Abstract
Based on a common belief, herbal medicine with the least possible side effects should be the center of attention in cancer care; however, in many cases they have not been properly studied with reliable clinical trials in human subjects. In this review, it was attempted to identify the available evidence on the use and clinical effects of herbs in cancer care. The research consists of two major parts including immunomodulator and chemopreventive herbal compounds whose mechanism, biological response, anticancer element of extract and related benefits were completely studied. Also, the safety of herbal anticancer compounds was discussed. Although the use of herbal medicines in treating cancer shows less chemotherapy-induced, toxicity, more researches are required to reach their full therapeutic potentials.
Keywords: Neoplasms, Plants, Immunologic factors, Prevention, Safety
Introduction
Cancer is a hyperproliferative disorder that involves transformation, dysregulation of apoptosis, proliferation, invasion, angiogenesis and metastasis. Cancers with alarming statistics, cause more than 7 million deaths per year worldwide, more than HIV/AIDS, malaria and tuberculosis combined [1]. It is estimated that the number of new cancer cases will reach 15 million every year by 2020; 70% of which will be in developing countries [2]. Patients confronting a diagnosis of advanced cancer face the statistical reality that conventional chemotherapy can affect a cure for only a tiny minority of all such cases. More often than not, the reasonable impulse of these patients to investigate alternative treatment options such as herbal medicine is met with physician's doubt.
For a long time, natural and herbal products have been considered as precise sources of treatment used in traditional medicine to treat a variety of diseases including infections and malignant diseases [3]. Several researches demonstrated the fact that extracts from a number of herbal plants exhibit anticancer activities both in vitro and in vivo [4-10]. A growing number of studies indicate that herbal medicine (looking at frequency, type and reasons for use) might have the anti-cancer effect by enhancing the immune system [11], inducing cell differentiation [12], inhibiting telomerase activities [13] and inducing apoptosis of cancer cells [14].
It is strongly believed that herbal medicines are natural and hence without significant side effects and less likely to cause dependency [15]. Nevertheless, many herbs can be toxic especially in higher quantities and with frequent use. Besides, herb-synthetic drug interactions are controversial [16].
The prevalence of herbs use ranges from 60 to 80% among cancer patients depending on the definition of herbal medicine used in each study, sample size, and the place where the study was conducted [17]. In UK, a population-based survey indicates that about 25% of cancer patients had consulted an herbal medicine practitioner in the past, although authors suggest that this number maybe underestimated [18]. A Canadian study shows that 20% of breast cancer patients used at least one herbal medicine treatment in the past[19], whereas American studies more consistently report rates well above 65% [20,21], such rates are considerably higher than those reported in general population [22] or among other cancer diagnostic groups [23].
Despite extensive use of herbal medicines in cancer care, most of the evidence is anecdotal and has not been properly studied with significant clinical trials, especially in human subjects [24]. Further, interaction of chemical drug-herbs should be considered as another important factor [25], because herbs cannot replace surgery or radiotherapy for early stages of cancer, even though it is believed that they do have merits of their own [26].
The objective of this review is to identify the available evidence on the use of herbs and their clinical effects in cancer care. The present work intends to make a review about this subject using ISI Web of Knowledge (Thomson Reuters) database from 2000 up to 2011. In some few cases, references other than 2000 to 2011(publishing date) can be cited as introductory to more recent works. Therefore, some references are mentioned whose dates do not match the period of the study. Search keys used for the study were a combination of: Cancer; Medicinal Herbs; Immunomodulator; Preventive therapy; Safety; 2000 to 2011 (year published). About 100 works were found including proceedings and articles. Patents, abstracts, and other scientific documents whose availability was restricted were not used.
Roles of Herbal Medicines in Cancer
From 200 to 1800 AD, following the teachings of Aristotle and Galen, which was believed that cancer, was a consequence of the coagulation of “black bile” till now when prevalence of biology has contributed to a 25% reduction in mortality [27], herbs play an important role in cancer symptom management, patients' quality of life and survival.
The main objectives of herbal therapies are:
Primary prevention of cancer; this is important for those who have a strong family history of cancer
Secondary presentation; prevention of a recurrence of cancer is therefore the objective for this group
To enhance body's immune system
To reduce the side effects resulting from conventional therapies such as chemotherapy or radiation therapy
In advanced stages of cancer, when conventional therapies have failed, many patients have no choice but resort to alternative treatments
The way herbal medicine fight cancer is significantly different from conventional chemical drugs, where no DNA mutation in surviving cell occurs. Specifically, natural compounds fight cancer by strengthening the immune system preventing the spread of cancer cells through inhibition of angiogenesis or growth of new blood vessels feeding the cancer cells, detoxifying the body and preventing further toxic build-up in the body, quenching free radicals that cause mutational changes that lead to cancer formation and supporting all targeted organs, especially those affected directly by the cancer. Besides creating an unfavourable environment for cancer growth is another benefit of herbal medicines, where, the ideal environment creates a high level of oxygen and temperature including increased metabolism rate, low sugar level and a high alkalinity space in the body [28].
Some of the herbs commonly used in traditional knowledge are listed in Figure 1, 2 and 3 based on active component, chemical structure and source.
Figure 1.
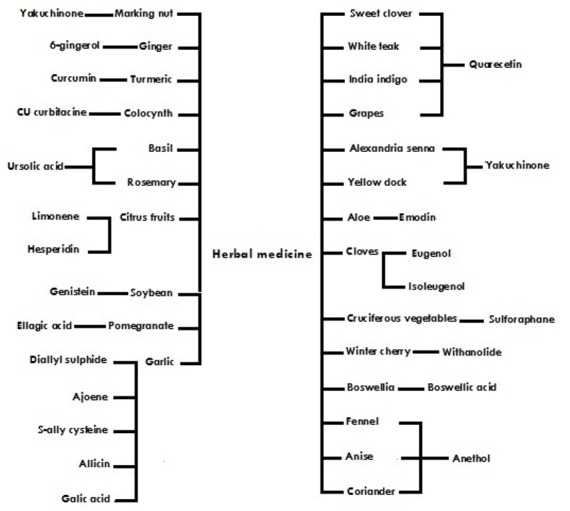
Active components from herbal medicine
Figure 2.
