Abstract
Polychlorinated biphenyl (PCB) congeners (PCB 52, 77, and 153) singly and in mixture were spiked and aged in soil microcosms and subsequently planted with switchgrass (Panicum virgatum) or poplar (Populus deltoids x nigra DN34). The planted reactors showed significantly greater reductions in PCB parent compounds when compared to unplanted systems after 32 weeks. There was evidence of reductive dechlorination in both planted and unplanted systems, but the planted microcosms with fully developed roots and rhizospheres showed greater biotransformation than the unplanted reactors. These dechlorination products accounted for approximately all of the molar mass of parent compound lost. Based on the transformation products, reductive dechlorination pathways are proposed for rhizospheric biotransformation of PCB 52, 77, and 153. This is the first report of rhizosphere biotransformation pathways for reductive dechlorination in marginally aerobic, intermittently flooded soil as evidenced by a mass balance on transformation products.
Keywords: Switchgrass, Poplar, Rhizosphere, PCB, Biotransformation
Introduction
Since production began in the 1930's an estimated 1.5 million tons of polychlorinated biphenyls (PCB) have been manufactured (Abraham et al., 2002). PCBs are one the most persistent organic pollutants in the environment today (Bedard 2008, Borja et al., 2005, Kacalkova and Tlustos, 2011). They are consistently included in the top 10 most toxic compounds on the United States Agency for Toxic Substances and Disease Registry (ATSDR) list of priority pollutants (Agency for Toxic Substances and Disease Registry, 2011). Lower chlorinated congeners (1-4 chlorines) usually are more amenable to degradation under aerobic conditions, while highly chlorinated congeners are more readily degraded under anaerobic conditions by reductive dechlorination (Abraham 2002, Borja et al., 2005, Pieper, 2005, Fagervold et al., 2007). Research has exploited the use of sequential aerobic and anaerobic treatment to optimize reduction of PCB mixtures (Fathepure and Vogel, 1991, Master et al., 2002,), and there have been reports of plant assisted microbial degradation of PCBs both in the lab and under field conditions (Leigh et al. 2006, Mackova et al., 2007, Slater et al., 2011).However, these and other studies have mostly focused on dissipation of parent compounds from mixtures or on phytoextraction (Aslund Whitfield et al., 2007,2008, Chekol et al., 2004, Dzantor et al., 2000, 2002, Ficko et al., 2011, Javorska et al., 2011, Singer et al., 2003, White et al., 2006, Zeeb et al., 2006). To date, no study has attained a mass balance or elucidated the transformation products and pathways in the rhizosphere.
Rhizoremediation is an attractive alternative to the current treatment options for PCB remediation, but the intermediates and pathways of transformation must be known. This study seeks to enhance rhizosphere biotransformation of selected PCB congeners by combining the benefits of plant assisted transformation with the manipulation of redox conditions in the rhizosphere to enhance dechlorination. Here, the rhizosphere is defined as the soil in the direct vicinity and influence of roots. Three representative congeners were used in single congener studies, and also combined in a mixture to determine if there are synergistic or antagonistic interactions and to better elucidate single congener pathways.
The most common route of degradation for lower chlorinated PCBs involves dioxygenase attack at the 2,3 or 5,6 positions on the carbon skeleton to form dihydrodiols which are further oxidized aerobically. The transformation of PCB 52 (2, 2’5, 5’ tetrachlorobiphenyl) is thought to be particularly difficult by this route. because in PCB 52, the normal sites of attack (the 2 and 2’ and 5 and 5’ ortho positions) are occupied by chlorine substituents. Ortho substituents are particularly difficult to remove due to steric hindrances which mitigate against an enzymatic approach to those sites. However, it is possible that PCB 52 can be degraded by a 3, 4 (4,5) dioxygenase attack. (Bedard et al., 1987, Komancova et al.,2003). Therefore, PCB 52 (2, 2’5, 5’ tetrachlorobiphenyl) was selected because it is difficult to degrade, due to the chlorine substituents in the ortho positions on both rings.
Due to their toxicity, studies involving coplanar PCBs are particularly important from a public health perspective (Ah-receptors). One microcosm study investigated the ability of microorganisms obtained from PCB contaminated sediments to degrade five coplanar congeners (Zanaroli et al., 2006). One congener, PCB 77 (3, 3’4, 4’ tetrachlorobiphenyl), showed 90% conversion, to products such as 3, 4 dichlorobiphenyl (PCB 12), 3, 4’dichlorobiphenyl (PCB 13) and 3, 3’ dichlorobiphenyl (PCB11). Therefore, PCB 77 was selected because it is one of the most toxic PCB congeners (with coplanar properties exhibiting dioxin-like properties), and it should be readily degraded because it is devoid of chlorine substituents in the ortho position.
The other congener selected was PCB 153 (2, 2’, 4, 4’, 5, 5’hexachlorobiphenyl), which has six chlorine moieties and a tendency to bioaccumulate in humans. In addition, it has ortho substituents on both rings, making it not readily amenable to microbial degradation. However, microbial degradation of PCB 153 has been observed previously with Burkholderia xenovorans LB400 and Alcaligenes eutrophus (now Ralstonia eutropha) H850 (Bedard et al., 1986, Leigh et al. 2006).
The plant species chosen for this study were switchgrass (Panicum virgatum) and hybridpoplar (Populus deltoids x nigra DN34). Switchgrass is perennial, deep rooted, hardy, widespread, and possesses a C4 mechanism for photosynthesis. Poplar is deep rooted, a model plant genetically and widely used in phytoremediation applications, and it has been shown to effectively stimulate the biotransformation of several xenobiotic compounds (Kacalkova and Tlustos, 2011,Liu et al., 2009, Zhai et al., 2010).
The objective of this study was to identify rhizospheric biotransformation products of the tested PCB congeners and determine transformation pathways based on the identified products. Furthermore the transformation of PCBs was investigated by subjecting the rhizosphere to intermittent cycles of flooding. We hypothesized that anoxic or anaerobic conditions induced by flooding would facilitate PCB dechlorination. Intermittent flooding was necessary to produce the redox conditions required for reductive dechlorination, without compromising the health of the plants. The manipulation of redox conditions should not have any deleterious effect on the plants as long as they are not subjected to prolonged flooding. Terrestrial plants respond to short term flooding by fermenting pyruvate to produce adenosine triphosphate (ATP) (Hasegawa and Locy, 2006). Switchgrass is hardy and is able to survive in a wide variety of environments and poplar has been widely used in hydroponics studies with PCBs and other xenobiotics (Burken and Schnoor, 1997, Thompson et al. 1998, Van Aken et al. 2004, Liu et al., 2009, Zhai et al., 2010) so the impact of short term flooding to achieve redox manipulation should not be detrimental to their overall health (Hughes,2003, Jackson, 2004, Jaeger, 2008,).
Poplar is a model plant for phytoremediation, but studies involving PCB and poplar thus far, have relied on hydroponic exposure (Liu et al. 2008, Liu et al., 2009). Most field-scale phytoremediation interventions involve planting in a soil matrix which is more complex than hydroponics, yet there are no soil based studies with PCB and poplar. On the other hand, a few rhizoremediation studies have looked at PCB amelioration utilizing switchgrass based systems, but these results have been mixed and have mostly focused on dissipation of parent compounds or mixtures (Dzantor et.al 2000, 2001,Chekol et al. 2004) and do not yield metabolite pathways or mass balances. In addition to dissipation of parent congeners, this study also looks at transformation products to gain insight into the mechanisms and pathways that may be at play.
To our knowledge, this study is the first to report transformation products and corresponding pathways in the rhizosphere of planted microcosms cycled at low redox conditions to enhance reductive dechlorination. It demonstrates dechlorination of PCB congeners under marginally aerobic conditions and provides closure or near-closure of the mass balance based on these dechlorination products alone.
