Abstract
Prostate cancer, the leading incidence of cancer in American males, is a disease in which treatment of nonlocalized tumors remains largely unsuccessful. These cancers lose expression of an arginine synthesis enzyme, argininosuccinate synthetase (ASS), and are susceptible to arginine deprivation by arginine deiminase (ADI). We show CWR22Rv1 prostate cancer cells are susceptible to ADI in a caspase-independent manner in vitro and in a xenograft model in vivo. We demonstrate that single amino acid deprivation by ADI is able to trigger autophagy. Inhibition of autophagy by chloroquine and siRNA enhances and accelerates ADI-induced cell death, suggesting that autophagy is a protective response to ADI, at least in the early phases. In addition, the co-administration of docetaxel, a caspase-dependent chemotherapy, with ADI inhibits tumor growth in vivo. Thus, targeting multiple cell death pathways, either through autophagy modulation or non-canonical apoptosis, may find expanded use as adjuvant chemotherapies, providing additional avenues for cancer treatment.
Keywords: autophagy, arginine deiminase, arginine deprivation, caspase-independent apoptosis, prostate cancer
Recently, there is renewed interest in agents that cause metabolic stress as an alternative or adjunctive therapy for cancer to overcome resistance to conventional genotoxic agents. Amino acid deprivation as an anticancer therapy has long been recognized. A well-known example is asparagine-deprivation by asparaginase for acute lymphoblastic leukemias. Similarly, arginase-based treatment for lymphosarcoma and hepatoma has been reported in experimental models. Arginase, however, has received little attention clinically, due to sub-optimal properties of the purified enzyme from bovine tissues. Arginine is a semi-essential amino acid that is manufactured by the enzyme ASS (argininosuccinate synthetase) (Fig. 1). For reasons not well understood, in the development of certain cancers there is a selection against ASS expression, rendering the cancer cells auxotrophic for arginine. Consequently, arginine depletion by either arginase or arginine deiminase (ADI) will lead to selective tumor cell death.
Figure 1.
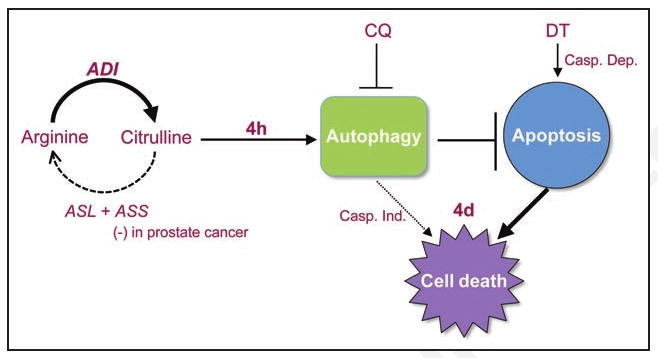
Proposed model of ADI-induced cell death. Arginine is synthesized in a two-step process catalyzed by the rate-limiting enzyme argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL). Arginine deiminase (ADI) hydrolyzes arginine back into its citrulline precursor (solid arrow). In prostate tumors that lack ASS, citrulline cannot re-enter the arginine synthesis pathway (dashed arrow), resulting in the depletion of intracellular arginine and the accumulation of citrulline upon ADI treatment. ADI treatment leads to a rapid induction of autophagy (4 hours) as a protective response to delay apoptosis at 4 days. Prolonged autophagy, however, may also contribute to caspase-independent cell death (dotted arrow). Cell death can be enhanced using chloroquine (CQ), an autophagic inhibitor, or docetaxel (DT), a caspase-dependent apoptotic inducer.
ADI is an enzyme isolated from Mycoplasma that effectively metabolizes arginine into citrulline. At physiological pH, ADI is 300× more effective than arginase at depleting arginine. Antigenicity is decreased by conjugation to polyethylene glycol (PEG), which also increases enzyme half-life. Pegylated modification of recombinant ADI (DesigneRx, California) has dramatically improved its prospects as a therapeutic agent. PEG-ADI is efficacious against hepatocellular carcinomas and melanomas in vitro and in vivo. In particular, phase I/II clinical trials in these patients yield significant response rates with mild side effects. The efficacy of ADI on hepatocellular carcinomas and melanomas is correlated with ASS deficiency. Strikingly, in our analysis of 88 prostate cancer specimens, none expressed ASS. Figure 2 illustrates the lack of ASS expression in tumor cells compared to the high expression found in the surrounding normal prostate epithelium.
Figure 2.
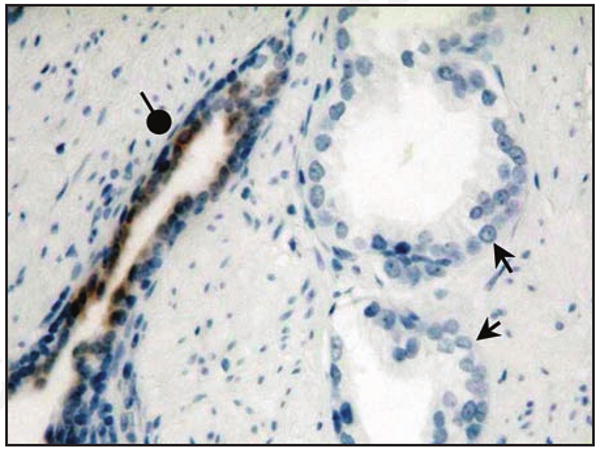
ASS expression in prostate cancer tissue by immunohistochemistry. ASS is readily expressed in benign prostate glands (round arrowhead) with heavy luminal staining by anti-ASS monoclonal antibody. In contrast, no ASS reactivity is detected in cancerous prostate glands (arrows).
