Abstract
Primary cilia are microtubule-based sensory organelles that coordinate signalling pathways in cell-cycle control, migration, differentiation and other cellular processes critical during development and for tissue homeostasis. Accordingly, defects in assembly or function of primary cilia lead to a plethora of developmental disorders and pathological conditions now known as ciliopathies. In this review, we summarize the current status of the role of primary cilia in coordinating receptor tyrosine kinase (RTK) signalling pathways. Further, we present potential mechanisms of signalling crosstalk and networking in the primary cilium and discuss how defects in ciliary RTK signalling are linked to human diseases and disorders.
Keywords: primary cilia, receptor tyrosine kinase (RTK), PDGFRα, EGFR, Tie1/2, IGF1R, FGFR, signal transduction and crosstalk, signalling networking, cell migration, cell cycle control, cell differentiation
Introduction
Primary cilia are slender microtubule (MT)-based organelles that exist in a single copy on the surface of almost every quiescent cell of the human body. Research within the last decade has shown that primary cilia are sensory organelles that coordinate a number of important signal transduction pathways that impinge on cell-cycle control, differentiation, migration and other cellular processes during embryonic development and in tissue homeostasis in the adult [1]. Therefore, mutations causing defective cilia assembly or function can lead to a variety of diseases and developmental disorders, referred to as ciliopathies [2-4]. Primary cilia contain a MT cytoskeleton, the axoneme, which is surrounded by a lipid bilayer membrane with a unique composition of signal transduction elements, such as specific receptor tyrosine kinases (RTKs), G-protein-coupled receptors (GPCRs), Notch receptors, receptors for extracellular matrix (ECM) proteins, transient receptor potential (TRP) ion channels and various transporter proteins [5-8]. Multiple lines of evidence have indicated that the appropriate targeting of these proteins into and out of the ciliary compartment is critical for their function, but much still remains to be learned about how primary cilia coordinate various signalling pathways in time and space. In this review, we first summarize the basic structure and mechanism of assembly of primary cilia. Next, we present an overview of the current status of RTK signalling in primary cilia and highlight some of the pathological conditions associated with dysfunctional cilia and RTK signalling.
Structure and assembly of primary cilia
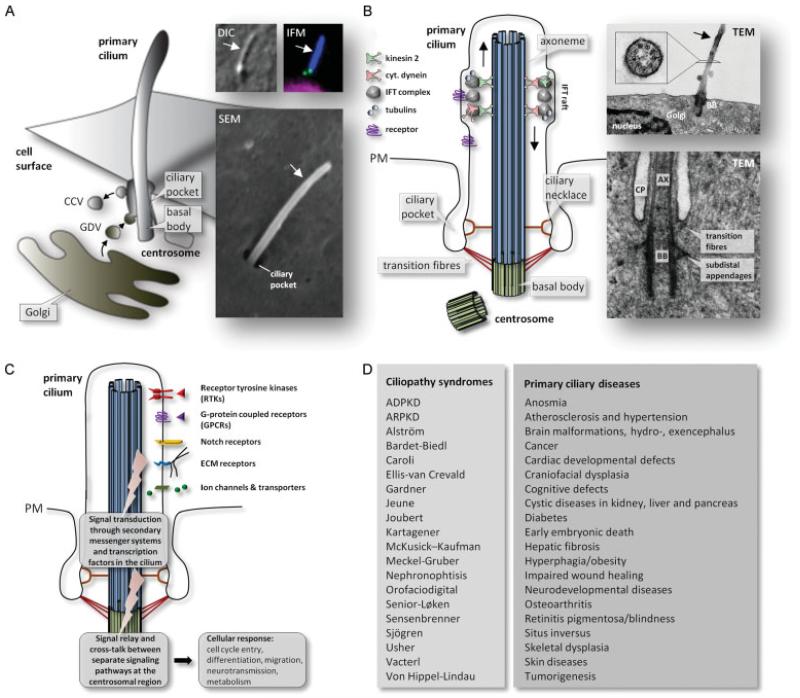
The core of primary cilia consists of a MT-based cytoskeleton, the axoneme, which is a direct extension of a modified centriole called a basal body. Similar to axonemes of most motile cilia, the primary cilium axoneme contains nine outer doublet MTs but lacks motility-related components, such as inner and outer dynein arms, radial spokes and central pair MTs. Primary cilia are hence referred to as 9 + 0 cilia [5,9-11]. The axoneme is surrounded by a bilayer lipid membrane that is continuous with the plasma membrane of the cell body (Figure 1A, B) but is enriched for specific membrane proteins and lipids (Figure 1C). Primary cilia are formed in quiescent cells and their assembly and disassembly occurs in tight coordination with the cell cycle (Figure 2) [12]. Ciliogenesis involves docking of post-Golgi vesicles to the distal end of the mother centriole, emergence of a ciliary bud within the lumen of the vesicle, and extension of the axoneme by intraflagellar transport (IFT) [13,14] (Figure 1B), a process that is also involved in cilia maintenance, disassembly and signalling [15]. In many cell types a large fraction of the mature cilium is localized deep in the cytoplasm, within an invagination of the plasma membrane known as the ciliary pocket, which is an endocytic domain for endocytosis by clathrin-coated vesicle formation (Figures 1B, C) [16,17].
Figure 1.
Overview on ciliary structures, signalling systems, diseases and disorders. (A) The primary cilium emerges from the ciliary pocket from the centrosomal mother centriole (basal body) that lies in close proximity to the Golgi apparatus at the plasma membrane in growth-arrested cells. Golgi-derived vesicles (GDVs) deliver ciliary axonemal and membrane proteins from the Golgi complex to the cilium, and clathrin-coated vesicles (CCVs) are formed from the ciliary pocket. Inserts to the right show primary cilia (arrows) visualized with differential interference contrast (DIC) microscopy, Immunofluorescence microscopy (IFM) and scanning electron microscopy (SEM; courtesy of Johan Kolstrup). In IFM, the cilium is localized with anti-acetylated α-tubulin (blue), the centrioles are marked with anti-centrin (green) and the nucleus is stained with DAPI (magenta). (B) The primary cilium is assembled by intraflagellar transport (IFT) from the basal body (see text for details). The upper inserts to the right show transmission electron microscopy (TEM) images of a longitudinal section of a mouse embryonic fibroblast primary cilium emerging from the ciliary basal body (BB) at the surface of the cell in close proximity to the Golgi and the nucleus (courtesy of Johan Kolstrup), and a cross-section of the cilium in a human embryonic stem cell that shows the axonemal arrangement of nine outer doublets of microtubules (9 + 0; from [139]), with permission from the Journal of Cell Biology). The lower insert shows a TEM image of a longitudinal section of a human foreskin fibroblast primary cilium emerging from the basal body (BB) in the ciliary pocket (CP; reproduced from [140] with permission from the Journal of Cell Science); AX: axoneme. (C) Schematic view on types of signal transduction systems that are coordinated by primary cilia and relayed through the cilium/centrosome axis to control cellular responses. (D) Examples on ciliopathy syndromes and primary cilia diseases and disorders associated with defects in ciliary assembly and function.
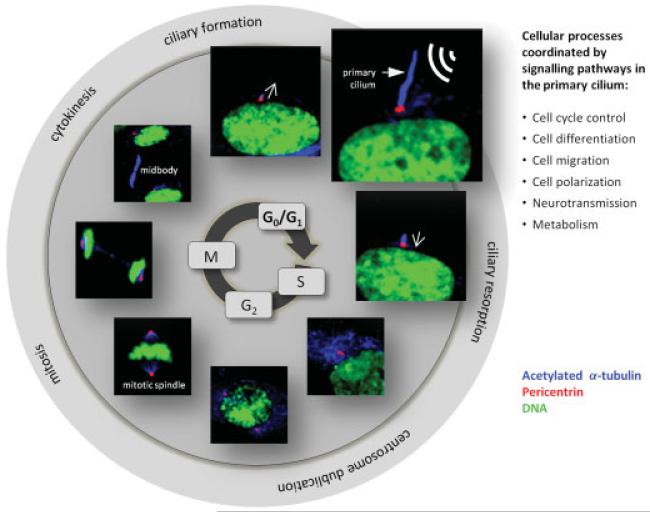
Figure 2.
