Abstract
Background
Acute lymphoblastic leukemia is a lymphoid malignancy, resulting from autonomous proliferation of monoclonal abnormal stem cell. The aim of this study was to evaluate the response rate and prognostic factor of adult patients suffering from acute lymphoblastic leukemia, who were treated with chemotherapy in south east of Iran and demographic methods were used for this study.
Methods
This study was conducted in Ali Ebne Abitaleb Hospital in south east of Iran (Zahedan) from 2003-2010. All adult patients with acute lymphoblastic leukemia in hematology ward received Vincristin, Daunorubicin, Cyclophosphamide, Prednisolone and high dose Methotrexate for induction therapy. All patients' information was recorded and multivariate analysis and survival studies were performed by using Kaplan-Meier statistics.
Results
Sixty six adult patients entered. Mean age of them was 33 years old (16-68), that 53 (80.3%) cases were male and 13 cases were female. Fifty one (77.3%) cases experienced complete remission and 15 (22.7%) cases had no remission state. In the following year 53% ( 33) was alive with complete remission and 47% ( 31) were dead. Median survival was 13 months. In the end of study 30 cases were in complete remission and alive and 36 (54.5%) were dead.
Conclusion
Our results were comparable with other studies and minimally better than those studies.
Keywords: Acute lymphoblastic leukemia, Stem cell, Outcome
Introduction
Acute Lymphoblastic Leukemia (ALL) is a lymphoid malignancy, which arises from bone marrow, appearing in marrow, blood circulation and other organs [1].
The incidence of leukemia is 44000 in 2008 [2] and is the most common hematologic malignancy in south east of Iran [3, 4]. Top five most common types of cancer in Ardabil were: stomach, esophagus, lung, colorectal, and bladder [5]. Other reports from south of Iran reveal the frequency of lymphocytic leukemia as the third most common malignancy in men [6]. The evaluation of cancer incidence in Fars province showed that top four most common malignancies in men were: ALL, stomach, Acute Myeloblastic Leukemia (AML), colon and in women the ALL was the second most common malignancy [7]. Clinical manifestation of ALL is related to bone marrow replacement. This pathophysiologic change is the cause of thrombocytopenia, anemia and leukocyte abnormal count. In all patients, cure rate and durable response in childhood period is better than adult patients and only 30-40% of adult had long term survival and exacted cure [8]. Disease-free survival in childhood period is more than 70% and this parameter in adult group is 20-38% [9]. The combination therapy with Vincristine (V), Daunorubicine (D) and Prednisolone (P) increased complete remission in adult ALL and median response increased to 17-18 months [10-12]. Many studies reported that more intensive treatment with or without stem cell transplantation increased the survival and cure rate in ALL patients [13, 14]. By using hematopoietic stem cell transplantation, the maximum cure rate in adult ALL patients seldom exceeded more than 40% [15]. The aim of current study is evaluation of clinical manifestation, prognostic factors and survival rate of ALL patients, treated with chemotherapy without stem cell support in south east of Iran (Zahedan).
Methods
In a single center study in Zahedan, Ali Ebne Abitaleb Hospital, sixty six newly diagnosed ALL patients with clinical manifestation as peripheral blood smear, and bone marrow aspiration entered in 2004-2011. Then, the complete physical examination, baseline laboratory and radiologic examination were performed. All patients after insertion of central venous line received maintenance fluid therapy and induction treatment was: Prednisolone (60 mg/m2 1-28), Cyclophosphamide (1200 mg/m2 stat), Daunorubicine (45 mg/m2, 1-3) and Vincristine (1.2 mg/m2 weekly for 4 weeks). In induction phase of treatment, all patients received Ranitidine, Fluconazol (anti fungal), and Cotrimoxazole. If the patients needed transfusion, RBC or PLT prescribed. In the induction therapy, all patients with fever and neutropenia, candidate for anti microbial therapy according to clinical manifestation and cultures. Fourteen to twenty days after initiation of treatment, all patients candidate for re-evaluation of bone marrow, cellularity and blast percentages. The patients who had favorable WBC, neutrophil count (>1500) and platelet count (>100000), candidate for high dose Methotrexate (2gr/m2) and after 16 hours Leucovorin rescue and intra techal with 50 mg Cytarabine and 15 mg Methotrexate for at least 4 times respectively. Consolidation therapy was 2 courses of Cytarabine 600 mg/m2/day, days 1,14, Etoposide 200 mg/m2/day, days 1,14 and Prednisolone 60 mg/m2/day 1-4 and 14-18 and then one single course of Cyclophosphamide (CTX) 1200 mg/m2 , Dounorubicine 45 mg/m2 Vincristine (VCR) 1.4 mg/m2 and Prednisolone 60 mg/m2. Maintenance therapy was: 6 Mercaptopurine (MP) 50 mg/daily and Methotrexate 20 mg/m2/week for 2 years. Routine follow up was every month (Table 1).
