Abstract
The purpose of this study was to evaluate the expression patterns of nicotinic and muscarinic ACh receptors (nAChRs and mAChRs, respectively) in relation to one another and to understand their effects on rabbit retinal ganglion cell response properties. Double-label immunohistochemistry revealed labeled inner-retinal cell bodies and complex patterns of nAChR and mAChR expression in the inner plexiform layer. Specifically, the expression patterns of m1, m4, and m5 muscarinic receptors overlapped with those of non-α7 and α7 nicotinic receptors in presumptive amacrine and ganglion cells. There was no apparent overlap in the expression patterns of m2 muscarinic receptors with α7 nicotinic receptors or of m3 with non-α7 nicotinic receptors. Patch-clamp recordings demonstrated cell type-specific effects of nicotinic and muscarinic receptor blockade. Muscarinic receptor blockade enhanced the center responses of brisk-sustained/G4 On and G4 Off ganglion cells, whereas nicotinic receptor blockade suppressed the center responses of G4 On-cells near the visual streak but enhanced the center responses of nonstreak G4 On-cells. Blockade of muscarinic or nicotinic receptors suppressed the center responses of brisk-sustained Off-cells and the center light responses of subsets of brisk-transient/G11 On- and Off-cells. Only nicotinic blockade affected the center responses of G10 On-cells and G5 Off-cells. These data indicate that physiologically and morphologically identified ganglion cell types have specific patterns of AChR expression. The cholinergic receptor signatures of these cells may have implications for understanding visual defects in disease states that result from decreased ACh availability.
Keywords: muscarinic acetylcholine receptors, nicotinic acetylcholine receptors, cholinergic agonists, cholinergic antagonists, retinal circuitry
in the retina, ACh is released from starburst amacrine cells tonically and in response to changes in illumination (Masland and Livingstone 1976; Peters and Masland 1996). ACh exerts its effects through the activation of ionotropic nicotinic ACh receptors (nAChRs) and G-protein-coupled muscarinic AChRs (mAChRs). The expression of nicotinic and/or muscarinic receptors by amacrine and ganglion cells has been described in the retinas of many species (Baldridge 1996; Kaneda et al. 1995; Kittila and Massey 1997; Liu et al. 2009; McBrien et al. 2009; Schmidt et al. 1987; Wasselius et al. 1998; Yamada et al. 2003; Zucker and Ehinger 1998). In rabbit, RT-PCR, Western blot, and immunohistochemistry (IHC) data demonstrate the expression of α3, α4, α6, α7, and β2–β4 containing nAChRs and m1–m5 mAChRs in subsets of rabbit bipolar, amacrine, and ganglion cells (Dmitrieva et al. 2001, 2003, 2007; Keyser et al. 2000; Strang et al. 2003, 2005, 2007, 2010). To date, no study has described the expression patterns of nicotinic and muscarinic receptors relative to one another in the mammalian retina.
mAChRs are G-protein-coupled receptors (GPCRs) that are associated with the Gq or Gi α subunits, and the effects of activation are mediated by K+ and/or Ca2+ channel activity. In neurons, the Gq-coupled m1, m3, and m5 mAChRs typically increase neuronal excitability by the activation of nonspecific cation channels, the release of Ca2+ from intracellular stores, or through the inhibition of Ca2+-activated K+ channels (Brown 2010). In contrast, Gi-coupled m2 and m4 mAChRs typically decrease neuronal activity through the activation of a subset of K+ channels or the inhibition of Ca2+ channels (Wess et al. 1997). Thus the activation of specific mAChR subtypes can directly increase or decrease the excitability of ganglion cells or other retinal neurons. In contrast, nAChR activation typically results in increased neuronal excitability via the influx of Na+ and Ca2+. Because AChRs are expressed by subsets of all inner-retinal cell types, ACh has the potential to affect ganglion cell responses through a number of different retinal circuits. Indeed, previous physiological studies have shown that ACh affects the firing rates and response properties of brisk-sustained, brisk-transient, and directionally selective (DS) ganglion cells via the activation of nicotinic (Ariel and Daw 1982; Kittila and Massey 1997; Strang et al. 2005, 2007) and/or muscarinic (Baldridge 1996; Kaneda et al. 1995; Sastry 1985; Schmidt et al. 1987; Strang et al. 2010) receptors.
Prior studies that described nicotinic and muscarinic effects on retinal ganglion cell response properties primarily focused on the effects of cholinergic agents on ganglion cell firing rates but did not investigate the underlying changes in ganglion cell light-evoked currents. The availability of subunit- or subtype-specific antibodies now allows for an expanded exploration of the relationship between nicotinic and muscarinic receptor expression patterns and how nAChR and mAChR activation together shape light-evoked currents that produce ganglion cell firing.