Schematic view on the formation and resorption of the primary cilium during the cell cycle. After mitosis and cytokinesis the two daughter cells enter the G1/G0 phase and begin their assembly of a primary cilium by IFT from the centrosomal mother centriole at the plasma membrane and into the extracellular environment for registration and relaying of extracellular cues. Upon stimulation with growth factors that triggers cell cycle entry at the G1/S phase transition the cilium is resorbed and the centrosome is duplicated to form the two spindle poles for mitotic spindle formation and mitosis (M). At the end of mitosis the midbody is created from the spindle microtubules to coordinate cytokinesis followed by cell cycle arrest and formation of a new primary cilium in each daughter cell. Images show immunofluorescence microscopy analysis of the different phases of the cell cycle, using anti-acetylated α-tubulin (blue) to mark primary cilia (bold arrow) and cytoplasmic microtubules. Centrosomes are marked with anti-Pericentrin (red) and DNA with DAPI (green). Open arrows indicate time points for formation and resorption of the primary cilium.
The region separating the basal body and cilium proper is called the transition zone. At the proximal end of this region transition fibres link the distal end of the basal body to the plasma membrane. A region known as the ‘ciliary necklace’ is located distal to the transition fibres and consists of champagne glass-like structures that link the base of the axoneme to the ciliary membrane (Figure 1B) [18]. The transition fibres and ciliary necklace appear to act as a selective filter or pore for cargo destined for the cilium [7,9-11]. For example, the ciliopathy protein CEP290 was shown to localize to the champagne glass-like structures of the ciliary necklace region in Chlamydomonas, and a mutant in Cep290 led to defective cilia assembly and altered protein composition of the cilia that did form [19]. Several other ciliopathy-related proteins were recently shown to be components of the ciliary necklace region in Caenorhabditis elegans [20], and there is also evidence that Septin 2 might be part of a diffusion barrier at the cilium base [21]. Selective transport through the ciliary necklace might resemble nuclear import and export through the nuclear pore complex [10,11]. For example, Dishinger and colleagues (2010) demonstrated that the nuclear import protein importin-β2 plays a role in the transport of KIF17 motor protein to the cilium in a Ran–GTP-dependent manner, and that KIF17 harbours a ciliary localization sequence (CLS) resembling a nuclear localization sequence [22]. CLSs have also been identified in a number of receptors and ion channels, but a clear consensus CLS has not been identified [23]. A noteworthy feature of CLSs is that they may sometimes act as nuclear localization signals, such that a ciliary protein (or a derivative thereof) can sometimes localize to the nucleus [22,24]. This dynamic localization pattern may be important in coupling ciliary signal transduction to specific changes in gene expression. Interestingly, defects in ciliary localization of somatostatin receptor 3 (Sstr3), melanin-concentrating hormone receptor 1 (Mchr1), leptin receptor (LepR) and rhodopsin (Rho) have been detected in different Bardet–Biedl syndrome (BBS) mouse models [25-27], highlighting the physiological importance of appropriate ciliary localization of these receptors, as well as the role of BBS proteins in this process. Indeed, BBS proteins were shown to form a complex, the BBSome, which cooperates with the IFT machinery to regulate trafficking of specific membrane proteins to and from the ciliary compartment [28-30].
Ciliary signalling and ciliopathies
A number of specific receptors, ion channels, transporters and downstream signalling proteins have been localized to primary cilia, thus allowing the cilium to detect and transduce extracellular signals to the inside of the cell. In this way the primary cilium functions as a unique cellular site for mechano-, chemo- and osmosensation to regulate cellular processes during development and in tissue homeostasis [5,31,32]. An important feature of ciliary signalling is the continuous interaction with regulatory signalling molecules at the ciliary base, ie the centrosomal region, which may coordinate the crosstalk between separate ciliary signalling pathways to activate specific cellular targets and gene arrays for specified cellular or tissue responses. The role of the primary cilium as a cellular command centre for signalling is evidently important, because defects in ciliary assembly (eg via mutations in IFT genes) or function (eg via mutations in genes coding for ciliary receptor proteins) have been linked to numerous developmental disorders and diseases, including early embryonic death, heterotaxy and situs inversus, craniofacial and skeletal patterning defects, heart defects, hypoplasia, renal and liver diseases, such as polycystic kidney disease, mental retardation, anosmia, obesity and tumourigenesis [3,31,33]. Light reception and transduction also occur through modified primary cilia, ie the outer segment of rod and cone photoreceptors in the vertebrate retina, where light-sensitive opsins are transported from the inner to the outer segment by IFT to detect light photons [34-36]. Hence, mutations in genes coding for IFT components or other proteins involved in cilia trafficking and photoreceptor formation lead to retinitis pigmentosa and blindness [37-39]. For a list of proposed ciliopathy syndromes and diseases, please see Figure 1D.
In addition to RTK signalling (see below), a number of other signalling pathways were reported to be specifically associated with primary cilia, including neurotransmission, Hedgehog (Hh), Wingless (Wnt), Notch, ECM and polycystin signalling. For example, in non-pathological vertebrate cells the primary cilium functions as a cellular switch, turning Hh signalling on and off through the coordinated trafficking and activation of Hh signalling components, Patched, Smoothened and Gli transcription factors in the cilium [32,40]. Hh signalling was originally connected to the cilium, when mouse IFT mutants were shown to display phenotypes typical of defective Hh signalling [41]. The primary cilium was also suggested to function as a switch in balancing Wnt signalling from the canonical Wnt/β-catenin to the non-canonical/planar cell polarity (PCP) signalling pathway, partly through activation of glycogen synthase kinase 3β (GSK3β) at the ciliary base for degradation of β-catenin. However, in contrast to Hh signalling, the link between Wnt signalling and cilia is somewhat controversial [32,42]. Other signalling pathways reported to be linked to primary cilia include vasopressin signalling in renal epithelial cells [43], as well as neuronal signalling systems such as somatostatin, serotonin and melanin-concentrating hormone signalling, which regulate human behavioural processes [44,45]. It is of note that loss of primary cilia in hypothalamic POMC neurons leads to obesity, hyperphagia and elevated levels of serum insulin, glucose and leptin in mice [46].
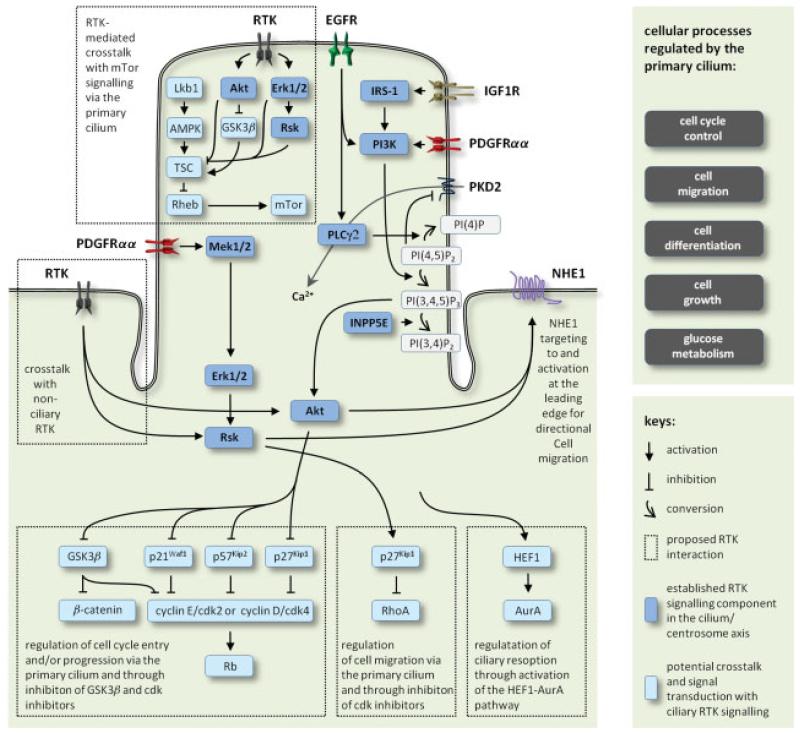
In this review, we mainly focus on the role of the primary cilium in coordinating different classes of RTK signalling, and we highlight illustrative examples on the potential crosstalk between ciliary RTK signalling and other signalling systems, which may regulate cell cycle entrance, differentiation, directional cell migration and further cellular processes critical during embryonic development and in tissue homeostasis in the adult. A summary of RTK signalling and potential signalling crosstalk in the primary cilium is presented in Figure 4.
