Abstract
Non-alcoholic steatohepatitis (NASH) is a disease that compromises hepatic function and the capacity to metabolize numerous drugs. Aryl hydrocarbon receptor (AhR), constitutive androstane receptor (CAR), pregnane X receptor (PXR), peroxisome proliferator-activated receptor alpha (PPARα), and nuclear factor-E2 related factor 2 (Nrf2) are xenobiotic activated transcription factors that regulate induction of a number of drug metabolizing enzymes (DMEs). The purpose of the current study was to determine whether experimental NASH alters the xenobiotic activation of these transcription factors and induction of downstream DME targets Cyp1A1, Cyp2B10, Cyp3A11, Cyp4A14 and NAD(P)H:quinone oxidoreductase 1 (Nqo1), respectively. Mice fed normal rodent chow or methionine-choline-deficient (MCD) diet for 8 weeks were then treated with microsomal enzyme inducers β-naphoflavone (BNF), 1,4-bis-[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP), pregnenolone-16α-carbonitrile (PCN), clofibrate (CFB) or oltipraz (OPZ), known activators of AhR, CAR, PXR, PPARα and Nrf2, respectively. Results of this study show that (1) Hepatic PXR mRNA levels were significantly increased (1.4-fold) in mice fed MCD diet, while AhR, CAR, PPARα and Nrf2 were not affected. (2) The MCD diet did not alter hepatic inducibility of Cyp1A1, Cyp2B10, Cyp3A11 mRNA levels by their respective microsomal inducers. (3) Constitutive levels of Cyp4A14 mRNA were significantly increased in mice fed the MCD diet, yet further induction by clofibrate was not observed. (4) Hepatic Nqo1 mRNA levels were significantly increased by the MCD diet; however, additional induction of Nqo1 was still achievable following treatment with the Nrf2 activator OPZ.
Keywords: Non-alcoholic fatty liver disease, Xenobiotic activated receptors, Drug metabolizing enzyme induction, Cytochrome P450 enzymes and NAD(P)H:quinone oxidoreductase 1
Introduction
Drug metabolizing enzymes (DMEs) play crucial roles in the biotransformation and detoxification of xenobiotics introduced to the human body, providing protection against potentially harmful insults from the environment. Xenobiotic metabolism is invariably affected by the expression of both phase I and phase II DMEs. In response to xenobiotic insults, the expression of many of these DMEs is regulated at the transcriptional level via activation of xenobiotic sensing receptors including aryl hydrocarbon receptor (AhR), constitutive androstane receptor (CAR), pregnane-X receptor (PXR), peroxisome proliferator-activated receptor alpha (PPARα), and nuclear factor-E2 related factor 2 (Nrf2) (Klaassen and Slitt 2005; Waxman 1999; Honkakoski and Negishi 2000; Pascussi et al. 2004). Indeed, in healthy humans, these signal transduction pathways provide a diverse and coordinate defense against potential xenobiotic insult. However, several studies using liver biopsy samples from patients with chronic active hepatitis, cholestasis and cirrhosis have noted reduced microsomal levels and/or activity of DMEs including CYP1A1, CYP2D6 and CYP3A4, suggesting that these induction pathways may be altered in various liver disease states (Wilkinson 1997).
The prevalence of non-alcoholic fatty liver disease (NAFLD) is an increasing concern in the world population, and though once thought an obese adult disease, it is now known to afflict both normal weight persons and children as well (Browning and Horton 2004). NAFLD encompasses a spectrum of symptoms ranging from simple steatosis (fatty liver) to the more severe nonalcoholic steatohepatitis (NASH, fatty liver with liver cell damage and inflammation) to progressive hepatic fibrosis and can eventually result in cirrhosis (Reynaert et al. 2005). NASH occurs in 2–3% of all adults and accounts for approximately 10% of all newly diagnosed cases of chronic liver disease (Neuschwander-Tetri and Caldwell 2003). Because this condition is increasing to remarkable proportions, NASH has rapidly become a significant clinical burden. The effect of this disease on the ability of the body to respond to external chemical stimuli through activation of xenobiotic sensing receptors is currently unknown.