Materials and Methods
Experimental Set up
Forty (40) kg of silty loam soil from the village of Middle Amana in Iowa,USA, was passed through a 60 mesh sieve and artificially contaminated with tetra chlorinated PCB 52, PCB77 and hexa chlorinated PCB 153(99% pure) separately and as a mixture of the three congeners. Prior to spiking the soil, the PCB congeners were dissolved in five liters of hexane and then added to the soil for a target final concentration of 1000 ng g−1 (3.42 nmol g−1 for PCB 52 and PCB 77 and 2.77 nmol g−1 for PCB 153) for each congener in the single congener treatments. The target concentration for each congener in the mixture was 500 ng g−1(1.71 nmol g−1 for PCB 52 and PCB 77 and 1.39 nmol g−1 for PCB 153). The soil was homogenized using the quartering technique. It consisted of dividing the soil into four quadrants on a quartering canvas, and making several diagonal trajectories to mix the soil components together. Twenty passes (diagonal trajectories) were made to homogenize the soil thoroughly. To account for heterogeneity, 20 subsets of soil were collected from different locations, homogenized and analyzed in triplicate. Triplicate concentration measurements were performed to establish the initial concentration, and it was compared to the target for the purpose of quality assurance. The contaminated soil was aged for two months at 25°C in sealed tubs to allow the PCBs to sequester themselves in the soil thereby reducing bioavailability, which would ensure conditions more representative of field sites (Alexander et al., 2000, Thompson et al., 1998, White et al., 2006).
The aged, contaminated soils were then planted separately with switchgrass (Panicum virgatum) seeds from Adams-Briscoe Seed Co. (Jackson, GA) and with 22.9 cm poplar (Populus deltoids x nigra DN34) cuttings from Segal Ranch Hybrid Poplars Nursery (Grand View, WA). RubbermaidTM, plastic shoe boxes (33.8 cm × 21.6 cm × 11.9 cm (LxWxD) with 1.9 cm holes drilled in the covers, lined with aluminum foil and containing 2500 g of soil were used as reactors. Holes (1.9 cm) were bored in the covers of the reactors to facilitate planting of the poplar cuttings, growth of the mature grass and measurement of redox potential and dissolved oxygen concentration during periods of flooding (low redox conditions). The use of covered reactors with holes drilled in the covers also reduced the potential for loss of PCBs by volatilization. Plants were grown under a 16 hour light/8 hour dark photo period with a light intensity of 200 μmol m−2s−1 and at a temperature of 25°C and humidity of 60% in a plant growth chamber. The experimental set up was carried out in triplicate. Unplanted spiked controls were also set up in triplicate.
Treatments consisting of switchgrass and poplar planted in uncontaminated soil was used as blanks and functioned as volatilization controls. The presence of PCBs in substantial concentrations in the uncontaminated soil, and material planted in it, at the end of exposure could indicate that any loss recorded in the spiked systems could be attributed to volatilization and subsequent deposition. The plants were allowed to establish themselves for four (4) weeks. After 4 weeks, the treatments were subjected to alternate cycles of seven (7) days flooding (to induce anaerobic conditions) and seven (7) days of no flooding (aerobic conditions). To achieve flooding, three liters of deionized water was initially added to the reactors and supplemented as dictated by transpiration rates. Transpiration typically functioned as an efficient mechanism for water withdrawal, so there was no need to withdraw excess water at the end of the flooding cycle. The experiment was conducted for thirty two (32) weeks and at the end of exposure, the soil and plant materials were analyzed for PCB content. Twelve (12), five (5) g samples of soil were collected randomly from each treatment using a Lock and Load™ syringe (Ben Meadows Company WI, USA) and homogenized prior to analysis.
A photograph and a schematic representation of the reactors used in the experimental setup can be seen in the supplementary information (Figure S1).
PCB Extraction
The extraction method is modified after Liu et al. (2009). Denaturation and extraction of PCB in soil and plant material was conducted by adding three (3) milliliters per gram of a 1:1 hexane/acetone mixture to 5 grams of grounded homogenized soil and sonicating for 1 hour. Prior to sonification, the samples were spiked with 50 ng of PCB14 (3,5-dichlorobiphenyl), deuterated PCB65 (2,3,5,6-tetrachlorobiphenyl) and PCB166 (2,3,4,4′,5,6-hexachlorobiphenyl) (Cambridge Isotope Laboratories, Inc.), which were used as surrogate standards. Surrogate recoveries ranged from 87.24±3.09% to 94.41±4.37% for PCB 14, 95.17±4.44% to 105.07±4.23% for PCB 65 and 93.19±2.49% to 106.37±2.46% for PCB 166.
The sonicated material was centrifuged at 3000 rpm for 5 minutes, after which the supernatant was transferred to a fresh vial. A second extraction was performed and the supernatants combined. The combined supernatant was evaporated to dryness using rotary evaporation and the solvent changed to hexane. Any loss by rotary evaporation was accounted for using the surrogate standard recoveries. Removal of lipids and other polar substances was achieved by double extraction with concentrated sulfuric acid (H2SO4) and hexane. This hexane extract was concentrated to approximately 0.5 ml under a gentle stream of nitrogen. The concentrate was eluted with 10 ml of hexane through a filter consisting of 0.1g of silica (70−230 mesh, Fisher Scientific, Inc.), 0.1g of anhydrous sodium sulfate (Na2SO4) and 0.9 g silica gel acidified with H2SO4 (2parts silica:1part H2SO4). The eluent was concentrated and PCB204 (2,2′,3,4,4′,5,6,6′ octachlorobiphenyl) was added as an internal standard to facilitate quantification before analysis by GC/MS/MS triple quadropole mass spectrometry (Agilent Technologies 6890N GC with an Agilent 7683 series autosampler coupled to a Waters Micromass Quattro micro GC mass spectrometer (Milford, MA)). The gas chromatogram (GC) was fitted with a Supelco SBP-Octyl capillary column (30 m × 0.25 mm ID, 0.25 μm film thickness) with helium as carrier gas at a constant flow rate of 0.8 ml min− 1. The GC operating conditions were as follows: injector temperature 270 °C, interface temperature 290 °C, initial temperature 75 °C, initial time 2 min. The GC temperature program was 75 to 150 °C at 15 °C min− 1, 150 to 290 °C at 2.5 °C min− 1, and final time 1 min. Identification and quantification of PCB congeners in the samples was performed using a calibration standard consisting of all 209 congeners and is described elsewhere (Hu et al. 2010).
Measurement of Redox Conditions
Dissolved oxygen (DO) concentrations were measured in the saturated soilwater with a Hach Sension 156 meter and probe, while redox measurements were obtained using a Hanna Instruments Redox/pH combination meter. The DO probe was calibrated in water saturated air according to the manufacturer's instructions. The accuracy was also checked by measuring and comparing the DO concentration in water at a specific temperature with a known saturation concentration. In addition, the probe was chemically zeroed to compensate for positive error readings of 0.02-0.05 mgl−1 which is possible when taking measurements below 1 mgl−1. The accuracy of the oxidation–reduction potential (ORP) meter was checked with 475 mv and 229 mv redox buffers (Aqua Solutions).
Statistical Analysis
Microsoft Excel Analysis Toolpak's ANOVA analysis and Students’ t-test were used for statistical testing. The significance level of 0.05 was utilized to indicate whether the treatments were significantly different than the controls.
Results
Dissolved Oxygen and Redox Measurement
Dissolved oxygen (D.O) measurements indicated that the water column in the planted systems was hypoxic during flooding, but oxygen was not sufficiently depleted to produce anaerobic conditions (supplementary information, Figure S2 which shows the typical D.O results when a reactor was flooded. D.O measurements could only be made during periods of flooding because the probe was not designed to take a DO reading in media that was not supersaturated). The dissolved oxygen measurements in the planted systems ranged from 0.45±0.17 mg l−1 to 0.52±0.15 mg l−1. The unplanted systems, which ranged from 0.15±0.11 mg l−1 to 0.21±0.17 mg l−1, had lower dissolved oxygen concentration than the planted systems and bordered on being anoxic. Soil redox measurements confirmed the absence of anaerobic or even anoxic conditions in the planted systems (supplementary information, Figures S3-S5). On the other hand, based on the soil redox measurements, anoxic conditions, specifically sulfidogenic conditions (0 to 200mv) were clearly present in the unplanted systems (supplementary information, Figures S2-S5). The absence of anoxic conditions in the planted systems was probably due to plant transfer of oxygen to the rhizosphere during photosynthesis. This hypothesis is supported by the fact that the unplanted systems showed greater propensity towards sulfidogenic conditions. Although it would have been desirable to have at least a methanogenic environment to facilitate reductive dechlorination, there have been reports of reductive dechlorination also occurring under sulfidogenic conditions (Fava et al., 2003, Kuo et al., 1999).