We first tested the ADI effect on various prostate cancer cell lines and found ADI sensitivity was inversely proportional to ASS protein levels. CWR22Rv1, a castration-resistant prostate cancer cell line that does not express ASS, is highly sensitive to ADI-induced killing. We then extended these studies in vivo with CWR22Rv1 xenografts in nude mice. Weekly injections of ADI resulted in the complete suppression of tumor growth, indicating the effectiveness of ADI as a treatment option for prostate cancer.
The ADI-induced cell killing of CWR22Rv1 is atypical in at least two aspects: first, it is caspase-independent, and second, it follows a delayed kinetics with very little cell killing in the first 48 hours. Although nutritional starvation is known to induce autophagy, the effect of single amino acid removal such as arginine has not been as well documented. We therefore set forth to test whether ADI induces autophagy in CWR22Rv1. By GFP-LC3 (a marker for autophago-somes) under fluorescence microscopy and the generation of LC3-II by western, the induction of autophagy was detected as early as 30 minutes after ADI treatment. Time-lapse images of CWR22Rv1 cells overexpressing GFP-LC3 and RFP-LAMP-1 (a marker for lysosomes) using live cell fluorescence microscopy revealed the rapid appearance of large, bright puncta as well as dynamic colocalization with lysosomes over time (Fig. 3). Advanced quantitative parameters extracted from 4D images of autophagosomes may reflect potentially different mechanistic pathways of autophagy. Detailed analysis of ADI-treated CWR22Rv1 cells revealed AMPK and ERK activation, and AKT, mTOR and S6K attenuation. Cells can, therefore, mount a strong autophagic response even to single amino acid depletion.
Figure 3.
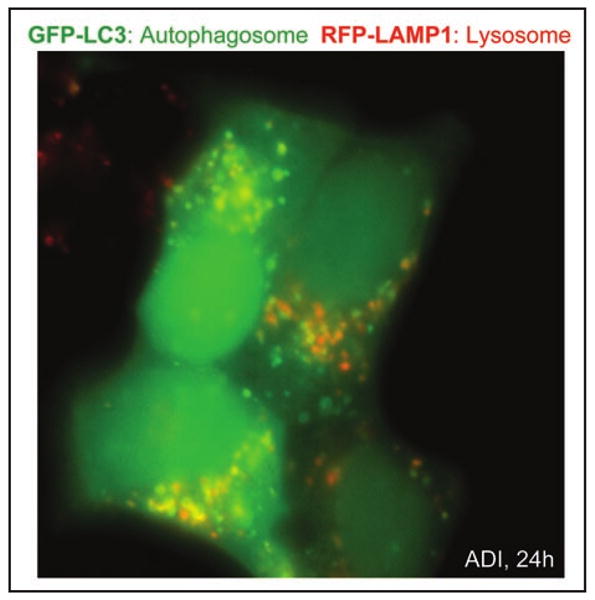
Autophagosome formation by ADI. CWR22Rv1 cells overexpressing GFP-LC3 and RFP-LAMP-1 labeled proteins were treated with 0.3 μg/mL ADI for 24 hours. Individual autophagosomes (green), lysosomes (red), and the co-localization of both structures (yellow) are readily observed.
The relationship between autophagy and apoptosis after ADI treatment was investigated. siRNA knockdown of beclin 1 enhanced ADI-induced apoptosis at both 24 and 48 hours, suggesting that autophagy, at least in the early phase, serves to protect cells from apoptosis. Additionally, we used chloroquine, a clinically approved antimalarial drug known to inactivate lysosomal functions, to interfere with the autophagic process. Chloroquine also accelerated apoptotic death induced by ADI. Our results suggest a protective role of autophagy in the initial phase of ADI-induced apoptosis, and the potential of using ADI/chloroquine combination therapy to enhance the killing of tumor cells.
The chemotherapeutic agent docetaxel represents one of the few options for treating hormone-resistant prostate cancer. Since docetaxel induces cell killing via caspases, leading to induction of traditional apoptosis, we determined in vivo whether ADI may complement docetaxel in cell killing via a caspase-independent mechanism. Whereas each therapy slowed growth of CWR22Rv1 xenografts, the ADI/docetaxel combination showed a dramatic reduction of tumor growth compared to the groups receiving individual therapies. Although we have yet to examine ADI/chloroquine combination therapy in vivo, chloroquine has emerged as an anti-cancer autophagy modulator in other in vivo models. Combination therapy is currently very attractive for several reasons. In addition to increased efficacy, combination therapy may circumvent or delay the emergence of resistant tumors or improve patient quality of life by reducing side effects associated with high concentrations of chemotherapy drugs.
In summary, we present a new therapy for prostate cancer, based on the interesting finding that most, if not all, prostate cancers are auxotrophic for arginine. At present, we do not understand why prostate cancer cells are selected against ASS expression, nor do we know the initial mechanism whereby ADI triggers autophagy and caspase-independent apoptosis. Increasing evidence suggests that autophagic death induced by metabolic stress utilizes pathways largely different from the caspase-dependent genotoxic agents. Targeting disparate mechanisms appears beneficial, implying that the interaction of autophagy and apoptosis has therapeutic rationale.