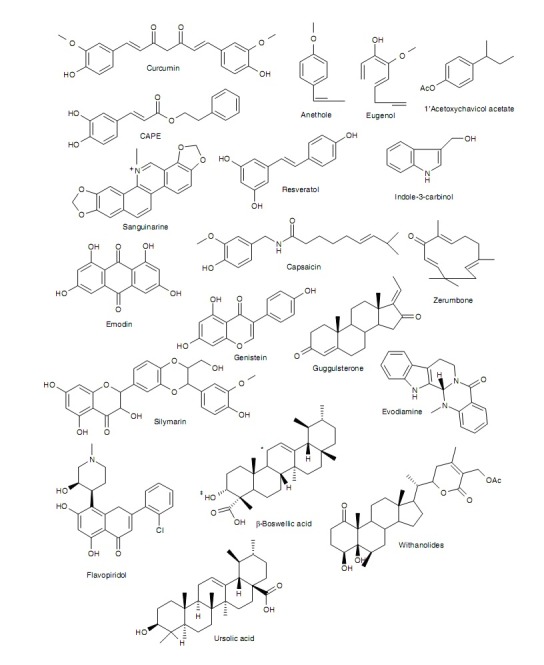
Chemical structures of selected active components in plants. (*can have a ketone group: 11-keto-ß boswellic acid. ‡can be acetylated: acetyl-ß boswellic acid. Both modifications together, result in acetyl- 11-keto-ß-boswellic acid.)
Figure 3.

Sources of traditional drugs
The approach to treat advanced cancer using natural medicines has consisted of two main different visions. Indeed many herbal medicines are widely used as immunomodulators, although another group was known as chemopreventive (adaptogenic) plant compounds [29].
Immunomodulation Versus Chemo Preventive Herbs
An important key role for plant medicines in cancer is immunomodulation. Such natural medicines have been reported to serve as biological response modifiers by activating, increasing and restoring the reactivity of immunological effector mechanisms that are involved in resistance to tumor growth and metastasis [30, 31].
In fact, cancer evades immune system surveillance because of low immunogenicity of most tumors. Nonetheless, many cancer patients with advanced malignancy do have lowered levels of innate (Th1) immunity, the branch of immune system whose cells, such as Natural Killer (NK) cells, directly kill the tumor. A variety of herbal medicines and plant compounds directly stimulate this innate immune response. These same agents can be used to help protect bone marrow against the myelosuppressive effects of conventional chemotherapy. As it is described in figure 4, the two most important classes of herbs here are immunomodulating and adaptogens.
Figure 4.
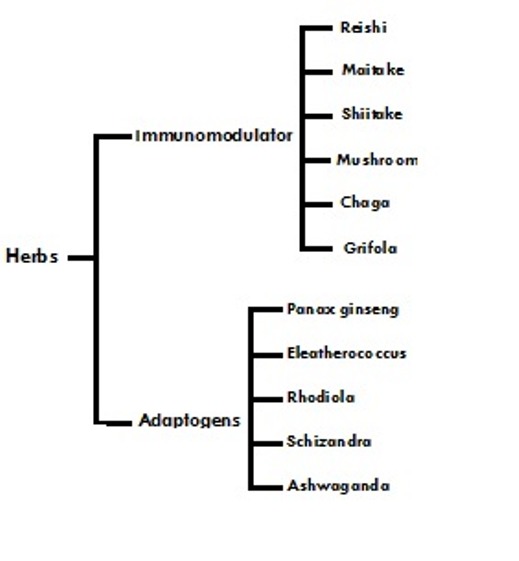
Adaptogenic and immunomdalaling herbs
As it is described in Figure 1, in neither of the cases the herbal medicines have equivalents among pharmaceutical drugs. The mushrooms contain polysaccharides, which are not only immunostimulating of anticancer effects; they also have non-specific effects of increasing longevity and reducing stress. The adaptogenic herbs such as Panax ginseng are even more unique. Adaptogens are nonspecific, nontoxic and normalizing. This means the effect they produce varies according to the physiopathologic state. For instance, ginseng is an angiogenic in wound healing, versus cancer, and it is also antiangiogenic [32]. This apparent paradox is typical of normalizing properties of adaptogens, which also have multiple anticancer effects as well as beneficial interactions with conventional chemotherapy and radiation. Some molecular targets of chemopreventive plants are illustrated in Figure 5. Most of these herbs operate on several of the mentioned targets simultaneously and often synergistically.
Figure 5.
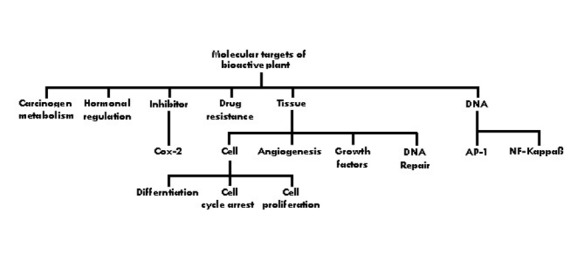
Molecular targets of chemopreventive compounds
Immunomodulation Herbs
Ganoderma lucidum, a highly ranked medicinal mushroom has potent enhancing effect on immune system and anticancer activity. Preclinical studies demonstrated its anti-tumor activity, and further studies indicated that the Polysaccharide (PS) fractions were the major active components for the anti-tumor action [33, 34].