PCB Dissipation: Exposure to a Mixture of PCB 52, PCB77 and PCB 153
Table 1 shows the results for the dissipation of parent PCB compounds in a mixture of PCB 52, PCB 77, and PCB 153 spiked and aged into soil and planted with switchgrass and poplar after 32 weeks of incubation. It also shows the results for the decline in parent PCB congeners when the soil was spiked with the individual congeners. In the mixture, the targeted initial concentration for each congener was 500 ng g−1 (1.71 nmol g−1 for PCB 52 and PCB 77 and 1.39 nmol g−1 for PCB153). However ,the measured initial concentration was different for all congeners; the difference likely due to the heterogeneity of the soil matrix despite efforts to obtain a homogenous mixture by repeatedly quartering the contaminated soil. A 57% decrease in PCB 52 was observed for both the switchgrass and poplar planted systems after 32 weeks of incubation (Table 1). This represented a statistically significant decline when compared to the initial PCB 52 concentration in the mixture (p<0.05).With respect to PCB 77 dissipation, the switchgrass-planted system containing the mixture recorded a 54% decline while the decrease in the poplar-planted system was effectively identical with a 53% diminution (Table 1) (p<0.05). Compared to the initial PCB 77 concentration, both the planted and unplanted systems had significant declines (p<0.05). There were simlar decreases in PCB 153 concentration in both the switchgrass and poplar planted systems. Both planted systems showed a dimunition of 55% in soil PCB 153 after 32 weeks exposure ( Table 1).
Table 1.
Summary Table for Transformation of Parent PCB in Switchgrass and Poplar Planted Soil after 32 weeks
| EXPOSURE Initial Conc. (ng g−1) [nmolg−1] | SYSTEM | REDUCTION | ||
|---|---|---|---|---|
| PCB 52 (%) | PCB 77 (%) | PCB153 (%) | ||
|
MIXTURE (PCB52,77,153) PCB 52 (573 ±29) [1.96± 0.10] PCB 77 (512 ±24) [1.96± 0.10] PCB 153 (499 ±26) [1.38± 0.07] |
Unplanted | 29.8 ±1.8 | 33.5 ±1.5 | 35.1 ±1.9 |
| Switchgrass | 57.2 ±0.6 | 54.3 ±0.7 | 55.2 ±0.2 | |
| Poplar | 57.5 ±3.2 | 52.6 ±0.8 | 55.4 ±0.8 | |
|
SINGLE CONGENER PCB 52 PCB 52 (1120 ±58) [3.84± 0.20] |
Unplanted | 29.1 ±3.9 | - | - |
| Switchgrass | 63.1 ±0.5 | - | - | |
| Poplar | 54.4 ±2.2 | - | - | |
|
SINGLE CONGENER PCB 77 PCB 77 (1012 ±3) [3.47±0.01] |
Unplanted | 20.1 ±2.0 | ||
| Switchgrass | - | 54.2 ±1.1 | - | |
| Poplar | - | 47.3 ±0.8 | - | |
|
SINGLE CONGENER PCB 153 PCB 153 (1033 ±28) [2.86.±0.01] |
Unplanted | - | - | 30.1 ±1.2 |
| Switchgrass | - | - | 51.5 ±0.4 | |
| Poplar | - | - | 50.4 ±0.4 | |
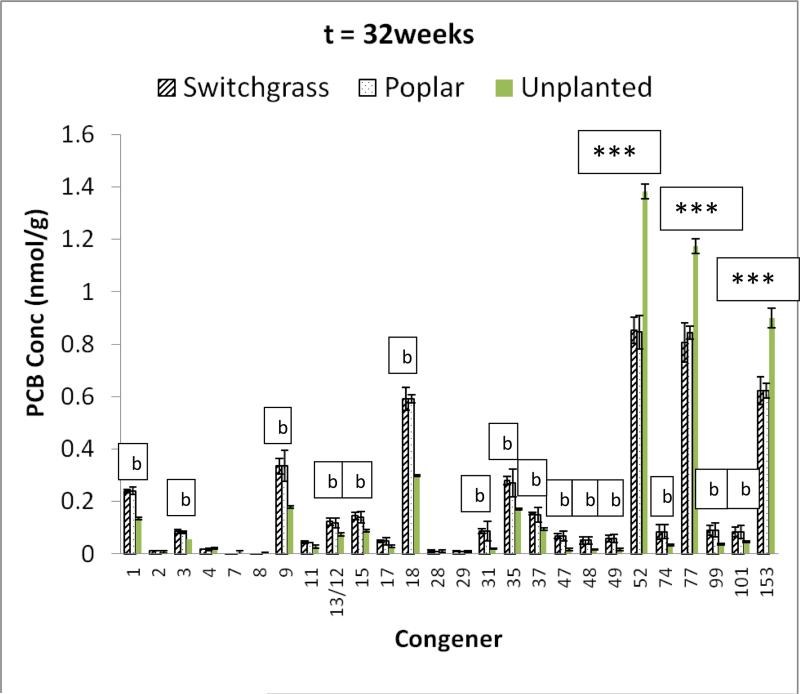
Figure 1 shows the congener profile for both the planted and unplanted systems in the soil contaminated with the mixture after 32 weeks of incubation. It illustrates that in both the planted and unplanted systems, there was significant decline in the concentration of the PCB congeners (PCB 52, PCB 77 and PCB 153 ) with which the soil was spiked. In addition, several transformation products from the degradation of PCB 52, PCB 77 and PCB 153 were obtained in both the planted and unplanted systems. For the unplanted systems, the yield of any single degradation product did not surpass 10% of the initial total PCB ( the sum of PCB 52, PCB 77 and PCB 153 ) concentration. In contrast, significantly higher yields (p<0.05) of similar transformation products were observed within the planted systems. PCBs 18 (2,2’,5 trichlorobiphenyl) and 9 (2,5 dichlorobiphenyl) which are potential degradation products of PCB 52 and 153 showed prominent increases in concentration. The trichlorinated PCB 37 (3,4,4’ trichlorobiphenyl) and PCB 35(3,3’,4 trichlorobiphenyl) which are potential products of PCB 77 were also present. Aerial deposition, from laboratory air to the soils, as a route for the appearance of transformation products was ruled-out because of the relatively high concentrations detected, and because the congener profile for the unspiked treatment was quite different from that observed in the spiked treatments.
Figure 1.
PCB congener profile for soil contaminated with mixture of PCB 52, PCB77, and PCB 153, and undergoing alternate cycles of flooding and no flooding after 32 weeks of exposure. b = significant increase in concentration of PCB congener for the planted systems when compared to unplanted system (p<0.05). The single “b” is for both switchgrass and poplar. The PCB congener concentrations in the switchgrass and poplar planted systems were statistically similar (p<0.05). *= significant decrease in parent PCB concentration in comparison to initial concentration (p<0.05). There was significant decrease in parent PCB congener in both the planted and unplanted systems, but significantly higher decline in the planted systems compared to the unplanted sytem.. Error bars are 1 standard deviation
PCB Dissipation : Single Congener Exposure to Plants with PCB 52
The result for the decrease in PCB 52 concentration in the single congener exposure is shown in Table 1. The targeted initial concentration for PCB 52 in the single congener exposure experiment was 1000 ng g−1 (3.42 nmol g−1), however the measured initial concentration was 1120 ±58 ng g−1 (3.84± 0.20 nmol g−1). The difference was likely due to the difficulty of accurately sampling the heterogeneous soil matrix. After 32 weeks of exposure, there was a 63% decline in the soil PCB 52 concentration for the switchgrass planted system , and a 54 % decrease for the poplar planted system. Overall, this represented a significant decrease in PCB 52 concentration in both the switchgrass and poplar planted systems, when compared to the initial concentration (p<0.05).
