Abstract
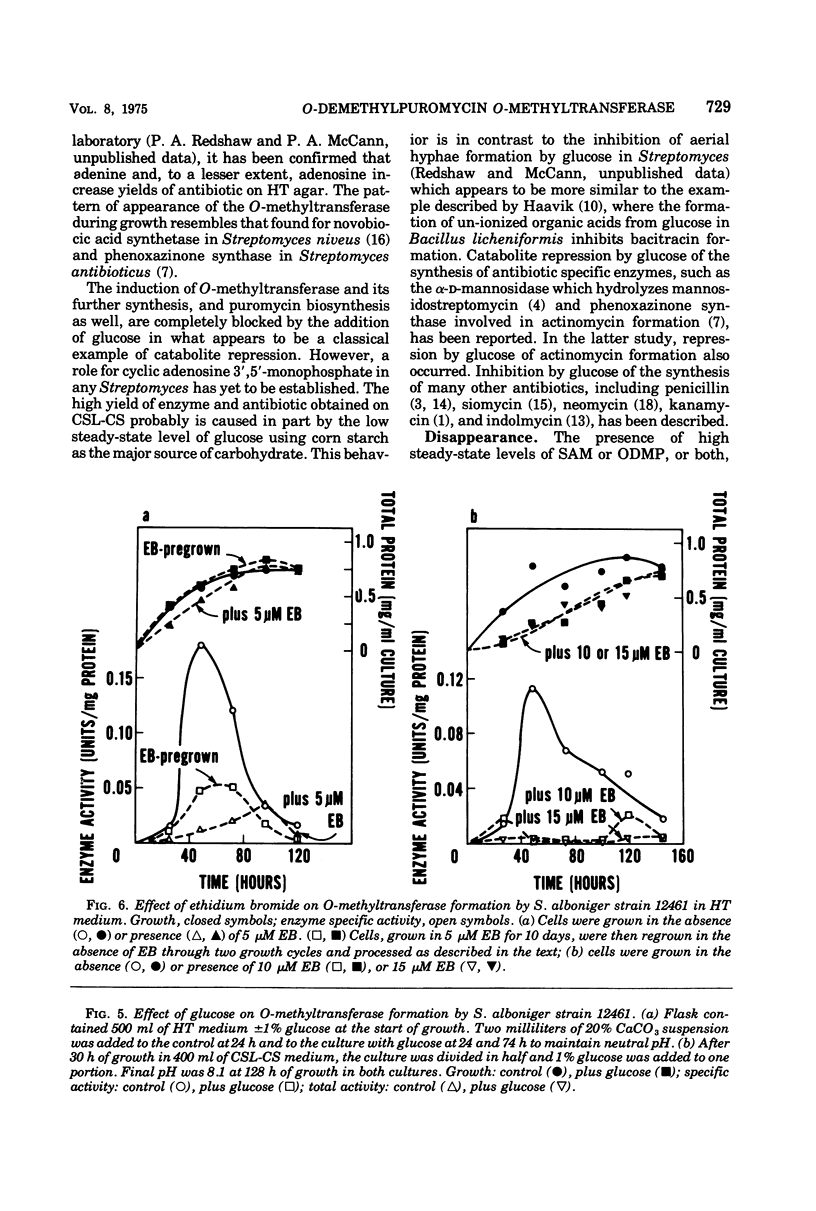
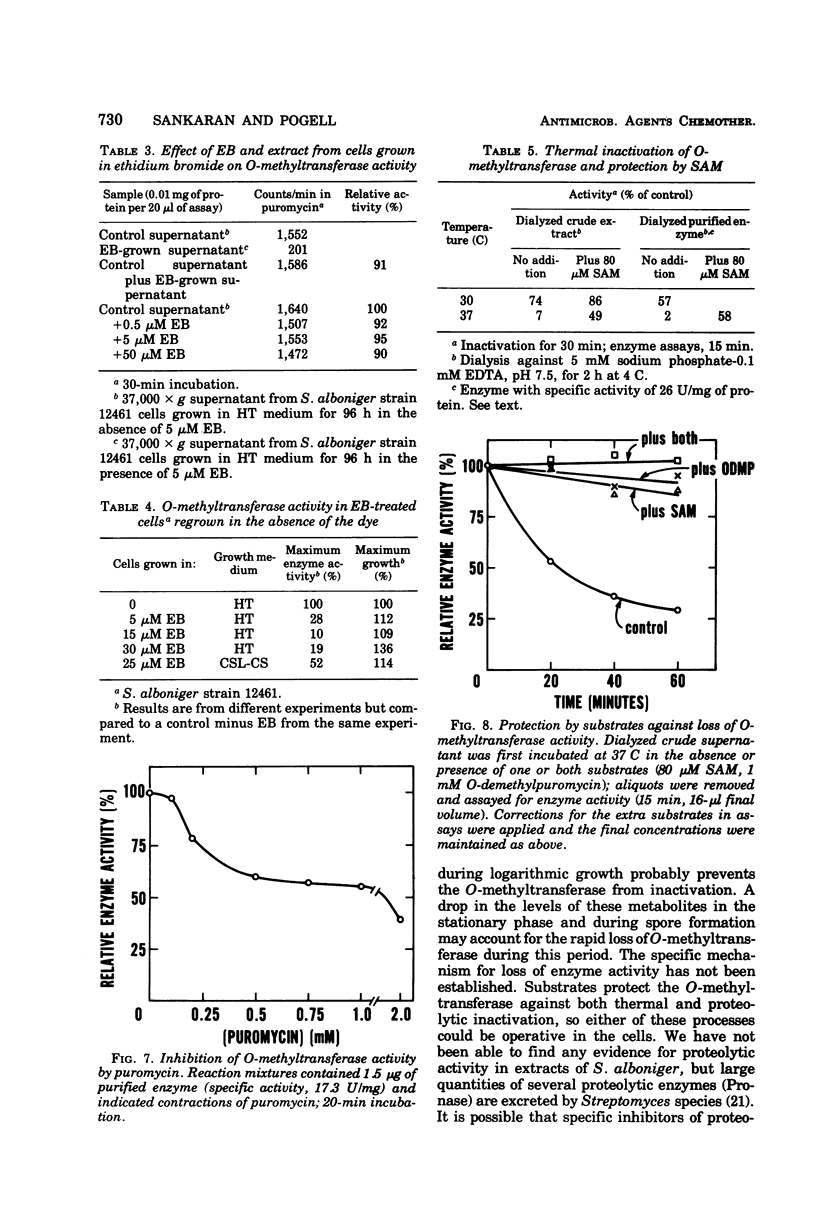
Mechanisms for regulation of puromycin biosynthesis in Streptomyces alboniger were studied by measuring the levels of S-adenosyl-l-methionine:O-demethylpuromycin O-methyltransferase. The enzyme was released in soluble form from mycelia by 3 to 5 min of sonication at 4 C. Maximal specific activities of 0.7 and 0.1 nmol/min per mg of protein were found in cells grown in corn steep liquor-corn starch and Hickey-Tresner media, respectively. In both media, the O-methyltransferase activity rose from low levels to a maximum during midlogarithmic growth and then declined or disappeared completely (in Hickey-Tresner medium) during stationary phase. Either glucose (1%) or ethidium bromide (5 μM) reduced O-methyltransferase formation to very low levels with no effect on overall growth. Complete glucose repression of antibiotic formation occurred on agar. Cells grown in the presence of ethidium bromide continued to produce low enzyme levels after regrowth in the absence of dye, but formed normal amounts of puromycin on Hickey-Tresner agar. The O-methyltransferase, either crude or purified, was rapidly inactivated at 37 C. Each substrate alone, or both together at lower concentrations, protected against this loss of activity. Puromycin inhibited the transferase. Regulation of O-methyltransferase synthesis in S. alboniger includes (i) induction early in growth that is susceptible to catabolite repression and differential inhibition by ethidium bromide, and (ii) protection of the enzyme from inactivation by increased intracellular levels of its substrates. The O-methyltransferase was purified 30- to 40-fold by a combination of protamine sulfate precipitation, ammonium sulfate fractionation, adsorption and gradient salt elution from diethylaminoethyl-cellulose and Sephadex G-200 gel filtration. The enzyme was very unstable, even at low temperatures, upon purification beyond the salt fractionation step, but was stabilized by the addition of S-adenosyl-l-methionine during later stages of purification.