/s0065-2571(96)00024-6. [DOI] [PubMed] [Google Scholar]
- 46.Bosmann HB. Camptothecin inhibits macromolecular synthesis in mammalian cells but not in isolated mitochondria or E. coli. Biochem Biophys Res Commun. 1970;41:1412–20. doi: 10.1016/0006-291x(70)90544-9. [DOI] [PubMed] [Google Scholar]
- 47.Gallo RC, Whang-Peng J, Adamson RH. Studies on the antitumor activity, mechanism of action,and cell cycle effects of camptothecin. J Nat Cancer Inst. 1971;46:789–95. [PubMed] [Google Scholar]
- 48.Hartwell JL, Abbott BJ. Antineoplastic principles in plants: recent developments in the field. Adv Pharmacol Chemother. 1969;7:117–209. [PubMed] [Google Scholar]
- 49.Gottlieb JA, Guarino AM, Call JB, Oliverio VT, Block JB. Preliminary pharmacologic and clinical evaluation of camptothecin sodium (NCS-100880). Cancer Chemother Rep. 1970;54:461–70. [PubMed] [Google Scholar]
- 50.Palnchon SM, Wuerzberger S, Frydman B, Witiak DT, Huston P, Church DR, et al. β-lapachone mediated apoptosis in human promyelocytic leukemia (HL-60) and human prostate cancer cells: a p53 independent response. Cancer Res. 1995;55:3706–11. [PMC free article] [PubMed] [Google Scholar]
- 51.Tolis C, Peters GJ, Ferreira CG, Pinedo HM, Giaccone G. Cell cycle distu rbances and apoptosis induced by topotecan and gemcitabine on human lung cancer cell lines. Eur J Cancer. 1999;35:796–807. doi: 10.1016/s0959-8049(98)00425-0. [DOI] [PubMed] [Google Scholar]
- 52.Reubei GH, Gareis M, Amselgruber WM. Cytotoxicity evaluation of mycotoxins by an MTT-bioassay. Mycotoxin Res. 1987;3:85–96. doi: 10.1007/BF03191994. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem. 1997;69:581–93. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- 54.Ebisuno S, Inagaki T, Kohjimoto Y, Ohkawa T. The cytotoxic effect of feroxacin and ciprofoxacin on transitional cell carcinoma in vitro. Cancer. 1997;80:2263–7. [PubMed] [Google Scholar]
- 55.Gürbay A, Garrel C, Osman M, Richard MJ, Favier A, Hincal F. Cytotoxicity in ciprofoxacin-treated human fibroblast cells and protection by vitamin E. Hum Exp Toxicol. 2002;21:635–41. doi: 10.1191/0960327102ht305oa. [DOI] [PubMed] [Google Scholar]
- 56.Gürbay A, Gonthier B, Barret L, Favier A, Hincal F. Cytotoxic effect of ciprofoxacin in primary culture of rat astrocytes and protection by vitamin E. Toxicology. 2007;229:54–61. doi: 10.1016/j.tox.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 57.Wijsman JA, Dekaban GA, Rieder MJ. Differential toxicity of reactive metabolites of clyndamicin and sulfonamides in HIV-infected cells: infuence of HIV infection on clyndamicin toxicity in vitro. J Clin Pharmacol. 2005;45:346–51. doi: 10.1177/0091270004272670. [DOI] [PubMed] [Google Scholar]
- 58.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 59.Richard R, Neubig R, Michael S, Terry K, Arthur C, Neubig RR, et al. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology . Pharmacol Rev. 2003;55:597–606. doi: 10.1124/pr.55.4.4. [DOI] [PubMed] [Google Scholar]
- 60.Osbome LS, Henley RW. Valuation of safer agrochem´s insecticidal soapfer the control of mites in the interior environment. J Foliage Digest. 2000;5:10–11. [Google Scholar]
- 61.Ciesielski MJ, Fenstermaker RA. Synergistic cytotoxicity, apoptosis and protein linked DNA breakage by etoposide and camptothecin in human U87 glioma cells: dependence on tyrosine phosphorylation. J Neurooncol. 1999;41:223–34. doi: 10.1023/a:1006129119460. [DOI] [PubMed] [Google Scholar]
- 62.Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicology in Vitro. 2005;19:975–83. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 63.Lin WS, Huang YW, Zhou XD, Ma YF. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicology and Applied Pharmacology. 2006;217:252–9. doi: 10.1016/j.taap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Tsujimoto Y. Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997;4:429–34. doi: 10.1038/sj.cdd.4400262. [DOI] [PubMed] [Google Scholar]
- 65.Nicotera P, Leist M, Ferrando-May E. Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol Lett. 1998;102:139–42. doi: 10.1016/s0378-4274(98)00298-7. [DOI] [PubMed] [Google Scholar]
- 66.Formigli L, Papucci L, Tani A, Schiavone N, Tempestini A, Orlandini GE, et al. Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J Cell Physiol. 2000;182:41–9. doi: 10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 67.Finstad HS, Kolset SO, Holme JA, Wiger R, Farrants AK, Blomhoff R, et al. Effect of n-3 and n-6 fatty acids on proliferation and differentiation of promyelocytic leukemic HL-60 cells. Blood. 1994;84:3799–809. [PubMed] [Google Scholar]
- 68.Kerr JFR, Wyllie AH, Currire AR. Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Graziani G, Szabo C. Clinical perspectives of PARP inhibitors. Pharmacol Res. 2005;52:109–18. doi: 10.1016/j.phrs.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Honda O, Kuroda M, Joja I, Asaumi J, Takeda Y, Akaki S, et al. Assessment of secondary necrosis of Jurkat cells using a new microscopic system and double staining method with annexin V and propidium iodide. Int J Oncol. 2000;16:283–8. doi: 10.3892/ijo.16.2.283. [DOI] [PubMed] [Google Scholar]
- 71.Ingalls CP, Barnes WS, Smith SB. Interaction between clenbuterol and run training: effects on exercise performance and MLC isoform content. J Appl Physiol. 1996;80:795–801. doi: 10.1152/jappl.1996.80.3.795. [DOI] [PubMed] [Google Scholar]
- 72.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–98. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 73.Blank M, Shiloh Y. Programs for Cell Death: Apoptosis is Only One Way to Go. Cell Cycle. 2007;6:686–95. doi: 10.4161/cc.6.6.3990. [DOI] [PubMed] [Google Scholar]
- 74.Baumann S, Krueger A, Kirchhoff S, Krammer PH. Regulation of T Cell Apoptosis During the Immune Response. Current Molecular Medicine. 2002;2:257–72. doi: 10.2174/1566524024605671. [DOI] [PubMed] [Google Scholar]
- 75.Nagata S. Apoptotic DNA fragmentation. Exp Cell Res. 2000;256:12–18. doi: 10.1006/excr.2000.4834. [DOI] [PubMed] [Google Scholar]
- 76.Wyllie AH. Glucorcorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–6. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]