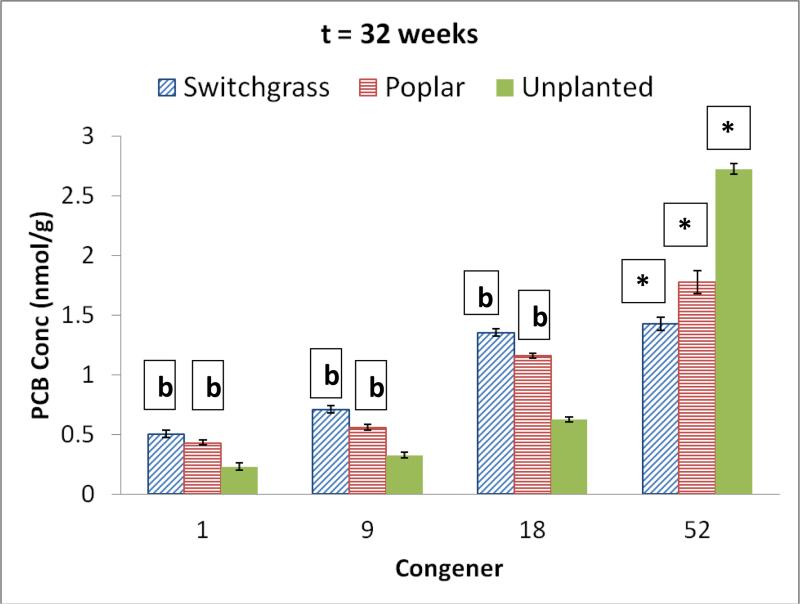
Figure 2 shows the congener profile in the soil contaminated with PCB 52 as the single congener for both the planted and unplanted sytems after 32 weeks of incubation. The only congener that was detected at the start of the experiment was PCB 52. However, there were significant increases in the concentration of various transformation products in both the switchgrass and poplar planted systems at the end of exposure. For both planted systems the yields of comparable transformation products in the single congener (PCB 52 ) treatment were significantly higher than what was obtained with the mixture (p<0.05). As with the experiment containing a mixture of congeners, the yields of PCB 52 transformation products in the single congener treatment were higher in the planted sytems than the unplanted system (p<0.05). Both the switchgrass and poplar planted systems mirrorred each other in terms of the mass conversion to major transformation products.
Figure 2.
PCB congener profile for soil contaminated with PCB 52 and undergoing alternate cycles of flooding and no flooding after 32 weeks of exposure. b = significant increase in concentration of PCB congener for the planted systems when compared to unplanted system (p<0.05). The PCB congener concentrations in the switchgrass and poplar planted systems were statistically similar (p<0.05). *= significant decrease in parent PCB concentration in comparison to initial concentration (p<0.05). There was significant decrease in parent PCB congener in both the planted and unplanted systems, but significantly higher decline in the planted systems compared to the unplanted system. Error bars are 1 standard deviation
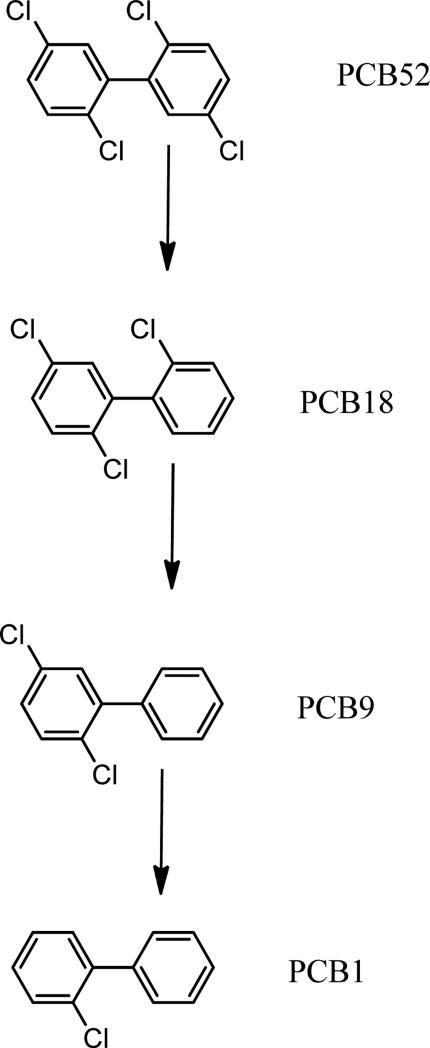
For example, in the switchgrass planted system, PCB 18 (2,2’,5 trichlorobiphenyl) was the major degradation product accounting for approximately 34% of the remaining PCB concentration in the soil with PCB 9 (2, 5 dichlorobiphenyl) and PCB-1 (2 chlorobiphenyl) accounting for 18% and 13%, respectively. Analagous to the switchgrass planted soil, PCB 18 was also the major degradation product in the poplar planted soil accounting for approximately 30 % of the remaining PCBs with PCB 9, and PCB 1 accounting for 14% and 11%, respectively. Based on the transformation products observed in the single congener exposure, a degradation pathway for the rhizospheric transformation of PCB 52 is proposed in Figure 3.
Figure 3.
Potential pathway for PCB 52 degradation based on transformation products observed.
PCB Dissipation: Single Congener Exposure with PCB 77
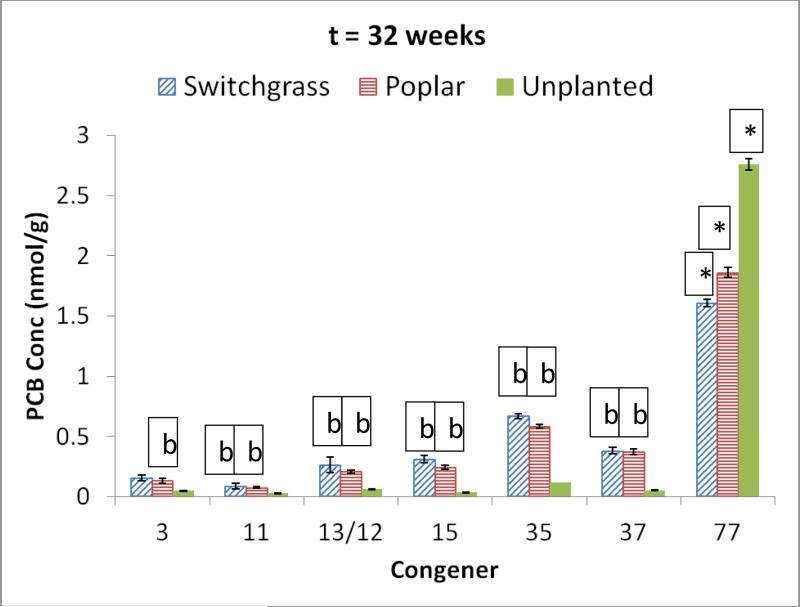
The target initial concentration for PCB 77 as the sole contaminant was 1000 ng g−1 (3.42 nmol g−1) and the measured concentration was 1012 ± 3 ng g−1 (3.47±0.01 nmol g−1). The soil PCB 77 concentration in the switchgrass planted system diminished by 54 % while the poplar planted system recorded a 47% decline (Table 1). Despite the significant difference between the decrease observed in the poplar and switchgrass planted systems (p< 0.05), both showed a significant loss when compared to the the initial PCB concentration (p<0.05).