Previous studies have reported that several cytochrome P450 (P450) enzymes are significantly altered in patients with NASH (Westphal and Brogard 1997; Farrell et al. 1978). Alterations in DMEs can predispose the body to xenobiotic insults and thus, patients with NASH are potentially more susceptible to environmental toxicants. The purpose of the present study was to determine the effect of experimental NASH on the expression of the xenobiotic sensing receptors including AhR, CAR, PXR, PPARα and Nrf2, as well as the constitutive expression and inducibility of their downstream DME targets Cyp1A1, Cyp2B10, Cyp3A11, Cyp4A14 and NAD(P)H:quinone oxidoreductase 1 (Nqo1), respectively.
Materials and methods
Chemicals
β-Naphoflavone (BNF), 1,4-bis-[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP), pregnenolone-16α-carbonitrile (PCN) and clofibrate (CFB) were purchased from Sigma (St. Louis, MO). Oltipraz (OPZ) was obtained from LKT Laboratories, Inc. (St. Paul, MN).
Animals
Male C57BL6/J mice (20–25 g) were obtained from Sprague Dawley (Indianapolis, IN). All animals were acclimated in 12 h light and 12 h dark cycles in a University of Arizona AAALAC-certified animal facility for at least 1 week prior to experiments and were allowed water and standard chow ad libitum. Housing and experimental procedures were in accordance with the Guide for the Care and Use of Laboratory Animals as determined by the U.S. National Institutes of Health. Mice (n = 4) were fed normal diet (Harlan Teklad, Indianapolis, IN) or a methioninecholine-deficient (MCD) diet (NASH-#518810 without L-methionine) (Dyets Inc., Bethlehem, PA) for 8 weeks. Following 8 weeks, mice (n = 4 control diet and n = 4 MCD diet/dose group) were either treated once with TCPOBOP (3 mg/kg, ip) or BNF (200 mg/kg, ip), PCN (200 mg/kg, ip), CFB (40 mg/kg, ip) OPZ (150 mg/kg, ip) or corn oil vehicle once per day for four consecutive days at a dose volume of 5 ml/kg. 24 h after the final dosage, mice were sacrificed, livers snap frozen in liquid nitrogen and stored at −80°C until mRNA analysis.
Total RNA isolation
Total RNA was isolated using RNA Bee reagent (Tel-Test Inc., Friendswood, TX) as per the manufacturer’s protocol. RNA concentrations were determined by UV spectrophotometry, and integrity was examined by ethidium bromide staining after agarose gel electrophoresis.
Branched DNA amplification assay
Probe sets for mouse AhR, CAR, PXR, PPARα and Nrf2, as well as mouse cytochrome P450 enzymes Cyp1A1, Cyp2B10, Cyp3A11, Cyp4A14 and Nqo1 were used as previously described (Petrick and Klaassen 2007; Fisher et al. 2007). Specific oligonucleotide probes were diluted in lysis buffer supplied in the Quantigene™ HV Signal Amplification Kit (Panomics, Inc., Freemont, CA). All reagents for analysis (i.e., lysis buffer, capture hybridization buffer, amplifier/label probe buffer, and substrate solution) were supplied in the Quantigene Discovery Kit. Total RNA (1 µg/µl; 10 µl) was added to each well of a 96-well plate containing capture hybridization buffer and 50 µl of each diluted probe set. Total RNA was allowed to hybridize to each probe set overnight at 53°C. Subsequent hybridization steps were carried out as per the manufacturer’s protocol, and luminescence was quantified with a Quantiplex™ 320 bDNA luminometer interfaced with Quantiplex™ Data Management Software Version 5.02 in 96-well plates.
Statistics
Statistical significance was determined between groups by Student’s t test or 1-way ANOVA followed by a Newman–Keuls post hoc test where appropriate and a statistical significance of p < 0.05 was set. Asterisks (*) represent a statistical difference between corn oil and xenobiotic inducer treatment within respective diet group and daggers (†) represent a statistical difference (p < 0.05) between control and MCD diet groups.