Figure 4.
Summary of ciliary RTK signalling pathways and examples on potential crosstalk between signalling pathways at the cilium-centrosome axis to regulate cell cycle control, cell migration, cell differentiation, cell growth and metabolism.
Receptor tyrosine kinases—an overview
Receptor tyrosine kinases (RTK) belong to a superfamily of about 20 different classes of single transmembrane high-affinity cell surface receptors for growth factors, cytokines and hormones during embryonic development and in tissue homeostasis [47]. When aberrantly regulated, these receptors play a critical role in tumourigenesis and progression of numerous cancer types. In most cases, the binding of ligand to the N-terminal, extracellular domain induces the formation of receptor dimers from a single subunit receptor, followed by the activation of the receptor complex through auto- or transphosphorylation of the intracellular catalytic domain and tyrosine phosphorylation of specific docking sites for adaptor and effector proteins in signal transduction. In a few cases, RTKs exist as multimeric complexes, such as the insulin receptor (IR) and insulin-like receptors 1 and 2 (IGFR1/2), which form disulphide-linked dimers in the absence of ligand. Activation of RTK and instigation of signal transduction proceeds through a series of signalling cascades, such as the PI3K–Akt (phosphatidylinositol 3-kinase–protein kinase B) pathway, phospholipase C (PLC) pathways and several members of the mitogen-activated protein kinase (MAPK) pathways, including the Mek1/2–Erk1/2 pathway, to control cell cycle entry, cell migration and differentiation processes [47].
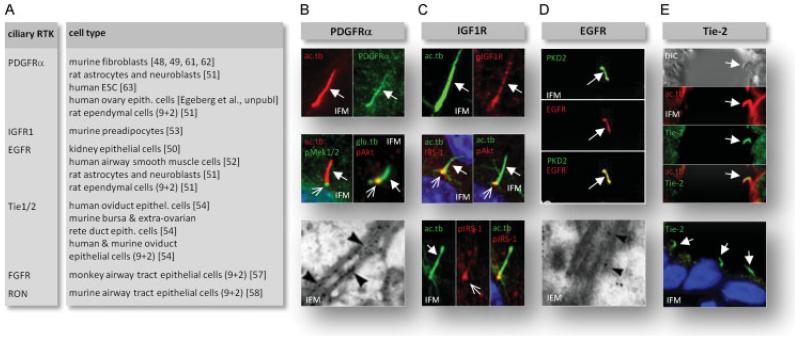
Platelet-derived growth factor-α (PDGFRα) is one of the first RTKs that was linked to primary cilia [48]. In quiescent fibroblasts this receptor localizes to primary cilia for ligand-dependent activation of cell cycle entry and directional cell migration [48,49] (Figure 3B). Additional classes of RTKs have now been linked to primary cilia and coordination of signalling events in cell cycle control, migration and differentiation. These include epidermal growth factor receptor (EGFR) [50-52], insulin-like growth factor 1 receptor (IGF1R) [53] and angiopoetin receptor (Tie-2) [54]. Additionally, fibroblast growth factor (FGF) signalling has been linked to motile cilia in various tissues in zebrafish, Xenopus and mammals [55-57]. Recepteur d’origine nantais (RON) localizes to motile cilia in the airway tract [58], EGFR and PDGFRα to motile cilia in the ependyma [51] and Tie1/2 to motile cilia in the oviduct [54]. The function of RTK signalling in motile cilia remains largely unknown, but could be linked to regulation of ciliary motility [5] or to tissue maintenance and differentiation. For a list of ciliary RTKs, please see Figure 3A.
Figure 3.
Overview on ciliary RTKs. (A) list of RTKs localizing to primary and motile cilia. Motile cilia are indicated by ‘9 + 2’. (B-E) immunofluorescence microscopy (IFM) and immunogold electron microscopy (IEM) analysis of RTK localization to primary+cilia (bold arrows) and to the ciliary base (open arrows). Cilia were localized with either anti-acetylated γ-tubulin (ac.tb) of detyrosinated α-tubulin (glu.tb). (B) Localization of PDGFRα to the primary cilium (upper images) and PDGF-AA-induced phosphorylaton of Mek1/2 (pMek1/2) and Akt (pAkt) in the cilium and at the ciliary base NIH3T3 cells (middle images) (reproduced from [48] with permission from Elsevier, and from [49] with permission from S. Karger AG Basel). Localization of PDGFRα to the primary ciliary membrane (lower images) in rat astrocytes (reproduced from [51] with permission from John Wiley and Sons). (C) insulin-induced phosphorylation of IGF1R in preadipocytes (upper images) and ciliary localization of IRS-1 and insulin-induced phosphorylation of Akt (pAkt) at the ciliary base (middle images). Lower images show insulin-induced phoshorylation of IRS-1 (pIRS-1) in the primary cilium and at the ciliary base (reproduced from [53] with permission from Journal of Cell Science). (D) Co-localization of EGFR and polycystin 2, PKD2, to primary cilia in kidney epithelial (LLC-PK1) cells (upper images; reproduced from [50] with permission from the American Society for Microbiology), and localization of EGFR to the primary ciliary membrane in rat astrocytes (lower image; reproduced from [51] with permission from John Wiley and Sons). (E) Localization of Tie-2 to epithelial cells in mouse extra-ovarian rete ducts (upper images with differential interference contrast, DIC) and to human ovary surface epithelium (lower image; reproduced from [54] with permission from Elsevier).
Many cells have multiple different classes of RTKs with special representatives localized to their primary cilia, while the remainder populates the non-ciliary cell membrane. For example, in fibroblasts PDGFRβ is localized in the plasma membrane, but not the cilium, while PDGFRα becomes almost exclusively localized to the primary cilium in quiescent cells [48]. Further, RTK signalling is well known to interact and extensively crosstalk with non-RTK signalling pathways. As examples, RTK signalling is tightly coupled to integrin receptors in ECM signalling in cancer cell growth, survival and invasion, GPCRs use RTKs as signalling intermediates and RTKs modulate G protein pathways. Since specific RTKs, GPCRs, ECM receptors and other membrane proteins in signal transduction are highly enriched in primary cilia, it is likely that the cilia/centrosome axis provides a unique site for the coordinated crosstalk between various signalling systems in human health and disease.
PDGFRα signalling
PDGFR is found as two isoforms, PDGFRα and PDGFRβ, which form homo- or heterodimers upon binding of dimers of PDGF-A, PDGF-B, PDGF-C and PDGF-D ligands with different affinities, such that PDGF-AA exclusively activates the homodimer of the α-receptor, PDGFRα while PDGF-BB, for example, activates all dimer combinations. PDGFRα is preferentially expressed in mesenchymal lung, skin, intestine and oligodentrocyte progenitors, and aberrant PDGFRα signalling contributes to the development of different types of cancers and has been associated with fibrotic diseases [59]. PDGFRβ is also widely distributed in tissues, often being present in the same cell as PDGFRα, but abundance of the two receptor isoforms may vary depending on the cell type. At least in fibroblasts, PDGFRα is a growth arrest-specific (GAS) gene and is up-regulated upon starvation upon formation of the primary cilium [48,60]. The newly synthesized PDGFRα is translocated, probably in Golgi-derived small vesicles, to the base of the cilium where exocytosis occurs and the molecule passes the transition zone barrier to populate the ciliary membrane. A basal level of PDGFRα is found in wild-type cells before ciliary induction and in Tg737orpk mouse embryonic fibroblasts (MEFs) that cannot grow primary cilia [48]. In addition to fibroblasts [48,61,62], PDGFRα localizes to primary cilia in a number of other cell types, including rat astrocytes and neuroblasts [51] (Figure 3B), human embryonic stem cells (hESCs) [63] and ovarian surface epithelial (OSE) cells [Egeberg et al., unpubl.]. In fibroblasts, the localization of the receptor to the cilium is necessary for dimerization and subsequent signalling upon ligand addition, and this is probably the case everywhere. In fibroblasts activation of the PDGFRαα receptor in the primary cilium leads to the onset of a number of downstream signalling pathways within the cilium, including Akt and Mek1/2-Erk1/2 pathways to regulate cell cycle control and directional cell migration [48,49].