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basak K., Majumdar S. K. Utilization of carbon and nitrogen sources by Streptomyces kanamyceticus for kanamycin production. Antimicrob Agents Chemother. 1973 Jul;4(1):6–10. doi: 10.1128/aac.4.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain A. L., Inamine E. Biochemistry and regulation of streptomycin and mannosidostreptomycinase (alpha-D-mannosidase) formation. Bacteriol Rev. 1970 Mar;34(1):1–19. doi: 10.1128/br.34.1.1-19.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D. A., Datta P. Catabolite inactivation of biodegradative threonine dehydratase of Escherichia coli. Biochemistry. 1975 Apr 22;14(8):1760–1767. doi: 10.1021/bi00679a031. [DOI] [PubMed] [Google Scholar]
- GREGORY K. F., HUANG J. C. TYROSINASE INHERITANCE IN STREPTOMYCES SCABIES. II. INDUCTION OF TYROSINASE DEFICIENCY BY ACRIDINE DYES. J Bacteriol. 1964 Jun;87:1287–1294. doi: 10.1128/jb.87.6.1287-1294.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo M., Katz E. Regulation of secondary metabolite biosynthesis: catabolite repression of phenoxazinone synthase and actinomycin formation by glucose. J Bacteriol. 1972 Feb;109(2):659–667. doi: 10.1128/jb.109.2.659-667.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HICKEY R. J., TRESNER H. D. A cobalt-containing medium for sporulation of Streptomyces species. J Bacteriol. 1952 Dec;64(6):891–892. doi: 10.1128/jb.64.6.891-892.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavik H. I. Studies on the formation of bacitracin by Bacillus licheniformis: role of catabolite repression and organic acids. J Gen Microbiol. 1974 Oct;84(2):321–326. doi: 10.1099/00221287-84-2-321. [DOI] [PubMed] [Google Scholar]
- Holzer H., Betz H., Ebner E. Intracellular proteinases in microorganisms. Curr Top Cell Regul. 1975;9:103–156. doi: 10.1016/b978-0-12-152809-6.50011-1. [DOI] [PubMed] [Google Scholar]
- Hurley L. H., Bialek D. Regulation of antibiotic production: catabolite inhibition and the dualistic effect of glucose on indolmycin production. J Antibiot (Tokyo) 1974 Jan;27(1):49–56. doi: 10.7164/antibiotics.27.49. [DOI] [PubMed] [Google Scholar]
