Abstract
The rostral ventromedial medulla (RVM) projects to the medullary and spinal dorsal horns and is a major source of descending modulation of nociceptive transmission. Traditionally, neurons in the RVM are classified functionally as ON, OFF, and NEUTRAL cells on the basis of responses to noxious cutaneous stimulation of the tail or hind paw. ON cells facilitate nociceptive transmission, OFF cells are inhibitory, whereas NEUTRAL cells are unresponsive to noxious stimuli and their role in pain modulation is unclear. Classification of RVM neurons with respect to stimulation of craniofacial tissues is not well defined. In isoflurane-anesthetized male rats, RVM neurons first were classified as ON (25.5%), OFF (25.5%), or NEUTRAL (49%) cells by noxious pinch applied to the hind paw. Pinching the skin overlying the temporomandibular joint (TMJ) altered the proportions of ON (39.2%), OFF (42.2%), and NEUTRAL (19.6%) cells. To assess the response of RVM cells to specialized craniofacial inputs, adenosine triphosphate (ATP; 0.01–1 mM) was injected into the TMJ and capsaicin (0.1%) was applied to the ocular surface. TMJ and ocular surface stimulation also resulted in a reduced proportion of NEUTRAL cells compared with hind paw pinch. Dose-effect analyses revealed that ON and OFF cells encoded the intra-TMJ concentration of ATP. These results suggest that somatotopy plays a significant role in the functional classification of RVM cells and support the notion that NEUTRAL cells likely are subgroups of ON and OFF cells. It is suggested that a portion of RVM neurons serve different functions in modulating craniofacial and spinal pain conditions.
Keywords: pain, temporomandibular joint, ATP, capsaicin, brain stem, pain modulation
it is well established that neurons in the rostral ventromedial medulla (RVM), which includes nucleus raphe magnus, nucleus gigantocellularis pars alpha, and lateral paragigantocellular nucleus, project to the medullary and spinal dorsal horns (Fields et al. 1995; Watkins et al. 1980) and modulate nociceptive transmission (Basbaum and Fields 1984; Heinricher et al. 2009; Mason 2001; Porreca et al. 2002; Ren and Dubner 2002).
Traditionally, RVM neurons have been defined as ON, OFF, NEUTRAL, and serotonergic cells on the basis of pattern of background activity and responses to noxious cutaneous stimulation (Fields et al. 2006; Gao and Mason 2000). ON cells are excited by noxious stimuli and are inhibited by morphine and proposed to facilitate nociception, while OFF cells exhibit a decrease in ongoing discharge during noxious stimulation, are excited by morphine, and are proposed to inhibit nociception (see Heinricher et al. 2009; Mason 2001). NEUTRAL cells are unaffected by noxious cutaneous stimuli or by exogenous opioids, and their role in nociception is unclear. Serotonergic cells are a separate group of RVM neurons defined by slow, regular discharge and distinct neurochemistry that may modulate autonomic nervous system functions (Mason 2001; Mason et al. 2007; Potrebic et al. 1994). The classification of ON, OFF, and NEUTRAL cells based on noxious cutaneous heat or mechanical stimulation of distal limbs or tail is generally consistent (Leung and Mason 1998). However, several studies have reported that RVM cell classification based on cutaneous limb stimulation differs from that based on craniofacial (Ellrich et al. 2001), visceral (Brink and Mason 2003), or vagus nerve (Thurston and Randich 1992) stimulation. Such dissociations suggest that the tissue origin of the afferent input is a significant factor in establishing the role of subclasses of RVM neurons in nociceptive processing. The present study addressed this hypothesis by comparing RVM cell classification after hind paw pinch to that found after stimulation of facial skin, the temporomandibular joint (TMJ), and the ocular surface. Stimulation of these latter tissues will provide new information on how trigeminal nerve input affects the classification of RVM neurons and on how RVM neurons respond to noxious stimulation of specialized craniofacial tissues.
Temporomandibular joint disorders (TMDs) represent a heterogeneous family of conditions associated with pain in the TMJ and masticatory muscles and are the most common form of nondental craniofacial pain (Maixner 2009). Nonarthritic TMD often is comorbid with chronic pain conditions such as fibromyalgia and inflammatory bowel syndrome, conditions that also present with no overt signs of injury or inflammation, suggesting a common etiology collectively referred to as central sensitization syndromes (Yunus 2007). Although descending modulation is thought to play a significant role in TMD (Sarlani and Greenspan 2003), the relationship between the RVM and TMJ nociception is poorly defined. These studies examined the relationships between the RVM and TMJ nociception at a cellular level by determining responses of RVM neurons following activation of nociceptors in the TMJ. Understanding how nociceptive afferent input from the TMJ drives descending facilitation and inhibition from the RVM may have implications for treating TMD pain.
The present study used electrophysiological recording methods to test two main hypotheses regarding the RVM and craniofacial pain. First, we determined whether there is a somatotopic organization to the classification of ON, OFF, and NEUTRAL cells based on the tissue origin of the sensory input. Second, we investigated how RVM cell classification is altered when based on stimulation of specialized craniofacial tissues (TMJ, ocular surface), tissues that are preferentially associated with pain sensation. Furthermore, we characterized and quantified the responses of RVM neurons to noxious stimulation of the TMJ and the ocular surface. These results indicated that sensory nerve origin is a key factor in the classification of RVM cells, as the percentage of NEUTRAL cells based on craniofacial skin and specialized structures was significantly reduced from that based on stimulation of distal limbs or tail. A preliminary report has appeared in abstract form (Khasabov et al. 2013).
METHODS
Animals.
Adult male Sprague-Dawley rats (n = 27, 250–375 g; Harlan Industries, Indianapolis, IN) were maintained in a climate-controlled room on a 12:12-h dark-light cycle, with food and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota and were performed in accordance with the guidelines recommended by the International Association for the Study of Pain.
Anesthesia and surgery.
Rats were initially anesthetized with pentobarbital sodium (60 mg/kg ip). After tracheotomy, anesthesia was maintained during surgery with isoflurane (1.5–2%). The carotid artery was catheterized for continuous monitoring of blood pressure, and the experiment was terminated if blood pressure fell below 70 mmHg. Core temperature was maintained at ∼37°C with a feedback-controlled heating pad (Harvard Apparatus, Holliston, MA). During experiments, the concentration of isoflurane was adjusted so that rats showed no sign of discomfort but a withdrawal response measured by electromyographic (EMG) activity could be evoked by application of noxious stimuli. EMG activity was recorded by paired fine needle electrodes placed in the ipsilateral biceps femoris or the contralateral masseter muscle. EMG activity evoked by noxious stimuli was defined as muscular activity that was >20% above any baseline activity prior to stimulation.