We report here that nAChRs and mAChRs are colocalized in populations of bipolar, amacrine, and ganglion cells. Consistent with immunohistochemical data, blockade of mAChRs and nAChRs differentially alters the light-evoked currents of specific ganglion cell types. Enhancement of ganglion cell responses appears to be mediated by nAChRs and m1, m3, and m5 mAChRs expressed directly by ganglion cells or by presynaptic bipolar cells, whereas inhibition may be mediated by nAChRs and/or excitatory mAChRs expressed by amacrine cells that also release GABA or glycine or by the inhibitory effects of m2 or m4 mAChRs expressed by ganglion and bipolar cells (Dmitrieva et al. 2007; Strang et al. 2005, 2010).
A more complete understanding of the role of the cholinergic system in complex visual processing is important, not only in the context of understanding normal retinal functioning but also for evaluation of vision in disease processes that affect the retinal cholinergic system and their treatments. For example, the decreased ACh levels in the retinas of individuals with Alzheimer's disease (Daniels et al. 1994; Strenn et al. 1991) have the potential to affect retinal processing. Additionally, the systemic acetylcholinesterase (AChE) inhibitors that are used to treat Alzheimer's disease decrease the activity of retinal AChE, leading to increased retinal concentrations of ACh (Alhomida et al. 2000) that can result in blurred vision and visual-field deficits (Carmine and Brogden 1985).
MATERIALS AND METHODS
Surgical Procedures
Rabbits were maintained in accordance with institutional guidelines and the Guide for the Care and Use of Laboratory Animals, set forth by the Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council (2011). All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Rabbits were dark adapted and anesthetized with urethane (2 g/kg; Sigma-Aldrich, St. Louis, MO), followed by Nembutal (30 mg/kg; Vedco, Saint Joseph, MO). When all reflexive action was abolished, the eyes were removed under dim, red light and placed into ice-cold Ames' Medium (Sigma-Aldrich) (Ames and Nesbett 1981). The rabbits were then overdosed with Fatal-Plus (Vortech, Dearborne, MI). The eyes were hemisected, and either the resulting retinal eye cups were mounted in a recording chamber and fixed or the retinas were isolated in Ames' Medium and incubated in labeled α bungarotoxin (αBgt).
Immunohistochemistry
For IHC, rabbit eyecups were fixed in 1, 2, or 4% paraformaldehyde, containing 0.34% L-lysine and 0.05% sodium metaperiodate (1% PLP). After fixation, retinas were cryoprotected in 30% sucrose, embedded in a 50/50 mixture of aquamount (VWR Scientific, West Chester, PA) and optimum cutting temperature medium (VWR Scientific), cryosectioned (12 μm sections), and stored at −20°C. Slides were washed for 3 × 10 min with 0.1 M PBS and blocked with 10% donkey normal serum in 0.1 M PBS with 0.3% Triton X-100 (PBS-triton), after which, the tissue was incubated overnight at 4°C with primary antibodies against mAChR subtypes and against nicotinic receptor subunits (Table 1). Some retina sections were blocked with avidin biotin-blocking solution (for nAChRs and m2 and m4 mAChRs) immediately before primary antibody incubation. For double-labeling studies with antibodies against α7 nAChRs, sections were incubated with 0.8% 2-mercaptoethanol for 1 h at room temperature for antigen retrieval before the blocking step (Dmitrieva et al. 2007). All antibodies were diluted in PBS-triton. After primary antibody incubation, sections were washed for 3 × 10 min in PBS and incubated in the appropriate FITC or rhodamine-conjugated secondary antibodies. For non-α7 nAChRs, m2 and m4 mAChRs, sections were incubated with biotinylated secondary antibodies, washed, and incubated in fluorophore-conjugated streptavidin. All double-labeling studies were done sequentially and repeated using retinas from at least three animals. Control sections were processed in parallel with experimental sections. To control for the specificity of the primary antibodies, IgG from the species of the primary antibody was substituted for the primary antibodies at matched protein concentrations. To control for the specificity of the secondary antibodies, PBS-triton was substituted for the primary antibodies (Table 1). No specific labeling was observed in the control slides.
Table 1.