Ganoderma lucidum was found to activate macrophages, T lymphocytes and NK cells and to induce the production of cytokines such as tumor necrosis factor, interleukins and interferons in in-vitro with human immune cells and in vivo in mice [35-37].
Herba taraxucum mongolicum is also shown immune stimulating effects [38]. Studies indicate that its chemical constituents such as taraxasterol, taraxacin, choline, inulin and pectin remove toxic heat, swelling and nodulation.
Sophora flavescens also increases leukocytes and promotes peripheral immune response. Scutellaria baicalensis is another potent heat and toxin- clearing with anti- tumor and immune-stimulating properties in vivo and in vitro that inhibits platelet aggregation and induces apoptosis [39]. Isatis tinctoria contains the compound indirubin, inhibits DNA synthesis in neoplastic cells, while simultaneously stimulating immune response [40].
Also, herbs such as Panax ginseng, Poria cocos, Atractylodes macrocephala, Angelica sinensis, Ligustici wallichii, Paeonia lactiflora, Rehmannia glutinosa and Astragalus membranaceus show an increase of white blood cell counts to normal levels in cancer patients [41].
Decades of pharmacological research have revealed that the polysaccharides and other compounds in Astragalus membranaceus promote cellular and humoral immune function and have vitro anti-tumor effects on cancer cell lines [42-44].
Multiple studies on patients with stomach cancer were conducted using formula Pishen Fang, which has immunostimulating properties. The formula contains: Codonopsis pilolusa, Atractylodes macrocephala, Lycium barbarum, Ligustrum lucidum, Cuscuta chinensis and Psoralea corylifolia [45].
Chemopreventive Herbs
The inhibitory effect of a herbal formula comprising Ginseng and Carthamus tinctorius on breast cancer was studied by Wings et al. [46]. This formula could be a useful anticancer compound against breast cancer by inhibiting proliferation in solid tumor. The compounds isolated from the pacific yew "Taxus brevifolia" has developed as the anticancer drug "Taxol". The extract from plant Scutellaria barbata has been shown to be cytotoxic to A549 human lung cancer cell lines [47, 48]. The synergetic effect on antiproliferative activity of chemotherapeutic agents (Doxorubicin) in combination with Thai herbal remedies (stem of Albizia procera, Diospyros mollis, Ficus hispida, smilax glabra, Gelonium multiflorum and Millingtonia hortensis) against lung cancer cells may induce DNA damage in lung cancer [49]. Amooranin extract (stem) which is a triterpene acid showed a strong inhibitory effect on survival of human breast carcinoma MDA-468 and breast adenocarcinoma MCF-7 cells compared to breast epithelial MCF-10A control cells [50].
Impact of herbal medicines on human breast cancer was studied by many research groups through reducing tumor burden by Resveratrol chemically modified extract [51], inhibition of estrogen-dependent gene transcription by Shikonin (gromwell)[52], cell cycle delay/arrest by Carcinosin, phytolacca, Conium and Thuja [53]and modulating signal pathway by cacalol which is a free radical scavenging compound from Cacalia delphinifolia plant [54]. Ganoderma lucidum, Astragalus mongholicus, polygonatum sibiricum, and Chinese sage herb, were observed to be effective on non-small-cell lung cancer for stage III or IV [55]. Rasagenthi Lehyam (RL) is a siddha medicine, which is a poly herbal formulation for the treatment of cancer in India. It is reported that the chloroform extract of RL inhibited the growth of prostate and lung cancers [56]; its governing mechanism is inhibition of pro-survival genes and up-regulating the pro-apoptotic genes.
Anti-proliferative effect of Melissa officinalis on human colon cancer cell line was well studied by Encalada et al. [57]. This herb’s hydroalcoholic extract also showed significant antioxidative activities by free radical scavenging. Thymoquinone (TQ) is the bioactive- constituent of the volatile oil of black seed whose anti-neoplastic and anti-inflammatory effects were studied by Gali-Muhtasib et al. [58]. The combination of TQ with clinically used anti-cancer drugs led to improvements in their therapeutic index and prevented non-tumor tissues from sustaining chemotherapy-induced damage.
Vinca alkaloids which are isolated from the periwinkle plant Catharanthus roseus, also known as Vinca rosea, possess many therapeutic effects including anti-tumor activity [59]. Vinca alkaloids are most commonly administrated weekly by short IV injection (1-15 min) more rarely by continuous infusion [60].
Other examples of plant-derived compounds fight cancer by inhibition of protein synthesis, and cell-cycle progression blocking are currently under investigation. Extracts isolated from the leaves and stem of Amoora rohituka, alkaloid isolated from Cephalotaxus harringtonia, -lapachone which is a quinine obtained from the bark of the Lapacho tree (Tabebuia avellanedae)and Combretastatin A4 which is isolated from the stem wood of south Africa tree Combretum caffrum are the most important objectives of related researches [61]. Curcumin is one of the most studied chemopreventive agents. It is a natural compound extracted from the rhizome of Curcuma longa that allows suppression, retardation or inversion of carcinogenesis [62-85].Evidence from numerous in vitro and in vivo studies have confirmed Resveratrol's(a polyphenol found in numerous plant species including peanuts and grapes) ability to modulate various targets and signalling pathways [86].
Table1 demonstrates governing mechanism of some herbs including test model and cancer types [87].
Table 1.