Rats were placed into a stereotaxic apparatus, a small craniotomy was made over the cerebellum, and dura mater was removed. A guide cannula (26 gauge) was inserted into the TMJ joint space (3 mm deep) to allow delivery of chemical stimuli as previously described (Takeshita et al. 2001; Tashiro et al. 2008). Injections of adenosine triphosphate (ATP) into the joint space were used to excite TMJ sensory fibers. For delivery of ATP (or vehicle) into the TMJ, polyethylene tubing was used to connect a microsyringe to an inner cannula (33 gauge) that protruded 1 mm from the end of the guide cannula.
Electrophysiological recording from RVM neurons.
Beginning at least 1 h after the completion of all surgical procedures, single RVM neurons were recorded extracellularly with stainless steel microelectrodes (10 MΩ; Frederick Haer, Brunswick, ME). The ranges of stereotaxic coordinates used were AP = −9.9 to −11.5 mm from bregma; DV = +9.4 to +10.6 mm from the cranial surface; L = −1.1 to +1.1 mm from midline (Paxinos and Watson 2007). Neurons were initially isolated by ongoing discharge, and action potentials were amplified, displayed on a storage oscilloscope, audio-monitored, and discriminated according to amplitude and waveform. Neuronal discharge, EMG activity, stimulus temperature, and time of mechanical and chemical stimulation were collected with Spike2 software (Cambridge Electronic Design, Cambridge, UK) and stored on a computer for off-line analyses. Brief tail pinch with the experimenter's fingers and/or unserrated forceps was used to search initially for RVM neurons. Once a cell was identified, it was classified functionally by pinching the hind paw. ON cells were defined by an abrupt increase in discharge, whereas OFF cells exhibited a pause in ongoing activity in response to noxious pinch that occurred just prior to a withdrawal response (EMG activity) and differed from the ongoing discharge rate by >20%. Cells whose ongoing discharge rate did not change during hind paw pinch prior to the withdrawal response were defined as NEUTRAL cells. Only one or two neurons were studied in each animal.
Drug solutions.
ATP was dissolved in normal saline to concentrations of 0.01, 0.1, and 1 mM. Capsaicin (Sigma, St. Louis, MO) was dissolved in 5% Tween 80 and saline and applied at a final concentration of 0.1%.
Evoked response measures and experimental design.
Once a neuron was classified according to the response evoked by firmly pinching the hind paw, responses to pinch of the forepaw and facial skin overlying the TMJ with unserrated forceps were obtained. Next, the response to gently stroking the eye with a blunt metal probe was determined. Responses of RVM neurons to stimulation of the TMJ were determined next by intra-TMJ injections of ATP (0.01, 0.1, and 1 mM; 10 μl) given at 20-min intervals to minimize tachyphylaxis. Finally, capsaicin (0.1%; 5 μl) was applied topically to the ocular surface.
Histological verification of recording sites.
At the end of each experiment, the RVM recording site was marked by passing current (30 μA for 20 s) through the recording electrode. Animals were euthanized with an overdose of pentobarbital sodium and perfused intracardially with physiological saline followed by 10% formalin with 1% potassium ferrocyanide. Recording sites were verified histologically in 50- or 100-μm sections. Lesion sites were reconstructed and mapped onto outlines adapted from the stereotaxic atlas of Paxinos and Watson (2007).
Data analyses.
The proportions of ON, OFF, and NEUTRAL cells classified according to responses evoked by noxious stimulation of the hind paw, facial skin, TMJ, or ocular surface were compared by χ2 analyses. Spontaneous discharge rates of neurons classed according to stimulation of the hind paw or facial skin were compared by ANOVA. Also, for each cell type differences in the percent change in discharge rates evoked by pinching the various body sites from ongoing spontaneous activity was evaluated by Kruskal-Wallis ANOVAs on ranks. Responses of individual RVM neurons to different concentrations of ATP injected into the TMJ and to application of capsaicin to the eye were determined by subtracting the mean ongoing discharge rate for 100 s before application from the mean discharge rate for 100 s after. A response to ATP or capsaicin was defined as an increase of >20% in the mean ongoing discharge rate for 100 s before application of ATP or capsaicin. Duration of responses to ATP and capsaicin was defined as the first of five consecutive 1-s bins that were <20% above the ongoing discharge rate prior to application of ATP and capsaicin. Responses to ATP were compared by one-way ANOVA corrected for repeated measures, and post hoc comparisons were made with Student-Newman-Keuls tests. The threshold dose of ATP sufficient to evoke ON and OFF cell responses was compared by χ2 analysis. A P value of <0.05 was considered significant in all cases. Mean (±SE) values are reported throughout the results unless otherwise stated. Since OFF cells exhibit ongoing activity that is decreased by noxious stimuli, their evoked responses are described as a negative number of impulses.
RESULTS
Functional characteristics of RVM neurons.
