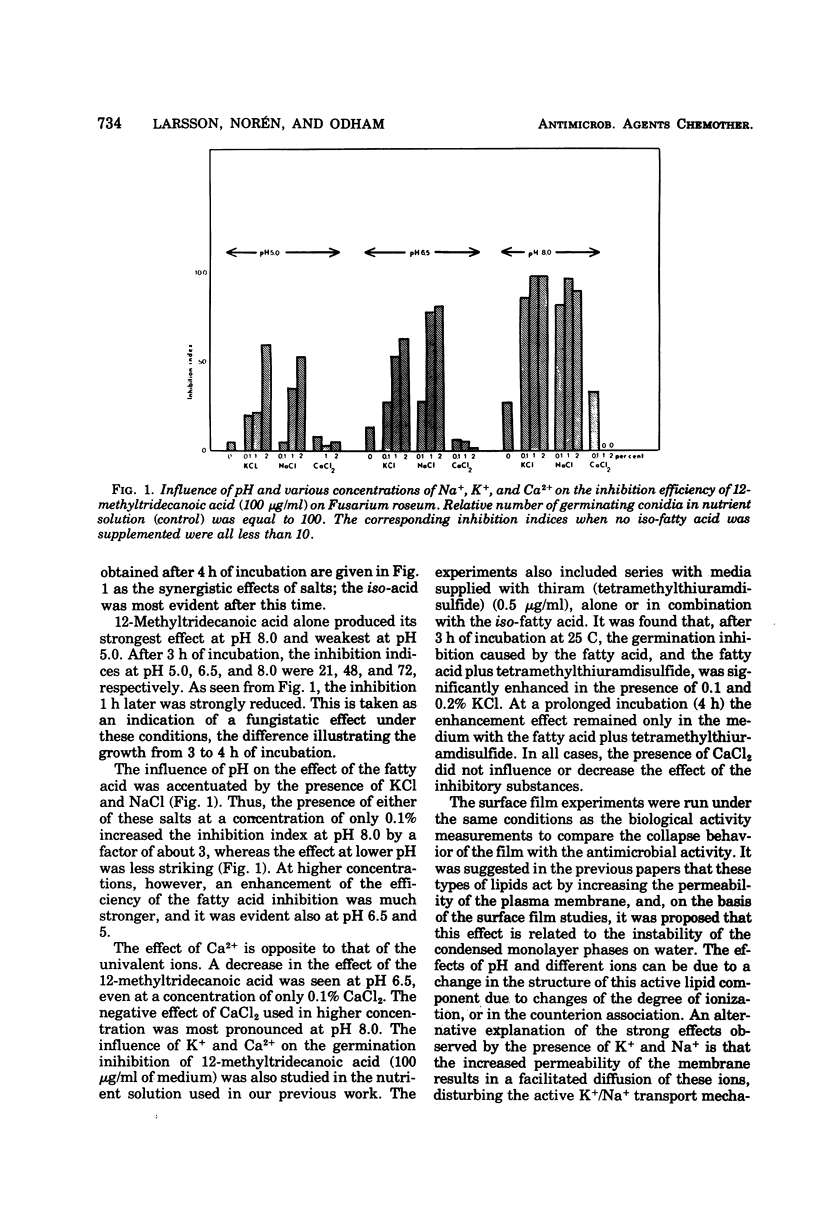
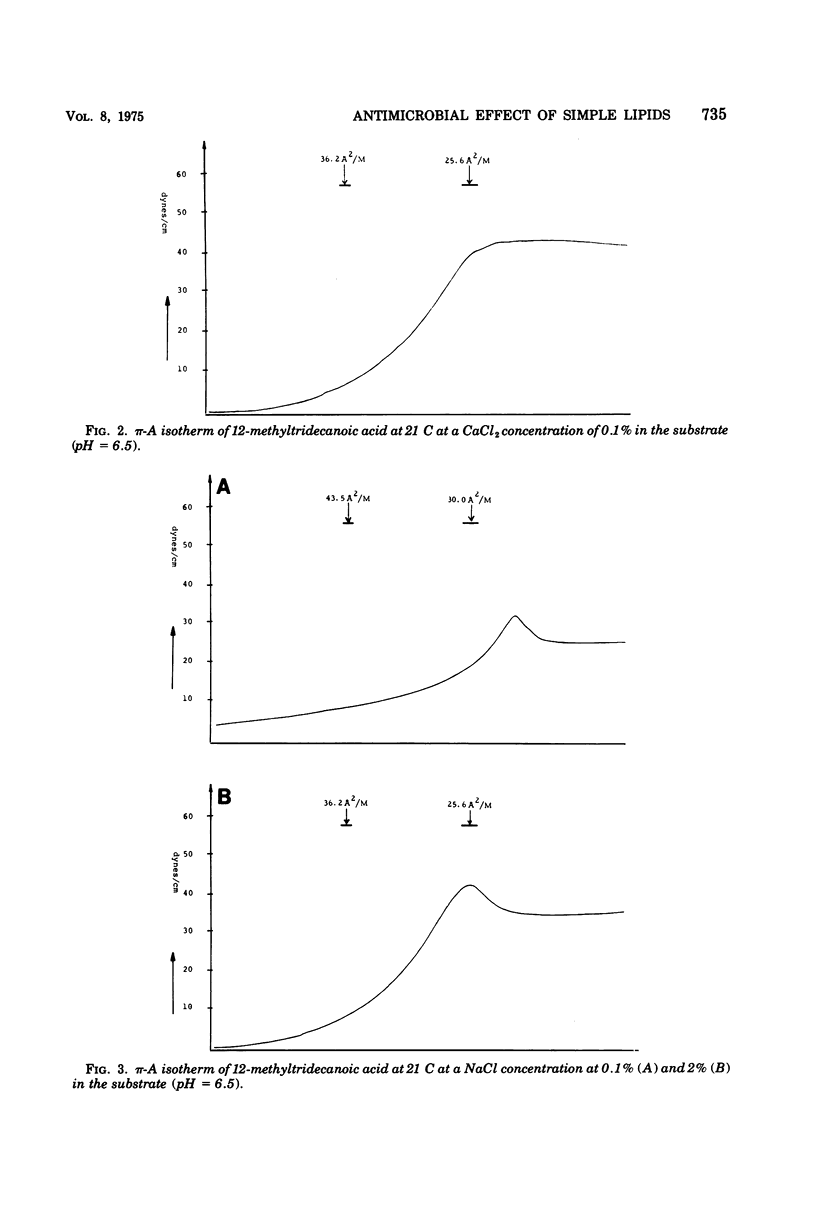
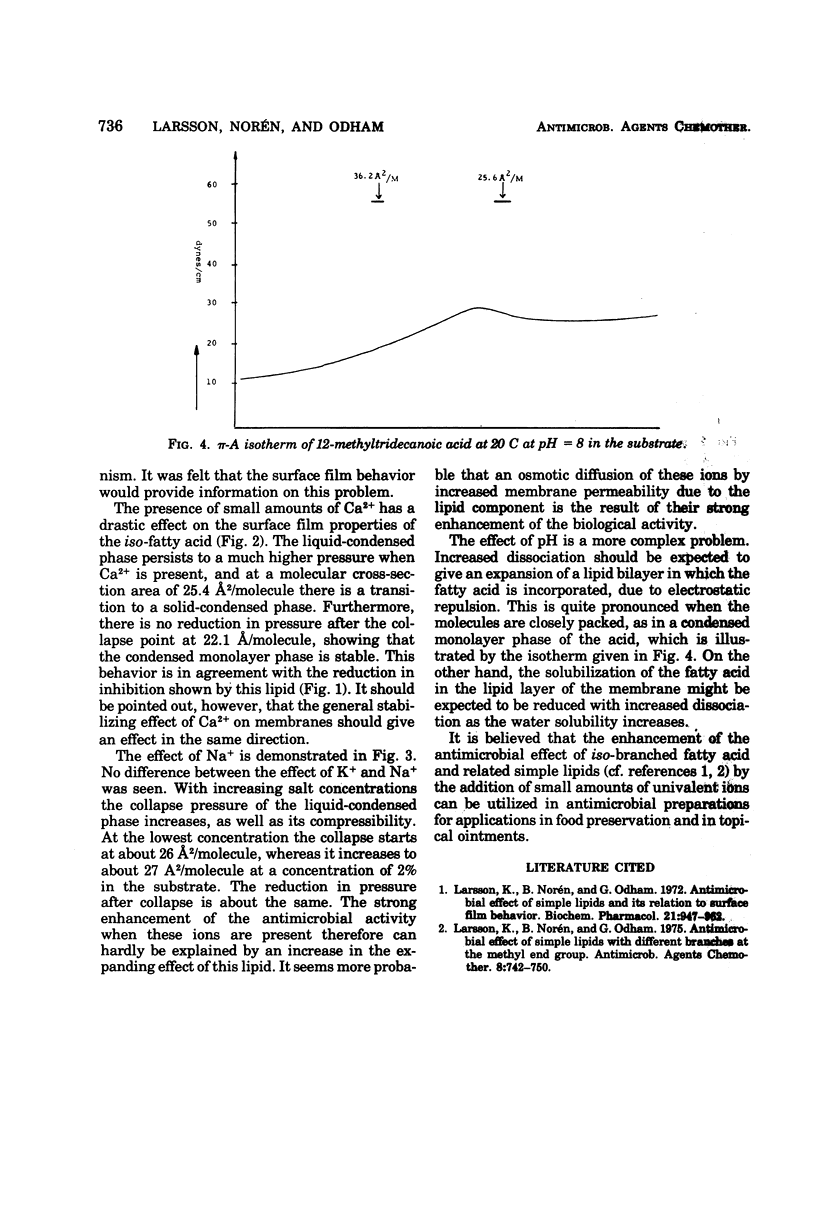
Abstract
Various branched fatty acids, particularly those of iso-configuration, have been shown to possess fungistatic and bacteriostatic properties. On the basis of their swelling effect on hyphae of Fusarium roseum it was suggested that this is due to an increase in the permeability of the plasma membrane. The solubilization of fatty acids in membranes should be expected to be influenced by the degree of dissociation and the presence of counter ions. Therefore, the effects of pH and K+, Na+, and Ca2+ ions were studied. It is demonstrated that the presence of the univalent ions, Na+ and K+, markedly enhances the fungistatic effect of iso-tetradecanoic acid, whereas the opposite effect is noted for the divalent ion, Ca2+. The effects are particularly pronounced at high pH. Furthermore, the antimicrobial effect obtained from the combination of fatty acid and tetramethylthiuramdisulfide is significantly enhanced in the presence of 0.1 and 0.2% KCl.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Larsson K., Noren B., Odham G. Antimicrobial effect of simple lipids and its relation to surface film behaviour. I. Biochem Pharmacol. 1972 Apr 1;21(7):947–962. doi: 10.1016/0006-2952(72)90399-1. [DOI] [PubMed] [Google Scholar]
- Larsson K., Norén B., Odham G. Antimicrobial effect of simple lipids with different branches at the methyl end group. Antimicrob Agents Chemother. 1975 Dec;8(6):742–750. doi: 10.1128/aac.8.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]