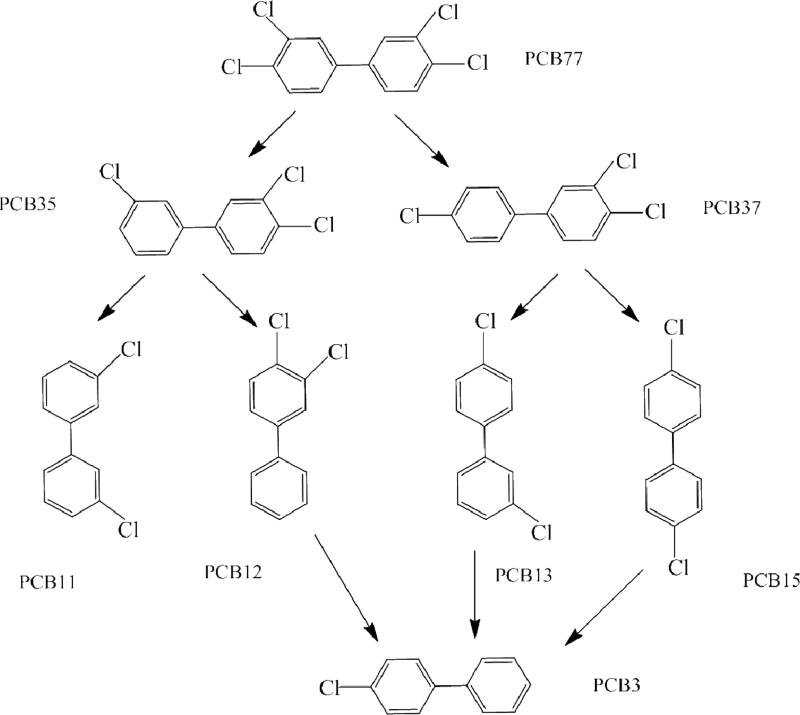
Compared to the congener profile at the beginning, there were several transformation products of PCB 77 in the soil rhizosphere after 32 weeks of exposure. The yield of the major degradation products was significantly higher in the single congener treatment than the mixture for both the planted and unplanted systems (p<0.05). Similar to the trend observed in other exposures discussed thus far, the yields obtained with the planted systems were much higher than with the unplanted system, at least 10 times as much. The major products of dechlorination were PCB 35 (3,3’,4 trichlorobiphenyl) and PCB 37 (3,3,4’ trichlorobiphenyl). PCB 35 accounted for 19 % of the final PCB concentration in the switchgrass planted soil and 17% in the poplar planted system. PCB 37 was responsible for 11% of the final soil PCB concentration in both the switchgrass and poplar planted systems. Figure 5 shows potential degradation pathways through PCB 35 and PCB 37 based on the transformation products observed.
Figure 5.
Potential pathway for PCB 77 degradation based on transformation products observed.
PCB Dissipation: Single Congener Exposure with PCB 153
The targeted initial concentration for PCB 153 in the single congener exposure experiment was 1000 ng g−1 (2.77 nmol g−1), and the measured initial concentration was nearly identical at 1033 ±28 ng g−1 (2.86.±0.01 nmol g−1). The soil PCB 153 concentration diminished by 51% in the switchgrass planted treatment and declined by 50% in the poplar planted treatment (Table 1). The dimunitions in both switchgrass and poplar planted systems were statistically significant compared to the the initial PCB concentration (p<0.05).
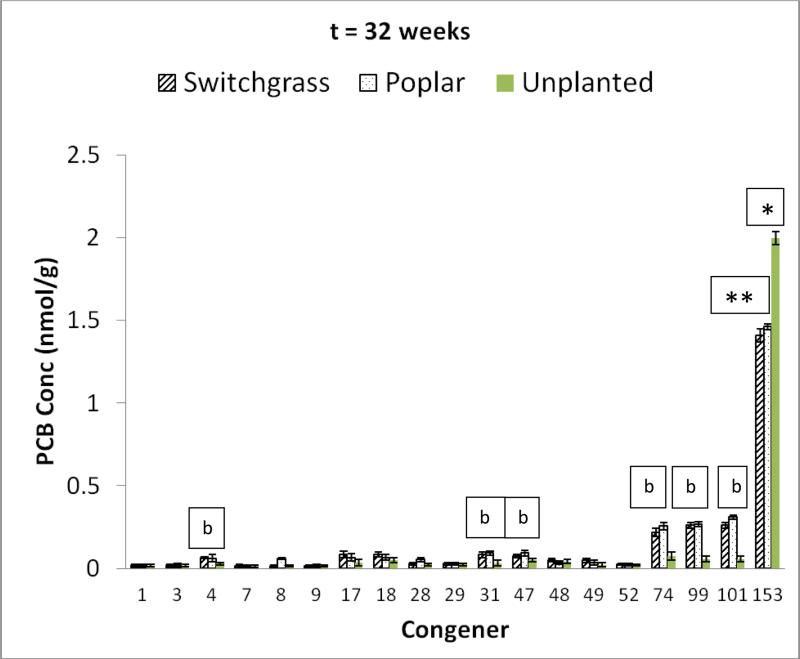
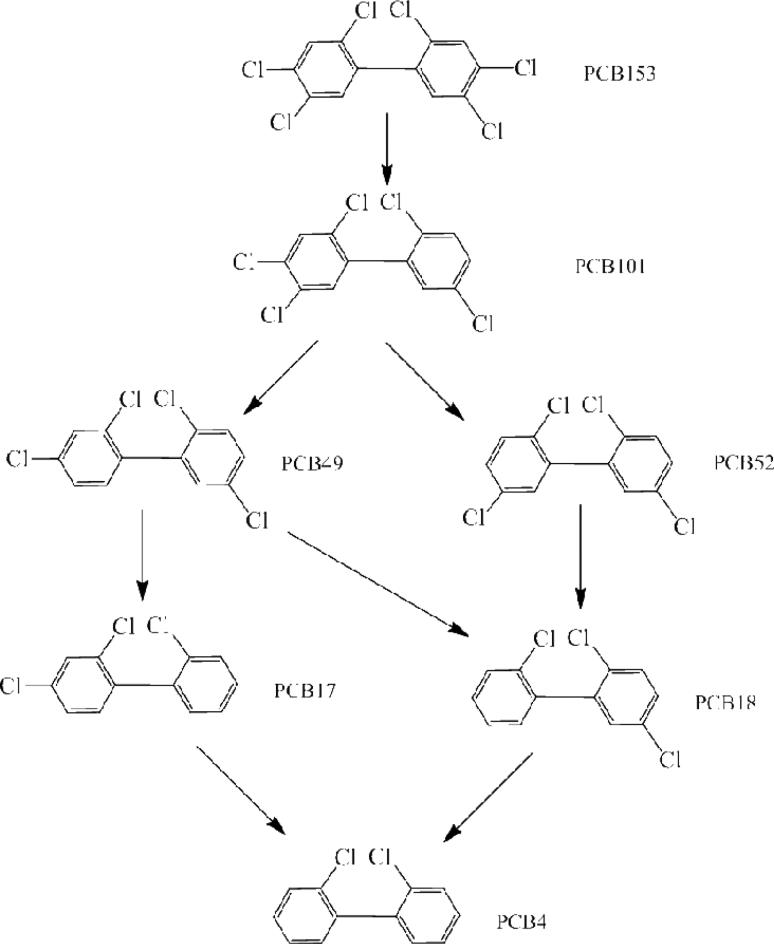
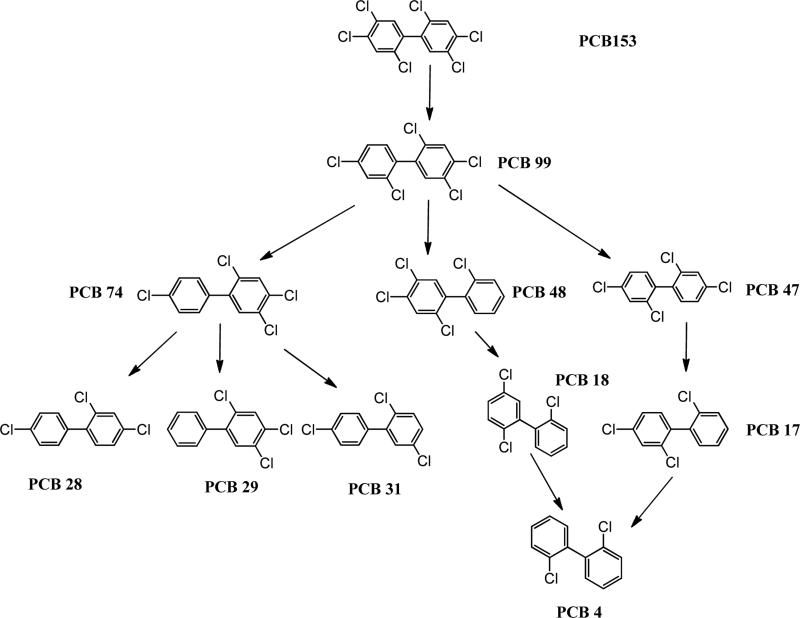
The congener profiles for the soil contaminated with PCB 153 as the sole contaminant and planted with switchgrass and poplar after 32 weeks of incubation are shown in Figure 6. Compared to the congener profile at the start of exposure, a wide range of degradation products were obtained spanning from pentachlorinated compounds to dichlorinated congeners in both the planted and unplanted systems (Figure 6). The yields were small, individually, and did not exceed 10% for any one congener. However, the summation of all the transformation products accounted for 99% of the total PCB 153 converted. The yields of pentachlorinated compounds PCB 101 (2,2’,4,5,5’pentachlorobiphenyl) and PCB 99 (2,2’,4,4’,5pentachlorobiphenyl) and tetrachlorinated PCB 74 were higher in the planted systems than in the unplanted system. Based on the the degradation products observed, two potential pathways for PCB 153 transformation are depicted in Figures 7 and 8. The first (Figure 7) shows the route through PCB 101 and the second (Figure 8) shows the transformation via the intermediary PCB 99.