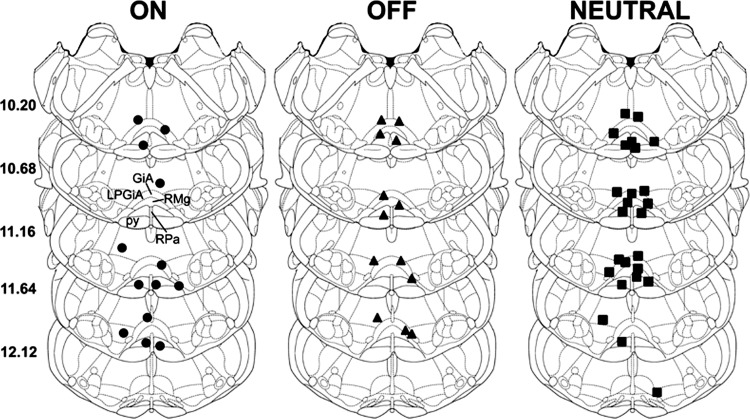
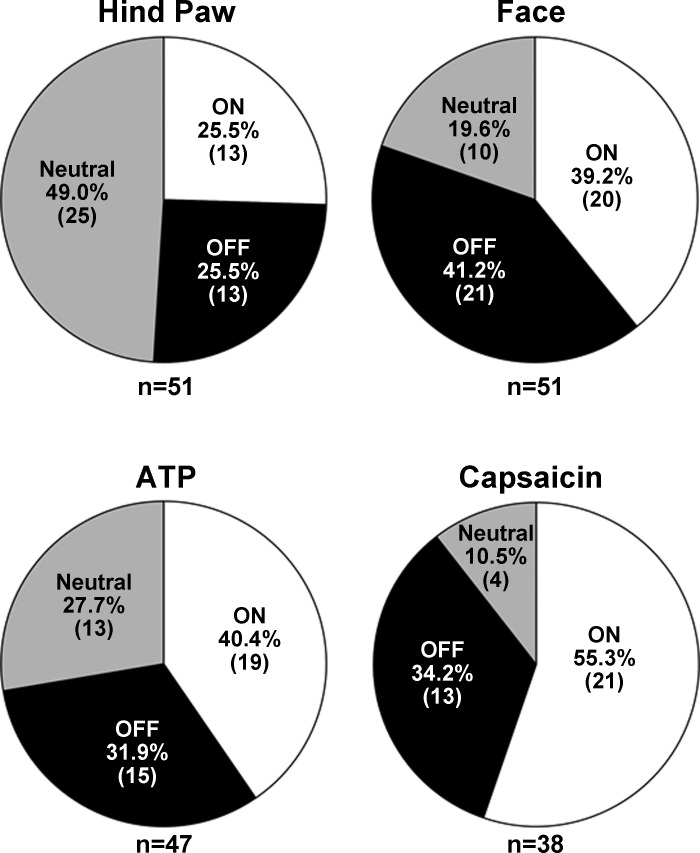
A total of 51 neurons were recorded within the RVM (Fig. 1). Each neuron was initially classified as ON, OFF, or NEUTRAL according to responses evoked by noxious pinch of the hind paw. This resulted in 13 neurons (25.5%) classified as ON cells, 13 (25.5%) as OFF cells, and 25 (49.0%) as NEUTRAL cells. Importantly, all ON and OFF cells always exhibited a change in discharge of at least 22% during noxious stimulation regardless of the site of stimulation. For all ON cells, the median percent changes (and 25th and 75th percentiles) in discharge evoked by stimulation of the tail, hind paw, forepaw, and facial skin were 119.8% (47% and 850%), 45.8% (33.6% and 113.6%), 59.4% (41.7% and 650%), and 126.9% (48.6% and 473.6%), respectively. For all OFF cells, the median percent change in activity evoked during noxious stimulation of the tail, hind paw, forepaw, and facial skin were −67.7% (−78.4% and −44.4%), −59.0% (−96.3% and −41.4%), −41.9% (−90.4% and −24.85), and −73.5% (−88% and −38.3%), respectively. Discharge rates of our NEUTRAL cells never changed more than ±18.8% relative to ongoing spontaneous activity. For all NEUTRAL cells, the median percent changes in discharge during noxious stimulation of the tail, hind paw, forepaw, and facial skin were −1.3% (−4% and 5.0%), 1.2% (−10% and 9.1%), 0.0% (−9.3% and 4.8%), and 0.0% (−9% and 4.1%), respectively. For each cell type, there were no differences in the evoked percent change in discharge rate as a function of the body site stimulated (Kruskal-Wallis ANOVAs on ranks).
Fig. 1.
Histological verification of the locations of all recording sites for ON (n = 13), OFF (n = 13), and NEUTRAL (n = 25) cells in the rostral ventromedial medulla (RVM). Schematics show cross sections of the medulla adapted from the stereotaxic atlas of Paxinos and Watson (2007) with permission. Distances from bregma are indicated on left. Structures in the medulla are indicated by dashed lines and are abbreviated: py, pyramidal tract; RMg, raphe magnus nucleus; RPa, raphe pallidus nucleus; GiA, gigantocellular reticular nucleus pars alpha; LPGiA, lateralis paragigantocellular reticular nucleus pars alpha.
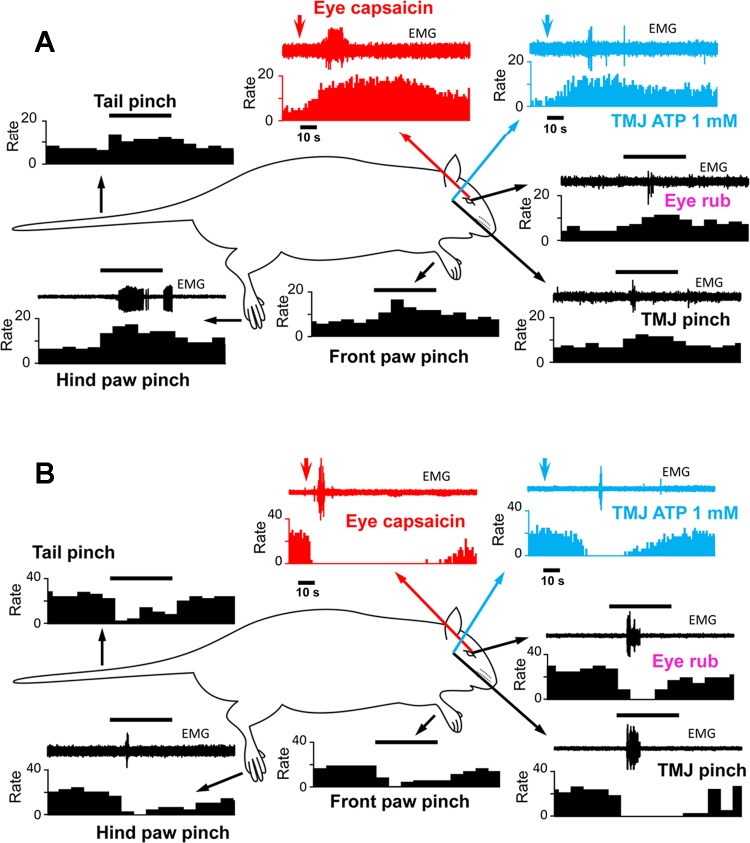
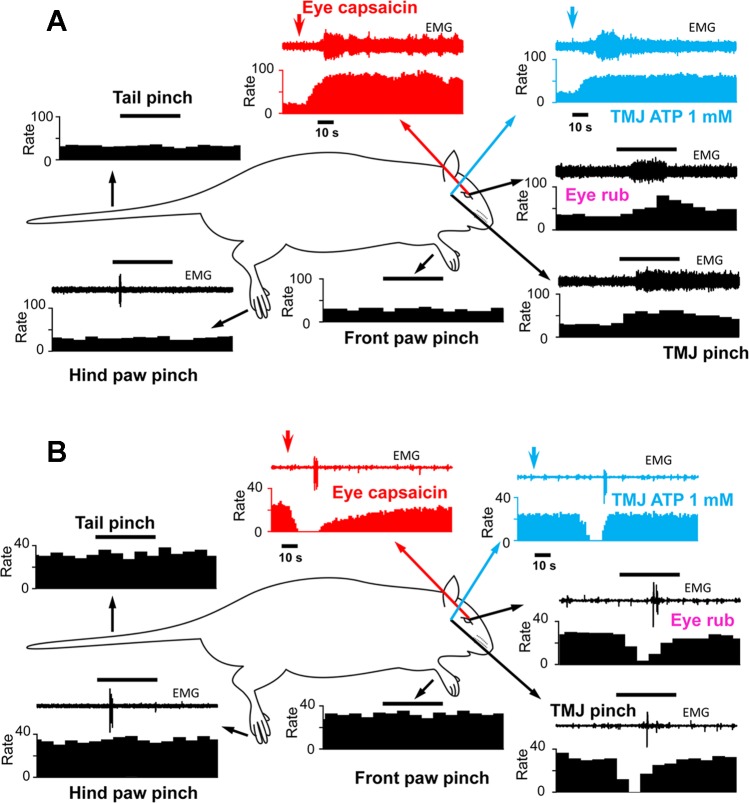
A primary objective of this study was to determine whether the classification of neurons as ON, OFF, or NEUTRAL cells remained constant when assessed by stimulation of multiple areas of the body, with emphasis on craniofacial tissues. The examples shown in Figs. 2 and 3 indicate the responses as well as the sequence of applied stimuli. Thus in Fig. 2A, the stimulus sequence, beginning in the top left panel, was pinch of tail, hind paw, forepaw, and face followed by gentle stroking of the ocular surface, intra-TMJ injection of ATP, and finally capsaicin (0.1%) applied to the ocular surface. The example in Fig. 2A is typical of an ON cell that retained this classification to all applied stimuli, while Fig. 2B is an example of a cell that behaved as an OFF cell to each stimulus. Although we cannot assume that the intensities of these various stimuli were equal, at least for cutaneous pinch of tail, hind paw, forepaw, and face, the ON and OFF cell responses appeared quantitatively similar. Figure 3 presents two examples of cells classified as NEUTRAL on the basis of hind paw pinch; however, craniofacial tissue stimulation evoked an ON cell-like (Fig. 3A) or an OFF cell-like (Fig. 3B) activity. A total of 38 neurons were successfully studied throughout the full sequence of stimuli. Of these 38 cells only 14 retained their initial classification based on hind paw pinch and all craniofacial stimuli. Interestingly, only 4 of 38 cells could be classified as NEUTRAL after ocular surface stimulation with capsaicin, and of those only 2 cells retained this classification throughout the entire stimulus sequence. Cell classification based on hind paw pinch, facial skin pinch, intra-TMJ injection of ATP, and ocular surface capsaicin is summarized in Fig. 4. χ2 Analyses revealed that cell classification based on hind paw pinch was significantly different from that seen after facial skin pinch (χ2 = 9.8, P < 0.007) and ocular surface capsaicin (χ2 = 15.52, P < 0.001) but was only marginally different compared with TMJ stimulation (χ2 = 4.11, P < 0.08). The proportions of cell classes compared across the three craniofacial tissues (i.e., skin, TMJ, eye) were not different (χ2 = 5.23, P < 0.26).
