Abstract
Protein adsorption on biomaterials provides them with cues for interaction with cells. A facile method is needed to control the protein adsorption onto biomaterials such as bone implants. Here we doped taurocholic acid (TCA), an amphiphilic biomolecule, into an array of 1D nano-architectured polypyrrole (NAPPy) on the implants. Doping TCA enabled the implant surface to show reversible wettability between 152° (superhydrophobic, switch-on state) and 55° (hydrophilic, switch-off state) in response to periodically switching two weak potentials (+0.50 and −0.80 V as a switch-on and switch-off potential, respectively). The potential-switchable reversible wettability, arising from the potential-tunable orientation of the hydrophobic and hydrophilic face of TCA, further led to potential-switchable preferential adsorption of proteins as well as cell adhesion and spreading. Such potential-switchable strategy may open up a new avenue to the control of biological activity on the implant surface.
Keywords: Protein adsorption, Conducting polymer, Potential, Bone implants, Wettability
The clinical success of orthopedic and dental implants, represented by biomedical titanium, heavily relies on the osteointegration at the interface between the implants and host tissue.[1] The optimal osteointegration, without fibrous tissue growth at the interface, stems from the optimal characteristics of the implant surface in close proximity with the bone tissue.[2] The surface characteristics of the implants, in particular the wettability, in conjunction with the cells, determine the biological responses at the implant surface.[3] The protein adsorption on the implants is the first biological response. It is often dependent on the wettability, which is determined by surface chemistry and topography.[4d,5,6] It tends to develope a “conditioning film” for modulating the celluar host response such as the adhesion, spreading, proliferation and differentiation, and consequently, bonic tissue growth on the implants.[4] Most implant materials have non-variable surface wettability. Therefore, tuning their surface wettability is being proposed to control their biological activity.[2b,4d,7] Ideally, an implant should be intelligent so that it is able to reversibly adsorb different proteins through reversible wettability. For instance, a protein that can be secreted by cells and promote cell adhesion and spreading, such as fibronectin (Fn),[8] can be selectively adsorbed onto the implant at the early stage of the implantation; A growth factor such as bone morphogenetic protein-2 (BMP-2)[9] that can promote osteogenesis and osteointegration can be selectively adsorbed onto the implant at the late stage of implantation. However, simply manipulating the surface chemistry and roughness cannot achieve reversible switch of surface wettability. Here we demonstrate the first-time use of a conducting polymer, polypyrrole (PPy), doped with a unique biomolecule, taurocholic acid (TCA), which can be found in bile and biosynthesized from cholesterol in the liver,[10] to achieve the reversible switch of surface wettability (Scheme 1) and the subseqent reversible protein adsorption as well as cell adhesion and spreading on the implants in response to a weak potential switch.