Results
Hepatic mRNA expression of xenobiotic activated receptors in experimental NASH
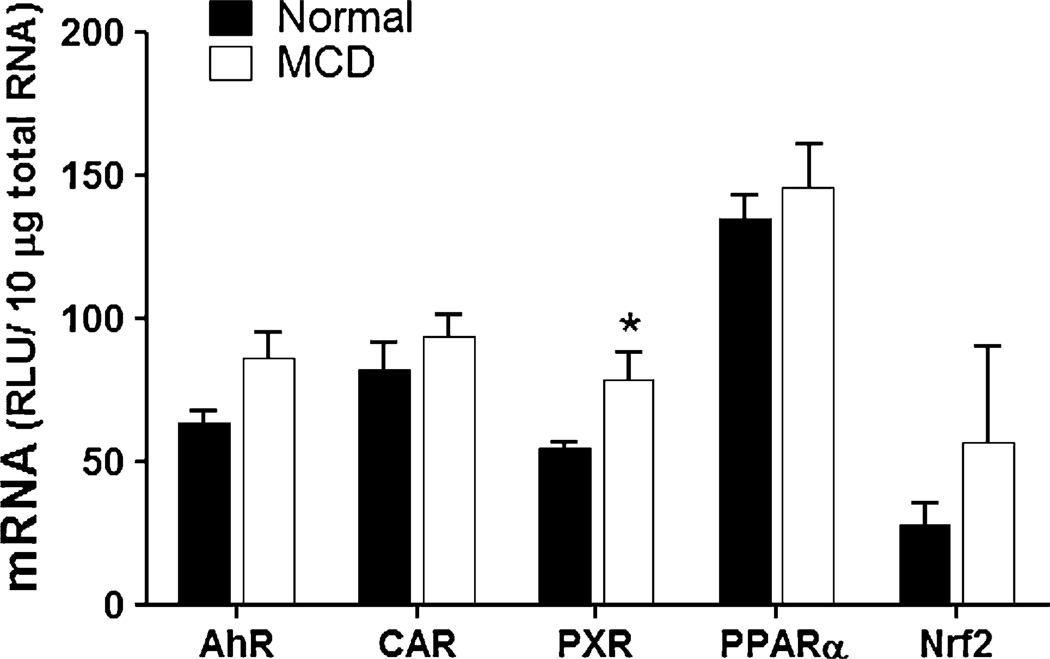
An important issue in regard to drug metabolism during disease states of the liver is whether the disease itself affects the expression of those transcription factors, e.g. nuclear receptors, which are critical to the transcriptional regulation of drug metabolism genes. Figure 1 shows hepatic mRNA levels of AhR, CAR, PXR, PPARα and Nrf2 in mice following 8 weeks on normal chow and MCD diet, respectively. Hepatic PXR mRNA was significantly increased (1.4-fold) in mice fed MCD diet compared to mice fed normal rodent chow. However, mRNA levels of AhR, CAR and PPARα were not significantly altered in MCD mouse livers compared to normal livers.
Fig. 1.
Hepatic mRNA levels of xenobiotic sensing receptors in experimental NASH. Total hepatic RNA from normal rodent diet-fed and MCD diet-fed mice was analyzed for AhR, CAR, PXR, PPARα and Nrf2 mRNA levels by branched DNA assay. Data is expressed as relative light units (RLU) ± standard error of mean. Asterisk indicates significant difference from normal livers (p < 0.05)
Inducibility of hepatic drug metabolizing enzymes in experimental NASH
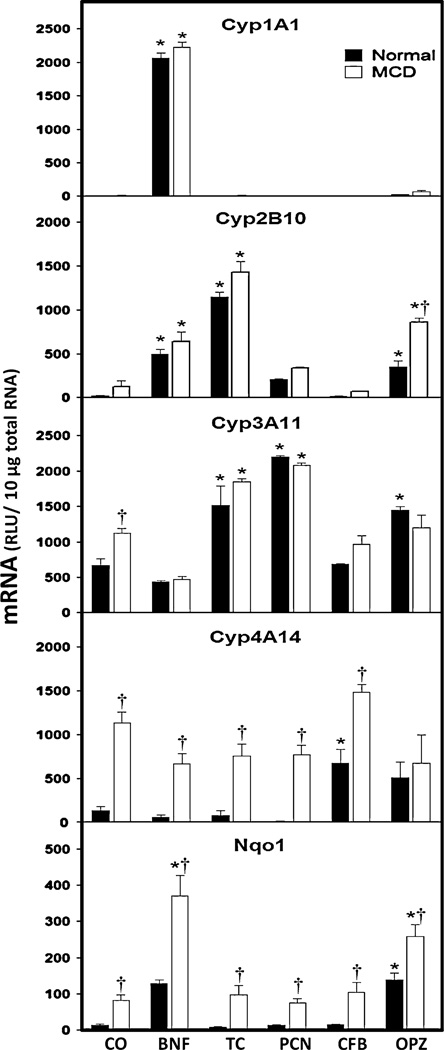
To determine whether experimental NASH disrupts the signaling pathways involved in the induction of hepatic drug metabolizing enzymes, mice were fed a normal or MCD diet were dosed with prototypical microsomal enzyme inducers that activate the xenobiotic sensing receptors AhR, CAR, PXR, PPARα and Nrf2, respectively. BNF treatment, a known activator of AhR, resulted in 594- and 167-fold increases in hepatic Cyp1A1 mRNA in both the normal and MCD diet groups (Fig. 2). In addition, BNF treatment significantly increased Cyp2B10 mRNA levels (21-fold) in normal livers and 5-fold in MCD livers, as well as Nqo1 mRNA levels by 8.5- and 4.5-fold in normal and MCD livers, respectively.
Fig. 2.
Hepatic mRNA levels of drug metabolizing enzymes in experimental NASH. Total hepatic RNA from normal diet-fed and MCD diet-fed mice was analyzed for Cyp1A1, Cyp2B10, Cyp3A11, Cyp4A14 and Nqo1 mRNA levels by branched DNA assay. Data is expressed as relative light units (RLU) ± standard error of mean. Asterisks indicate significant difference between corn oil and xenobiotic inducer treatment within respective diet group (*) (p < 0.05). Daggers indicate significant difference between normal and MCD diet groups when administered a specific xenobiotic inducer (†) (p < 0.05)