Antibodies
| Antibody (Antigen) | Species | Catalogue | Supplier (Location) | Specificity (Dilution) |
|---|---|---|---|---|
| ChAT (human placental enzyme; NM_020549.3) | Goat | AB144P | Chemicon (Temecula, CA) | Firth et al. 2003 (1:200) |
| α3β2 Nicotinic AChRs (Bovine and mouse, recognizes an epitope AA 66–76 of the nicotinic AChR α1 subunit; also binds to native human α1, α3, and α5.) | Rat | MAb210 | Generous gift of Dr. Jon Lindstrom, University of Pennsylvania (Philadelphia, PA) | Keyser et al. 2000 (1:200) |
| α7 Nicotinic AChRs (Sequence wkfaacvvdrlclmafsvft maps to the cytoplasmic loop between the TM3 and -4 and partially into TM4 of the human α7 subunit.) | Goat | SC1447 | Santa Cruz Biotechnology (Santa Cruz, CA) | Dmitrieva et al. 2007 (1:200) |
| m1 Muscarinic AChR (GST fusion protein and part of i3 intercellular loop of human m1 muscarinic AChR; AA 227–353; accession P11229) | Rabbit | AB5164 | Chemicon | Levey et al. 1990; Dorje et al. 1991; Hersh et al. 1994; Strang et al. 2010 (1:50) |
| m2 Muscarinic AChR (clone M2-2-B3, i3 loop of m2 receptor; AA 225–359; fused to GST) | Rat | MAb367 | Chemicon | Levey et al. 1995; Strang et al. 2010 (1:100) |
| m3 Muscarinic AChR (peptide analogue of the carboxyl terminal of muscarinic m3 receptor covalently bonded to carrier protein) | Rabbit | AS-3741S | Research & Diagnostic Antibodies (Las Vegas, NV) | Ndoye et al. 1998 |
| WB-3741S | Webber Biotechnology (Las Vegas, NV) | Strang et al. 2010 (1:300) | ||
| m4 Muscarinic AChR (clone 18C7.2, m4 receptor i3 loop; human; fused to GST; NM_000741.2) | Mouse | MAb1578 | Chemicon | Wang et al. 2001 Strang et al. 2010 (1:200) |
| m5 Muscarinic AChR (synthetic peptide from the 3rd cytoplasmic domain of human muscarinic AChR m5; NM_012125.2) | Rabbit | AB9454 | Chemicon | Strang et al. 2010 (1:200) |
ChAT, choline acetyltransferase; AChR, ACh receptor; AA, amino acids; TM, transmembrane; GST, glutathione-S-transferase.
αBgt Labeling
A previous study established the specificity of αBgt labeling in the inner plexiform layer (IPL) of rabbit retina (Renna et al. 2007), using labeling methods adapted from those of Pourcho (1979). Briefly, retinas were isolated from the choroid and incubated in oxygenated Ames' Medium containing 6.7 μM tetramethylrhodamine-conjugated αBgt (αBgt-Rho; Molecular Probes, Eugene, OR) for 2 h, rinsed in oxygenated Ames' Medium for 1 h, and fixed by immersion in 1% PLP for 2 h. Labeled retinas were then processed for whole-mount IHC with antibodies against choline acetyltransferase (ChAT) and m1–m5 mAChRs at the same concentrations as were used for frozen sections. Incubation times were lengthened to allow for antibody penetration through the entire retina.
Imaging
Fluorescent images were collected with a Leica TCS 4D laser-scanning confocal microscope (Leica Microsystems, Mannheim, Germany), equipped with argon, krypton, helium-neon, and UV lasers. Images were typically collected with a 40× oil-immersion objective, with a numerical aperture of 1.25. Each channel was scanned separately, and merged images were created from overlay of the separate channels. Projection images of up to five optical sections were used to study the distribution of labeled cells and processes. The images were processed for contrast and brightness, and all figures were created using Photoshop (Adobe Systems, Mountain View, CA).
The ImageJ Colocalization Finder plug-in (http://rsb.info.nih.gov/ij/plugins/colocalization-finder.html) was used to establish colocalization for double-label IHC experiments. Pixels from each channel of raw, single-optical sections were combined into a scatterplot of pixel intensities, from which a region of interest (ROI) that contained equivalent minimum and maximum pixel intensities and equal contributions from each channel was identified. Pixels contained within that ROI were masked as white pixels back onto an overlay of the original images. The average overlap coefficient, as generated by ImageJ software, for each masked image (n = 3) was between 0.86 and 0.98.
For triple-label experiments, we used the ImageJ Colocalization Finder plug-in to mask areas of overlap among αBgt-Rho labeling, mAChR subtype immunoreactivity (IR), and ChAT IR. The overlap coefficients ranged from 0.85 to 0.96. Colocalization between αBgt-Rho and ChAT IR was pseudocolored yellow, colocalization between αBgt-Rho and mAChR IR was pseudocolored magenta, and colocalization between ChAT IR and mAChR IR was pseudocolored cyan. The psuedocolored, masked pixel layers were then merged into a single image, in which, areas of triple overlap were masked with white.
Electrophysiology and Pharmacology
Retinal eye cups were flat mounted in a perfusion chamber ganglion cell, side up, and superfused (2–4 ml/min) with Ames' Medium (Sigma-Aldrich; pH 7.4, equilibrated with 95% O2 and 5% CO2), heated with an inline heater (Warner Instruments, Hamden, CT) to 34–36°C. To expose the ganglion cells for patch-clamp recordings, the inner-limiting membrane was removed from the inner-retinal surface using gentle fluid pressure from glass capillaries filled with Ames' solution.