Figure 6.
PCB congener profile for soil contaminated with PCB 153 and undergoing alternate cycles of flooding and no flooding after 32 weeks of exposure. b = significant increase in concentration of PCB congener for the planted system when compared to unplanted system (p<0.05). The single “b” is for both switchgrass and poplar. The PCB congener concentrations in the switchgrass and poplar planted systems were statistically similar (p<0.05). *= significant decrease in parent PCB concentration in comparison to initial concentration (p<0.05). There was significant decrease in parent PCB congener in both the planted and unplanted systems, but significantly higher decline in the planted systems compared to the unplanted system. Error bars are 1 standard deviation
Figure 7.
Some products observed with PCB 153 transformation and potential degradation through the PCB 101 pathway.
Figure 8.
Some products observed with PCB 153 transformation and potential degradation through the PCB 99 pathway.
Discussion
Significantly higher reductions in parent PCB congeners were obtained with the planted systems when compared to unplanted systems. This is demonstrated by data in Figures 1,2, 4 and 6. It is likely the result of rhizosphere stimulation by plant exudates (Jordahl et al., 1997). The most important role for the plants in PCB phytoremediation is in creating an enabling environment for increased microbial activity through the production of microbial growth substrates and biostimulants which serve as electron donors for the microbes (Singer et al 2003a,2003b, Leigh et al 2002, Hernandez et al.,1997,Ficko et al. 2011). Plant exudates such as flavonoids and terpenes have been shown to stimulate growth of competent microrganisms and have increased PCB degradation by organisms in the lab and the field (Donnelly et al.,1994, Hernandez et al.,1997, Ionescu et al., 2009, Leigh et al., 2002,2006, Slater et al., 2011 de Carcer et al., 2007). It is possible that the plant exudates derive their abilty to stimulate microbial metabolism of PCBs due to structural similarities with biphenyl, a well known inducer of microbial PCB degradation (Singer et al.,2003a).
Figure 4.
PCB congener profile for soil contaminated with PCB 77 and undergoing alternate cycles of flooding and no flooding after 32 weeks of exposure. b = significant increase in concentration of PCB congener for the planted system when compared to unplanted system (p<0.05). The PCB congener concentrations in the switchgrass and poplar planted systems were statistically similar (p<0.05). *= significant decrease in parent PCB concentration in comparison to initial concentration (p<0.05). There was significant decrease in parent PCB congener in both the planted and unplanted systems, but significantly higher decline in the planted systems compared to the unplanted system. Error bars are 1 standard deviation
The decrease in the concentration of the spiked PCBs were largely a consequence of enhanced rhizosphere transformation, rather than a direct function of plant uptake and biotransformation. The reason for this conclusion is two fold. Based on the log Kow and water solubility of these congeners, they will not be significantly taken-up by plants and enter the transpiration stream (Liu et al., 2008, 2009, Schnoor et al.,1995) although there are reports that pumpkin , zuchinni and tall fescue grass take up and translocate PCBs (White et al. 2006,Aslund et al., 2008, Weber and Mrozek,1979) . Second, on a mass basis, all the parent PCBs detected in the plant material resided in the roots (Table 2) and were not translocated to shoots, and at least 95% of the PCBs detected at the end of exposure was resident in the soil (Table 2 ).
Table 2.
Molar Mass Balance in Poplar and Switchgrass Planted Soil (Average of n=3)
| Parent Compound In Soil (mole %) | Transformation Products in Soil (mole %) | Roots (mole%) | Shoots (%) | Total Recovered (mole%) | |
|---|---|---|---|---|---|
| PCB52 Poplar | 45.9 | 56.2 | 3.4 | <0.1 | 105.6 |
| PCB52 Switchgrass | 37.2 | 63.0 | 3.5 | <0.1 | 103.7 |
| PCB77 Poplar | 53.5 | 47.4 | 2.6 | <0.1 | 103.5 |
| PCB77 Switchgrass | 46.4 | 55.5 | 4.5 | <0.1 | 106.4 |
| PCB153 Poplar | 51.0 | 47.7 | 4.3 | <0.1 | 102.4 |
| PCB153 Switchgrass | 49.0 | 46.9 | 2.2 | <0.1 | 98.1 |
Transformation products seen in the planted systems are similar to those in the unplanted systems (Figures 1,2,4&6). This is likely the result of the structure of the three congeners chosen and the relatively few likely pathways available for transformation by the microbial community.
The transformation pathways are consistent with reductive dechlorination seen in other studies. Two different cultures of Dehalococcoides were able to degrade PCB 153 to PCB 47 and PCB 52 in Aroclor 1260 mixture going through PCB 99 and 101 respectively (Adrian et al., 2009,Bedard et al., 2007). Regarding PCB transformation in the literature, dechlorination of PCB 77 to biphenyl was observed in one study (Rhee et al., 1993). In terms of degradation products, Kuo and colleagues reported that there was active dechlorination of PCB 77 in sediments, but only saw dechlorination to the dichlorinated PCB 11 and nothing further (Kuo et al., 1999). Looking at the congener profiles for PCB 77 in this study, both in the mixture and as a single congener, reduction to monochlorinated PCB 3 was evident, which extends the pathway further than reported by Kuo and collaborators. Kuo et al. (1999) did not report the precursors of PCB 11, whereas in the current study, the progression from tri- to di- and to monochlorinated PCBs was clearly evident in PCB 77 degradation.
For PCB 77 degradation products, Zanaroli and coworkers reported the presence of PCB 11, 12, and 13, but did not report any trichlorinated precursors in the reductive dechlorination process (Zanaroli et al., 2006). However, these trichlorinated intermediates may have been rapidly degraded to less chlorinated products observed in the study. PCBs 11, 12 and 13 were also detected in this study as transformation products of PCB 77, including detection of some direct precursors. However, PCBs 12 and 13 were co-eluted in our study; therefore it is difficult to ascribe quantifiable proportions to each. The high conversion rate (90%) of the parent compound by Zanaroli and colleagues was much higher than observed in this study, but it is quite possibly that these high rates were a consequence of the methanogenic conditions in the previously PCB polluted estuarine sediments with which they were working.
Our ability to detect aerobic products was limited by use of GC/MS/MS, and as such we could not measure polar metabolites (hydroxyl-PCBs, ring cleavage products) or mineralization to carbon dioxide and HCl. But the fact that the reductive dechlorination transformation products summed to approximately the total mole fraction (lost) suggests that these aerobic processes (oxidation, mineralization) were not large. In spite of the soil remaining marginally aerobic during flooding, it is possible that highly reduced microzones (as suggested by the oxygen concentration in bulk pore water, which ranged from <0.1 to 0.5 mg/L) within soil particles could still exist and provide the opportunity for reductive dechlorination to take place. Typically, ring cleavage and mineralization would require a different consortium of aerobic organisms with dioxygenase enzymes, to be predominant. Nonetheless, dechlorination seems to have predominated in these experiments, and dechlorination is a vital and necessary first step in the achievement of ring cleavage and mineralization.
Reductive dechlorination reactions were consistent with the products observed even though strict anaerobic conditions did not prevail. Microzones of low redox conditions in the rhizosphere are likely responsible for the dechlorination which occurred. As it stands, the results from this experiment show clearly that reductive dechlorination can occur even where soils are marginally aerobic in the root zones of plants. There were few differences in rhizosphere transformation rates or pathways between the two plant species examined here (Populus spp. and Panicum virgatum). But there was a clear and statistically significant tendency for planted microcosms to achieve more substantial biotransformation than unplanted controls, although the pathways were similar in both systems.