Laboratory Experiments of Herbs Anticancer Effects
| Type of Cancer | Model | Herb | Mechanism |
|---|---|---|---|
| HNSCC | SCC-25 and KB cell lines, four nude mice with s.c. inoculation of KB cells | Scutellaria baicalensis | Inhibition of cell growth in vitro and in vivo, inhibition of PGE2 synthesis via suppression of COX-2 expression |
| KB, KB v200 cell lines | Asiaticoside | Induction of apoptosis and enhancement of the anti-tumor activity of Vincristine | |
| Leukemia | U937 cell line | Mylabris phalerlata, Scutellaria barbata, | Induction of apoptosis |
| NB4, HL60 cell line | Red orpiment | Induction of apoptosis | |
| AKR/J mice | Echinacea purpurea | Enhancement of nonspecific immune or cellular immune systems (or of both). | |
| CCRF-CEM,CEM/E1000, CEM/VLB(100)cell lines | Artesunate(ART), Bufalin | ART significantly increased Daunorubicin accumulation in CEM/E1000 cells, but not in CEM/VLB (100) or CCRF-CEM parental cells, Bufalin caused a small, but significant increase in Daunorubicin accumulation in CEM/VLB (100) and CEM/E1000 cells. | |
| NB4 cell line | Arsenic trioxide | Induction of apoptosis | |
| HL-60 cell line | Hydrolysable tannis from Eugenia jambos L. | Induction of apoptosis | |
| HL-60,NB4,U937 and THP-1 cell line | PC-SPES | Inhibition of growth, induction of differentiation and apoptosis. | |
| Colorectal Carcinoma | CoLo205 cell line Mice bearing colon26/ clone 20 carcinRed orpiment oma cells | Magnolol Coptidis rhizome and Berberine | Induction of apoptosis Reduction of IL-6 mRNA levels and protein levels in tumors and sera |
| Gastric Cancer | MGC-803 cell line AGS cell line MNK45 and KATO-III cell line | Isoliquiritigenin Astragali(AR) Anemarrhena asphodeloides | Induction of apoptosis Cytostatic Induction of apoptosis |
| Hepatic Cancer | Hep-G2 cell line SMMC-7221 cell line | Mognolol Isoverbascoside | Induction of apoptosis Induction of differentiation |
| Lung Cancer | A549 cell line Lung cancer cells | Bupleuri radix Triptolide | Inhibition of telomerase activity and activation of apoptosis Induction of apoptosis in combination with Apo2L/ trAIL |
| Breast Cancer | 95-D cell line F344 rats MCF-7 cell line MCF-7cell line MCF-7 and MCF-7/ADM cell line | Acutiaporberine Anticancer-number-one Rosemary Tea and tea polyphenols Asiaticoside | Induction of apoptosis Increasement of NK cell activity increasing and inhibition of tumor metastasis. Reversing MDR Suppression of fatty acid synthase (a key enzyme in lipogenesis) Enhancement of the anti-tumor activity of Vincristine |
| Ovarian Cancer | SKOV3,CAOV3 and OVCAR-cell lines | Scutellaria barbatae | Induction of apoptosis |
| Prostate Cancer | LNCaP cell lines Prostate carcinoma cells Embryoid bodies and multicellular DU-145 prostate tumor spheroids | PC-SPES Equiguard Baicalein, Epicatechin, Berberine,Acteoside | Activation of the JNK/c-Jun/AP-1 signal pathway resulting in growth arrest and apoptosis of prostate cancer cells Down-regulation of expression of androgen receptor and prostate- specific antigen, induction of apoptosis Down- regulation of MMp expression, inhibition of angiogenesis. |
| Glioma | Rat C6 glioma cells Mice that injected with LZEJ-C3 cells subcuta-neously | Saikosaponin a,b Dang-gui-bu- xai-tang | Induction of differentiation increasing the population of activated T helper cells (CD4+CD25+)in spleen and tdLN |
HNSCC: head and neck squamous cell carcinoma; PGE2: prostaglandin E2; COX-2: cyclooxygenase 2; EC: endotheliocytes; CTLs: cytotoxic T lymphocytes; TDLN: tumor-draining lymph nodes.
Anticancer-number-one: Panax ginseng, Poria cocos, Atractylodes macrocephala, Anglica sinensis, A. membranaceus, Curcuma zedoaria,Scutellaria baicalensis, Phellodenron chinense, Coptis chinensis, Glycyrrhiza uralensis, Crataegus pinnatifida, Hordeum ?ulgare, Sal?ia miltiorrhiza, Schisandra chinensis, Hedyotis diffusa, Ophiopogon japonicus, Lobelia chinesis lour, Scutellaria barbaba, Massa fermentata medicinalis; PC-SPES, Reishi mushroom, Baikal skullcap, Rabdosia, Dyer’s woad, Chrysanthemum, Saw palmetto, Panax ginseng, and Licorice; Dang-gui-bu-xai-tang, Radix Angelicae sinensis and Radix Astragali membranaceus.
In addition, some vivo (human) studies are summarized in table2; this table demonstrates herbal medicine effectiveness in cancer treatment [88].
Table 2.
Human Study of Herbs Anticancer Effects
| Herb name | Cancer type | Reported outcome |
|---|---|---|
| Essiac | Prostate | Decrease PSA levels from 87.19 to 0.12 ng/ml |
| PC-SPES |
Prostate |
Less than 50% decrease in PSA level |
| PC-SPES | Prostate | Decrease PSA levels from 100 to 24 ng/ml and386 to114 ng/ml |
| PC-SPES |
Prostate |
Decrease PSA levels from 8.8 to 0.1 ng/ml |
| PC-SPES |
Prostate |
Increase serum PSA levels ranging from345% to 880% after discontinuation of PC-SPES |
| Chinese herbal medicine a | Lung | Complete regression |
| Oriental herbal medicine and Lyophyllum decates sing |
Lung |
Partial response |
| Ninjin yoei To (Traditional Chinese Medicine herbal medicine) | Lung | Decreased tumor marker levels CEA: 14.6 to11.3 ng/ml; CA19-9: 55 to39.2 U/ml |
| Chinese herbal extract (specific herbal component not identified) |
CLL |
Complete remission |
| Ganoderma lucidum | Gastric large B-cell Lymphoma | Complete regression |
| Green Tea | CLL | Partial response |
| Mixture of 36 herbs |
Intracranial tumor(teratoid/rhabdoid tumor) |
Complete response |
| Hochu-ekki-to | Lymphoma (Mycosis fungoides) | Partial improvement of skin eruption |
| Mistletoe |
Malignant melanoma |
Complete remission of liver metastasis |
| Mistletoe | CD 30+ cutaneous lymphoproliferative lymphoma | Complete regression |
| Morinda citrifolia (noni) | Gastric Cancer | Tumor suppression |
| Peruvian herbal tea |
Barrett's adenocarcinoma |
Seven year survival |
| Mixture of 9 herbs | Hepatocellular | Complete regression |
PSA: Prostate Specific Antigen
a Components of Chinese herbal medicine: Herba Hedyotis diffusae, Maidony, Radix ophiopnis, pugongying Herba taraxaci, Sanqi Radix notoginseng, shancigu pseudobulbus, Cremastrae seupleiones, Xiyangshen Radix Panacis quinqufolii, Yuxingcao Herba houttuyniae, Zhebeimu Bulbus Fritillariae thunbergii, Zhibanxia Rhizoma pinelliae perparata.