PDGFRα signalling in cell cycle control
Multiple lines of evidence have indicated a role for primary cilia and ciliary PDGF signalling in regulating cell cycle entry and progression. Tucker and co-workers originally showed that PDGF induces the resorption of the cilium and the competence of cells to replicate their DNA in cultures of growth-arrested fibroblasts [64], and activation of PDGFRαα in the fibroblast primary cilium leads to cyclin-dependent kinase 4 (Cdk4)-mediated phosphorylation of retinoblastoma protein (Rb) [48], which marks the cell cycle G1–S phase transition. This implies the presence of a highly balanced signalling network that organizes multiple signalling events through activation and/or deactivation of key regulatory proteins in the cilia/centrosome axis. A key regulator in these joint processes may include Akt, which controls multiple events in the cell cycle, such as activation of the cyclin D–cdk4 and cyclinE–cdk2 complexes by inhibition of cdk inhibitors (CKIs), p21Waf1, p27Kip1 and p57Kip2 [65,66] and through activation of the Wnt–β-catenin pathway by inhibition of GSK3β (Figure 4). Aberrant GSK3β and CKI signalling is critically associated with tumourigenesis and cancer cell proliferation [67-69], thus linking defects in ciliary assembly and resorption to malignant neoplasm. Further and upstream in relation to Akt in the primary cilium, inactivated or mislocalized PtdIns(3,4,5)P3 5-phosphatase (INPP5E) leads to increased ciliary PDGFRαα signalling and premature disassembly of the cilium, followed by accelerated cell cycle entry [61]. Dysfunctional INPP5E causes a series of different ciliopathies, including cystic kidneys [61,70]. Additional pathways may be implicated in regulation of cell-cycle events and cell migration through RTK-mediated Akt and Mek1/2–Erk1/2 activation, including Hh signalling and the mammalian target of rapamycin (mTOR) pathway, as discussed below. Previously, human enhancer of filamentation 1 (HEF1)–Aurora A kinase (AurA) pathway, Pitchfork (Pifo) and the dynein light chain, Tctex-1, were shown to promote ciliary disassembly [71-77]. It is therefore tempting to speculate that ciliary RTK signalling acts upstream in relation to one or more of these key molecules during ciliary resorption, where the centrosomal region at the ciliary base functions as a plinth for the continued crosstalk between the different signalling systems in cell-cycle checkpoint regulation.
PDGFRα signalling in directional cell migration
Defective cell migration leads to developmental defects, defective tissue regeneration, tumourigenesis and metastasis [78]. A number of observations have shown that primary cilia and ciliary RTK signalling control cell migration and regenerative processes [75], such that mutant mice with defects in ciliary assembly have significant defects in in vivo wound closure compared to wild-type animals [49]. Further, in many cell types, the primary cilium specifically orients parallel to the path of migration and towards the leading and mobile edge of the cell, ie the lamellipodium, which is formed by extensive reorganization of the actin cytoskeleton [49,52,79-81]. This implies a tight connection between the direction of cell migration and the orientation of the cilium, which may function as a cellular GPS that coordinates the right positioning of the cell in connective and polarized tissues [75,82].
In adult skin, fibroblasts are embedded in ECM, are usually in growth arrest and display primary cilia, presumably with PDGFRαα localized in the ciliary membrane. Little or no PDGF is normally present unless the tissue is wounded, when PDGF is released, mainly from platelets. PDGF was initially characterized as a potent mitogenic stimulant and chemoattractant for fibroblasts to promote wound healing [83]. All forms of PDGF bind to PDGFRαα and could operate by binding to the ciliary membrane not only to induce mitogenesis, as above, but also in directional cell migration (chemotaxis). Micropipette analyses revealed that in Tg737 orpk MEFs, where ciliary assembly is defective, normal chemosensory response to PDGF-AA is absent compared to wild-type MEFs, and in vitro wound-healing assays revealed that mutant fibroblasts have unregulated speed and uncontrolled directional cell displacement during wound closure. In wild-type cells, PDGF-AA induces tyrosine phosphorylation of the receptor at several motifs, followed by phosphorylation of Mek1/2 and Akt, as discussed above [48,49]. Mek1/2 is localized along the cilium, presumably moving basally to activate Erk1/2 and its downstream target, p90 ribosomal s6 kinase (Rsk), whereas Akt is localized at the ciliary base. As they are in mitogenesis, both pathways are probably involved in chemotaxis. Inhibition of either the Mek1/2–Erk1/2–Rsk or PI3K–Akt signalling blocks the initiation of directional migration by PDGF-AA, but this does not mean that their actions are identical [Clement et al., unpubl.].
Since, in the presence of a PDGF-AA gradient, the primary cilium specifies where the lamellipodium is to form, information must be conveyed from the cilium to the leading edge. Two prominent molecules in conveying this information is p27Kip1 and the Na+–H+ exchange protein 1, NHE1 (Figure 4). Besides cell cycle regulation, p27Kip1 is phosphorylated by Rsk to inhibit the Rho GTPase (RhoA) that controls actin stabilization [84,85]. NHE1 is up-regulated during quiescence [86] and localized primarily in numerous vesicles that normally move through the cytoplasm in both wild-type and mutant MEFs. Upon addition of PDGF-AA in wild-type MEFs, NHE1 translocates to the leading edge of the cell, then incorporates into the cell membrane with Ezrin/Moesin/Radizin (ERM) proteins and the actin cytoskeleton becomes organized in the lamellipodium for motility [Clement et al., unpubl., 86]; this translocation is abrogated by inhibitors of Akt. Although details of how the directionality of movement is linked to the direction in which the cilia point are unknown, the RTK cascade proteins, when phosphorylated, must have effects on NHE1 vesicle translocation, so that the vesicles move in the direction in which the cilium points to form the leading edge of the migrating fibroblast. Likely substrates include the motor or scaffold proteins of the NHE1-containing vesicles necessary for translocation along specific stable microtubules that extend from the centrosome region around the ciliary basal body. Rsk is an activator of NHE1 [87,88], suggesting that PDGF-AA-induced Mek1/2–Erk1/2–Rsk signalling may phosphorylate and activate incorporated NHE1 in the leading edge membrane [Clement et al., unpubl.].
IGF-1R signalling
In vertebrates, there are three receptors of the larger RTK superfamily that can bind with high affinity to insulin and insulin-like growth factors (IGFs) to regulate cell proliferation, growth, migration, differentiation, metabolism, reproduction and longevity [89-92]. The three receptors include insulin receptor (IR) and insulin-like growth factor receptors 1 and 2 (IGFR1/2) [93]. The three receptors can form a dimeric structure, which can be either a homodimer, composed of two identical α/β monomers, or a heterodimer formed by two different α/β monomers, eg IRαβ/IGF1Rαβ [94]. IGF1R signalling through primary cilia has been shown to play an essential role in preadipocyte differentiation. An important step in adipocyte differentiation in 3T3-L1 preadipocytes is the achievement of confluence by contact inhibition, which allows the formation of primary cilia. Disruption of primary cilia assembly by using IFT88 siRNA blocks adipocyte differentiation and the expression of two key adipogenic transcription factors, C/EBPα and PPARγ. The IGF1R is localized in the primary cilium, but not exclusively; it is also located in the plasma membrane. However, the receptors in the cilium are more sensitive to insulin stimulation and are activated prior to the receptors in the plasma membrane [53]. As a response to insulin stimulation, several signalling molecules, including Akt and insulin receptor substrate 1 (IRS-1; a direct target of IGF1R and IR tyrosine kinases [95]), which initiate differentiation and are tyrosine-phosphorylated at the basal body. IFT88 siRNA and loss of primary cilia significantly reduces the phosphorylation of Akt at the base of the cilium [53]. We surmise that other signalling pathways, such as Mek1/2–Erk1/2, are activated by IGF-R1 signalling in the primary cilium to control processes other than cell differentiation, including cell-cycle control and directional cell migration, such as in ciliary PDGFRα signalling, although this has not yet been investigated.