The high affinity CAR agonist, TCPOBOP has been show to activate CAR and subsequently induce Cyp2B10 expression in mice. TCPOBOP administration resulted in an increase of hepatic Cyp2B10 mRNA by 50-fold in normal mice and 11-fold in MCD mice. TCPOBOP treatment also caused a significant twofold increase in Cyp3A11 mRNA in both normal and MCD livers.
In experimental NASH, constitutive expression of hepatic Cyp3A11 mRNA levels was significantly increased by 1.7-fold in MCD livers when compared to control diet. Transcriptional regulation of the CYP3A subfamily is of particular importance due to the fact that in humans, CYP3A4 is responsible for the metabolism of approximately 50% of all therapeutic drugs (Harmsen et al. 2007). PCN is a well-established activator of mouse PXR and ultimately induces Cyp3A11 mRNA expression. PCN treatment significantly increased Cyp3A11 mRNA levels in normal and MCD group livers by 2.3- and 2-fold, respectively.
Similar to Cyp3A11, the constitutive expression of Cyp4A14 mRNA was significantly increased (8.6-fold) in MCD diet mice when compared to normal diet-fed mice. Treatment with the PPARα agonist clofibrate has been shown to induce hepatic Cyp4A14 mRNA in mice. As expected, clofibrate treatment produced a significant increase (5.1-fold) in Cyp4A14 mRNA levels in normal mouse livers. However, clofibrate treatment did not induce hepatic Cyp4A14 mRNA in mice with experimental NASH (MCD diet-fed).
Finally, oltipraz has been shown to activate Nrf2 resulting in the nuclear translocation and induction of Nqo1 mRNA. Hepatic Cyp2B10, Cyp3A11 and Nqo1 mRNA levels were significantly increased in OPZ-treated normal mice by 17-, 2.2- and 9.3-fold, respectively. OPZ treatment of experimental NASH mice also caused a 6.7-fold increase in Cyp2B10 mRNA levels, while Cyp3A11 mRNA induction was absent in MCD livers. Similar to Cyp3A11 and Cyp4A14 mRNA levels, constitutive expression of Nqo1 mRNA was significantly increased (5.4-fold) by the MCD diet compared to normal diet-fed mice. However, in contrast to clofibrate, OPZ treatment further induced hepatic Nqo1 mRNA levels (twofold) in MCD diet mice when compared to MCD diet mice treated with vehicle.