Both switchgrass and poplar were able to tolerate the intermittent flooding to which they were subjected. However, qualitatively ,the switchgrass seemed to thrive to a greater extent, and there was less yellowing of their shoots compared to the faint leaf discoloration seen in the poplar plants. Switchgrass had more root mass than poplar . Therefore, when the reductions are normalized to rootmass, poplar outperformed switchgrass with PCB mass reductions in the soil ranging from 1.5 to 3 times more in the poplar planted systems than the switchgrass planted treatments.
The significant decreases in parent compound and concomitant increases in transformation products obtained in this research are encouraging, but reproducibility in the field will be highly site specific because of variability in the microbial community associated with each site. On the other hand PCB contaminated sites will have organisms that are well acclimated to PCB and the introduction of plants in such an environment could stimulate their activities. What is not clearly understood is the extent of the effect that plants will have on the redox conditions in the rhizosphere in the field since this research utilized small confined reactors. Potentially, this could negatively impact the anaerobic degradation of the higher chlorinated PCBs by bacteria. Potential candidates for the application of this research could be engineered storage facilities like confined disposal facilities which store dredged PCB contaminated material. Poplar has produced good results with hydroponics (Liu et al. 2008, 2009, Zhai et al., 2011) and the concept of redox manipulation could be incorporated in the design of these engineered systems. The results in terms of percentage reduction of soil concentration obtained with switchgrass in the current study straddled the range (30%- 77%) of other studies ( Dzantor et al., 2000, 2001, Chekol,2004) conducted with grasses, despite differences in initial concentrations and length of exposure. It suggests that there may be some limiting effective range when using grass species over the short time frames tested. The high end of the range represents a substantial reduction and argues well for the potential use of grasses in phytoremediation. The occurrence of large reductions in the root zone for both poplar and switchgrass (rhizoremediation) is highly desirable in comparison to systems using phytoextraction (uptake,translocation, and degradation) because it limits the transfer of contaminants to the aerial compartment and the food web.
Supplementary Material
Highlights.
Soil was spiked and aged and then planted with poplar and switchgrass.
Planted microcosms showed significant reductive dechlorination and greater biotransformation than unplanted reactor.
Rhizospheric reductive dechlorination pathways are proposed.
Acknowledgements
Funding for this research was provided by NIEHS Superfund Research Program (SRP) Grant No. P42ES13661. Special recognition is also due to Dr. Hans Lehmler for providing PCB congeners. Special thanks to all the anonymous reviewers whose valuable comments substantially improved the quality of the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Capsule
This study provides insight into rhizospheric transformation of PCBs.
Contributor Information
Richard E. Meggo, Department of Civil and Environmental Engineering 4105 Seamans Center University of Iowa, IA, 52242, USA.
Jerald L. Schnoor, Department of Civil and Environmental Engineering 4105 Seamans Center University of Iowa, IA, 52242, USA (jerald-schnoor@uiowa.edu)
Dingfei Hu, Department of Civil and Environmental Engineering 4105 Seamans Center University of Iowa, IA, 52242, USA (dingfei-hu@uiowa.edu).
References
- Adrian L, Dudkova V, Demnerova K, Bedard DL. Dehalococcoides” sp Strain CBDB1 Extensively Dechlorinates the Commercial Polychlorinated Biphenyl Mixture Aroclor 1260. Applied and Environmental Microbiology. 2009;75:4516–4524. doi: 10.1128/AEM.00102-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Alexander M. [January 11,2012];2000 http://www.atsdr.cdc.gov/spl/index.html.
- Aging, bioavailability, and overestimation of risk from environmental pollutants. Environmental Science & Technology. 34:4259–4265. [Google Scholar]
- Aslund MLW, Rutter A, Reimer KJ, Zeeb BA. The effects of repeated planting, planting density, and specific transfer pathways on PCB uptake by Cucurbita pepo grown in field conditions. Science of the Total Environment. 2008;405:14–25. doi: 10.1016/j.scitotenv.2008.07.066. [DOI] [PubMed] [Google Scholar]
- Aslund MLW, Zeeb BA, Rutter A, Reimer KJ. In situ phytoextraction of polychlorinated biphenyl - (PCB) contaminated soil. Science of the Total Environment. 2007;374:1–12. doi: 10.1016/j.scitotenv.2006.11.052. [DOI] [PubMed] [Google Scholar]
- Bedard DL. A Case Study for Microbial Biodegradation: Anaerobic Bacterial Reductive Dechlorination of Polychlorinated Biphenyls-From Sediment to Defined Medium. Annual Review of Microbiology. 2008;62:253–270. doi: 10.1146/annurev.micro.62.081307.162733. [DOI] [PubMed] [Google Scholar]
- Bedard DL, Ritalahti KA, Loffler FE. The Dehalococcoides population in sediment-free mixed cultures metabolically dechlorinates the commercial polychlorinated biphenyl mixture aroclor 1260. Applied and Environmental Microbiology. 2007;73:2513–2521. doi: 10.1128/AEM.02909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard DL, Unterman R, Bopp LH, Brennan MJ, Haberl ML, Johnson C. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated-biphenyls. Applied and Environmental Microbiology. 1986;51:761–768. doi: 10.1128/aem.51.4.761-768.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard DL, Wagner RE, Brennan MJ, Haberl ML, Brown JF. Extensive degradation of aroclors and environmentally transformed polychlorinated-biphenyls by Alcaligenes-Eutrophus H850. Applied and Environmental Microbiology. 1987;53:1094–1102. doi: 10.1128/aem.53.5.1094-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borja J, Taleon DM, Auresenia J, Gallardo S. Polychlorinated biphenyls and their biodegradation. Process Biochemistry. 2005;40:1999–2013. [Google Scholar]
- Chekol T, Vough LR, Chaney RL. Phytoremediation of polychlorinated biphenyl-contaminated soils: the rhizosphere effect. Environment International. 2004;30:799–804. doi: 10.1016/j.envint.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Correa PA, Lin L, Just CL, Hu D, Hornbuckle KC, Schnoor JL, Van Aken B. The effects of individual PCB congeners on the soil bacterial community structure and the abundance of biphenyl dioxygenase genes. Environment International. 2010;36:901–906. doi: 10.1016/j.envint.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly PK, Hegde RS, Fletcher JS. Growth of pcb-degrading bacteria on compounds from photosynthetic plants. Chemosphere. 1994;28:981–988. [Google Scholar]
- Dzantor E, Woolston J, Momen B. PCB Dissipation and Microbial Community Analysis in Rhizosphere Soil under Substrate Ammendment Conditions. International Journal of Phytoremediation. 2002;4:283–295. [Google Scholar]
- Dzantor EK, Chekol T, Vough LR. Feasibility of using forage grasses and legumes for phytoremediation of organic pollutants. Journal of Environmental Science and Health Part a-Toxic/Hazardous Substances & Environmental Engineering. 2000;35:1645–1661. [Google Scholar]
- Fagervold SK, May HD, Sowers KR. Microbial reductive dechlorination of aroclor 1260 in Baltimore Harbor sediment microcosms is catalyzed by three phylotypes within the phylum Chloroflexi. Applied and Environmental Microbiology. 2007;73:3009–3018. doi: 10.1128/AEM.02958-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathepure BZ, Vogel TM. Complete degradation of polychlorinated hydrocarbons by a 2-stage biofilm reactor. Applied and Environmental Microbiology. 1991;57:3418–3422. doi: 10.1128/aem.57.12.3418-3422.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava F, Gentilucci S, Zanaroli G. Anaerobic biodegradation of weathered polychlorinated biphenyls (PCBs) in contaminated sediments of Porto Marghera (Venice Lagoon, Italy). Chemosphere. 2003;53:101–109. doi: 10.1016/S0045-6535(03)00438-7. [DOI] [PubMed] [Google Scholar]