Anticancer Herbs' Safety
Herb safety and herb-drug interactions are complex and controversial issues. With the increasing use of herbs, their potential abuse and toxicity effects should be considered legitimately. The safety of a drug, herb or a complex compound is always relative and contextual. Safety is determined by defining the conditions under which a substance is considered to be safe or dangerous and weighing potential benefits against possible short and long-term adverse effect.
As a matter of fact, compared to the record of approved pharmaceutical drugs with a few well-known exceptions, medicinal herbs are safer [89]. Common use of herbs is rarely associated with adverse effects that are not easily reversible. These effects are seldom serious and include such transient reactions as: hot flashes, dizziness, headache, indigestion and rashes that are rapidly abated by discontinued use or dose reduction [90].
The preponderance of evidence shows that when used as an adjunct to conventional medicine, herbs both enhance the desired effects and mitigate the harmful ones.
Conclusion
It is estimated that more than 70% of the world's population cannot afford modern cancer medicines. In addition to cost, current cancer therapies are minimally effective and exhibit toxicities that are intolerable in most cases.
By this review, evidence presents that agents derived from plants used in herbal medicine can be used not only to prevent cancer but also to treat it. Because of their pharmacological safety, these agents can be used alone or as adjacent to current chemotherapeutic agents to enhance therapeutic effects and minimize chemo therapy-induced toxicity.
This research indicates that the molecular targets of chemopreventive agents are similar to those currently used to treat cancer. It is also evident that more research is required on herbal medicine to ensure and reach their full therapeutic potential.
Acknowledgments
The authors thank Kish Sayan Herbal Company for funding this research.
Footnotes
Conflicts of Interest
The authors have no conflict of interest in this article.
Authors' Contribution
Javad Tavakoli designed the study and wrote the manuscript. Solaleh Miar contributed to the data entry, literature review and writing-up process. Mohammad Majid Zarezadeh and Hossein Akbari contributed to searching process.
REFERENCES
- 1.Vorobiof DA, Abratt R. The cancer burden in Africa. South African Medicine Journal. 2007;97:937–9. [PubMed] [Google Scholar]
- 2.Kuete V, Efferth T. Pharmacogenomics of Cameroonian traditional herbal medicine for cancer therapy. Journal of Ethnopharmacology. 2011;137:752–66. doi: 10.1016/j.jep.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Yin X, Zhou J, Jie C, Xing D, Zhang Y. Anticancer activity and mechanism of Scutellaria barbata extract on human lung cancer cell line A549. Life Sci. 2004;75:2233–44. doi: 10.1016/j.lfs.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Gill CI, Boyd A, McDermott E, McCann M, Servili M, Selvaggini R, et al. Potential anticancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int J Cancer. 2005;117(1):1–7. doi: 10.1002/ijc.21083. [DOI] [PubMed] [Google Scholar]
- 5.Yevhen F, Oksana F, Rostyslav S. Transforming growth factor beta-1 enhances cytotoxic effect of doxorubicin in human lung adenocarcinoma cells of A549 line. Cell Biol Int. 2007;31:851–5. doi: 10.1016/j.cellbi.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Rockwell S, Liu Y, Higgins SA. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 2005;90:233–9. doi: 10.1007/s10549-004-4260-x. [DOI] [PubMed] [Google Scholar]
- 7.Notani PN. Global variation in cancer incidence and mortality. Curr Sci. 2001;81:465–74. [Google Scholar]
- 8.Vatanasapt V, Sriamporn S, Vatanasapt P. Cancer control in Thailand. Jpn J Clin Oncol. 2002;32:82–91. doi: 10.1093/jjco/hye134. [DOI] [PubMed] [Google Scholar]
- 9.Pongnikorn S, Fongmoon D, Kasinrerk W, Limtrakul PN. Effect of bitter melon (Momordica charantia Linn) on the level and function of natural killer cells in cervical cancer patients with radiotherapy. J Med Assoc Thai. 2003;86(1):61–8. [PubMed] [Google Scholar]
- 10.Lawrence CM, Chiu Ho, Elanine YL, Vincent EC. Ethyl acetate extract of Patrinia scabiosaefolia downregulates anti-apoptotic Bcl-2/Bcl-XL expression, and induces apoptosis in human breast carcinoma MCF-7 cells independent of caspase-9 activation. J Ethno. 2006;105:263–8. doi: 10.1016/j.jep.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Hu Z, YangX X, Huang M, Gao Y, Tang W, et al. Monitoring of immune responses to a herbal immunomodulator in patients with advanced colorectal cancer. International Immunopharmacology. 2006;6:499–508. doi: 10.1016/j.intimp.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Jing Y, Nakajo S, Xia L, Nakaya K, Fang Q, Waxman S, et al. Boswellic acid acetate induces differentiation and apoptosis in leukemia cell lines. Leukemia Research . 1999;23(1):43–50. doi: 10.1016/s0145-2126(98)00096-4. [DOI] [PubMed] [Google Scholar]