Borosilicate glass pipettes (A-M Systems, Sequim, WA) with 4–10 mΩ tip resistances, pulled using a P-97 puller (Sutter Instrument, Novato, CA), were used for voltage-clamp recordings. Pipette solutions contained the following (in mM): K+ gluconate (125), NaCl (10), CaCl2 (0.5), EGTA (5), adenosine-triphosphate (magnesium salt; 4.0), guanosine-triphosphate (trisodium salt; 0.5), HEPES (5), pH 7.32, 275–282 mOsm. Alexa 488, Alexa 594, or Lucifer yellow (1–2%) was added to the pipette solutions and used for morphological identification of ganglion cells at the end of the recordings. Liquid-junction potentials for all solutions were calculated using pCLAMP 9.0 software (Molecular Devices, Sunnyvale, CA), and measured membrane potentials were corrected accordingly.
Physiological data were collected using the PC-ONE patch amplifier (Dagan, Minneapolis, MN), with low-pass Bessel filtering at 1 KHz, digitized at 1–3 kHz with Digidata 1322A (Molecular Devices). LabVIEW software (National Instruments, Austin, TX) was used for data collection. Whole-cell configuration was obtained under visual control in dim, red light. In a whole-cell configuration, resting membrane potential was measured at zero current, i.e., the point at which no current is required to clamp voltage. Under the conditions described above, whole-cell configuration was routinely maintained for 2–3 h. Membrane potentials and input resistance were monitored throughout the experiment. Recordings from cells that did not maintain at least ±75% of initial input resistance or depolarized to >40 mV were not included in the analyses. Data were analyzed offline with Clampfit 9.2 (Molecular Devices), and voltage plots were generated using SigmaPlot (Systat Software, San Jose, CA). Peak inward currents (pA) were used to measure transient components of the light responses, whereas area under the curve (AUC; average nA·1 s) was calculated to measure the sustained components of the light responses. Due to the short time course, the transient component contributed only minimally to the AUC. Friedman's nonparametric repeated-measures ANOVAs, followed by Dunn's post hoc comparisons (GraphPad Prism; GraphPad Software, San Diego, CA), were used for significance testing of changes in peak responses and AUC of each cell after pharmacological manipulations. P < 0.05 was considered to be statistically significant. The averaged responses of cells with the same morphology and the same physiological responses were reported in tabular form.
Ganglion cell light responses were first tested with full-field light flashes (70 cd/m2), delivered via computer monitor. Receptive fields were mapped with spot and annular stimuli, controlled by LabVIEW (National Instruments). The light responses of each cell were tested with a range of eight spot sizes and two annulus sizes to determine the cell type, the extent of the center and surround receptive field, and the optimally sized spot for each cell. Optimal spot size was empirically defined as the spot size for a given cell that elicited the largest inward current or number of spikes at light onset (or offset) without the activation of a surround response. After receptive-field mapping, cholinergic antagonists were bath applied for at least 10 min, and light responses were again tested. All cells were tested with muscarinic and nicotinic antagonists, which were washed for a minimum of 20 min and the light responses tested for recovery to control levels before the application of the next agent. In several cases, the second antagonist application contained both nicotinic and muscarinic agents to test the effects of full cholinergic blockade. Only cells that maintained light responses and ±75% of initial input resistance after the final wash were included in the analyses. Atropine (ATR; 1–3 μM) was used to block muscarinic-mediated responses. Hexamethonium bromide (HEX; 100 μM) or a combination of HEX and methyllycaconitine (MLA; 100 nM) was used to block nicotinic-mediated responses. Antagonists were bath applied individually and in combination, and the order of application was alternated.
After recording, retinas were fixed and imaged as described above. The morphology of the filled cells was compared with published descriptions of rabbit retinal ganglion cell types (Amthor et al. 1989; Rockhill et al. 2002). Combined physiological and morphological criteria were used to determine cell type.
RESULTS
Immunohistochemistry
We performed pairwise immunohistochemical comparisons among four of the five muscarinic subtypes and heteromeric β2-containing nAChRs, as revealed by MAb210 IR, and among all five muscarinic subtypes and α7 nicotinic receptors, as revealed by SC1447 IR. The specificity of the antibodies has been demonstrated previously in rabbit retina (Dmitrieva et al. 2007; Keyser et al. 2000; Strang et al. 2010). Double-labeling for m2 muscarinic and β2-containing nAChRs was not possible, because the antibodies against these AChRs were both made in the same species. For a further comparison of the spatial relationships of AChR-labeled dendrites to starburst amacrine cell dendrites in the IPL, we compared muscarinic m1–m5 AChR IR, ChAT IR, and synaptic sites labeled with fluorescent αBgt.