Table 1.
General Characteristics of Patients
| Characteristic | No | Percent% |
|---|---|---|
| M/F | 53/13 | 80.3/19.7 |
| Age (18-60) | Mean =33.59 | |
| Pro B cell | 58 | 87.8 |
| T Cell | 8 | 12. 2 |
| Mature B cell | 0 | 0 |
| Fatigue and malaise | 31 | 47 |
| Fever and chills | 41 | 62.1 |
| Musculoskeletal pain | 24 | 36.4 |
| pallor | 39 | 59.1 |
| Clinical bleeding | 29 | 43.9 |
| Petechia and purpura | 15 | 22.7 |
| Bone pain | 12 | 18.2 |
| Lymphadenopathy | 17 | 25.8 |
| Mediastinal widening | 3 | 4.5 |
| Organomegaly | 11 | 16.7 |
| CNS involvement | 0 | 0 |
| Leukocyte >50000 | 15 | 22.7 |
M: Male, F: Female
Results
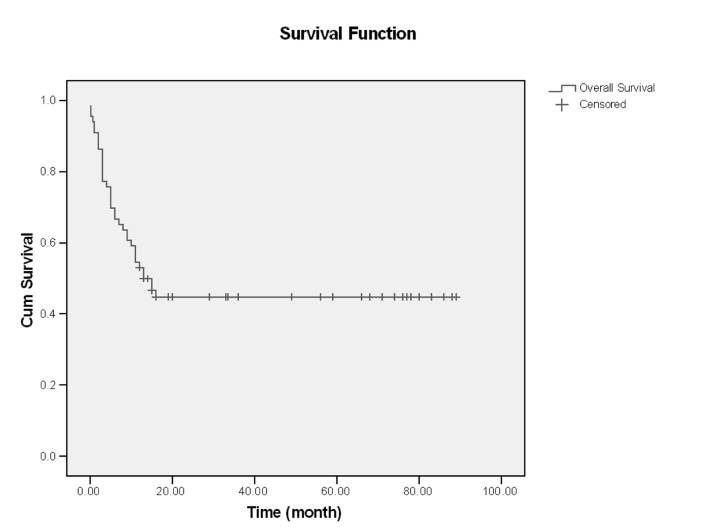
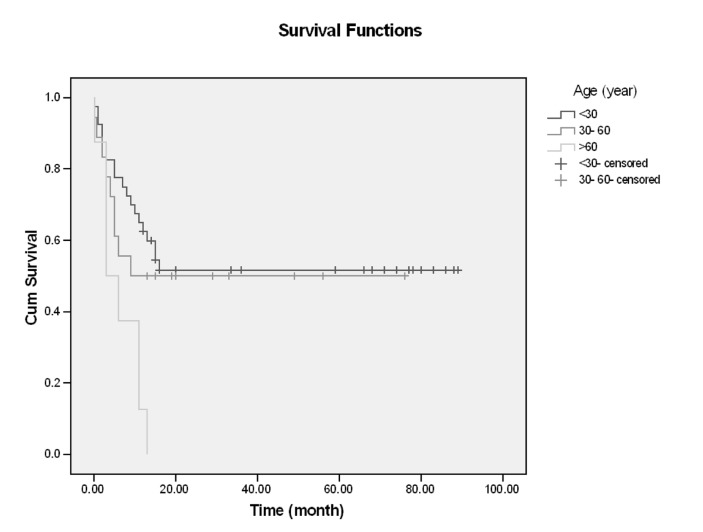
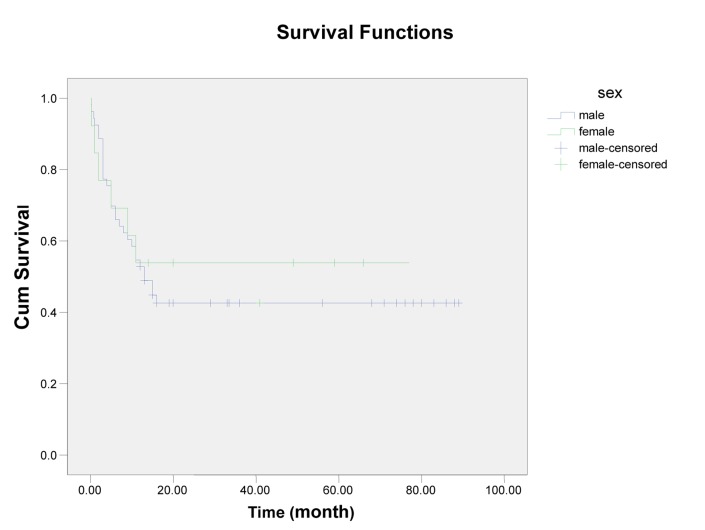
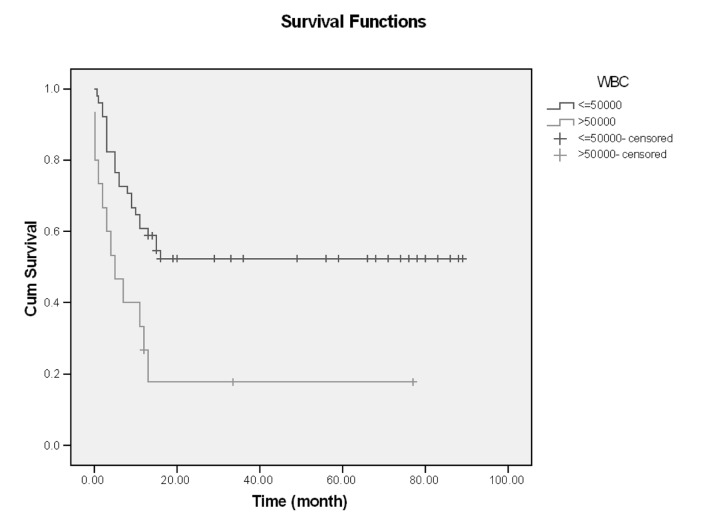
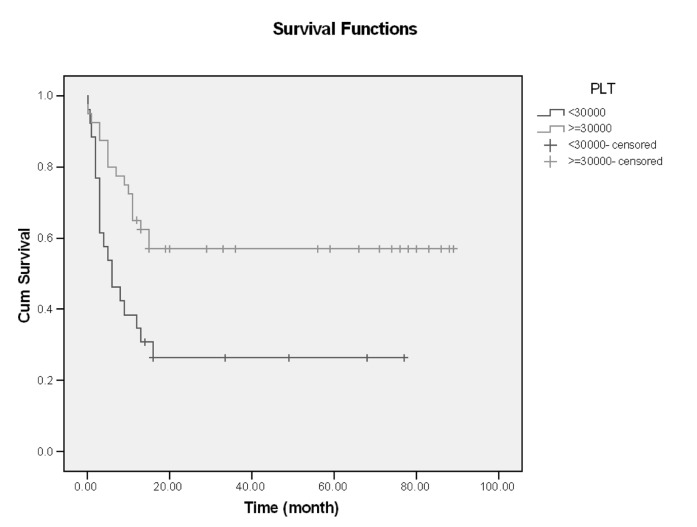
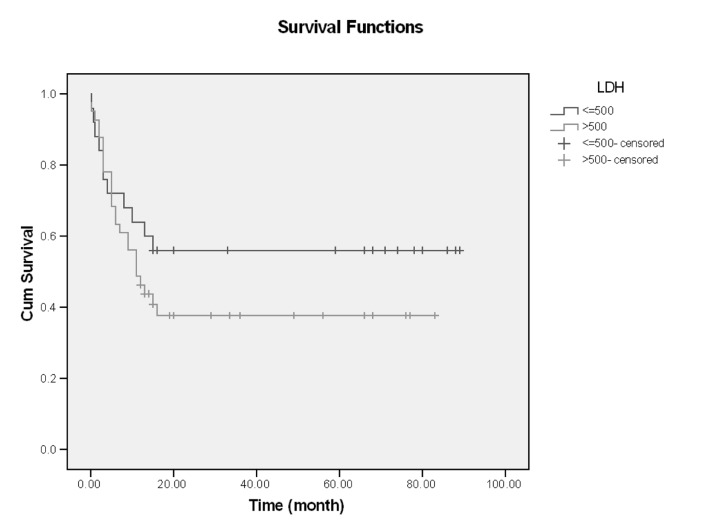
In this single center of study, 66 patients with ALL entered and were evaluated. About 60% had age <30 years (18-68), and mean age of them was 33.59, Male/Female (M/F) was 53/13 (80% /20%).The more common clinical manifestation were: fever (62.1%), paleness (59.1%), bleeding (43.9%), bone pain (36.4%) and lymphadenopathy and organomegaly; 25.8% and 16.7% respectively. Gastrointestinal bleeding occurred in 3 cases and every 3 cases had hematologic abnormality (thrombocytopenia) and resolved with supportive care, without upper or lower GI endoscopy (Table1). Of all patients 77.3% experienced Complete Remission (CR) and 22.7% with Non Remission (NR) state. During the first year follow up, 53% alive and 47% death occurred. In the end of study 45.5% was alive and median survival was 13 months (4-2670 days). Outcome of patients who had CR was significantly different from NR patients. After 15 months, survival rate in CR group was 52.9% and in NR group was 20% (p=0.001) (Figure1-6). Response rate decreased with age increment. After 13 months follow up, survival in age>60 was 0% and in age<30 was 51.6% (p=0.008). M/F didn't have statistical effect on survival and response rate. Leukocyte count < 30000 had no significant effect in outcome, but leukocyte count > 50000 was with poor outcome (p=0.003) (Figure1-6). Platelet count had statistical efficacy on outcome of ALL patients. Outcome in PLT<30000 to PLT> 30000 was 26.9% and 52.5% (p=0.022). Correlation between hemoglobin level, Lactate Dehydrogenase (LDH), kidney function and liver function and outcome were important but without significant statistical analysis. Fever was documented in 50% of patients. Survival and response rate were not different in both groups. About 82% received empirical antibiotic therapy but intravenous antifungal therapy, such as Amphotericin B was limited to 3 cases. All 3 cases had suspicious fungal infection without any documented site of this infection. The duration of Amphotricin B administration was 6.33±1.52 days. Non- requirement to anti fungal therapy and non- documented fungal infection were the most important findings in clinical manifestation of our experienced. In our study, expected 1- year survival was 53% and 2 and 3 years survival was 44.8% respectively. The overall median survival was 13 (7.9-18.1) months. In current study, three- year disease- free survival was 32% (Table 2).
Figure 1.
Overall survival
Figure 2.
Survival and Age
Figure 3.
Survival and Sex
Figure 4.
Survival and WBC count
Figure 5.
Survival and PLT
Table 2.