EGFR signalling
Epidermal growth factor receptors extensively regulate cellular processes during development and in tissue homeostasis, and they are critically associated with tumourigenesis and cancer cell invasion [96]. EGFR signalling in primary cilia was first reported in studies on mechanosensation in cultures of kidney epithelial cells. In these cells, EGF may sensitize polycystin-2 (PKD2) to mechanical stimuli, such that EGFR signalling in the primary cilium activates PKD2 by releasing this TRP ion channel from its inhibition by PIP2, partly through PI3K-induced phosphorylation of PI(4,5)P2 into PI(3,4,5)P3 and partly through the hydrolysis of PIP2 by PLCγ2 [50] (Figure 4). Mutations in the genes coding for either EGFR or PKD2 lead to cystic kidneys in the mouse [97,98]. Since then, EGFR has been localized to the primary cilium/centrosome axis in other cell types, including astrocytes and neuroblasts in the rat ventricular zone [51] and in human airway smooth muscle cells (ASMs) [52]. While little is known on the function of EGFR signalling in neural cells, EGFR was suggested to play a major role in mechanosensation and directed cell migration in ASMs through the interaction with integrins and polycystins 1 and 2 in the primary cilium [52]. In this scenario, the cilium could directly interact with the ECM to detect mechanical load, as was originally shown for chondrocytes in the cartilage [99,100]. Further, by using a method for deciliation of growth-arrested cells with chloral hydrate [101], ASMs showed a reduced rate of migration in in vitro wound-healing assays in response to EGF, supporting the conclusion that ciliary EGFR signalling could function in ASM injury repair [52].
FGFR signalling
Fibroblast growth factors (FGFs) comprise a family of structurally related polypeptides that play important roles during embryonic development and in tissue homeostasis in the adult. In vertebrates, 22 different FGF family members have been identified, most of which signal through a family of RTKs, known as FGFRs, that are encoded by four different genes (FGFR1–4) [102,103]. The first link between FGF signalling and cilia came from two independent studies in zebrafish [55,56]. In the first study, morpholino knock-down of Fgfr1 or the redundant ligands Fgf8 and Fgf24 was shown to cause shortening of cilia in the laterality organ Kupffer’s vesicle, as well as in the otic vesicle and pronephric ducts [55]. In the same study, expression of dominant negative Fgfr1 in Xenopus led to shortening of monocilia in the gastrocoel roof plate [55], which is reminiscent of the zebrafish Kupffer’s vesicle and the embryonic node of mammals [104]. These organs play a critical role in establishing the left–right asymmetry axis in the developing embryo via a mechanism that involves dynein-dependent movement of monocilia to create a directional flow across the ventral surface of the node [105,106]. Indeed, knockdown of Fgfr1 (or Fgf8 /Fgf24) was associated with left–right patterning defects in zebrafish [55]. Similarly, knockdown of two FGF target genes in zebrafish, Ier1 and Fibp2, were shown to cause cilia shortening and laterality defects [56], and recent studies have confirmed similar roles for Fgfr2 [107] and Fgf4 [108]. Importantly, the effect of Fgf8 knockdown on ciliogenesis could be rescued by injection of ier1 and fib1 mRNA, indicating that ier1 and fib1 act downstream of Fgf8 in ciliogenesis [56]. Both Ier1 and Fib1 localize to the nucleus [56], and abrogation of Fgfr1 led to down-regulation of two ciliogenic transcription factors, foxj1 and rfx2, as well as the gene coding for IFT protein 88 [55]. These findings suggest that FGF signalling positively regulates ciliogenesis by promoting transcription of ciliogenic genes. Additional players in FGF-mediated regulation of ciliogenesis include the SET domain containing protein Setdb2 [109] and the regulatory protein Capony 1 [110]. In addition, FGF signalling may also be coupled to cilia in a more direct way, as FGFRs were shown to localize to motile cilia in the airway of rhesus monkey during postnatal development of the tracheal basement membrane zone [57], and extensive crosstalk between FGF signalling and other signalling pathways connected to cilia has been reported to occur, eg during skeletogenesis [111] (see also below).
RTK crosstalking and signalling networking
It is becoming increasingly clear that crosstalking between separate signalling pathways and the continuous networking between individual signalling components are critical in the coordinated regulation of cellular processes [112-115]. Integrins and GPCRs use RTKs as signalling intermediates and vice versa to regulate, for example, cell cycle control, differentiation and migration, and when this crosstalk is aberrantly coordinated it leads to tumourigenesis as well as cancer cell proliferation and invasion [113-115]. In neurogenesis, IGF1R is an essential component of the signalling network that includes Hh, Wnt and Notch signalling. Since Integrins, Notch receptors and several members of GPCRs in Hh and Wnt signalling, as well as neurotransmission, are localized to primary cilia, it is tempting to speculate that ample signalling crosstalk and networking takes place in the cilium–centrosome axis. In connective, muscle and skeletal tissues, the cilium may directly interact with multiple components of ECM to regulate the length and orientation of primary cilia to control cellular positional information [52,81,100,116-119] and, when defective in chondrocytes, may lead to osteoarthritis [120] and osteochondroma [121]. In this regard, the further crosstalk with TRP ion channels, such as polycystins, adds an additional layer of complexity in signalling networking in ciliary mechanosensation [122].
Recently, the primary cilium was proposed to function as a regulator of mTor signalling [123,124], which controls cell growth, cell volume, cell proliferation, cell migration, metabolism and cell survival via the mTOR complex 1 and 2 (mTORC1, mTORC2) [125,126]. TORC1 activation stimulates increase in glucose uptake, glycolysis and de novo lipid biosynthesis [127], which are often seen in human cancers [128]. Essential upstream regulators of this pathway, including tumour suppressor protein (Lkb1) and AMP-activated protein kinase (AMPK), which inhibit mTor signalling through activation of the tuberous sclerosis complex (TSC) and inhibiton of the small G-protein (Rheb), have been localized to the primary cilium–centrosome axis in kidney epithelial cells [123]. Loss of cilia in kidney-specific Kif3a-mutant mice thus results in enlarged cells when compared with control animals [123]. In Tg737 orpk mutant mice, metabolic control after glucose challenge is severely impaired, therefore creating a possible functional link between glucose metabolism and cilia [129]. Most importantly, there are multiple RTK signalling cascades that contribute to mTor signalling, including the Mek1/2–Erk1/2–Rsk and PI3K–Akt pathways that either directly activate the mTor pathway, eg through inhibiton of TSC, or indirectly via inhibition of GSK3β, which is an activator of TSC [130,131]. Further, different chemotactic inputs from both RTKs and GPCRs regulate together mTORC1 and mTORC2 signalling to coordinate the organization of F-actin and focal adhesions, eg through the expression and activation of multiple cell polarity proteins in directional cell migration [126]. Here, PDGFRα, EGFR, FGFR and Tie-2 all contribute to regulation of mTor signalling, and defects in this contribution set off a multitude of different cancer types [132-135]. A number of RTKs, including PDGFRα, are also negatively regulated by a feedback loop from mTor signalling [136], adding a further level to the crosstalk between RTK and mTOR signalling. It is therefore not unlikely that mTOR signalling is directly linked to the ciliary action of RTK and other receptor systems to control cellular processes in cell cycle control, cell migration and differentiation, as outlined in the above.
As a final case in point on the latent complexity of signalling crosstalk in the primary cilium, networking between individual RTK classes comes into play, not only in the primary cilium, but potentially also between ciliary and non-ciliary RTKs. For example, FGF signalling extensively crosstalks with EGFR and PDGFRα signalling during skeletogenesis, and disorganized crosstalking between these signalling systems leads to craniosynostosis [111], which is associated with cranioectodermal dysplasia. Interestingly, this developmental disorder is also caused by defects in the IFT machinery [137,138], supporting the idea that primary cilia take part in the coordinated networking between separate RTK systems.