Discussion
The liver has a unique physiologic role to protect against a vast array of potentially harmful compounds. Hepatocytes are generally the first line of defense against toxic insults and as such are required to adapt to a complex and ever-changing intracellular environment in a timely manner (Karpen 2002). Hepatocytes are equipped with a number of transcription factors that act as biosensors which, in response to a xenobiotic, are able to induce the expression of genes involved in biotransformation and transport (Elias and Mills 2007). Activation of these biosensor transcription factors must occur in order to trigger their respective signal transduction pathways. However, several studies indicate that disease states such as septic cholestasis, obstructive cholestasis and alcoholic and viral hepatitis cause alterations in cytochrome P450 expression (Westphal and Brogard 1997; Farrell et al. 1978). Coincidentally, several of these P450s are regulated by the aforementioned transcription factors, including PXR and Nrf2. These studies suggest that disease states may alter the ability of biosensors to respond to toxic xenobiotics and coordinate hepatoprotective responses at the transcriptional level.
In the present study, we investigated whether a dietary-induced model of NASH alters the xenobiotic activation of transcription factors which may provide a defense against environmental insults. Classical microsomal enzyme inducers were administered to mice fed a normal diet or an MCD diet. Xenobiotic activation of AhR, CAR, PXR, PPARα, and Nrf2 was assessed in the liver via induction of their downstream target genes, Cyp1a1, Cyp2b10, Cyp3a11, Cyp4a14, and Nqo1, respectively. Both control and MCD diet mice showed a robust induction of Cyp1A1 mRNA levels following BNF administration. These results suggest that experimental MCD does not disrupt the activation and signal transduction pathway mediated by AhR.
The xenobiotic receptors CAR and PXR represent two important members of the nuclear receptor super family and function as sensors of toxic compounds in order to enhance their elimination (Timsit and Negishi 2007). Because CAR and PXR bind to both the phenobarbital response element module (PBREM) and PXR response element (PXRE) of gene promoter regions, ligands of these nuclear receptors robustly increase hepatic Cyp2B10 and Cyp3A11 mRNA expression. As expected, a significant increase in hepatic Cyp2B10 mRNA was observed in normal mice following administration of the known CAR agonist TCPOBOP as previously observed (Koike et al. 2007; Pustylnyak et al. 2007; Qatanani and Moore 2005). The inducibility of Cyp2B10 mRNA levels by TCPOBOP was maintained in experimental NASH mice as similar levels were observed in normal and MCD diet groups. Additionally, TCPOBOP administration to normal and MCD mice resulted in significant increases in hepatic Cyp3A11 mRNA levels due to the cross-talk between CAR and PXR response elements (Anakk et al. 2004; Kast et al. 2002). PCN, a well established PXR agonist, equally induced hepatic expression of Cyp3A11 in both normal and MCD diet mice. Taken together, these data suggest that CAR and PXR activation and subsequent induction of Cyp2B10 and Cyp3A11 mRNA, respectively, remain intact in experimental NASH. Interestingly, hepatic mRNA levels of PXR itself were increased in MCD mice when compared to normal diet mice. This may explain why the constitutive expression of Cyp3A11 was increased in these animals. A previous study by Yang et al. reported decreased levels of CYP3A4 in patients with cirrhosis (Yang et al. 2003), but to our knowledge we are the first to report alterations of this important drug metabolizing enzyme in experimental NASH.
PPARα regulates the expression of genes involved in lipid metabolism, fatty acid oxidation, and glucose homeostasis (Zandbergen and Plutzky 2007) and more recently also implicated in the modulation inflammatory responses linked to liver fibrogenesis (Tanaka and Aoyama 2006). Nagawasa et al. showed that administration of the PPAR-pan agonist, benzofibrate, to MCD mice resulted in decreased hepatocyte steatosis, liver inflammation and number of activated hepatic stellate cells (Nagasawa et al. 2006). In the present study constitutive basal Cyp4A14 mRNA levels were significantly increased suggesting that the pathogenesis of this model results in activation of PPARα. This is not particularly unexpected as CYP4A has been shown to be increased in NAFLD and implicated in the second hit of the hepatic progression of steatosis to NASH (Chitturi and Farrell 2001) However, unlike AhR, CAR or PXR activators, clofibrate administration resulted in no additional induction of Cyp4A14 mRNA levels in MCD mice, inferring that this model causes maximal induction of PPARα.