The Spatial Relationships of Non-α7 nAChRs Relative to mAChRs
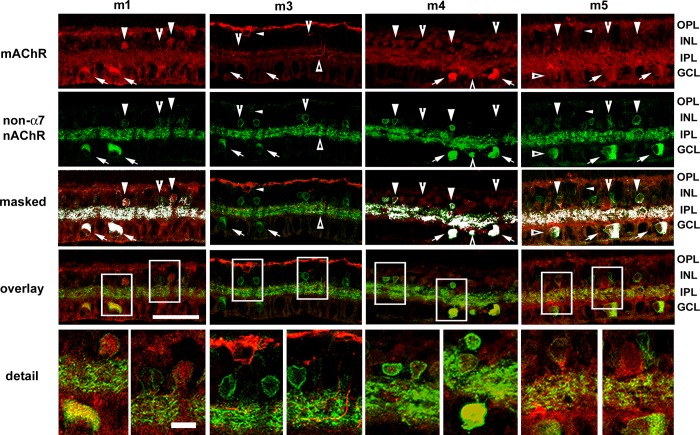
Double-labeling studies of mAChRs and β2-containing nAChRs showed distinct patterns of colocalization in the inner retina. Colocalization was assessed from single confocal optical sections (0.5 μm steps) and masked onto overlay images. Overall expression patterns were assessed using five optical sections (2.5 μm tissue depth). Many cell bodies in the inner nuclear layer (INL), presumably amacrine cells, displayed nicotinic IR and/or m1 mAChR IR (Fig. 1; Pearson R correlation = 0.23; overlap R = 0.91 ± 0.004), whereas most m1-positive amacrine cells were also immunoreactive for β2-containing nAChRs, but not all amacrine cells that were positive for β2-containing nAChRs were m1 immunoreactive. Additionally, whereas both m1 mAChR and β2 nAChR IR were broadly distributed through the IPL, the labeling for m1 AChRs was less dense than the nAChR labeling, consistent with the observation that there were fewer m1-immunoreactive somas. Subsets of cells in the ganglion cell layer (GCL) were also positive for both m1 mAChRs and β2-containing nAChRs. Muscarinic m1 IR was present in the outer plexiform layer (OPL), possibly indicating m1 expression by bipolar cells or horizontal cells.
Fig. 1.
Expression patterns of muscarinic ACh receptors (mAChRs) relative to the expression patterns of non-α7 nicotinic AChRs (nAChRs). Confocal image stacks of projections of 5 optical sections (0.5 μm steps) of m1 and m3–m5 mAChRs relative to β2-containing nAChRs. Colocalization was assessed from single confocal optical sections (0.5 μm steps) and masked onto overlay images. Column 1: many ganglion cells (arrows) and subsets of amacrine cells were immunoreactive for both m1 mAChRs and β2-containing nAChRs (arrowheads). Whereas most m1-positive amacrine cells also displayed β2-containing nAChR immunoreactivity (IR), not all amacrine cells that were positive for β2-containing nAChRs were immunoreactive for m1 receptors (notched arrowheads). Whereas both m1 and β2-containing nAChR IR was broadly distributed throughout the inner plexiform layer (IPL), the limited colocalization in the IPL was consistent with the somatic-labeling patterns. Muscarinic m1 IR was present in the outer plexiform layer (OPL), possibly indicating m1 expression by bipolar cells or horizontal cells. Column 2: there was no apparent double-labeling of m3 mAChRs and β2-containing nAChRs. Arrows and notched arrowheads indicate ganglion and amacrine cell somas that displayed β2-containing nAChR IR, whereas small arrowheads indicate bipolar cells that displayed m3 mAChR IR. m3 IR processes were localized to the OPL and to innermost and outermost sublamina (triangles) of the IPL, whereas β2-containing nAChR IR was broadly distributed throughout the IPL. Column 3: m4 muscarinic IR and β2-containing nAChR IR were colocalized almost completely in cell bodies in the ganglion cell layer (GCL; arrows) but were only colocalized to subsets of cell bodies in the inner nuclear layer (INL; arrowheads), whereas other m4-immunoreactive amacrine cell somata did not display β2-containing nAChR IR (notched arrowheads). Double-labeled processes were evident throughout the IPL. Column 4: ganglion cells in the GCL (arrows) and subsets of amacrine cells in the INL (arrowheads) were double labeled for m5 muscarinic and β2-containing nAChRs, and double-labeled processes were clearly visible throughout the IPL. Additional ganglion (triangles) and amacrine cells (notched arrowheads) were immunoreactive for β2-containing nicotinic but not m5 mAChRs. Bipolar cells (small arrowheads) were immunoreactive for m5 mAChRs but not β2-containing nAChRs. Original scale bar, 50 μm. Bottom: boxed areas in detail; original scale bar, 10 μm.