Fig. 2.
Examples of responses of individual RVM neurons initially classified as ON cells (A) or OFF cells (B) to pinching of the hind paw. Stimuli and the order in which they were applied: tail pinch, hind paw pinch, forepaw pinch, facial skin pinch [temporomandibular joint (TMJ) pinch], gentle stroking of the ocular surface, injection of ATP into the TMJ, and application of capsaicin to the ocular surface. Accompanying EMG activity was recorded from the ipsilateral biceps femoris or the contralateral masseter muscle. Arrows point to responses evoked by stimulation at the site of each arrow's origin. Arrowheads indicate time of chemical application. Horizontal bar above the EMG activity is equal to 5 s. Bin widths for histograms are either 0.5 s or 1 s.
Fig. 3.
Examples of responses of individual RVM neurons initially classified as NEUTRAL cells after hind paw pinch that behaved as an ON cell (A) or an OFF cell (B) after all craniofacial stimuli. See Fig 2 for other descriptions and abbreviations.
Fig. 4.
Proportions of functional classes of RVM neurons based on pinching of the hind paw, facial skin pinch, intra-TMJ injection of ATP, and capsaicin applied to the ocular surface. Note that the majority of NEUTRAL cells based on the response to hind paw pinch responded as ON or OFF cells to stimulation of craniofacial tissues.
Among ON cells, the ongoing spontaneous activity of cells classified by pinching facial skin was nearly twice that of ON cells classified by pinch applied to the hind paw (14.9 ± 3.3 and 7.1 ± 1.76 imp/s, respectively; t-test, P < 0.05). Spontaneous activity of OFF and NEUTRAL cells classified by pinching the hind paw or facial skin were not different.
The same 51 neurons we classified by hind paw pinch were classified by pinching facial skin. Of these, 20 (39.2%) were ON cells, 21 (41.2%) behaved as OFF cells, and 10 (19.6%) were NEUTRAL cells. Thus more than half of the NEUTRAL cells as classified by pinching the hind paw behaved as ON or OFF cells to facial skin pinch (Fig. 4). Importantly, there were no differences in the level of spontaneous activity of NEUTRAL cells just prior to stimulation at each body site between those cells that exhibited an ON or OFF response to facial pinch and those that did not. For example, the mean spontaneous activity of NEUTRAL cells that had an ON response to facial pinch (n = 8) just prior to pinching of the tail, hind paw, and face was 25.5 ± 5.72, 21.3 ± 5.25, and 19.9 ± 4.85 imp/s, respectively. For NEUTRAL cells that had an OFF response to facial pinch, their spontaneous activity just prior to pinching of the tail, hind paw, and face was 24.4 ± 4.94, 22.9 ± 5.4, and 25.6 ± 6.15 imp/s, respectively. These values did not differ from those of NEUTRAL cells that did not exhibit ON or OFF responses to facial pinch (13.7 ± 3.48 imp/s prior to tail pinch, 11.2 ± 2.34 imp/s prior to hind paw pinch, and 12.2 ± 1.65 imp/s prior to facial pinch). Although the majority of ON and OFF cells (92.3%) classified by noxious hind paw pinch retained that classification after facial skin pinch, two neurons exhibited complex responses with an apparent change in phenotype. One neuron classified as an ON cell to pinching of the hind paw exhibited OFF-like responses to noxious pinch applied to the face, while one OFF cell based on hind paw pinch exhibited ON-like responses to facial skin pinch. Notably, neurons initially classified as ON or OFF cells based on hind paw pinch never exhibited NEUTRAL-like responses to noxious pinch applied to the face.