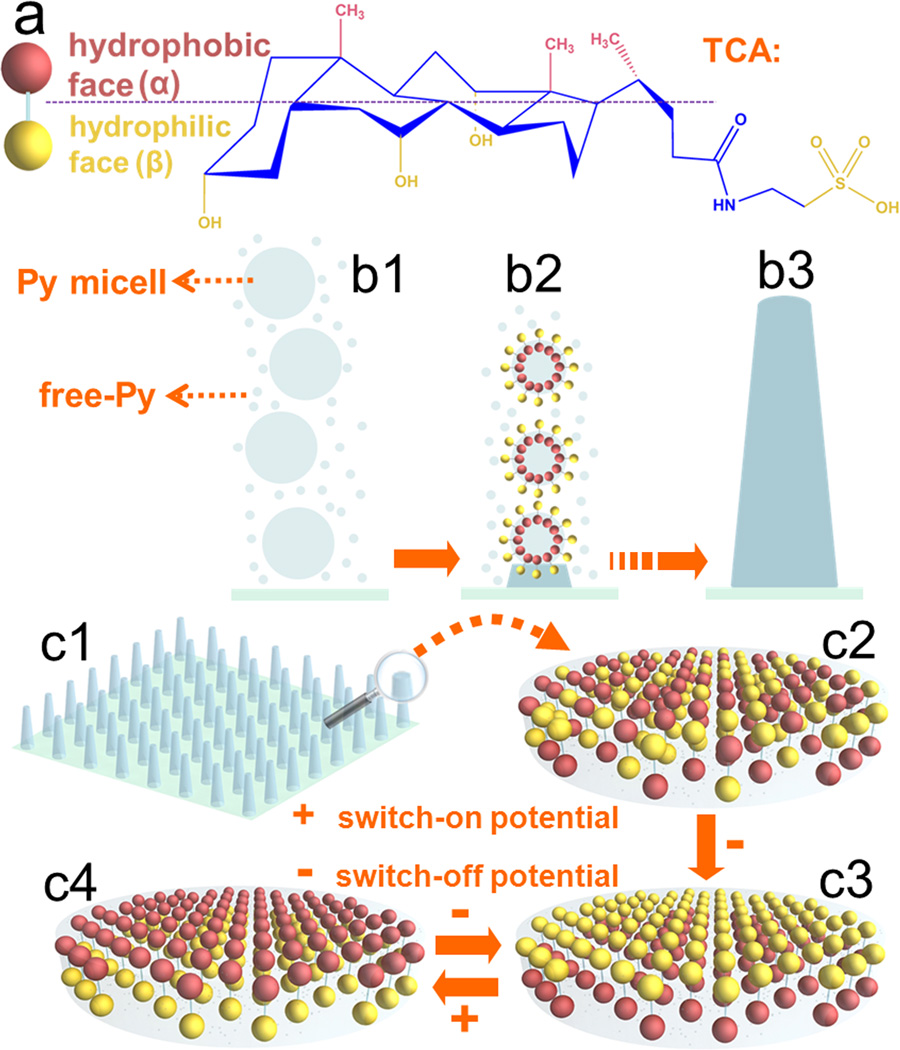
Scheme 1.
(a) The chair conformation of TCA. (b) The possible mechanism of forming 1D NAPPy/TCA through the self-assembly and polymerization of Py droplets and free-Py. (c) Possible mechanism of the potential-switchable wettability of 1D NAPPy/TCA: (c1–c2) The orientation of hydrophobic α-face and hydrophilic β-face of TCA was random in the absence of potential. (c3) The hydrophilic β-face of TCA was exposed on the surface of the implants when a switch-off potential was applied to the implants. This state was termed as switch-off. (c4) When a switch-on potential was applied, the hydrophobic α-face would be exposed on the surface, making the implant surface become hydrophobic. This state was termed as switch-on.
PPy is a class of functional polymers with electroactivity derived from their unique molecular structures [11] and a promising biomaterial due to its excellent tissue compatibility.[12] A dopant is needed to control its properties.[13] However, the perfluorinated dopant (like perfluorooctanesulfonic acid) currently being used in PPy is not suitable for biomedical use as it is an organic pollutant.[14] Hence, from the perspective of biomedical applications, there is a pressing need in a new approach to tuning the properties such as wettability through the use of non-toxic molecules.[15]
TCA was proposed as such a non-toxic molecule in this work. It has special pharmaceutical effects such as anti-inflammation and cough relief. The chair conformation of TCA (Scheme 1a) indicates a unique facial amphiphilicity. Namely, it bears a distinguishable hydrophobic face (α-face) and hydrophilic face (β-face)[16] due to the exposure of -CH3 and -OH/-SO3H groups, respectively. Here TCA was employed as a surfactant-like dopant molecule to guide the self-assembly and polymerization of pyrrole (Py) nanodroplets (i.e., Py micelles) along with free-Py into conical 1D nano-architectured polypyrrole (NAPPy) (Scheme 1b) on the biomedial titanium implants. The TCA molecule was expected to possess tunable orientation of α-face and β-face in PPy matrix in response to periodic switching potentials (Scheme 1c). We found that an appropriate concentration of TCA was indispensable in the fabrication of 1D NAPPy on the titanium. Moreover, potential-induced molecular orientation switch between α-face and β-face of TCA was used to achieve potential-induced surface wettability of the 1D NAPPy/TCA. To demonstrate the possible use of the potential-induced wettability and thus the intelligent response of the conducting polymers on biomedical titanium, we showed that the potential-induced wettability led to the potential-induced preferential adsorption of three model proteins (Fn, bovine serum albumin (BSA) and protamine sulfate (PAS)) as well as controlled adhesion and spreading of MC3T3-E1 osteoblasts on 1D NAPPy/TCA.