There was no apparent coexpression of m3 mAChRs and β2-containing nAChRs (Fig. 1; Pearson R correlation = −0.05; overlap R = 0.96 ± 0.01). Many ganglion and amacrine cell somata displayed β2-containing nAChR IR, whereas muscarinic m3 IR was limited to a subset of somata in the outer INL and a small set of presumptive amacrine cells in the INL. m3-immunoreactive processes were localized to the OPL and to the inner- and outermost sublamina of the IPL, whereas β2 IR was broadly distributed throughout the IPL. β2- and m3-immunoreactive processes appeared to stratify near one another in the outer margins of the IPL but did not overlap.
β2-containing nAChR and m4 mAChR IR in the INL were colocalized in a small subset of cell bodies. In contrast, m4 mAChR and β2-containing AChR IR overlapped almost completely in the GCL (Fig. 1; Pearson R correlation = 0.35; overlap R = 0.93 ± 0.01). Double-labeled cells included both presumptive ganglion and displaced amacrine cells. Double-labeled processes were evident throughout the IPL.
Muscarinic m5 IR was similar to that of m1, with subsets of m5-positive, presumptive amacrine cells in the INL double-labeled, β2-containing nAChRs (Fig. 1; Pearson R correlation = 0.14; overlap R = 0.86 ± 0.05). Most labeled cells in the GCL displayed IR for both receptor types, and double-labeled processes were clearly visible, broadly through the middle of the IPL. Additional amacrine cell bodies were labeled for m5 mAChR but not β2-containing nAChRs or for β2-containing nAChRs but not m5 mAChRs. Additionally, a subset of bipolar cells was immunoreactive for m5 mAChRs but not β2-containing nAChRs.
The Spatial Relationships of α7 nAChRs Relative to mAChRs
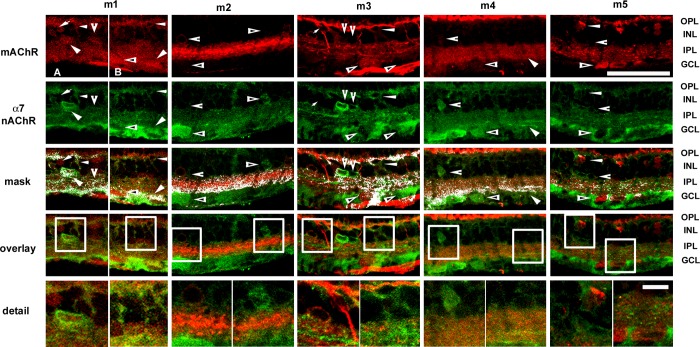
To identify the spatial relationships of cells that expressed α7 nAChRs relative to cells that expressed m1–m5 mAChRs, retina sections were double labeled with antibodies against α7 nAChR subunits and antibodies against m1–m5 mAChRs. Figure 2 shows the results of these experiments. Colocalization was assessed from single confocal optical sections (0.5 μm steps) and masked onto overlay images. Overall expression patterns were assessed using five optical sections (2.5 μm tissue depth), and the stacked images (2.5 μm tissue depth) were used to provide greater morphological information. Colocalization between muscarinic receptors and α7 nicotinic subunits was less extensive than between mAChRs and non-α7 nicotinic receptors. Consistent with previous reports (Dmitrieva et al. 2007), α7 nicotinic receptor IR (Fig. 2) was localized to subsets of bipolar, amacrine, and ganglion cells and broadly distributed throughout the IPL, with greater density in the inner IPL.
Fig. 2.