Results and Outcome of Study
| Factor | Number (%) | CR (Complete Remission) | Median survival time(95%CI) | Last comparable survival time (probability) | Last calculated survival time (probability) | P-Value |
|---|---|---|---|---|---|---|
| Age | ||||||
| <30 | 40 (60.6) | 34 (85%) | - | 9 (705) | 13 (51.6%) | 0.008 |
| 30-60 | 18 (27.3) | 12 (66%) | - | 9 (50%) | - | |
| >60 | 8 (12.1) | 5 (62.5%) | 3 (0.0,7.0) | 9 (37.5%) | 13 (0%) | |
| Sex | ||||||
| Male | 53 (80.3) | 42 (79.24%) | 13 (8.1,17.9) | 11 (54.7%) | 16 (42.6%) | 0.650 |
| Female | 13 (19.7) | 9 (69.2%) | 11(53.8%) | |||
| WBC | ||||||
| ≤ 50000 | 51 (77.3) | 41 (80.39%) | - | 13 (58.8%) | 16 (52.3%) | 0.003 |
| >50000 | 15 (22.7) | 10 (66.6%) | 5 (0.0,10.0) | 13 (17.8%) | - | |
| PLT | ||||||
| ≤30000 | 26 (39.4) | 18 (69.2%) | 6 (1.0,11.0) | 15 (30.8%) | 16 (26.4%) - | 0.005 |
| >30000 | 40 (60.6) | 33 (82.5%) | - | 15 (57.0%) | ||
| LDH | ||||||
| ≤ 500 | 25 (37.9) | 19 (76%) | - | 15 (56.0%) | - | 0.243 |
| >500 | 45 (62.1) | 32 (78%) | 11 (6.0,16.0) | 15 (40.8%) | 16 (37.7%) |
Discussion
Our study showed acceptable response and survival rate to this protocol, although we didn't have stem cell support. Study of Gokbuget and Hoelzer that was reported in American Society of Hematology, focused on patients treated with chemotherapy and stem cell support and other group that had Philadelphia chromosome- positive [16]. CR in every group was 84%, 83% and survival rate was 35, 36% (in this study), but in our study CR was 77.3% and survival rate was 45.5% and median survival was 13 months. Prognostic factors in Gokbuget and Hoelzer were advanced age and leukocyte count > 30000-50000, and in our study the prognostic factors were: platelet count <30000, leukocyte count > 50000 and advanced age. In Larsen study the most important prognostic factor which compromise CR and survival rate was advanced age [17]. According to this study CR in age <30 , 30-60 and >60 was 90%, 81%, and 52% and 3 years survival in these 3 ages was 58%, 38%,and 12%. In our study age was one of the most important risk factors for CR and survival rate in age >60 was 0%. In other study [18], 922 cases with ALL in 4 groups (standard risk, high risk, Philadelphia positive and CNS involvement) entered. In this study, patients' characteristics and clinical manifestations were: mean age 33 years, mediastinal mass 18%, organomegally 67%, CNS involvement 7%, bleeding 25%, fever 30%, WBC count >30000, 38%, and PLT <50000 46% respectively. Results showed: CR, failure, and death in group 1 were; 85%, 15% and 2%. These parameter in other 3 groups were; 66%, 28%, 6%, 53%, 45%, 2%, and in group 4 were 73%, 16% and 11 % [18]. Three year survival in every groups were; 41%, 38%, 24% and 44% and 5 year survival were; 35%, 30%, 20% and 41%. The most common prognostic factors were: age, WBC count and time to achievement of complete remission. Total cases who candidate for salvage therapy were 192 cases [18].