Responses of RVM neurons to intra-TMJ injections of ATP.
Nineteen of forty-seven neurons tested with intra-TMJ injection of ATP exhibited ON-like responses (Fig. 4). Fifteen of these cells also demonstrated ON-like responses to pinching facial skin, one cell responded as an OFF cell to pinch, and three cells were NEUTRAL to pinching. Similarly, 15 of 47 cells were classified as OFF cells to injection of ATP into the TMJ. Of these cells, 12 also had OFF-type response to pinching facial skin, 2 exhibited ON-like responses to pinch, and 1 cell was NEUTRAL to pinching facial skin. Thirteen cells behaved as NEUTRAL cells to injection of ATP into the TMJ. Of these, five cells were also unresponsive to pinching facial skin, while three cells were excited (ON-like response) and five cells responded with an OFF-like response to pinching facial skin. Thus the majority of ON and OFF cells based on facial skin pinch retained that classification based on intra-TMJ injection of ATP, while nearly 50% of NEUTRAL cells exhibited ON- or OFF-like responses to ATP.
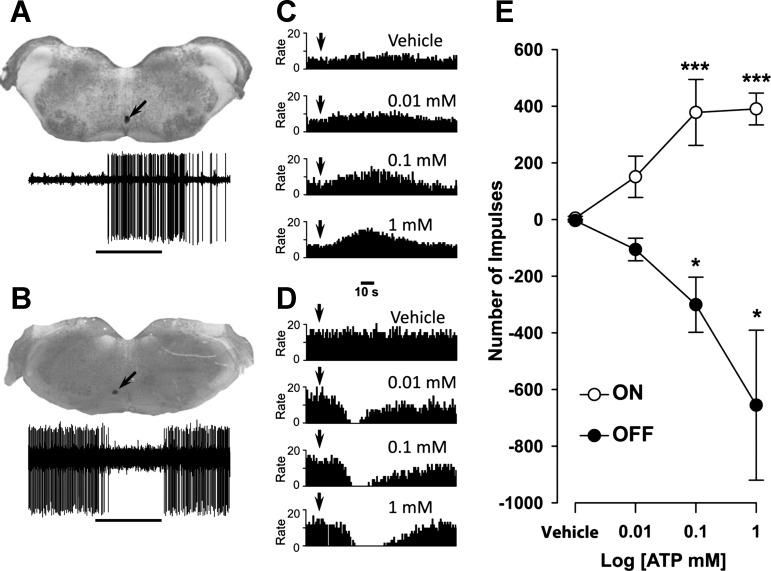
Intra-TMJ injections of ATP evoked dose-dependent increases in ON cells (F3,68 = 6.31, P < 0.001) and dose-dependent decreases in evoked OFF cell responses (F3,56 = 4.07, P < 0.001) (Fig. 5). The threshold ATP concentrations required to evoke ON and OFF cell-like activity were similar (χ2 = 0.57, P = 0.75). The mean durations of ON cell responses to 0.01, 0.1, and 1 mM ATP were 72.7 ± 15.3, 64.9 ± 14.2, and 85.3 ± 12.6 s, respectively, and were not different (F2,29 = 0.78, P = 0.47). Similarly, the durations of inhibitory responses of OFF cells to 0.01, 0.1, and 1 mM ATP averaged 60.4 ± 9.2, 79.0 ± 22.4, and 90.9 ± 24.5 s, respectively, and did not differ from each other (F2,26 = 0.71, P = 0.51). There was a trend for the pause response of OFF cells to increase in duration with increasing doses of ATP. This suggested that nociceptive stimulation of intra-TMJ afferents was capable of recruiting anti- as well as pronociceptive descending signals from the RVM.
Fig. 5.
Responses of ON and OFF cells evoked by injection of vehicle or ATP into the TMJ are dose dependent. A and B: recording sites (arrows) in the RVM for individual ON (A) and OFF (B) cells and their functional characterization based on responses to pinching facial skin (horizontal line). C and D: for the cells in A and B, frequency histograms show examples of responses of the ON (C) and OFF (D) cells to injections of vehicle and ATP into the TMJ. Dose-related responses of OFF cells are characterized by an increase in the duration of the pause and an increase in the time in which ongoing activity returns to preinjection level. Bin widths are 1 s. Arrowheads indicate the time of injection. E: mean (±SE) number of impulses evoked by injection of vehicle or ATP (0.1–1 mM). Responses for each cell were determined by subtracting the ongoing spontaneous activity before injection (number of impulses for 100 s) from the number of impulses evoked for 100 s after injection. Responses of OFF cells are presented as a negative number of impulses since they exhibit a decrease in ongoing activity during noxious stimulation. Significant differences from vehicle: *P < 0.05, ***P < 0.005.
Responses of RVM neurons to application of capsaicin to ocular surface.
A total of 38 of the 51 RVM neurons classified after facial skin pinch were assessed for responses to topical application of capsaicin (0.1%) to the ocular surface. As summarized in Fig. 4, 21 of 38 cells (55.3%) displayed ON-like cell responses, 13 (34.2%) displayed OFF-like responses, while only 4 cells (10.5%) were unresponsive to capsaicin and were classified as NEUTRAL cells.