Nrf2 is an oxidative stress inducible transcription factor that regulates transcriptional activation of target genes via the antioxidant response element (ARE). Nrf2 is known to induce a number of antioxidant genes including its classical target gene Nqo1 (Venugopal and Jaiswal 1996; Petrick and Klaassen 2007; McMahon et al. 2001) Similar to our previous findings (Lickteig et al. 2007), constitutive Nqo1 mRNA levels were significantly increased by the MCD diet. However, unlike the PPARα agonist clofibrate, the prototypical Nrf2 activator oltipraz was still able to further induce Nqo1 mRNA levels in MCD diet mice beyond the initial induction observed due to the MCD diet alone. These data suggest that the activation pathway of Nrf2 is still functional during experimental NASH. Furthermore, these data may indicate that patients suffering from NASH may retain a functional Nrf2 signaling response and still benefit from antioxidant/Nrf2 activation therapies.
In the present study, it was investigated whether a diet-induced model of NASH alters the expression or function of xenobiotic activated transcription factors that may provide a defense against environmental insults. Novel data presented in the current manuscript suggest that: (1) experimental NASH results in increased mRNA levels of PXR; (2) the signaling pathways regulated by the xenobiotic receptors AhR, CAR, PXR maintain their function in experimental NASH; PPARα induction of Cyp4A14 by clofibrate was no longer possible due to putative maximal induction in experimental NASH; and (4) while Nqo1 is induced by experimental NASH, further induction through pharmacologic therapy is still possible. Since xenobiotic activation of Nrf2 and downstream antioxidant drug metabolizing enzymes is maintained during NASH, it is likely NASH patients can still benefit from antioxidant treatment.
Acknowledgments
We are grateful to Ms. Lisa Beilke and Mr. Matthew Merrell for their reading of the manuscript. The project described was supported by NIH grants DK068039, ES06694, and AT002842. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes Digestive and Kidney Diseases, the National Institute for Environmental Health Sciences, the National Center for Complementary & Alternative Medicine of the National Institutes of Health.
Contributor Information
Craig D. Fisher, Email: cfisher@pharmacy.arizona.edu.
Nathan J. Cherrington, Email: cherrington@pharmacy.arizona.edu.
References
- Anakk S, Kalsotra A, Kikuta Y, Huang W, Zhang J, Staudinger JL, Moore DD, Strobel HW. CAR/PXR provide directives for Cyp3a41 gene regulation differently from Cyp3a11. Pharmacogenomics J. 2004;4:91–101. doi: 10.1038/sj.tpj.6500222. [DOI] [PubMed] [Google Scholar]
- Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis. 2001;21:27–41. doi: 10.1055/s-2001-12927. [DOI] [PubMed] [Google Scholar]
- Elias E, Mills CO. Coordinated defence and the liver. Clin Med. 2007;7:180–184. doi: 10.7861/clinmedicine.7-2-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell GC, Cooksley WG, Hart P, Powell LW. Drug metabolism in liver disease. Identification of patients with impaired hepatic drug metabolism. Gastroenterology. 1978;75:580–588. [PubMed] [Google Scholar]
- Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, Lehman-McKeeman LD, Cherrington NJ. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive and rostane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos. 2007;35:995–1000. doi: 10.1124/dmd.106.014340. [DOI] [PubMed] [Google Scholar]
- Harmsen S, Meijerman I, Beijnen JH, Schellens JH. The role of nuclear receptors in pharmacokinetic drug–drug interactions in oncology. Cancer Treat Rev. 2007;33:369–380. doi: 10.1016/j.ctrv.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Negishi M. Regulation of cytochrome P450 (CYP) genes by nuclear receptors. Biochem J. 2000;347:321–337. doi: 10.1042/0264-6021:3470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen SJ. Nuclear receptor regulation of hepatic function. J Hepatol. 2002;36:832–850. doi: 10.1016/s0168-8278(02)00129-0. [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane × receptor, farnesoid x-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Slitt AL. Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab. 2005;6:309–328. doi: 10.2174/1389200054633826. [DOI] [PubMed] [Google Scholar]