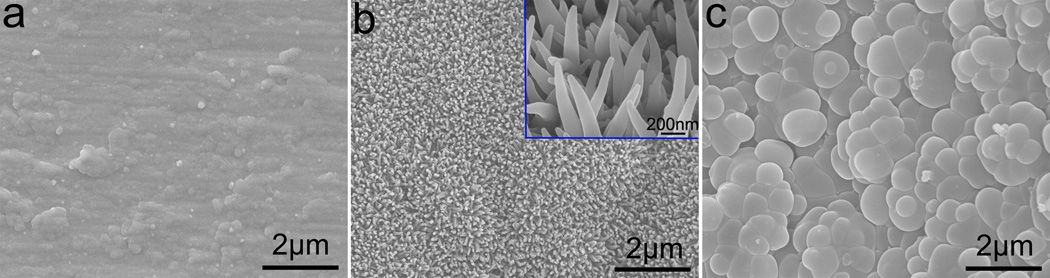
In the attempt to study the effect of TCA as a bio-surfactant on the fabrication of NAPPy/TCA through template-free electrochemical polymerization, it was found that the nano-architectures of PPy were dependent on the concentration of TCA in PBS. When the concentration of TCA was increased from 0.01 M to 0.07 M, and finally to 0.20 M, the PPy evolved from irregular films (Figure 1a) to 1D conical nanowires (Figure 1b), and to tightly packed microparticles (Figure 1c). We believe that it was very important for anionic dopants such as TCA to act like surfactants to stabilize the dispersion of Py nanodroplets in PBS (as electrolyte), which self-assembled into column-like 1D structures and polymerized to form 1D NAPPy (Scheme 1b). When the TCA of lower concentration (0.01 M) was captured by Py nanodroplets, they were not stablizied by enough TCA molecules and failed to self-assemble along one direction into columns. Consequently, an irregular film (Figure 1a) was obtained by the polymerization of the nanodroplets and dissolved free Py. When the TCA concentration was very high (0.2 M), exceeding its critical micelle concentration, TCA formed microscale micelles, which contained dissolved free Py and even Py nanodroplets. As a result, tightly packed microparticles were obtained (Figure 1c). Py nanodroplets could self-assemble along the direction perpendicular to the substrates to from ordered 1D nano-architectures (Scheme 1b) only if TCA was in an appropriate concentration (0.07 M). In addition, the growth of 1D nanoarchitectures started from the surface of the substrates. During its elongation, the dissolved free Py monomers were simultaneously polymerized on its outer surface, allowing them to grow along the lateral direction to increase its diameter and consquently leading to the formation of conical nanowires nearly vertically oriented on the substrates (Figure 1b). We studied the adhesion of MC3T3-E1 osteoblasts on the three different PPy/TCA structures (Figure 1) as well as on the uncoated Ti substrates. We found that 1D NAPPy/TCA could promote the adhesion when compared to the irregular PPy/TCA films and showed similar capability in promoting cell adhesion as un-coated and PPy/TCA microparticle-coated substrates (Figure S1). Furthemore, we found that doping TCA in PPy increased the biocompatibility of the 1D NAPPy/TCA (Figure S2).
Figure 1.
Filed emission scanning electron microscopy (FE-SEM) images of NAPPy/TCA obtained in PBS containing TCA of 0.01 M (a), 0.07 M (b) and 0.20 M (c). Inset in (b): higher magnification.