In reports of Jabbou and co-workers, high level of LDH was correlated with poor prognosis and with CNS involvement [19], but in our study LDH didn't have an effect of CR or survival rate. Fungal infection is one of the most important causes of mortality and morbidity in leukemic patients [20, 21]. In a study by Haan and co-workers [22], documented Invasive Fungal Infection (IFI) occurred in 23.5% of all patients. But in our study suspicious fungal infection was in 3 cases and documented IFI was 0%, and only these 3 cases needed intravenous Amphotericin B administration. In other study [23], postinduction CR was 86.6%. Treatment-related mortality was 13.2% ( 3.8% in refractory disease, and 9.4% due to severe infection). Median Overall Survival (OS) for all cases was 16.7 months and estimate 4 years survival rate was 25.4%±8.9% respectively. Central Nervous System (CNS) involvement was 4%, Precursor B cell ALL, T cell ALL, mature B cell ALL were 70.6%, 23.5% and 5.9% [23]. CR in 86.8%, 3.8% had refractory ALL and 9.4% died of infection. In comparison with our study, CR was higher than our study; infection related mortality was higher than our study. Median survival was 16.7 months, and Relapse-Free Survival (RFS) was 12.2 months which were comparable to our results. T cell phenotype was the major prognostic factor, but in contrast with our study that Tcell was not associated with poor prognosis. In other study in Singapore [24], more common clinical manifestations were: fever (82%), bleeding (25.5%), anemia (79%), Lymphadenopathy (LAP) (35.5%), and organomegaly (18.5%), when compared with our study was different. Poor prognostic factors in this study were: male gender, WBC counts > 50000, splenomegaly, and Lactate Dehydrogenase (LDH) > 1000. In comparison with our study, prognostic factors as WBC and PLT count and advanced age were different. CR in 85%, higher than our results, in our study and in 18 months follow up 44.4% had relapse disease and died , 30% was alive ( 26% with CR state and 4% with disease) .Median survival was 12.7 months [24].
Our study revealed the acceptable results of this protocol in treatment of ALL patients with minimal toxicity and favorable outcome with comparable results with other studies.
Figure 6.
Survival and LDH
Acknowledgments
This study was supported by Research Deputy of Zahedan University of Medical Sciences, Zahedan, Iran. The authors thank all nurses of Oncology ward of Ali Ebne Abitaleb Hospital especially Miss Khademi and Sheikhi for their assistance.
Footnotes
Conflicts of Interest
The authors have no conflict of interest in this article.
Authors' Contribution
Mohammad Ali Mashhadi designed the study, analysed and wrote the manuscript. Mohammad mahdi Koushyar helped in writing and editing of manuscript. Mehdi Mohammadi designed the study and analysed the data.
REFERENCES
- 1.Faderl S, Jeha S, Kantarjian H. The biology and therapy of adult acute lymphoblastic leukaemia. Cancer. 2003;98:1337–54. doi: 10.1002/cncr.11664. [DOI] [PubMed] [Google Scholar]
- 2. Stat bite: Estimated new leukaemia cases in 2008. J Natl Cancer Inst. 2008;16:100–531. doi: 10.1093/jnci/djn111. [DOI] [PubMed] [Google Scholar]
- 3.Mashhadi MA, Zakeri Z, Abdollahinejad MJ. Cancer Incidence in South East of Iran: Results of a Population- ased Cancer Registry. J Shiraz E-Med. 2010;11(3) [Google Scholar]
- 4.WaielAl-Kahiry A. Hematological Malignancies in Al-Amal Oncology Unit, Aden. Indian J Hematol Blood Transfuse. 2011 doi: 10.1007/s12288-011-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadjadi A, Malekzadeh R, Derakhshan MH, Sepehr A, Nouraie M, Sotoudeh M, et al. Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int J cancer. 2003;107(1):113–8. doi: 10.1002/ijc.11359. [DOI] [PubMed] [Google Scholar]
- 6.Masoompour SM, Yarmohammadi H, Rezaianzadeh A, Lankarani KB. Cancer incidence in southern Iran, 1998-2002: results of population-based cancer registry. Cancer pidemiol . 2011;35(5):42–7. doi: 10.1016/j.canep.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Mehrabani D, Tabei SZ, Heydari ST, Shamsina SJ, Shokrpour N, Amini M. Cancer Occurrence in Fars Province. Southern Iran IRCMJ. 2008;10(4):314–22. [Google Scholar]