- Koike C, Moore R, Negishi M. Extracellular signal-regulated kinase is an endogenous signal retaining the nuclear constitutive active/androstane receptor (CAR) in the cytoplasm of mouse primary hepatocytes. Mol Pharmacol. 2007;71:1217–1221. doi: 10.1124/mol.107.034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickteig AJ, Fisher CD, Augustine LM, Cherrington NJ. Genes of the antioxidant response undergo upregulation in a rodent model of nonalcoholic steatohepatitis. J Biochem Mol Toxicol. 2007;21:216–220. doi: 10.1002/jbt.20177. [DOI] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The cap’n’collar basic leucine zipper transcription factor Nrf2 (NFE2 P45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- Nagasawa T, Inada Y, Nakano S, Tamura T, Takahashi T, Maruyama K, Yamazaki Y, Kuroda J, Shibata N. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006;536:182–191. doi: 10.1016/j.ejphar.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steato-hepatitis: summary of an AASLD single topic conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Gerbal-Chaloin S, Drocourt L, Assenat E, Larrey D, Pichard-Garcia L, Vilarem MJ, Maurel P. Cross-talk between xenobiotic detoxication and other signalling pathways: clinical and toxicological consequences. Xenobiotica. 2004;34:633–664. doi: 10.1080/00498250412331285454. [DOI] [PubMed] [Google Scholar]
- Petrick JS, Klaassen CD. Importance of hepatic induction of constitutive androstane receptor (CAR) and other transcription factors that regulate xenobiotic metabolism and transport. Drug Metab Dispos. 2007;35(10):1806–1815. doi: 10.1124/dmd.107.015974. [DOI] [PubMed] [Google Scholar]
- Pustylnyak VO, Lebedev AN, Gulyaeva LF, Lyakhovich VV, Slynko NM. Comparative study of CYP2B induction in the liver of rats and mice by different compounds. Life Sci. 2007;80:324–328. doi: 10.1016/j.lfs.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Qatanani M, Moore DD. CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metab. 2005;6:329–339. doi: 10.2174/1389200054633899. [DOI] [PubMed] [Google Scholar]
- Reynaert H, Geerts A, Henrion J. Review article: the treatment of non-alcoholic steatohepatitis with thiazolidinediones. Aliment Pharmacol Ther. 2005;22:897–905. doi: 10.1111/j.1365-2036.2005.02682.x. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Aoyama T. PPAR and NASH. Nippon Rinsho. 2006;64:1089–1094. [PubMed] [Google Scholar]
- Timsit YE, Negishi M. CAR and PXR: the xenobiotic-sensing receptors. Steroids. 2007;72:231–246. doi: 10.1016/j.steroids.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and C-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H: quinone oxidoreductase1 gene. Proc Natl Acad Sci USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ. P450 Gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch Biochem Biophys. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- Westphal JF, Brogard JM. Drug administration in chronic liver disease. Drug Saf. 1997;17:47–73. doi: 10.2165/00002018-199717010-00004. [DOI] [PubMed] [Google Scholar]
- Wilkinson GR. The effects of diet, aging and disease-states on presystemic elimination and oral drug bioavailability in humans. Adv Drug Deliv Rev. 1997;27:129–159. doi: 10.1016/s0169-409x(97)00040-9. [DOI] [PubMed] [Google Scholar]
- Yang LQ, Li SJ, Cao YF, Man XB, Yu WF, Wang HY, Wu MC. Different alterations of cytochrome P450 3A4 isoform and its gene expression in livers of patients with chronic liver diseases. World J Gastroenterol. 2003;9:359–363. doi: 10.3748/wjg.v9.i2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbergen F, Plutzky J. PPARalpha in atherosclerosis and inflammation. Biochim Biophys Acta. 2007;1771(8):972–982. doi: 10.1016/j.bbalip.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]