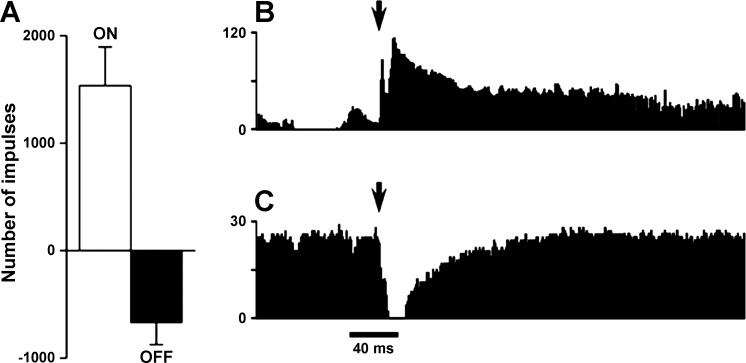
RVM cells classified by their response to capsaicin applied to the ocular surface resulted in the greatest change in cell classification compared with that produced by hind paw pinch, with an increase in the percentage of ON-like cells and a reduction in NEUTRAL-like cells compared with that seen after pinching the hind paw (χ2 = 15.52, P < 0.001). Although somatotopy (i.e., trigeminal vs. spinal nerve input) was likely a key determinant of RVM cell classification, it could not be excluded that stimulus intensity and/or modality also were contributing factors. Responses of RVM neurons to application of capsaicin were immediate and intense. The mean number of impulses evoked during 100 s after application of capsaicin was 1,535.4 ± 358.9 impulses for ON cells and −669.7 ± 205.8 impulses for OFF cells (Fig. 6). Interestingly, the mean duration of responses for ON cells was longer than the pause in ongoing activity of OFF cells (220.9 ± 18.9 and 97.5 ± 19.1 s, respectively; t-test, P < 0.0001). These results suggest that capsaicin engages outputs from the RVM that are primarily pronociceptive. The peak discharge rate (per 1-s bin width) for ON cells evoked by capsaicin was 65.5 ± 21.7 imp/s, and this was greater than the peak discharge rates evoked by pinching facial skin (21.9 ± 8.72 imp/s) and the hind paw (15.1 ± 6.21 imp/s) (1-way ANOVA, P < 0.05).
Fig. 6.
Responses of ON and OFF cells to capsaicin applied to the ocular surface. A: mean (±SE) number of impulses evoked by capsaicin for all ON and OFF cells. Responses for each cell were determined by subtracting the ongoing spontaneous activity before injection (number of impulses for 100 s) from the number of impulses evoked for 100 s after capsaicin application. Responses of OFF cells are presented as a negative number of impulses since they exhibit a decrease in ongoing activity during noxious stimulation. B and C: representative examples of responses of single ON (B) and OFF (C) cells evoked by application of capsaicin to the eye.
DISCUSSION
The main finding of this study was that the proportions of RVM neurons classified as ON, OFF, and NEUTRAL cells as determined by stimulation of facial skin or specialized craniofacial structures were significantly different from those seen after hind paw pinch. Notably, the proportion of NEUTRAL cells was greatly reduced, while that of both ON and OFF cells was increased. These data suggested that RVM cell classification based on trigeminal nerve input was significantly different from that seen after spinal nerve input. However, since stimulation of different craniofacial tissues resulted in similar proportions of cell classes, these data support the notion that somatotopy is a key determinant of functional classification of RVM neurons.
The RVM is a major source of descending control of nociceptive input to spinal and medullary dorsal horn (Vc) neurons (Fields et al. 2006). Although nonselective activation of RVM outflow significantly reduces Vc neural responses to noxious stimulation of facial skin (Dostrovsky et al. 1983) and specialized craniofacial tissues such as tooth pulp (Chiang et al. 1989), jaw muscles and TMJ (Chiang et al. 1994, 1995), and the ocular surface (Meng et al. 2000), the properties of different functional classes of RVM cells that underlie modulation of trigeminal nociception are not well defined. The present study addressed this issue by first classifying RVM cells as ON, OFF, and NEUTRAL by the method of hind paw pinch and then comparing the results to those seen after noxious stimulation of different body areas, including craniofacial tissues. Although a technical limitation was the nonuniform pinch stimulus with handheld forceps, responses evoked by pinching different body sites (tail, hind paw, and forepaw) were fairly consistent. The results revealed a significant reduction in the proportion of NEUTRAL cells after all craniofacial stimuli. Fewer than 50% of cells classified as ON or OFF cells after paw pinch retained their classification across all craniofacial stimulus sites. Several studies have reported that stimulus site greatly influences the proportion of classes of RVM cells. For example, early studies in cats showed that reticulospinal neurons were occasionally excited or inhibited by pinching of different body sites (Wolstencroft 1964). Ellrich et al. (2000) found in a small sample (n = 14) of NEUTRAL cells classified by noxious heat applied to the tail that 43% were excited (ON-like) and 57% inhibited (OFF-like) by pinch of the ear and none retained its NEUTRAL cell status. Among a population of ON cells based on heat stimulation of the tail, 10 of 13 cells behaved as ON cells after pinch of the nose, brush of the cornea, and brush of the exposed dura; however, only 12 of 23 OFF cells retained this classification across all applied stimuli (Ellrich et al. 2001). Visceral stimuli also have been shown to be randomly associated with RVM cells of different classes, since only 16 of 45 (36%) of NEUTRAL cells classified based on heat stimuli applied to the tail remained unresponsive to colorectal distension (Brink and Mason 2003). These results and those of the present study strongly suggest that tissue location or type is a key determinant in classifying RVM cells.
It cannot be excluded that other factors such as depth of anesthesia, stimulus modality, or stimulus intensity play role in determining RVM cell class. High concentrations of isoflurane can blunt or eliminate responses of ON and OFF cells, making them appear as NEUTRAL cells (Leung and Mason 1995), while the presence or absence of withdrawal reflexes may affect the magnitude of the responses of ON and OFF cells independent of isoflurane concentration (Jinks et al. 2004). However, in the present study neural activity was recorded during the presence of a visible withdrawal response (EMG activity) from both the ipsilateral biceps femoris and the contralateral masseter muscle, thus reducing the likelihood that anesthesia had a profound influence on the pattern of activity of RVM neurons. The role of stimulus modality on RVM cell classification was not addressed specifically here; however, the proportions of RVM cells of different classes were similar after facial skin pinch, intra-TMJ injection of ATP, and ocular surface-applied capsaicin. Stimulus intensity also could have affected the functional classification of RVM cells; however, others have reported that acute injection of capsaicin (Brink et al. 2012) or hindpaw inflammation (Cleary and Heinricher 2013) did not cause NEUTRAL cells to behave as ON or OFF cells (however, see Miki et al. 2002). Similarly, increasing the concentration of ATP had no effect on the functional class of the recorded RVM cell. Furthermore, the peak discharge rates evoked by pinching of the hind paw or facial skin were similar, although pinching of facial skin resulted in a greater proportion of neurons classed as ON cells compared with pinching of the hind paw, and, although capsaicin application to the ocular surface produced the greatest rate of discharge, there were no differences in the proportions of cell classes characterized across craniofacial tissues. These results argue against stimulus intensity alone as being a key factor in the functional classification of RVM cells. These results could not exclude that the response magnitude of RVM cells of different classes, rather than simply the frequency of occurrence, was affected by stimulus modality or intensity. Indeed, it has been proposed that the magnitude of somatic sensory-evoked phasic activity of RVM cells rather than tonic discharge rates determines the extent of modulatory influence on pain behavior (Mason 2012).