Expression patterns of mAChRs relative to the expression of α7 nAChRs. Confocal image stacks of projections of 5 optical sections (0.5 μm steps) of m1, m3, and m5 mAChRs relative to α7 nAChRs. Colocalization was assessed from single confocal optical sections and masked onto overlay images. Column 1, A and B: subsets of amacrine cells and ganglion cells (arrowheads) and subsets of bipolar cells (small arrowheads) were immunoreactive for both m1 mAChRs and α7 nAChRs. Other bipolar cells (arrows), amacrine cells (notched arrowheads), and ganglion cells (triangles) were m1 immunoreactive but not α7 immunoreactive. Muscarinic m1 and nicotinic α7 receptor IR were also colocalized in a band in the On sublamina of the IPL. Colocalization of IR throughout the rest of the IPL was limited. Column 2: ganglion cells and subsets of amacrine cells (triangles) were immunoreactive for the α7 nAChR subunit, whereas other amacrine cells were immunoreactive for m2 mAChRs (notched arrowheads). However, there was no apparent colocalization between m2 IR and α7 IR in cell bodies. m2 labeling in cell somas was very dim (notched arrowheads), even though IR was broadly distributed throughout the IPL, with intense labeling in a broad center band. There were double-labeled processes throughout the IPL, with strong masking at the boundary of GCL in the On sublamina of the IPL. Column 3: whereas a small number of bipolar cells were immunoreactive for both α7 nAChR subunits and m3 mAChRs (small arrowheads), the majority of immunoreactive bipolar cells was immunoreactive either for m3 mAChRs (small arrows) or α7 nAChRs (notched arrowheads), although there was a narrow band of colocalization in the OPL. Likewise, immunoreactive cells in the inner INL and GCL, presumptive amacrine and ganglion cells, displayed either α7 or m3 IR but not both. m3 IR was distributed primarily in 2 bands in the inner and outer IPL and sparsely colocalized with α7 IR. Column 4: cells in the GCL were m4 immunoreactive and α7 immunoreactive (arrowheads), whereas ganglion cells (triangles) and amacrine cells (notched arrowheads) were immunoreactive only to antibodies against α7 nAChR subunits. Dendritic stratification of α7-immunoreactive ganglion cells appeared to be in the On sublamina of the IPL, as colocalization through the rest of the IPL was minimal. Column 5: there was no apparent colocalization of m5 IR and α7 IR in ganglion cells (triangles) or amacrine cells (notched arrowheads), but bipolar cells with small cell bodies appeared to be immunoreactive for both m5 mAChR and α7 nAChR subunits (small arrowheads). Double-labeled processes were sparsely distributed throughout the IPL. Original scale bar, 50 μm. Bottom: boxed areas in detail; original scale bar, 10 μm.
Colocalization of muscarinic m1 and nicotinic α7 receptor IR (Fig. 2; Pearson R correlation = 0.14; overlap R = 0.93 ± 0.01) was evident in subsets of bipolar, amacrine, and ganglion cells. Other bipolar, amacrine, and ganglion cells were m1 but not α7 immunoreactive. Muscarinic m1 and nicotinic α7 receptor IR also colocalized in a band in the On sublamina of the IPL. Colocalization of IR throughout the rest of the IPL was limited.
There was no apparent colocalization between m2 IR and α7 IR in cell bodies (Fig. 2). Cells in the GCL and subsets of amacrine cells were immunoreactive to antibodies against the α7 nAChR subunit, whereas other amacrine cells were immunoreactive to antibodies against m2 mAChRs. m2 labeling in cell somas was very dim, even though IR was broadly distributed throughout the IPL, with intense labeling in a broad center band. The dim m2 labeling of cell somas might have been responsible for the lack of colocalization in the INL and GCL, as there were double-labeled processes throughout the IPL, with strong masking at the boundary of GCL in the On sublamina of the IPL (Pearson R correlation = 0.19; overlap R = 0.84 ± 0.003).
Whereas a small number of bipolar cells were immunoreactive for both α7 nAChR subunits and m3 mAChRs (Fig. 2; Pearson R correlation = 0.18; overlap R = 0.96 ± 0.01), the majority of immunoreactive bipolar cells displayed either α7 nAChR IR or m3 muscarinic IR, although there was a narrow band of colocalization in the OPL. Likewise, immunoreactive cells in the inner INL and GCL, presumptive amacrine and ganglion cells, displayed either α7 or m3 IR but not both. m3 IR was primarily distributed in two bands in the inner and outer IPL and sparsely colocalized with α7 IR. Cells in the GCL were both m4 immunoreactive and α7 immunoreactive (Fig. 2; Pearson R correlation = 0.14; overlap R = 0.89 ± 0.02). Because IPL colocalization was strongest in the inner IPL, these cells likely stratified in the On sublamina. Other ganglion cells and amacrine cells were immunoreactive for α7 nAChR subunits. Unlike in Fig. 1, m4 IR was present in the OPL; however, it had minimal colocalization with α7 IR.
Bipolar cells with small cell bodies appeared to be immunoreactive for both m5 mAChRs and α7 nAChR subunits (Fig. 2), but there was no apparent colocalization of m5 IR and α7 IR in ganglion cells or amacrine cells. Double-labeled processes were sparsely distributed throughout the IPL (Pearson R correlation = 0.35; overlap R = 0.94 ± 0.01).