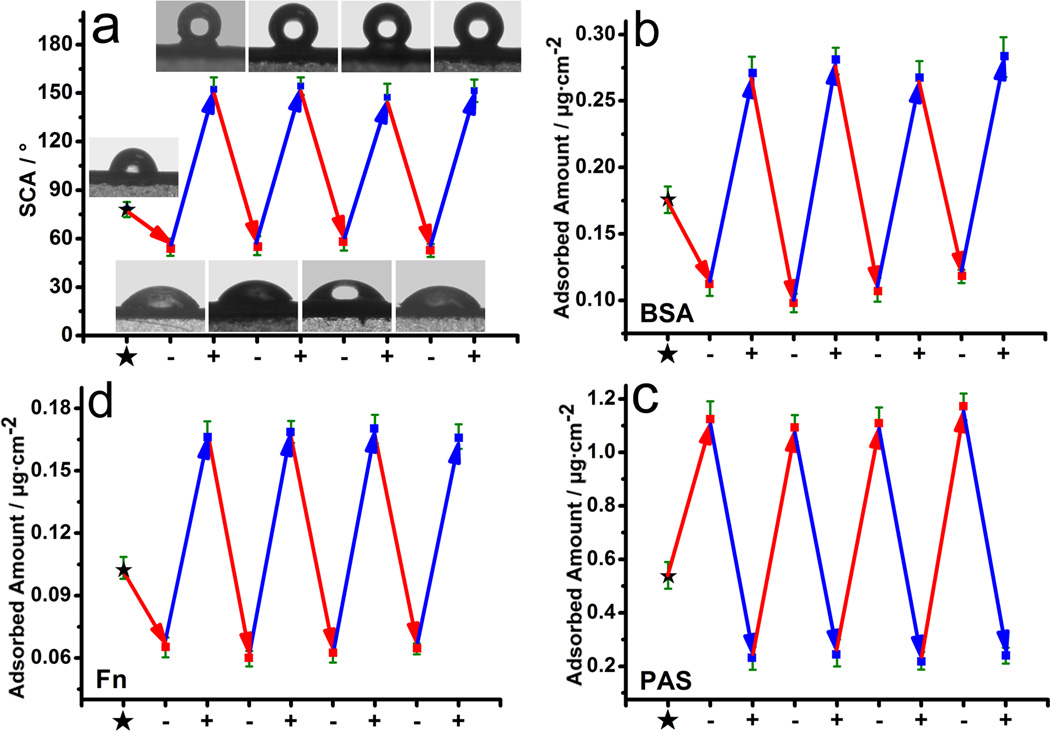
We then tested the reversible switch in the wettability of 1D NAPPy/TCA (Figure 1b) by applying periodic potentials. −0.80 V (as a switch-off potential) and +0.50 V (as a switch-on potential) were applied to generate the reduction state (switch-off state) and oxidation state (switch-on state) of 1D NAPPy/TCA, respectively. A reversible switch (about 152° in switch-on state and 55° in switch-off state) in wettability was achieved (Figure 2a). When the dopant was changed to Cl−, the wettability of the doped 1D NAPPy was not switched in response to the periodic potential as significanlty as 1D NAPPy/TCA (Figure S3), suggesting the unique role of the TCA dopant. In addition, the cell viability on 1D NAPPy/TCA after applying periodic switching potentials with different magnitudes showed that the weak potentials (−0.80 V/+0.50 V) we used did not kill ostoeblasts (Figure S4).
Figure 2.
(a) The plots of SCA of 1D NAPPy/TCA and (b–d) the plots of reversible amount of adsorbed BSA (b), PAS (c) and Fn (d) on 1D NAPPy/TCA versus switch-off potentials (−0.80 V) and switch-on potentials (+0.50 V) in situ. Inset: SCA images.
Through 500 cycles of periodic potential switches, both static contact angle (SCA) in switch-on and switch-off states remained relatively stable (Figure S5). Moreover, the wettability of switch-on and switch-off states after immersion in physiological medium was nearly unchanged (Figure S6). The TCA was not found to release from 1D NAPPy/TCA in physiological medium (Figure S7). These data showed that 1D NAPPy/TCA was very stable.