Conclusions
Recent research has indicated an important role of primary cilia in coordinating a variety of different receptor systems and signalling pathways, including RTK signalling, through the activation of PDGFRα, EGFR and IGF1R to control cell cycle entry, migration and differentiation. Activation of these receptors leads to the onset of a diversity of signalling pathways, which are linked to the cilium–centrosome axis that may function as a major site for the coordinated crosstalking between separate receptor systems to activate specific cellular targets and gene arrays for specified cellular or tissue responses. In this way, the primary cilium offers a unique cellular site for extensive signalling networking critical during development and in tissue homeostasis. Future research should therefore focus on how RTK signalling is integrated with GPCR signalling, TRP ion channel activity, ECM receptors and other signalling systems at the cilium–centrosome axis, and how aberrant organization of the different sets of signalling connections is associated with pathological conditions, including developmental disorders and cancer.
Acknowledgment
STC and LBP acknowledge funding from the Danish Natural Science Research Council (Grant Nos 09-070398 and 10-085373), the Lundbeck Foundation (Grant Nos R54-A5642 and R54-A5375), the Danish Cancer Society (Grant No. DP08096), the Novo Nordisk Foundation, and Nordforsk (Grant No. 27480). We thank Mr Johan Kolstrup for providing us with the EM images in Figure 1. We apologize to those authors whose primary works have not been cited owing to space constraints.
Footnotes
No conflicts of interest were declared.
Author contributions
All authors (STC, LBP, CAC and PS) jointly wrote and edited the manuscript. STC produced the accompanying figures.
Supporting information on the internet
Teaching material of the figures from this review is supplied as supporting information in the online version of this article.
References
- 1.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badano JL, Mitsuma N, Beales PL, et al. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genom Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 3.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 4.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen ST, Pedersen LB, Schneider L, et al. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 6.Rohatgi R, Snell WJ. The ciliary membrane. Curr Opin Cell Biol. 2010;22:541–546. doi: 10.1016/j.ceb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezratty EJ, Stokes N, Chai S, et al. A role for primary cilia in Notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–1141. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2006;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen LB, Schrøder JM, Satir P, et al. The ciliary cytoskeleton. Comprehens Physiol. 2011 doi: 10.1002/cphy.c110043. in press. [DOI] [PubMed] [Google Scholar]
- 12.Quarmby LM, Parker JD. Cilia and the cell cycle? J Cell Biol. 2005;169:707–710. doi: 10.1083/jcb.200503053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen LB, Veland IR, Schrøder JM, et al. Assembly of primary cilia. Dev Dyn. 2008;237:1993–2006. doi: 10.1002/dvdy.21521. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 16.Molla-Herman A, Ghossoub R, Blisnick T, et al. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. 2010;123:1785–1795. doi: 10.1242/jcs.059519. [DOI] [PubMed] [Google Scholar]
- 17.Ghossoub R, Molla-Herman A, Bastin P, et al. The ciliary pocket: a once-forgotten membrane domain at the base of cilia. Biol Cell. 2011;103:131–144. doi: 10.1042/BC20100128. [DOI] [PubMed] [Google Scholar]
- 18.Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972;53:494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craige B, Tsao CC, Diener DR, et al. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams CL, Li C, Kida K, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Q, Milenkovic L, Jin H, et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dishinger JF, Kee HL, Jenkins PM, et al. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-β2 and RanGTP. Nat Cell Biol. 2010;12:703–710. doi: 10.1038/ncb2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Dev Biol. 2008;85:115–149. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- 24.Chauvet V, Tian X, Husson H, et al. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest. 2004;114:1433–1443. doi: 10.1172/JCI21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berbari NF, Johnson AD, Lewis JS, et al. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell. 2008;19:1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura DY, Fath M, Mullins RF, et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci USA. 2004;101:16588–16593. doi: 10.1073/pnas.0405496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo S, Guo DF, Bugge K, et al. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18:1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blacque OE, Reardon MJ, Li C, et al. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 2004;18:1630–1642. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nachury MV, Loktev AV, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 30.Lechtreck KF, Johnson EC, Sakai T, et al. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol. 2009;187:1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veland IR, Awan A, Pedersen LB, et al. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol. 2009;111:p39–53. doi: 10.1159/000208212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. 2011;26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sung CH, Tai AW. Rhodopsin trafficking and its role in retinal dystrophies. Int Rev Cytol. 2000;195:215–267. doi: 10.1016/s0074-7696(08)62706-0. [DOI] [PubMed] [Google Scholar]
- 36.Keady BT, Le YZ, Pazour GJ. IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol Biol Cell. 2011;22:921–930. doi: 10.1091/mbc.E10-09-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roepman R, Wolfrum U. Protein networks and complexes in photoreceptor cilia. Subcell Biochem. 2007;43:209–235. doi: 10.1007/978-1-4020-5943-8_10. [DOI] [PubMed] [Google Scholar]
- 38.Hosch J, Lorenz B, Stieger K. RPGR: role in the photoreceptor primary cilium, human retinal disease, and gene therapy. Opthalmic Genet. 2011;32:1–11. doi: 10.3109/13816810.2010.535889. [DOI] [PubMed] [Google Scholar]
- 39.Sedmak T, Wolfrum U. Intraflagellar transport proteins in ciliogenesis of photoreceptor cells. Biol Cell. 2011;103:449–466. doi: 10.1042/BC20110034. [DOI] [PubMed] [Google Scholar]
- 40.Christensen ST, Ott CM. Cell signaling. A ciliary signaling switch. Science. 2007;317:330–331. doi: 10.1126/science.1146180. [DOI] [PubMed] [Google Scholar]
- 41.Huangfu D, Liu A, Rakeman AS, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 42.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raychowdhury MK, Ramos AJ, Zhang P, et al. Vasopressin receptor-mediated functional signaling pathway in primary cilia of renal epithelial cells. Am J Physiol Renal Physiol. 2009;296:F87–97. doi: 10.1152/ajprenal.90509.2008. [DOI] [PubMed] [Google Scholar]
- 44.Green JA, Mykytyn K. Neuronal ciliary signaling in homeostasis and disease. Cell Mol Life Sci. 2010;67:3287–3297. doi: 10.1007/s00018-010-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JE, Gleeson JG. Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol. 2011;24:98–105. doi: 10.1097/WCO.0b013e3283444d05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davenport JR, Watts AJ, Roper VC, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 48.Schneider L, Clement CA, Teilmann SC, et al. PDGFRaa signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Schneider L, Cammer M, Lehman J, et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010;25:279–292. doi: 10.1159/000276562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma R, Li WP, Rundle D, et al. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol. 2005;25:8285–8298. doi: 10.1128/MCB.25.18.8285-8298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danilov AI, Gomes-Leal W, Ahlenius H, et al. Ultrastructural and antigenic properties of neural stem cells and their progeny in adult rat subventricular zone. Glia. 2009;57:136–152. doi: 10.1002/glia.20741. [DOI] [PubMed] [Google Scholar]