Several earlier studies have suggested that noxious sensory input to spinal (Yu and Mense 1990) and medullary dorsal horn (Chiang et al. 1994, 1995) neurons from deep tissues was under stronger descending control than input from cutaneous tissues. The basis for this difference is not certain. The present study used intra-TMJ injections of ATP, a stimulus that reliably activates nociceptive Vc neurons (Okamoto et al. 2012), evokes nocifensive behaviors after TMJ injection (Oliveira et al. 2005), and excites articular sensory nerves in a dose-related manner (Dowd et al. 1998). Dose-effect analyses revealed that ON and OFF cells encoded the concentration of the ATP stimulus and the threshold to activate ON and OFF cells was similar. This suggested that TMJ afferents were able to recruit pro- as well as antinociceptive RVM circuitry equally, and that the final net effect on behavior depended on sensory integration at sites outside the RVM.
The majority of ON and OFF cells retained their functional classification when tested by pinching the tail, hind paw, forepaw, or face. However, several cells displayed an apparent phenotypic switch in the class depending on the applied stimulus. This was particularly apparent for cells tested with capsaicin applied to the ocular surface. Compared with other stimulus sites, ocular surface stimulation by capsaicin resulted in the lowest percentage of NEUTRAL cells. The basis for a phenotypic switch among RVM cells, particularly between ON and OFF cell classes, is likely complex and as noted above may be due to experimental methodologies. So-called atypical response properties of RVM neurons have been reported previously for differences in cell class due to stimulus site, modality, or responses to analgesic drugs (Schnell et al. 2002). During the onset of inflammation and continuous RVM cell recording, 11 of 15 NEUTRAL cells developed ON or OFF cell-like behavior (Miki et al. 2002; however, see Cleary and Heinricher 2013), suggesting that environmental factors or history of activity may affect the functional classification of RVM cells. Thus the function of such atypical cells remains difficult to interpret since it is likely that RVM cells contribute to other homeostatic functions as well as modifying painlike behaviors (Heinricher et al. 2009; Mason 2012).
The ascending trigeminal and spinal pathways that recruit descending controls from the RVM have traditionally been thought to terminate initially in regions outside the RVM such as the periaqueductal gray (PAG) or parabrachial area (Fields et al. 2006). This was supported by studies in cats in which no spinal neurons were found to project directly to the RVM (Abols and Basbaum 1981). By contrast, moderate to weak direct projections were found from the trigeminal brain stem nuclear complex (TBNC) to the RVM in the rat (Hermann et al. 1997; Sugiyo et al. 2005), albeit far weaker than the projections from TBNC to the PAG (Chang et al. 2012). Furthermore, recent evidence indicates significant direct projections from the RVM to rostral and caudal regions of Vc that process sensory input from the TMJ and ocular surface (Aicher al. 2012). Strong reciprocal direct projections between the RVM and TBNC may help explain why we found a greater proportion of ON and OFF cells and a reduced proportion of NEUTRAL cells after craniofacial stimulation versus after spinal stimulation.
In conclusion, a significant percentage of NEUTRAL cells in the RVM, defined functionally by noxious stimulation of the hind paw, exhibited robust ON- or OFF-like responses to noxious stimulation of cutaneous and specialized craniofacial structures. The data suggested that somatotopic location (craniofacial vs. lower body) was a critical determinant of RVM cell classification. Additional studies will be needed to determine whether the intensity and modality of craniofacial stimuli as well as environmental factors such as prior injury, inflammation, or psychophysical stress also play significant roles in determining the functional classification of RVM cells and their influence on pain of craniofacial origin.
GRANTS
This work was supported by National Institute of Dental Research Grant DE-12758 and National Eye Institute Grant EY-021447 (D. A. Bereiter).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.G.K., K.O., D.A.B., and D.A.S. conception and design of research; S.G.K., P.M., J.N., J.T., and D.A.S. performed experiments; S.G.K., D.A.B., and D.A.S. analyzed data; S.G.K., K.O., D.A.B., and D.A.S. interpreted results of experiments; S.G.K., P.M., J.N., J.T., and D.A.S. prepared figures; S.G.K., K.O., D.A.B., and D.A.S. drafted manuscript; S.G.K., K.O., D.A.B., and D.A.S. edited and revised manuscript; D.A.B. and D.A.S. approved final version of manuscript.
REFERENCES
- Abols IA, Basbaum AI. Afferent connections of the rostral medulla of the cat: a neural substrate for midbrain-medullary interactions in the modulation of pain. J Comp Neurol 201: 285–297, 1981. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Hermes SM, Whittier KL, Hegarty DM. Descending projections from the rostral ventromedial medulla (RVM) to trigeminal and spinal dorsal horns are morphologically and neurochemically distinct. J Chem Neuroanat 43: 103–111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 7: 309–338, 1984. [DOI] [PubMed] [Google Scholar]
- Brink TS, Mason P. Raphe magnus neurons respond to noxious colorectal distension. J Neurophysiol 89: 2506–2515, 2003. [DOI] [PubMed] [Google Scholar]
- Brink TS, Pacharinsak C, Khasabov SG, Beitz AJ, Simone DA. Differential modulation of neurons in the rostral ventromedial medulla by neurokinin-1 receptors. J Neurophysiol 107: 1210–1221, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z, Okamoto K, Bereiter DA. Differential ascending projections of temporomandibular joint-responsive brainstem neurons to periaqueductal gray and posterior thalamus of male and female rats. Neuroscience 203: 230–243, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CY, Hu JW, Dostrovsky JO, Sessle BJ. Changes in mechanoreceptive field properties of somatosensory brainstem neurons induced by stimulation of nucleus raphe magnus in cats. Brain Res 485: 371–381, 1989. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Hu JW, Sessle BJ. Parabrachial area and nucleus raphe magnus-induced modulation of nociceptive and nonnociceptive trigeminal subnucleus caudalis neurons activated by cutaneous or deep inputs. J Neurophysiol 71: 2430–2445, 1994. [DOI] [PubMed] [Google Scholar]
- Chiang CY, Hu JW, Sessle BJ. Parabrachial area and nucleus raphe magnus-induced modulation of electrically evoked trigeminal subnucleus caudalis neuronal responses to cutaneous or deep A-fiber and C-fiber inputs. Pain 62: 61–68, 1995. [DOI] [PubMed] [Google Scholar]