Electron probe micro-analysis (EPMA) (Figure S8a) identified no significant difference in TCA concentration between the two switching states. However, attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR) of specimens in different states (Figure S8b) showed that not only the peaks resulting from -OH/-SO3H groups on the β-face of TCA were blueshifted in switch-off state compared to other states, but also the reduction potential used to generate the switch-off state caused partial deprotonation of -C-OH (on the β-face) to form -C-O−. Furthermore, Kelvin probe force microscopy (KFM) showed that the surface potential (Figure S8c) of specimen in the switch-off state possessed a stronger strength of negative charge arising from the polar groups on the β-face of TCA whereas a negligible strength of negative charge was present in the switch-on state. In addition, the surface potential distribution of 1D NAPPy/TCA in the switch-off and switch-on states was more even than that in the original state (Figure S8c), suggesting that the orientation of TCA was uniform (i.e., mainly either α-face or β-face is exposed) across the surface of the 1D NAPPy/TCA under the switching potentials. Together these results indicated the expected orientational change of TCA during potential switching (Scheme 1).
We then proposed a model to understand the potential-induced reversible switch in the wettability of 1D NAPPy/TCA (Scheme 1c). In the original state (Scheme 1c2), TCA with α-face and β-face randomly oriented was uniformly incorporated into the PPy matrix, resulting in a SCA of approximately 78°. Unlike common small molecules, TCA has a unique and large spatial structure (Scheme 1a). Thus TCA would not move out of PPy matrix but could change its orientation during potential switching. Under a switch-off potential (Scheme 1c3), the PPy matrix became negative, repelling and thus forcing the -OH/-SO3H polar groups on the β-faces to be exposed on the surface of PPy matrix and consequently generating a hydrophilic surface. It was noteworthy that, polar groups such as -OH and -SO3H formed intermolecular hydrogen bond, and deprotonation of -OH occurred under the switch-off potential as well, which could be evidenced by the blueshifts of the three peaks associated with the -OH/-SO3H groups and the presence of the C-O− peak at 905 cm−1 in swtich-off states (Figure S8b2), respectively. When a switch-on potential was applied (Scheme 1c4), the PPy matrix became positive, thus attracting the polar groups on the β-face of TCA to turn toward the titanium substrate while driving the α-faces of TCA to be uniformly turned toward PPy surface. Consequently, quite a few of α-faces in lieu of β-faces were exposed on the PPy surface, making 1D NAPPy/TCA exhibit a superhydrophobic surface. As such, potential-induced reversible switch in wettability (Figure 2a) was in fact a cycle between the states shown in Scheme 1c3 (switch-off state) and Scheme 1c4 (switch-on state). The surface potential characterized by KFM almost did not change for the switch-on and switch-off states before (Figure S8c) and after (Figure S9) MC3T3-E1 osteoblasts were cultured on the 1D NAPPy/TCA, suggesting that cell growth did not alter the orientation of TCA.
Herein, BSA and PAS with an isoelectric point (pI) of about 4.8 and 12.0, respectively, were chosen as model proteins to test the potential-switchable protein adsorption. It was found that the potential-switchable wettability could be used to control the adsorption of BSA and PAS (Figure 2b and 2c). The amount of adsorbed protein was determined by bicinchoninic acid (BCA) assay.[17] Both proteins were reversibly adsorbed on the 1D NAPPy/TCA in response to periodic potentials. However, the two proteins show inverse adsorption profiles (Figure 2b and 2c). The pI values of BSA and PAS suggested that BSA and PAS were negatively and positively charged at pH 7.4, respectively. The α-faces and β-faces of TCA were exposed on the surface of 1D NAPPy in switch-on and switch-off states, respectively. Therefore, BSA at pH 7.4 was difficult to be adsorded on 1D NAPPy/TCA in hydrophilic switch-off state due to the repellence by polar groups (-OH/-SO3H) of β-faces, while more BSA molecules could be preferentially adsorbed in hydrophobic switch-on state due to the significantly reduced repellence. Conversely, PAS at pH 7.4 was apt to be adsorbed on 1D NAPPy/TCA in hydrophilic swtich-off state due to their attraction with the -OH/-SO3H of β-faces, while fewer PAS molecules could be adsorbed in hydrophobic switch-on state due to the largely reduced attraction. In this way, a reversible switch in preferential protein adsorption can be realized by potential-induced switch in wettability, which is expected to modulate the biological response on the implant surface in real time. Our work indicates that it is possible to control which protein will preferentially adsorb onto the implants, which in turn will control which biological activity will be activated. As such, a functional adhesion protein, Fn (pI=5.5), was employed to validate the feasibility of achieving periodically reversible adsorption by potential-induced switch in wettability. Indeed, Fn showed a similar adsoption profile as BSA because their pI values were very close (Figure 2d).