- Ficko SA, Rutter A, Zeeb BA. Effect of pumpkin root exudates on ex situ polychlorinated biphenyl (PCB) phytoextraction by pumpkin and weed species. Environmental Science and Pollution Research. 2011;18:1536–1543. doi: 10.1007/s11356-011-0510-8. [DOI] [PubMed] [Google Scholar]
- Hasegawa PM, Locy RD. Stress Physiology. In: Taiz L, Zeiger E, editors. Plant Physiology. Sinauer Associates,Inc., Publishers; 2006. pp. 672–705. [Google Scholar]
- Hernandez BS, Koh SC, Chial M, Focht DD. Terpene-utilizing isolates and their relevance to enhanced biotransformation of polychlorinated biphenyls in soil. Biodegradation. 1997;8:153–158. [Google Scholar]
- Hu D, Lehmler H-J, Martinez A, Wang K, Hornbuckle KC. Atmospheric PCB congeners across Chicago. Atmospheric Environment. 2010;44 doi: 10.1016/j.atmosenv.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes FMR. FLOBAR2. Department of Geography, University of Cambridge UK; 2003. The Flooded Forest: Guidance for policy makers and river managers in Europe on the restoration of floodplain forests. p. 96. [Google Scholar]
- Ionescu M, Beranova K, Dudkova V, Kochankova L, Demnerova K, Macek T, Mackova M. Isolation and characterization of different plant associated bacteria and their potential to degrade polychlorinated biphenyls. International Biodeterioration & Biodegradation. 2009;63:667–672. [Google Scholar]
- Jackson MB. [January 10, 2013];The Impact of Flooding Stress on Plants and Crops. 2004 http://www.plantstress.com/Articles/(waterlogging)
- Jaeger C. Faculty of Forest and Environmental Sciences. Albert-Ludwigs-University at Freiburg im Breisgau; Germany: 2008. Ecophysiological studies on the flood tolerance of common ash (Fraxinus excelsior L.) -impact of root-zone hypoxia on central parameters of C metabolism; p. 184. [Google Scholar]
- Javorska H, Tlustos P, Kaliszova R. Distribution of Polychlorinated Biphenyl Congeners in Root Vegetables. Polish Journal of Environmental Studies. 2011;20:93–99. [Google Scholar]
- Jordahl JL, Foster L, Schnoor JL, Alvarez PJJ. Effect of hybrid poplar trees on microbial populations important to hazardous waste bioremediation. Environmental Toxicology and Chemistry. 1997;16:1318–1321. [Google Scholar]
- Kacalkova L, Tlustos P. The uptake of persistent organic pollutants by plants. Central European Journal of Biology. 2011;6:223–235. [Google Scholar]
- Komancova M, Jurcova I, Kochankova L, Burkhard J. Metabolic pathways of polychlorinated biphenyls degradation by Pseudomonas sp 2. Chemosphere. 2003;50:537–543. doi: 10.1016/s0045-6535(02)00374-0. [DOI] [PubMed] [Google Scholar]
- Kuo C, Liu S, Liu C. Biodegradation of Coplanar Polychlorinated Biphenyl by Anaerobic Microorganisms from Estuarine Sediments. Chemosphere. 1999;39:1, 445–441, 458. doi: 10.1016/s0045-6535(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Leigh MB, Fletcher JS, Fu XO, Schmitz FJ. Root turnover: An important source of microbial substrates in rhizosphere remediation of recalcitrant contaminants. Environmental Science & Technology. 2002;36:1579–1583. doi: 10.1021/es015702i. [DOI] [PubMed] [Google Scholar]
- Leigh MB, Prouzova P, Mackova M, Macek T, Nagle DP, Fletcher JS. Polychlorinated biphenyl (PCB)-degrading bacteria associated with trees in a PCB-contaminated site. Applied and Environmental Microbiology. 2006;72:2331–2342. doi: 10.1128/AEM.72.4.2331-2342.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Hu DF, Jiang GB, Schnoor JL. In Vivo Biotransformation of 3,3′,4,4′-Tetrachlorobiphenyl by Whole Plants-Poplars and Switchgrass. Environmental Science & Technology. 2009;43:7503–7509. doi: 10.1021/es901244h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Schnoor JL. Uptake and translocation of lesser-chlorinated polychlorinated biphenyls (PCBs) in whole hybrid poplar plants after hydroponic exposure. Chemosphere. 2008;73:1608–1616. doi: 10.1016/j.chemosphere.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackova M, Vrchotova B, Francova K, Sylvestre M, Tomaniova M, Lovecka P, Demnerova K, Macek T. Biotransformation of PCBs by plants and bacteria - consequences of plant-microbe interactions. 2007:233–241. [Google Scholar]
- Master ER, Lai VWM, Kuipers B, Cullen WR, Mohn WW. Sequential anaerobic-aerobic treatment of soil contaminated with weathered aroclor 1260. Environmental Science & Technology. 2002;36:100–103. doi: 10.1021/es001930l. [DOI] [PubMed] [Google Scholar]
- Pieper DH. Aerobic degradation of polychlorinated biphenyls. Applied Microbiology and Biotechnology. 2005;67:170–191. doi: 10.1007/s00253-004-1810-4. [DOI] [PubMed] [Google Scholar]
- Registry A.f.T.S.a.D. Priority List of Hazardous Substances. 2011 http://www.atsdr.cdc.gov/spl/index.html 2011.
- Rhee GY, Sokol RC, Bethoney CM, Bush B. A long-term study of anaerobic dechlorination of PCB congeners by sediment microorganisms - pathways and mass-balance. Environmental Toxicology and Chemistry. 1993;12:1829–1834. [Google Scholar]
- Schnoor JL, Licht LA, McCutcheon SC, Wolfe NL, Carreira LH. Phytoremediation of organic and nutrient contaminants. Environmental Science & Technology. 1995;29:A318–A323. doi: 10.1021/es00007a747. [DOI] [PubMed] [Google Scholar]
- Singer AC, Crowley DE, Thompson IP. Secondary plant metabolites in phytoremediation and biotransformation. Trends in Biotechnology. 2003a;21:123–130. doi: 10.1016/S0167-7799(02)00041-0. [DOI] [PubMed] [Google Scholar]
- Singer AC, Smith D, Jury WA, Hathuc K, Crowley DE. Impact of the plant rhizosphere and augmentation on remediation of polychlorinated biphenyl contaminated soil. Environmental Toxicology and Chemistry. 2003b;22:1998–2004. doi: 10.1897/02-471. [DOI] [PubMed] [Google Scholar]
- Slater H, Gouin T, Leigh MB. Assessing the potential for rhizoremediation of PCB contaminated soils in northern regions using native tree species. Chemosphere. 2011;84:199–206. doi: 10.1016/j.chemosphere.2011.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PL, Ramer LA, Schnoor JL. Uptake and transformation of TNT by hybrid poplar trees. Environmental Science & Technology. 1998;32:975–980. [Google Scholar]
- Weber JB, Mrozek E. Polychlorinated biphenyls - phytotoxicity, absorption and translocation by plants, and inactivation by activated carbon. Bulletin of Environmental Contamination and Toxicology. 1979;23:412–417. doi: 10.1007/BF01769980. [DOI] [PubMed] [Google Scholar]
- White JC, Parrish ZD, Isleyen M, Gent MPN, Iannucci-Berger W, Eitzer BD, Kelsey JW, Mattina MI. Influence of citric acid amendments on the availability of weathered PCBs to plant and earthworm species. International Journal of Phytoremediation. 2006;8:63–79. doi: 10.1080/15226510500507102. [DOI] [PubMed] [Google Scholar]
- Zanaroli G, Perez-Jimenez JR, Young LY, Marchetti L, Fava F. Microbial reductive dechlorination of weathered and exogenous co-planar polychlorinated biphenyls (PCBs) in an anaerobic sediment of Venice Lagoon. 2006:19–27. doi: 10.1007/s10532-005-3752-7. [DOI] [PubMed] [Google Scholar]
- Zeeb BA, Amphlett JS, Rutter A, Reimer KJ. Potential for phytoremediation of polychlorinated biphenyl-(PCB-)contaminated soil. International Journal of Phytoremediation. 2006;8:199–221. doi: 10.1080/15226510600846749. [DOI] [PubMed] [Google Scholar]
- Zhai G, Lehmler F-J, Schnoor JL. Identification of hydroxylated metabolites of 3,3′,4,4′-tetrachlorobiphenyl and metabolic pathway in whole poplar plants. Chemosphere. 2010;81:523–528. doi: 10.1016/j.chemosphere.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.