- 13.Baum M, Ernst E, Lejeune S, Horneber M. Role of complementary and alternative medicine in the care of patients with breast cancer: Report of the European Society of Mastology (EUSOMA) Workshop, Florence, Italy, December 2004. European journal of cancer. 2006;42:1702–10. doi: 10.1016/j.ejca.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Lian Z, Niwa K, Gao J, Tagami K, Mori H, Tamaya H. Association of cellular apoptosis with anti-tumor effects of the Chinese herbal complex in endocrine-resistant cancer cell line. Cancer Detection and Prevention. 2003;27:147–154. doi: 10.1016/s0361-090x(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 15.Mahady G. global harmonisation of herbal health claims. journal of nutrition. 2001;131:162–96. doi: 10.1093/jn/131.3.1120S. [DOI] [PubMed] [Google Scholar]
- 16.Fong H. Interaction of herbal medicine into modern medical practices: issues and prospects. Integrative cancer therapies. 2002;1:3287–93. doi: 10.1177/153473540200100313. [DOI] [PubMed] [Google Scholar]
- 17.Digianni LM, Garber JE, Winer EP. Compelementary and alternative medicine use amang women with breast cancer. J clin oncol. 2002;20(18):34–8. [PubMed] [Google Scholar]
- 18.Rees RW, Feigel I, Vickers A, Zollman C, Mc Gurk R, Smith C. prevalence of complementary therapy use by women with breast cancer:a population based survey. Eur J Cancer. 2000;36:1354–64. doi: 10.1016/s0959-8049(00)00099-x. [DOI] [PubMed] [Google Scholar]
- 19.Gray RE, Fitch M, Goel V, Franssen E, labrecquem A. Otizilation of complementary/alternative services by women with breast cancer. J Health Soc Policy. 2003;16:75–84. doi: 10.1300/J045v16n04_04. [DOI] [PubMed] [Google Scholar]
- 20.Ashikaga T, Bosompark A, OBrien P, Nelson L. Use of complementary and alternative medicine in breast cancer patients: prevelance, patterns and communication with physicians. Support care cancer. 2002;10:542–8. doi: 10.1007/s00520-002-0356-1. [DOI] [PubMed] [Google Scholar]
- 21.Henderson JW, Donattele RJ. Complementary and alternative medicine use by women after completion of allopatice treatment for breast cancer. Altern ther health med. 2004;10(1):1052–7. [PubMed] [Google Scholar]
- 22.Vander Creek L, Rogers E, Lester J. Use of alternative therapies among breast cancer outpatients compared with the general population. Altern ther health med. 1998;5:71–6. [PubMed] [Google Scholar]
- 23.Morris KT, Johnson N, Homer L, Walts D. A comparison of complementary therapy use between breast cancer patients and with other primary tumor sites. Am J Surg. 2009;179:407–11. doi: 10.1016/s0002-9610(00)00358-5. [DOI] [PubMed] [Google Scholar]
- 24.Wachtel-Galor S, Tomlinson B, Benzie I. Ganoderma lucidum (‘‘Lingzhi’’), a Chinese medicinal mushroom: biomarker responses in a controlled human supplementation study. British Journal of Nutrition. 2004;91:263–9. doi: 10.1079/BJN20041039. [DOI] [PubMed] [Google Scholar]
- 25.Yarnell E, Abascal K. Overview of drug-herb interactions. Alternative & Complementary Therapies. 2002;8:87–96. [Google Scholar]
- 26.Taixiang W, Wei X, Yang X, Zhiyu C. Medicinal herbs for esophageal cancer. Cochrane Database System Review. 2007;24(1):CD004520. doi: 10.1002/14651858.CD004520.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20-69 years. Lancet. 2000;355(9217):1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- 28.Lam M. Beating Cancer with Natural Medicine: Printed in the United States of America Bloomington, IN. Natural medicine. 2003:80–85. [Google Scholar]
- 29.Treasure J. Herbal medicine and cancer: an introductory overview. Seminars in Oncology Nursing. 2005;21(3):177–83. doi: 10.1016/j.soncn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Wasser SP, Weis AL. Therapeutic effects of substances occurring in higher basidiomycetes mushrooms: a modern perspective. Crit Rev Immuno. 1999;19:65–96. [PubMed] [Google Scholar]
- 31.Werner GH, Jolles P. Immunostimulating agents-what next-a review of their present and potential medical applications. Eur J Biochem. 1996;242:1–19. doi: 10.1111/j.1432-1033.1996.0001r.x. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, et al. Modulating angiogenesis: The yin and the yang in ginseng. Circulation. 2004;110:1219–25. doi: 10.1161/01.CIR.0000140676.88412.CF. [DOI] [PubMed] [Google Scholar]
- 33.Chang R. Seoul7 II Yang Press.; 1996. The central importance of the beta-glucan receptor as the basis of immunologic bioactivity of Ganoderma polysaccharides. pp. 177–9. [Google Scholar]
- 34.Gao Y, Gao H, Chan E, Tang W, Xu A, Yang H, et al. Antitumor activity and underlying mechanisms of Ganopoly, the refined polysaccharides extracted from Ganoderma lucidum, in mice. Immunol Invest. 2005;34:171–98. [PubMed] [Google Scholar]
- 35.Wang SY, Hsu ML, Hsu HC, Tzeng CH, Lee SS, Shiao MS. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer. 1997;70:699. doi: 10.1002/(sici)1097-0215(19970317)70:6<699::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Zhou SF, Gao YH. The immunomodulating effects of Ganoderma lucidum (Curt. Fr) P. Karst (Ling Zhi, Reishi mushroom) (Aphyllophoromycetideae). Int J Med Mushroom. 2002;4:1–11. [Google Scholar]
- 37.Gao YH, Zhou SF, Jiang WQ, Huang M, XH D. Effect of Ganopoly, a Ganoderma lucidum polysaccharide extract on the immunological function in advanced-stage cancer patients. Immunol Invest. 2003;32:201–15. doi: 10.1081/imm-120022979. [DOI] [PubMed] [Google Scholar]
- 38.Beinfield H, Korngold E. Chinese medicine and cancer care. Alternative therapies. 2003;9(5):38–52. [PubMed] [Google Scholar]