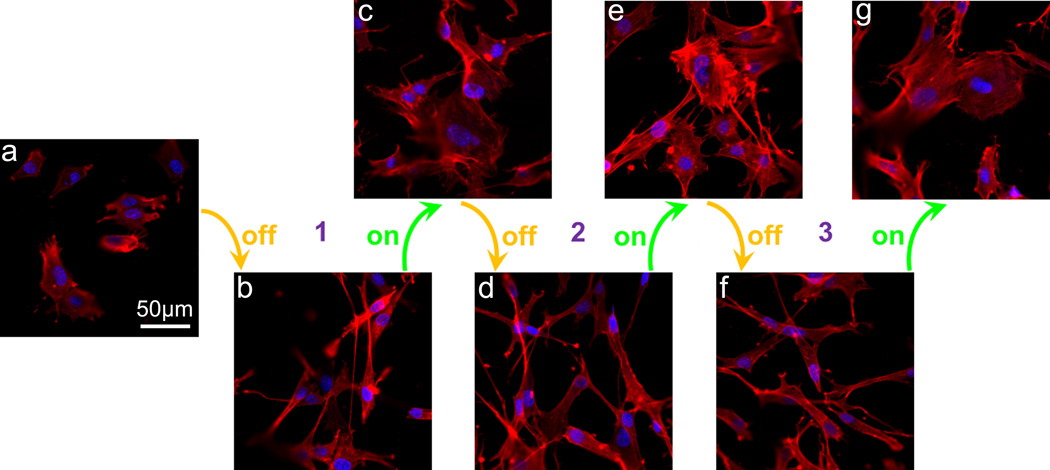
Cells such as osteoblasts will secrete Fn and deposit it on the bone implants in bone repair,[8a] which can in turn promote the cell adhesion and spreading on the implants.[8b,18] Ideally osteoblasts are expected to adhere onto and spread on the implants.[3b,19] Hence, we hypothesized that the potential-induced reversible adsorption of Fn secreted by osteoblasts (Figure 2d) could lead to potential-induced reversible adhesion and spreading of osteoblasts on the 1D NAPPy/TCA. To test this hypothesis, MC3T3-E1 osteoblasts were cultured on the 1D NAPPy/TCA in periodic switch-off and switch-on states and their adhesion and spreading were monitored by immunofluorescence imaging. As expected, the potential-induced reversible Fn adsorption onto the 1D NAPPy/TCA (Figure 2d) indeed resulted in the potential-induced reversible preferential cell adhesion (Figure S10) and spreading (Figures 3 and S11), probably because Fn secreted by osteoblasts was preferentially adsorbed onto or desorbed from the 1D NAPPy/TCA in the swtich-on or switch-off states, respectively.
Figure 3.
Immunofluorescence staining images of MC3T3-E1 osteoblasts after being seeded on 1D NAPPy/TCA in original state (a), periodic switch-off states (b, d and f) and switch-on states (c, e and g) for 8 h.
In summary, in order to form intelligent implant surface that can potentially govern interfacial biological response in real time, the 1D NAPPy was fabricated by doping TCA. The resultant coating displayed reversible switch in wettability by in situ applying cell-safe periodic weak potentials; its switch-on and switch-off states exhibited SCA of 152° and 55°, respectivley, which was due to the switching of the orientation between α-face and β-face of TCA on PPy surface. Moreover, such potential-switchable reversible wettability resulted in a reversible switch in the adsorption of various proteins as well as in the adhesion and spreading of osteoblasts. The facile potential-switchable wettability and protein adsorption will enable us to manipulate the biological response on the implant surface in real time as needed at different implantation stages. Such potential-induced switch in surface chemistry may also be achieved in other conducting polymers, opening up a new avenue to potential-responsive intelligent materials.
Experimental Section
Experimental details are described in supplementary information.