- 8.Pui CH. Acute lymphoblastic leukemia in children. Curr Opin Oncol. 2000;12:3–12. doi: 10.1097/00001622-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Hoelzer D, Ludwig WD, Thiel E. Improved outcome in adult B-cell acute lymphoblastic leukemia. Blood. 1996;87:495–508. [PubMed] [Google Scholar]
- 10.Annino L, Vegna ML, Camera A. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMAALL 0288 randomized study. Blood. 2002;99:863–71. doi: 10.1182/blood.v99.3.863. [DOI] [PubMed] [Google Scholar]
- 11.Go¨kbuget N, Hoelzer D, Arnold R. Treatment of adult ALL according to protocols of the German Multicenter Study Group for Adult ALL (GMALL). Hematol Oncol Clin North Am. . 2000;14:1307–25. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- 12.Schaison G, Sommelet D, Bancillon A. Treatmentof acute lymphoblastic leukemia French protocol Fralle 83- 87. Leukemia. 1992;6 Suppl 2:148–52. [PubMed] [Google Scholar]
- 13.Kantarjian HM, O’Brien S, Smith TL. Results of treatment with Hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–61. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 14.Durrant I, Prentice H, Richards S. Intensification of treatment for adults with acute lymphoblastic leukaemia: Results of U. K. Medical Research Council randomized trial U K A L L X A: Medical Research Council Working Party on Leukaemiai n Adults . Br J Haemato. 1997;l99:84–92. doi: 10.1046/j.1365-2141.1997.3613175.x. [DOI] [PubMed] [Google Scholar]
- 15.Ching-Hon P, William E. Evans. Treatment of Acute Lymphoblastic Leukemia. N Engl J Med. 2006;354:166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 16.Gokbuget N, Hoelzer D. Treatment of adult acute lymphoblastic leukemia. Am Soc Hematol. 2006:133–41. doi: 10.1182/asheducation-2006.1.133. [DOI] [PubMed] [Google Scholar]
- 17.Larson RA. Acute lymphoblastic leukemia: older patients and newer drugs. Hematol Am Soc Hematol Educe Program. 2005:131–6. doi: 10.1182/asheducation-2005.1.131. [DOI] [PubMed] [Google Scholar]
- 18.Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of Treatment in Adults With Acute Lymphoblastic Leukemia: Analysis of the LALA-94 Trial. J Clin Oncol. 2004;22(20):4075–986. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 19.Jabbour E, Faderl E, Kantarjian H. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2005;80(11):1517–27. doi: 10.4065/80.11.1517. [DOI] [PubMed] [Google Scholar]
- 20.Kabayashi R, Kaneda M, Sato T, Ichikava M, Suzuki D. The clinical feature of invasive fungal infection in pediatric patients with hematologic malignancy: A 10-years analysis at a single insitution at japan. J pediatr Hematol Oncol. 2008;30:886–90. doi: 10.1097/MPH.0b013e3181864a80. [DOI] [PubMed] [Google Scholar]
- 21.Caira M, Girmenia C, fadda RM, mitra ME, picardi M, Van Lint MT. Invasive fungal infection in patients with AML in those submited to allogenic hematopoietic stem cell transplant: who is at highest risk? Eur J haematol. 2008;81:242–3. doi: 10.1111/j.1600-0609.2008.01096.x. [DOI] [PubMed] [Google Scholar]
- 22.Haan-Ast C, Glasmacher A, Muckter S. Overall survival and fungal infection-related mortality in patients with invasive fungal infection and neutropenia after myelosuppresive chemotherapy and a tertiary care center from 1995-2006. J Antimicrob Chemother. 2010;65:761–8. doi: 10.1093/jac/dkp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S, Kim J, Kim D, Lee S, lee S, Youn S. Treatment outcome of adult acute lymphoblastic leukemia with VPD (L) regimen: Analysis of prognostic factors. The Korean J int med. 2003;18:21–8. doi: 10.3904/kjim.2003.18.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaikh M, Ali N, Adil S, Khurshid M. Outcome of adult patients with acute lymphoblastic leukaemia receiving the MRC UKALL XII protocol: a tertiary care centre experience. Singapore Med J. 2011;52(5):370. [PubMed] [Google Scholar]