- 39.Chang HM, But PPH. Pharmacology and Applications of Chinese Materia Medica. Vol 1. Teaneck, NJ: World Scientific Publishing Company; 1986. p. 112. [Google Scholar]
- 40.Chang HM, But PPH. Pharmacology and Applications of Chinese Materia Medica. Vol 2. Teaneck, NJ: World Scientific Publishing Company; 1987. p. 695. [Google Scholar]
- 41.Shen R, Zhan Z. Clinical study of the use of ginseng and tang-kuei ten combination in the treatment of leukopenia. Int J Oriental Med. 1997;22:30–1. [Google Scholar]
- 42.Sun Y, Hersh EM, Lee SL, McLaughlin M, Loo TL, Mavligit GM. Preliminary observations on the effects of the Chinese medicinal herbs Astragalus membranaceus and Ligustrum lucidum on lymphocyte blastogenic responses. J Biol Response Mod. 1983;2(3):227–37. [PubMed] [Google Scholar]
- 43.Chu D, Wong W, Giora M, Mavligit GM. Immunotherapy with Chinese medicinal herbs I, Immune restoration of local xenogenic graft-versus-host reaction in cancer patients by fractionated astragalus membranaceus in vitro. J Clin Lab Immunol. 1988;25:119–23. [PubMed] [Google Scholar]
- 44.Chu DT, Lin JR, Wong W. The in vitro potentiation of LAK cell cytotoxicity in cancer and AIDS patients induced by F3-a fractionatedextract of astragalus membranaceus. Zhonghua Zhong Liu Za Zhi. 1994;16:167–71. [PubMed] [Google Scholar]
- 45.Mingi P, Xiuzhuang C. Cancer Treatment with Fu Zheng Pei Ben Principle. Fuzho, China: Fujian Science and Technology Publishing House; 1992. p. 34. [Google Scholar]
- 46.Loo W, Cheung M, Chow L. The inhibitory effect of a herbal formula comprising ginseng and carthamus tinctorius on breast cancer. Life Sciences. 2004;76:191–200. doi: 10.1016/j.lfs.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 47.Hennenfent KL, Govindan R. Novel formulations of taxanes: a review. Old wine in a new bottle? . Ann Oncol. 2006;17:735–49. doi: 10.1093/annonc/mdj100. [DOI] [PubMed] [Google Scholar]
- 48.Rowinsky EK, Calvo E. Novel agents that target tubulin and related elements. Semin Oncol. 2006;33:421–35. doi: 10.1053/j.seminoncol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Srisapoomi T, jiratchariyakul W, O-partkiatikul N. effect of tow thai herbal remedies on the sensitivity of chemotherapeutic agents in human cancer cells. Asian journal of traditional medicines. 2008;3(4):144–52. [Google Scholar]
- 50.Rabi T, Wang L, Banerjee S. Novel triterpenoid 25-hydroxy-3-oxoolean-12-en-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Breast Cancer Res Treat. 2007;101:27–36. doi: 10.1007/s10549-006-9275-z. [DOI] [PubMed] [Google Scholar]
- 51.E Aiyar, Park H, B Aldo, Mor G, J Gildea, L Miller, et al. TMS, a chemically modified herbal derivative of Resveratrol, induces cell death by targeting Bax. Breast Cancer Res Treat. 2010;124:265–77. doi: 10.1007/s10549-010-0903-2. [DOI] [PubMed] [Google Scholar]
- 52.Yao Y, Zhou Q. A novel antiestrogen agent Shikonin inhibits estrogen-dependent gene transcription in human breast cancer cells. Breast Cancer Res Treat. 2010;121:233–40. doi: 10.1007/s10549-009-0547-2. [DOI] [PubMed] [Google Scholar]
- 53.Frenkel M, Mishra B, Sen S, Yang P, Pawlus A, Vence L, et al. Cytotoxic effects of ultra-diluted remedies on breast cancer cells. International journal of oncology. 2010;36:395–403. [PubMed] [Google Scholar]
- 54.Liu W, Furuta E, Shindo K, Watabe M, Xing F, Pandey P, et al. Cacalol, a natural sesquiterpene, induces apoptosis in breast cancer cells by modulating Akt-SREBP-FAS signaling pathway. Breast Cancer Res Treat. 2011;128:57–68. doi: 10.1007/s10549-010-1076-8. [DOI] [PubMed] [Google Scholar]
- 55.Xu ZY, Jin CJ, Zhou CC, Wang ZQ, Zhou WD, Deng HB, et al. Treatment of advanced non-small-cell lung cancer with Chinese herbal medicine by stages combined with chemotherapy. J Cancer Res Clin Oncol. 2011;137:1117–22. doi: 10.1007/s00432-011-0975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabi T, Wang L, Banerjee S. Novel triterpenoid 25-hydroxy-3-oxoolean-12-en-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Breast Cancer Res Treat. 2007;101:27–36. doi: 10.1007/s10549-006-9275-z. [DOI] [PubMed] [Google Scholar]
- 57.Encalada MA, Hoyos KM, Rehecho S, Berasategi I, García-Íñiguez de Ciriano M, Ansorena D, et al. Anti-proliferative Effect of Melissa officinalis on Human Colon Cancer Cell Line. Plant Foods Hum Nutr. 2011;66(4):328–34. doi: 10.1007/s11130-011-0256-y. [DOI] [PubMed] [Google Scholar]
- 58.Gali-Muhtasib H, Roessner A, Schneider-Stock R. Thymoquinone: A promising anti-cancer drug from natural sources. The International Journal of Biochemistry & Cell Biology. 2006:1249–53. doi: 10.1016/j.biocel.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Fahy J. Modifications in the “upper” velbenamine part of the Vinca alkaloids have major implications for tubulin interacting activities. Curr Pharm Des. 2001;7:1181–97. doi: 10.2174/1381612013397483. [DOI] [PubMed] [Google Scholar]
- 60.Leveque D, Jehl F. Molecular pharmacokinetics of catharanthus (vinca) alkaloids. J Clin Pharmacol. 2007;47:579–88. doi: 10.1177/0091270007299430. [DOI] [PubMed] [Google Scholar]