- Cleary DR, Heinricher MM. Adaptations in responsiveness of brainstem pain-modulating neurons in acute compared with chronic inflammation. Pain 154: 845–855, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostrovsky JO, Shah Y, Gray BG. Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. II. Effects on medullary dorsal horn nociceptive and nonnociceptive neurons. J Neurophysiol 49: 948–960, 1983. [DOI] [PubMed] [Google Scholar]
- Dowd E, McQueen DS, Chessell IP, Humphrey PP. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. Br J Pharmacol 125: 341–346, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellrich J, Messlinger K, Chiang CY, Hu JW. Modulation of neuronal activity in the nucleus raphe magnus by the 5-HT1-receptor agonist naratriptan in rat. Pain 90: 227–231, 2001. [DOI] [PubMed] [Google Scholar]
- Ellrich J, Ulucan C, Schnell C. Are “neutral cells” in the rostral ventro-medial medulla subtypes of on- and off-cells? Neurosci Res 38: 419–423, 2000. [DOI] [PubMed] [Google Scholar]
- Fields H, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: Textbook of Pain, edited by McMahon SB, Koltzenburg M. Edinburgh: Churchill Livingstone, 2006, p. 125–142. [Google Scholar]
- Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol 74: 1742–1759, 1995. [DOI] [PubMed] [Google Scholar]
- Gao K, Mason P. Serotonergic raphe magnus cells that respond to noxious tail heat are not ON or OFF cells. J Neurophysiol 84: 1719–1725, 2000. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev 60: 214–225, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b). J Chem Neuroanat 13: 1–21, 1997. [DOI] [PubMed] [Google Scholar]
- Jinks SL, Carstens E, Antognini JF. Isoflurane differentially modulates medullary on and off neurons while suppressing hind-limb motor withdrawals. Anesthesiology 100: 1224–1234, 2004. [DOI] [PubMed] [Google Scholar]
- Khasabov SG, Brink TS, Schupp M, Noack J, Simone DA. Changes in response properties of rostral ventromedial medulla neurons during prolonged inflammation: modulation by neurokinin-1 receptors. Neuroscience 224: 235–248, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabov SG, Okamoto K, Katagiri A, Simone DA, Bereiter DA. Activation of “NEUTRAL” cells in the RVM by noxious craniofacial stimulation (Abstract). Soc Neurosci Abstr 2013: 461.–04., 2013. [Google Scholar]
- Leung CG, Mason P. Effects of isoflurane concentration on the activity of pontomedullary raphe and medial reticular neurons in the rat. Brain Res 699: 71–82, 1995. [DOI] [PubMed] [Google Scholar]
- Leung CG, Mason P. Physiological survey of medullary raphe and magnocellular reticular neurons in the anesthetized rat. J Neurophysiol 80: 1630–1646, 1998. [DOI] [PubMed] [Google Scholar]
- Maixner W. Temporomandibular joint disorders. In: Functional Pain Syndromes: Presentation and Pathology, edited by Mayer E, Bushnell M. Seattle, WA: IASP, 2009, p. 55–69. [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci 24: 737–777, 2001. [DOI] [PubMed] [Google Scholar]
- Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol 77: 1087–1098, 2009. [DOI] [PubMed] [Google Scholar]
- Mason P. Medullary circuits for nociceptive modulation. Curr Opin Neurobiol 22: 640–645, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P, Gao K, Genzen JR. Serotonergic raphe magnus cell discharge reflects ongoing autonomic and respiratory activities. J Neurophysiol 98: 1919–1927, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Bereiter DA. Parabrachial area and nucleus raphe magnus inhibition of corneal units in rostral and caudal portions of trigeminal subnucleus caudalis in the rat. Pain 87: 241–251, 2000. [DOI] [PubMed] [Google Scholar]
- Miki K, Zhou QQ, Guo W, Terayama R, Dubner R, Ren K. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol 87: 750–760, 2002. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Tashiro A, Chang Z, Thompson R, Bereiter DA. Temporomandibular joint-evoked responses by spinomedullary neurons and masseter muscle are enhanced after repeated psychophysical stress. Eur J Neurosci 36: 2025–2034, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira MC, Parada CA, Veiga MC, Rodrigues LR, Barros SP, Tambeli CH. Evidence for the involvement of endogenous ATP and P2X receptors in TMJ pain. Eur J Pain 9: 87–93, 2005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 2007. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci 25: 319–325, 2002. [DOI] [PubMed] [Google Scholar]
- Potrebic SB, Fields HL, Mason P. Serotonin immunoreactivity is contained in one physiological cell class in the rat rostral ventromedial medulla. J Neurosci 14: 1655–1665, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain 100: 1–6, 2002. [DOI] [PubMed] [Google Scholar]
- Sarlani E, Greenspan JD. Evidence for generalized hyperalgesia in temporomandibular disorders patients. Pain 102: 221–226, 2003. [DOI] [PubMed] [Google Scholar]
- Schnell C, Ulucan C, Ellrich J. Atypical on-, off- and neutral cells in the rostral ventromedial medulla oblongata in rat. Exp Brain Res 145: 64–75, 2002. [DOI] [PubMed] [Google Scholar]
- Sugiyo S, Takemura M, Dubner R, Ren K. Trigeminal transition zone/rostral ventromedial medulla connections and facilitation of orofacial hyperalgesia after masseter inflammation in rats. J Comp Neurol 493: 510–523, 2005. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Hirata H, Bereiter DA. Intensity coding by TMJ-responsive neurons in superficial laminae of caudal medullary dorsal horn of the rat. J Neurophysiol 86: 2393–2404, 2001. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Bereiter DA. Morphine modulation of temporomandibular joint-responsive units in superficial laminae at the spinomedullary junction in female rats depends on estrogen status. Eur J Neurosci 28: 2065–2074, 2008. [DOI] [PubMed] [Google Scholar]
- Thurston CL, Randich A. Effects of vagal afferent stimulation on and OFF cells in the rostroventral medulla: relationships to nociception and arterial blood pressure. J Neurophysiol 67: 180–196, 1992. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Griffin G, Leichnetz GR, Mayer DJ. The somatotopic organization of the nucleus raphe magnus and surrounding brain stem structures as revealed by HRP slow-release gels. Brain Res 181: 1–15, 1980. [DOI] [PubMed] [Google Scholar]
- Wolstencroft JH. Reticulospinal neurons. J Physiol 174: 91–108, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Mense S. Response properties and descending control of rat dorsal horn neurons with deep receptive fields. Neuroscience 39: 823–831, 1990. [DOI] [PubMed] [Google Scholar]
- Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum 36: 339–356, 2007. [DOI] [PubMed] [Google Scholar]