Supplementary Material
Acknowledgements
This work was supported by gratefully acknowledge the financial support of National Basic Research Program of China (Grant No. 2012CB619100) and the National Natural Science Foundation of China (Grant Nos. 51372087, 51072057). YZ and CBM would like to thank the financial support from National Institutes of Health (EB015190), National Science Foundation (CBET-0854414, CMMI-1234957, and DMR-0847758), Department of Defense Peer Reviewed Medical Research Program (W81XWH-12-1-0384), Oklahoma Center for Adult Stem Cell Research (434003) and Oklahoma Center for the Advancement of Science and Technology (HR11-006). We would like to thank Xuliang Deng’s group (Peking University School and Hospital of Stomatology) for their assistance in biological tests.
Contributor Information
Guoxin Tan, Email: tanguoxin@126.com.
Chengyun Ning, Email: imcyning@scut.edu.cn.
Chuanbin Mao, Email: cbmao@ou.edu.
References
- 1.Albrektsson T, Brånemark P-I, Hansson H-A, Lindström J. Acta Orthop. 1981;52:155. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- 2.a) Variola F, Vetrone F, Richert L, Jedrzejowski P, Yi JH, Zalzal S, Clair S, Sarkissian A, Perepichka DF, Wuest JD. Small. 2009;5:996. doi: 10.1002/smll.200801186. [DOI] [PubMed] [Google Scholar]; b) Padial-Molina M, Galindo-Moreno P, Fernández-Barbero JE, O’Valle F, Jódar-Reyes AB, Ortega-Vinuesa JL, Ramón-Torregrosa PJ. Acta Biomater. 2011;7:771. doi: 10.1016/j.actbio.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 3.a) Divya Rani V, Vinoth-Kumar L, Anitha V, Manzoor K, Deepthy M, Shantikumar VN. Acta Biomater. 2012;8:1976. doi: 10.1016/j.actbio.2012.01.021. [DOI] [PubMed] [Google Scholar]; b) Geetha M, Singh A, Asokamani R, Gogia A. Prog. Mater. Sci. 2009;54:397. [Google Scholar]
- 4.a) Jun S-H, Lee E-J, Yook S-W, Kim H-E, Kim H-W, Koh Y-H. Acta Biomater. 2010;6:302. doi: 10.1016/j.actbio.2009.06.024. [DOI] [PubMed] [Google Scholar]; b) Okada S, Ito H, Nagai A, Komotori J, Imai H. Acta Biomater. 2010;6:591. doi: 10.1016/j.actbio.2009.07.037. [DOI] [PubMed] [Google Scholar]; c) Anselme K. Biomaterials. 2000;21:667. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]; d Xu LC, Siedlecki CA. Biomaterials. 2007;28:3273. doi: 10.1016/j.biomaterials.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosales-Leal J, Rodríguez-Valverde M, Mazzaglia G, Ramon-Torregrosa P, Diaz-Rodriguez L, Garcia-Martinez O, Vallecillo-Capilla M, Ruiz C, Cabrerizo-Vilchez M. Colloids Surf. A. 2010;365:222. [Google Scholar]
- 6.a) Niepel MS, Peschel D, Sisquella X, Planell JA, Groth T. Biomaterials. 2009;30:4939. doi: 10.1016/j.biomaterials.2009.06.014. [DOI] [PubMed] [Google Scholar]; b) Israelachvili J, Wennerstrom H. Nature. 1996;379:219. doi: 10.1038/379219a0. [DOI] [PubMed] [Google Scholar]