- 52.Wu J, Du H, Wang X, et al. Characterization of primary cilia in human airway smooth muscle cells. Chest. 2009;136:561–570. doi: 10.1378/chest.08-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu D, Shi S, Wang H, et al. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J Cell Sci. 2009;122:2760–2768. doi: 10.1242/jcs.046276. [DOI] [PubMed] [Google Scholar]
- 54.Teilmann SC, Christensen ST. Localization of the angiopoietin receptors Tie-1 and Tie-2 on the primary cilia in the female reproductive organs. Cell Biol Int. 2005;29:340–346. doi: 10.1016/j.cellbi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Neugebauer JM, Amack JD, Peterson AG, et al. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature. 2009;458:651–654. doi: 10.1038/nature07753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong SK, Dawid IB. FGF-dependent left-right asymmetry patterning in zebrafish is mediated by Ier2 and Fibp1. Proc Natl Acad Sci USA. 2009;106:2230–2235. doi: 10.1073/pnas.0812880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evans MJ, Fanucchi MV, Van Winkle LS, et al. Fibroblast growth factor-2 during postnatal development of the tracheal basement membrane zone. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1263–1270. doi: 10.1152/ajplung.00180.2002. [DOI] [PubMed] [Google Scholar]
- 58.Manzanares D, Monzon ME, Savani RC, et al. Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON. Am J Respir Cell Mol Biol. 2007;37:160–168. doi: 10.1165/rcmb.2006-0413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lih CJ, Cohen SN, Wang C, et al. The platelet-derived growth factor α-receptor is encoded by a growth-arrest-specific (gas) gene. Proc Natl Acad Sci USA. 1996;93:4617–4622. doi: 10.1073/pnas.93.10.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacoby M, Cox JJ, Gayral S, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet. 2009;41:1027–1031. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- 62.Anderson CT, Stearns T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr Biol. 2009;19:1498–1502. doi: 10.1016/j.cub.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Awan A, Oliveri RS, Jensen PL, et al. Immunoflourescence and mRNA analysis of human embryonic stem cells (hESCs) grown under feeder-free conditions. Methods Mol Biol. 2010;584:195–210. doi: 10.1007/978-1-60761-369-5_11. [DOI] [PubMed] [Google Scholar]
- 64.Tucker RW, Pardee AB, Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17:527–535. doi: 10.1016/0092-8674(79)90261-7. [DOI] [PubMed] [Google Scholar]
- 65.Shin I, Yakes FM, Rojo F, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 66.Guo H, Tian T, Nan K, et al. p57: A multifunctional protein in cancer (review) Int J Oncol. 2010;36:1321–1329. doi: 10.3892/ijo_00000617. [DOI] [PubMed] [Google Scholar]
- 67.Borriello A, Bencivenga D, Criscuolo M, et al. Targeting p27Kip1 protein: its relevance in the therapy of human cancer. Expert Opin Therapeut Targets. 2011;15:677–693. doi: 10.1517/14728222.2011.561318. [DOI] [PubMed] [Google Scholar]
- 68.Pateras IS, Apostolopoulou K, Niforou K, et al. p57KIP2: ‘Kip’ing the cell under control. Mol Cancer Res. 2009;7:1902–1919. doi: 10.1158/1541-7786.MCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 69.Gartel AL. p21(WAF1/CIP1) and cancer: a shifting paradigm? Biofactors. 2009;35:161–164. doi: 10.1002/biof.26. [DOI] [PubMed] [Google Scholar]
- 70.Bielas SL, Silhavy JL, Brancati F, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kinzel D, Boldt K, Davis EE, et al. Pitchfork regulates primary cilia disassembly and left-right asymmetry. Dev Cell. 2010;19:66–77. doi: 10.1016/j.devcel.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim S, Zaghloul NA, Bubenshchikova E, et al. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat Cell Biol. 2011;13:351–360. doi: 10.1038/ncb2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pugacheva EN, Jablonski SA, Hartman TR, et al. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li A, Saito M, Chuang JZ, et al. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat Cell Biol. 2011;13:402–411. doi: 10.1038/ncb2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christensen ST, Pedersen SF, Satir P, et al. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol. 2008;85:279–292. doi: 10.1016/S0070-2153(08)00810-7. [DOI] [PubMed] [Google Scholar]
- 76.Kim S, Tsiokas L. Cilia and cell cycle re-entry: More than a coincidence. Cell Cycle. 2011;10 doi: 10.4161/cc.10.16.17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plotnikova OV, Golemis EA, Pugacheva EN. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008;68:2058–2061. doi: 10.1158/0008-5472.CAN-07-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kurosaka S, Kashina A. Cell biology of embryonic migration. Birth Defects Res C Embryo Today. 2008;84:102–122. doi: 10.1002/bdrc.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albrecht-Buehler G. Phagokinetic tracks of 3T3 cells: parallels between the orientation of track segments and of cellular structures which contain actin or tubulin. Cell. 1977;12:333–339. doi: 10.1016/0092-8674(77)90109-x. [DOI] [PubMed] [Google Scholar]
- 80.Katsumoto T, Higaki K, Ohno K, et al. The orientation of primary cilia during the wound response in 3Y1 cells. Biol Cell. 1994;81:17–21. doi: 10.1016/0248-4900(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 81.Lu CJ, Du H, Wu J, et al. Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press Res. 2008;31:171–184. doi: 10.1159/000132462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benzing T, Walz G. Cilium-generated signaling: a cellular GPS? Curr Opin Nephrol Hypertens. 2006;15:245–249. doi: 10.1097/01.mnh.0000222690.53970.ca. [DOI] [PubMed] [Google Scholar]
- 83.Ross R. Platelet-derived growth factor. Annu Rev Med. 1987;38:71–79. doi: 10.1146/annurev.me.38.020187.000443. [DOI] [PubMed] [Google Scholar]
- 84.Larrea MD, Wander SA, Slingerland JM. p27 as Jekyll and Hyde: regulation of cell cycle and cell motility. Cell Cycle. 2009;8:3455–3461. doi: 10.4161/cc.8.21.9789. [DOI] [PubMed] [Google Scholar]
- 85.Besson A, Gurian-West M, Schmidt A, et al. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schneider L, Stock CM, Dieterich P, et al. The Na+/H+ exchanger NHE1 is required for directional migration stimulated via PDGFRα in the primary cilium. J Cell Biol. 2009;185:163–176. doi: 10.1083/jcb.200806019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cuello F, Snabaitis AK, Cohen MS, et al. Evidence for direct regulation of myocardial Na+/H+ exchanger isoform 1 phosphorylation and activity by 90-kDa ribosomal S6 kinase (RSK): effects of the novel and specific RSK inhibitor fmk on responses to α1-adrenergic stimulation. Mol Pharmacol. 2007;71:799–806. doi: 10.1124/mol.106.029900. [DOI] [PubMed] [Google Scholar]
- 88.Luo J, Kintner DB, Shull GE, et al. ERK1/2-p90RSK-mediated phosphorylation of Na+/H+ exchanger isoform 1. A role in ischemic neuronal death. J Biol Chem. 2007;282:28274–28284. doi: 10.1074/jbc.M702373200. [DOI] [PubMed] [Google Scholar]
- 89.Tissenbaum HA, Ruvkun G. An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans. Genetics. 1998;148:703–717. doi: 10.1093/genetics/148.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brogiolo W, Stocker H, Ikeya T, et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 91.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 92.Nef S, Verma-Kurvari S, Merenmies J, et al. Testis determination requires insulin receptor family function in mice. Nature. 2003;426:291–295. doi: 10.1038/nature02059. [DOI] [PubMed] [Google Scholar]
- 93.Hernandez-Sanchez C, Mansilla A, de Pablo F, et al. Evolution of the insulin receptor family and receptor isoform expression in vertebrates. Mol Biol Evol. 2008;25:1043–1053. doi: 10.1093/molbev/msn036. [DOI] [PubMed] [Google Scholar]
- 94.Moxham CP, Duronio V, Jacobs S. Insulin-like growth factor I receptor β-subunit heterogeneity. Evidence for hybrid tetramers composed of insulin-like growth factor I and insulin receptor heterodimers. J Biol Chem. 1989;264:13238–13244. [PubMed] [Google Scholar]
- 95.White MF, Yenush L. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Curr Top Microbiol Immunol. 1998;228:179–208. doi: 10.1007/978-3-642-80481-6_8. [DOI] [PubMed] [Google Scholar]
- 96.Takeuchi K, Ito F. EGF receptor in relation to tumor development: molecular basis of responsiveness of cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J. 2010;277:316–326. doi: 10.1111/j.1742-4658.2009.07450.x. [DOI] [PubMed] [Google Scholar]