- 61.Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, et al. Natural compounds for cancer treatment and prevention. Pharmacological Research. 2009;59:365–78. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 62.Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181–90. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 63.Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170–81. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Bharti A, Donato N, Aggarwal B. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–71. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 65.Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002;23:143–50. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- 66.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal B. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene . 2001;20:7597–609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 67.Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852–61. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 68.Aggarwal B, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anti-Cancer Res. 2003;23:363–98. [PubMed] [Google Scholar]
- 69.Shishodia S, Potdar P, Gairola CG, Aggarwal BB. Curcumin (diferuloylmethane) downregulates cigarette smoke-induced NfkappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003;24:1269–79. doi: 10.1093/carcin/bgg078. [DOI] [PubMed] [Google Scholar]
- 70.Bharti AC, Takada Y, Aggarwal B. Curcumin (diferuloylmethane) inhibits receptor activator of NF-kappa B ligand-induced NF-kappa B activation in osteoclast precursors and suppresses osteoclastogenesis. J Immunol. 2004;172:5940–47. doi: 10.4049/jimmunol.172.10.5940. [DOI] [PubMed] [Google Scholar]
- 71.Bharti AC, Shishodia S, Reuben JM, Weber D, Alexanian R, Raj-Vadhan S, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–84. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 72.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factorkappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–62. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 73.Aggarwal S, Takada Y, Singh S, Myers JN, Aggarwal BB. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer. 2004;111:679–92. doi: 10.1002/ijc.20333. [DOI] [PubMed] [Google Scholar]
- 74.Aggarwal BB, Takada Y, Oommen OV. From chemoprevention to chemotherapy: common targets and common goals. Expert Opin Investig Drugs. 2004;13:1327–38. doi: 10.1517/13543784.13.10.1327. [DOI] [PubMed] [Google Scholar]
- 75.Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–62. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 76.Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–40. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 77.Takada Y, Bhadwaj A, Potdar P, Aggarwal BB. Nonsteroidal antiinflammatory agents differ in their ability to suppress NF-kappaB activation, inhibition of expression of cyclooxygenase-2 and cyclin D1, and abrogation of tumor cell proliferation. Oncogene. 2004;23:9247–58. doi: 10.1038/sj.onc.1208169. [DOI] [PubMed] [Google Scholar]
- 78.Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann NY Acad Sci. 2004;1030:434–41. doi: 10.1196/annals.1329.054. [DOI] [PubMed] [Google Scholar]
- 79.Bharti AC, Takada Y, Aggarwal BB. PARP cleavage and caspase activity to assess chemosensitivity. Methods Mol Med. 2005;111:69–78. doi: 10.1385/1-59259-889-7:069. [DOI] [PubMed] [Google Scholar]
- 80.Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogenactivated/ extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104:879–90. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- 81.Shishodia S, Amin H, LAI R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–13. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 82.Yan C, Jamaluddin MS, Aggarwal B, Myers J, Boyd DD. Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol Cancer Ther. 2005;4:233–41. [PubMed] [Google Scholar]
- 83.Shishodia S, Sethi G, Aggarwal BB. Curcumin: Getting back to the roots. Ann N Y Acad Sci. 2005;1056:206–17. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 84.Aggarwal BB, Kumar A, Bharti AC. Packe L, Ong CN, Halliwell B. Herbal and Traditional Medicine. New York: Marcel Dekker; 2004. Therapeutic potential of curcumin derived from turmeric (Curcuma longa). pp. 103–20. [Google Scholar]
- 85.Aggarwal BB, Kumer S, Aggarwal S, Shishodia S. Curcumin derived from turmeric (Curcuma longa): A spice for all seasons. Phytochmeicals in Cancer Chemoprevention, Bagchi D, Preuss HG (Eds.). CRC press. 2005:90–113. [Google Scholar]
- 86.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–83. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wen-jing R, Mao-de L, Jian-guang Z. Anticancer effects of Chinese herbal medicine, science or myth? Journal of Zhejiang University Science B. 2006;7(12):1006–14. doi: 10.1631/jzus.2006.B1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olaku O, White JD. Herbal therapy use by cancer patients: A literature review on case reports. European journal of cancer. 2011;47(4):508–14. doi: 10.1016/j.ejca.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lazarou J, Pomeranz BH, Corey PN. Incidence of Adverse Drug Reactions in Hospitalized Patients: A Meta-analysis of prospective studies. JAMA. 1998;279(15):1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 90.Oh B, Hu G, Kao S, Gebski V, Walls R, Truong L, et al. The Safety and Tolerability of Chinese Herbal Medicine in Cancer Patients Receiving Chemotherapy: Pilot Study. WebmedCentral CHINESE MEDICINE. 2011;2(3):WMC001671. [Google Scholar]