- 7.a) Arima Y, Iwata H. Biomaterials. 2007;28:3074. doi: 10.1016/j.biomaterials.2007.03.013. [DOI] [PubMed] [Google Scholar]; b) Ranella A, Barberoglou M, Bakogianni S, Fotakis C, Stratakis E. Acta Biomater. 2010;6:2711. doi: 10.1016/j.actbio.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 8.a) Faghihi S, Azari F, Zhilyaev AP, Szpunar JA, Vali H, Tabrizian M. Biomaterials. 2007;28:3887. doi: 10.1016/j.biomaterials.2007.05.010. [DOI] [PubMed] [Google Scholar]; b) Scheideler L, Rupp F, Wendel HP, Sathe S, Geis-Gerstorfer J. Dent. Mater. 2007;23:469. doi: 10.1016/j.dental.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 9.a) Goodman SB, Yao Z, Keeney M, Yang F. Biomaterials. 2013;34:3174. doi: 10.1016/j.biomaterials.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hunziker EB, Enggist L, Kueffer A, Buser D, Liu Y. Bone. 2012;51:98. doi: 10.1016/j.bone.2012.04.004. [DOI] [PubMed] [Google Scholar]; c) Lee D-W, Yun Y-P, Park K, Kim SE. Bone. 2012;50:974. doi: 10.1016/j.bone.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Zhu X. Sci.China Chem. 2009;52:849. [Google Scholar]
- 11.a) Stavrinidou E, Leleux P, Rajaona H, Khodagholy D, Rivnay J, Lindau M, Sanaur S, Malliaras GG. Adv. Mater. 2013;25:4488. doi: 10.1002/adma.201301240. [DOI] [PubMed] [Google Scholar]; b) Liao J, Wu S, Yin Z, Huang S, Ning C, Tan G, Chu PK. ACS Appl. Mater. Interfaces. 2014 doi: 10.1021/am5017478. [DOI] [PubMed] [Google Scholar]
- 12.a) Guimard NK, Gomez N, Schmidt CE. Prog. Polym. Sci. 2007;32:876. [Google Scholar]; b) Liao J, Pan H, Ning C, Tan G, Zhou Z, Chen J, Huang S. Macromol. Rapid Comm. 2014;35:574. doi: 10.1002/marc.201300843. [DOI] [PubMed] [Google Scholar]; c) Abidian MR, Ludwig KA, Marzullo TC, Martin DC, Kipke DR. Adv. Mater. 2009;21:3764. doi: 10.1002/adma.200900887. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Darmanin T, Guittard F. ChemPhysChem. 2013;14:2529. doi: 10.1002/cphc.201300283. [DOI] [PubMed] [Google Scholar]
- 13.a) Xu L, Chen Z, Chen W, Mulchandani A, Yan Y. Macromol. Rapid Comm. 2008;29:832. [Google Scholar]; b) Xin B, Hao J. Chem. Soc. Rev. 2010;39:769. doi: 10.1039/b913622c. [DOI] [PubMed] [Google Scholar]; c) Darmanin T, Givenchy ETd, Amigoni S, Guittard F. Adv. Mater. 2013;25:1378. doi: 10.1002/adma.201204300. [DOI] [PubMed] [Google Scholar]; d) Zhu Y, Feng L, Xia F, Zhai J, Wan M, Jiang L. Macromol. Rapid Comm. 2007;28:1135. [Google Scholar]; e) Isaksson J, Tengstedt C, Fahlman M, Robinson N, Berggren M. Adv. Mater. 2004;16:316. [Google Scholar]
- 14.a) Pernites RB, Ponnapati RR, Advincula RC. Adv. Mater. 2011;23:3207. doi: 10.1002/adma.201100469. [DOI] [PubMed] [Google Scholar]; b) Xiao F, Halbach TR, Simcik MF, Gulliver JS. Water Res. 2012;46:3101. doi: 10.1016/j.watres.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Liao J, Ning C, Yin Z, Tan G, Huang S, Zhou Z, Chen J, Pan H. ChemPhysChem. 2013;14:3891. doi: 10.1002/cphc.201300746. [DOI] [PubMed] [Google Scholar]
- 16.a) Mukhopadhyay S, Maitra U. Curr Sci. 2004;87:1666. [Google Scholar]; b) Zhu X-X, Nichifor M. Acc. Chem. Res. 2002;35:539. doi: 10.1021/ar0101180. [DOI] [PubMed] [Google Scholar]
- 17.Sampson DL, Chng YL, Upton Z, Hurst CP, Parker AW, Parker TJ. Anal. Biochem. 2013;442:110. doi: 10.1016/j.ab.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 18.CR W, JA P, WM S, PR VT. Biomaterials. 2007;28:851. [Google Scholar]
- 19.Khang D, Choi J, Im YM, Kim YJ, Jang JH, Kang SS, Nam TH, Song J, Park JW. Biomaterials. 2012;33:5997. doi: 10.1016/j.biomaterials.2012.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.