- JOHNSON M. J. Recent advances in penicillin fermentation. Bull World Health Organ. 1952;6(1-2):99–121. [PMC free article] [PubMed] [Google Scholar]
- Kominek L. A. Biosynthesis of novobiocin by Streptomyces niveus. Antimicrob Agents Chemother. 1972 Feb;1(2):123–134. doi: 10.1128/aac.1.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Majumdar M. K., Majumdar S. K. Synthesis of neomycin by washed mycelium of Streptomyces fradiae and some physiological considerations. Folia Microbiol (Praha) 1971;16(4):285–292. doi: 10.1007/BF02872809. [DOI] [PubMed] [Google Scholar]
- Malik V. S., Vining L. C. Effect of chloramphenicol on its biosynthesis by Streptomyces species 3022a. Can J Microbiol. 1972 Feb;18(2):137–143. doi: 10.1139/m72-023. [DOI] [PubMed] [Google Scholar]
- Molano J., Gancedo C. Specific inactivation of fructose 1,6-bisphosphatase from Saccharomyces cerevisiae by a yeast protease. Eur J Biochem. 1974 May 2;44(1):213–217. doi: 10.1111/j.1432-1033.1974.tb03475.x. [DOI] [PubMed] [Google Scholar]
- PRIDHAM T. G., ANDERSON P., FOLEY C., LINDENFELSER L. A., HESSELTINE C. W., BENEDICT R. G. A selection of media for maintenance and taxonomic study of Streptomyces. Antibiot Annu. 1956:947–953. [PubMed] [Google Scholar]
- Pattabiraman T. N., Pogell B. M. Biosynthesis of puromycin in Streptomyces alboniger. Possible precursors of the antibiotic in a commercial sample. Biochim Biophys Acta. 1969 May 20;182(1):245–247. doi: 10.1016/0005-2787(69)90539-5. [DOI] [PubMed] [Google Scholar]
- Rao M. M., Rebello P. F., Pogell B. M. Biosynthesis of puromycin in Streptomyces alboniger. Enzymatic methylation of O-demethylpuromycin. J Biol Chem. 1969 Jan 10;244(1):112–118. [PubMed] [Google Scholar]
- Sankaran L., Pogell B. M. A simple, one-step assay for methyltransferase activity: direct partitioning of radioactive product into scintillation fluid. Anal Biochem. 1973 Jul;54(1):146–152. doi: 10.1016/0003-2697(73)90257-1. [DOI] [PubMed] [Google Scholar]
- Sankaran L., Pogell B. M. Differential inhibition of catabolite-sensitive enzyme induction by intercalating dyes. Nat New Biol. 1973 Oct 31;245(148):257–260. doi: 10.1038/newbio245257a0. [DOI] [PubMed] [Google Scholar]