- 97.Threadgill DW, Dlugosz AA, Hansen LA, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 98.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jensen CG, Poole CA, McGlashan SR, et al. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int. 2004;28:101–110. doi: 10.1016/j.cellbi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 100.McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- 101.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 102.Dorey K, Amaya E. FGF signalling: diverse roles during early vertebrate embryogenesis. Development. 2010;137:3731–3742. doi: 10.1242/dev.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149:121–130. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Essner JJ, Vogan KJ, Wagner MK, et al. Conserved function for embryonic nodal cilia. Nature. 2002;418:37–38. doi: 10.1038/418037a. [DOI] [PubMed] [Google Scholar]
- 105.Nonaka S, Tanaka Y, Okada Y, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating left-ward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 106.Hirokawa N, Tanaka Y, Okada Y, et al. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 107.Liu DW, Hsu CH, Tsai SM, et al. A variant of fibroblast growth factor receptor 2 (fgfr2) regulates left-right asymmetry in zebrafish. PLoS One. 2011;6:e21793. doi: 10.1371/journal.pone.0021793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamauchi H, Miyakawa N, Miyake A, et al. Fgf4 is required for left-right patterning of visceral organs in zebrafish. Dev Biol. 2009;332:177–185. doi: 10.1016/j.ydbio.2009.05.568. [DOI] [PubMed] [Google Scholar]
- 109.Xu PF, Zhu KY, Jin Y, et al. Setdb2 restricts dorsal organizer territory and regulates left-right asymmetry through suppressing fgf8 activity. Proc Natl Acad Sci USA. 2010;107:2521–2526. doi: 10.1073/pnas.0914396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matsui T, Thitamadee S, Murata T, et al. Canopy1, a positive feedback regulator of FGF signaling, controls progenitor cell clustering during Kupffer’s vesicle organogenesis. Proc Natl Acad Sci USA. 2011;108:9881–9886. doi: 10.1073/pnas.1017248108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miraoui H, Marie PJ. Fibroblast growth factor receptor signaling crosstalk in skeletogenesis. Sci Signal. 2010;3:re9. doi: 10.1126/scisignal.3146re9. [DOI] [PubMed] [Google Scholar]
- 112.Annenkov A. The insulin-like growth factor (IGF) receptor type 1 (IGF1R) as an essential component of the signalling network regulating neurogenesis. Mol Neurobiol. 2009;40:195–215. doi: 10.1007/s12035-009-8081-0. [DOI] [PubMed] [Google Scholar]
- 113.Hao H, Naomoto Y, Bao X, et al. Focal adhesion kinase as potential target for cancer therapy (review) Oncol Rep. 2009;22:973–979. doi: 10.3892/or_00000524. [DOI] [PubMed] [Google Scholar]
- 114.Liebmann C. EGF receptor activation by GPCRs: an universal pathway reveals different versions. Mol Cell Endocrinol. 2011;331:222–231. doi: 10.1016/j.mce.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 115.Pyne NJ, Pyne S. Receptor tyrosine kinase-G-protein-coupled receptor signalling platforms: out of the shadow? Trends Pharmacol Sci. 2011;32:443–450. doi: 10.1016/j.tips.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 116.Whitfield JF. The solitary (primary) cilium—a mechanosensory toggle switch in bone and cartilage cells. Cell Signal. 2008;20:1019–1024. doi: 10.1016/j.cellsig.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 117.Knight MM, McGlashan SR, Garcia M, et al. Articular chondrocytes express connexin 43 hemichannels and P2 receptors—a putative mechanoreceptor complex involving the primary cilium? J Anat. 2009;214:275–283. doi: 10.1111/j.1469-7580.2008.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McGlashan SR, Knight MM, Chowdhury TT, et al. Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol Int. 2010;34:441–446. doi: 10.1042/CBI20090094. [DOI] [PubMed] [Google Scholar]
- 119.Farnum CE, Wilsman NJ. Orientation of primary cilia of articular chondrocytes in three-dimensional space. Anat Rec (Hoboken) 2011;294:533–549. doi: 10.1002/ar.21330. [DOI] [PubMed] [Google Scholar]
- 120.McGlashan SR, Cluett EC, Jensen CG, et al. Primary cilia in osteoarthritic chondrocytes: from chondrons to clusters. Dev Dyn. 2008;237:2013–2020. doi: 10.1002/dvdy.21501. [DOI] [PubMed] [Google Scholar]
- 121.de Andrea CE, Wiweger M, Prins F, et al. Primary cilia organization reflects polarity in the growth plate and implies loss of polarity and mosaicism in osteochondroma. Lab Invest. 2010;90:1091–1101. doi: 10.1038/labinvest.2010.81. [DOI] [PubMed] [Google Scholar]
- 122.Drummond IA. Polycystins, focal adhesions and extracellular matrix interactions. Biochim Biophys Acta. 2011;1812:1322–1326. doi: 10.1016/j.bbadis.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boehlke C, Kotsis F, Patel V, et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol. 2010;12:1115–1122. doi: 10.1038/ncb2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bell PD, Fitzgibbon W, Sas K, et al. Loss of primary cilia upregulates renal hypertrophic signaling and promotes cystogenesis. J Am Soc Nephrol. 2011;22:839–848. doi: 10.1681/ASN.2010050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Caron E, Ghosh S, Matsuoka Y, et al. A comprehensive map of the mTOR signaling network. Mol Syst Biol. 2010;6:453. doi: 10.1038/msb.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu L, Parent CA. TOR kinase complexes and cell migration. J Cell Biol. 2011;194:815–824. doi: 10.1083/jcb.201102090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duvel K, Yecies JL, Menon S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27(suppl 2):S43–51. doi: 10.1038/onc.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Q, Davenport JR, Croyle MJ, et al. Disruption of IFT results in both exocrine and endocrine abnormalities in the pancreas of Tg737(orpk) mutant mice. Lab Invest. 2005;85:45–64. doi: 10.1038/labinvest.3700207. [DOI] [PubMed] [Google Scholar]
- 130.van Veelen W, Korsse SE, van de Laar L, et al. The long and winding road to rational treatment of cancer associated with LKB1/AMPK/TSC/mTORC1 signaling. Oncogene. 2011;30:2289–2303. doi: 10.1038/onc.2010.630. [DOI] [PubMed] [Google Scholar]
- 131.Dobashi Y, Watanabe Y, Miwa C, et al. Mammalian target of rapamycin: a central node of complex signaling cascades. Int J Clin Exp Pathol. 2011;4:476–495. [PMC free article] [PubMed] [Google Scholar]
- 132.Brader S, Eccles SA. Phosphoinositide 3-kinase signalling pathways in tumor progression, invasion and angiogenesis. Tumori. 2004;90:2–8. doi: 10.1177/030089160409000102. [DOI] [PubMed] [Google Scholar]
- 133.Sapi Z, Fule T, Hajdu M, et al. The activated targets of mTOR signaling pathway are characteristic for PDGFRA mutant and wild-type rather than KIT mutant GISTs. Diagn Mol Pathol. 2011;20:22–33. doi: 10.1097/PDM.0b013e3181eb931b. [DOI] [PubMed] [Google Scholar]
- 134.Wen HY, Abbasi S, Kellems RE, et al. mTOR: a placental growth signaling sensor. Placenta. 2005;26(suppl A):S63–69. doi: 10.1016/j.placenta.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 135.Moritz A, Li Y, Guo A, et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal. 2010;3:ra64. doi: 10.1126/scisignal.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang H, Bajraszewski N, Wu E, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–738. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arts HH, Bongers EM, Mans DA, et al. C14ORF179 encoding IFT43 is mutated in Sensenbrenner syndrome. J Med Genet. 2011;48:390–395. doi: 10.1136/jmg.2011.088864. [DOI] [PubMed] [Google Scholar]
- 138.Walczak-Sztulpa J, Eggenschwiler J, Osborn D, et al. Cranioectodermal dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet. 2010;86:949–956. doi: 10.1016/j.ajhg.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kiprilov EN, Awan A, Desprat R, et al. Human embryonic stem cells in culture possess primary cilia with hedgehog signaling machinery. J Cell Biol. 2008;180:897–904. doi: 10.1083/jcb.200706028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schrøder JM, Larsen J, Komarova Y, et al. EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. J Cell Sci. 2011;124:2539–2551. doi: 10.1242/jcs.085852. [DOI] [PMC free article] [PubMed] [Google Scholar]