The Spatial Relationships of αBgt Binding Relative to mAChR IR in the IPL
The α7 nAChR antibody SC1447 binds at the third transmembrane domain of the α7 subunit (Dmitrieva et al. 2007), and permeabilization of the tissue with Triton X-100 during incubation with the antibodies leads to the labeling of both surface receptors and intracellular receptors being trafficked to and from the plasma membrane. The competitive α7 nAChR antagonist αBgt binds only to cell-surface receptors in living tissue. To determine the spatial relationships of membrane surface nAChRs and mAChRs at the presumptive synaptic locations in the IPL, we used fluorescently labeled αBgt in triple-label studies with antibodies against m1–m5 mAChRs and against ChAT. For these studies, live retinas were isolated, incubated with fluorescently labeled αBgt in oxygenated Ames' Medium, and then fixed and processed for IHC as whole mounts. Figure 3 shows single optical confocal sections at the level of the ChAT-positive dendrites in the On sublamina of the IPL. Pseudocolored masks of colocalized pixels for each pair of fluorophores were obtained from single optical sections (Pearson R correlation ranged from −0.3 to 0.09; overlap R ranged from 0.85 to 0.97). The three pairwise masks were then merged into a single map of pixels that contained fluorescence from either two or three fluorophores (Fig. 3). Colocalization of αBgt and mAChR labeling within a single pixel was pseudocolored magenta, mAChR and ChAT labeling was pseudocolored cyan, αBgt and ChAT labeling was pseudocolored yellow, and all three colors in a single pixel appear white. The images show differential patterns of expression that were consistent with the overall labeling patterns shown by antibodies against mAChR and α7 nAChR subunits.
Fig. 3.
Triple-label studies of mAChR IR, α bungarotoxin (αBgt) labeling, and choline acetyltransferase (ChAT) IR in the IPL. Confocal images of single optical sections of m1–m5 mAChR and αBgt labeling at the level of the labeled ChAT band (green) in the inner IPL. Density and expression patterns of muscarinic receptor subtypes m1–m5 (row 1, blue) varied, whereas the αBgt labeling (row 2, red) and ChAT labeling (row 3, green) were more uniform across experiments. Pseudocolored masks of colocalized pixels for each pair of fluorophores were obtained from single optical sections. The 3 pairwise masks were then merged into a single map of pixels that contained fluorescence from either 2 or 3 fluorophores (row 4). Colocalization of αBgt and mAChR labeling within a single pixel was pseudocolored magenta, mAChR and ChAT labeling was pseudocolored cyan, αBgt and ChAT labeling was pseudocolored yellow, and all 3 colors in a single pixel appear white. m1 IR was punctate with regions of larger puncta that did not colocalize with αBgt or ChAT (arrowheads). In a smaller area, m1 IR colocalized with αBgt labeling but rarely with ChAT IR (notched arrowheads). Colocalization of all 3 markers was minimal (arrows). Although m2 IR was closely apposed to αBgt-labeled puncta (notched arrowheads), m2 had limited colocalization with αBgt labeling or ChAT IR (arrowheads). m3-immunoreactive puncta at the level of the ChAT bands were frequently colocalized with both αBgt labeling and ChAT IR (arrows), although there were regions of m3-immunoreactive puncta without ChAT IR but with (notched arrowheads) and without (arrowheads) αBgt. m4 IR was almost always colocalized with αBgt labeling at this level of the retina (notched arrowheads) but rarely with ChAT IR (arrows). m5 IR was punctate with regions of larger puncta that did not colocalize with αBgt or ChAT. In a smaller area, m5 IR colocalized with αBgt labeling but not with ChAT IR (notched arrowheads). Colocalization of all 3 markers was minimal (arrows). Original scale bar, 20 μm. Optical depth is indicated by a double-headed arrow in vertical reconstructions of Z-stack (row 5) and lower magnification (row 6), with boxed areas demarking regions of interest; original scale bar, 50 μm.
m1 IR was punctate with regions of larger puncta that did not colocalize with either αBgt or ChAT. In other areas, m1 IR colocalized with αBgt labeling but rarely with ChAT IR, and colocalization of all three markers was minimal. Although m2 IR was closely apposed to αBgt-labeled puncta (Fig. 3), it had limited colocalization with αBgt labeling or ChAT IR. m3 IR puncta at the level of the ChAT bands were frequently colocalized with αBgt labeling and ChAT IR (Fig. 3), although there were other regions in which m3-immunoreactive puncta were not observed with either ChAT IR or αBgt labeling. m4 IR was almost always closely apposed to or colocalized with αBgt at this level of the retina but rarely with ChAT IR (Fig. 3). Muscarinic m5 IR was similar to m1 IR. It was punctate with regions of larger puncta that did not colocalize with either αBgt or ChAT (Fig. 3). In a smaller area, m5 IR colocalized with αBgt labeling but not with ChAT IR. Colocalization of all three markers was minimal. Each of the AChR subtypes appeared to be in a position to receive direct cholinergic input in the On sublamina of the IPL.
Taken together, the immunohistochemical data provide anatomical evidence that nAChRs and mAChRs are both positioned to respond to ACh release in the inner retina. The differential distribution of AChRs also suggests that different ganglion cell types are likely to have different patterns of cholinergic input, including both nicotinic and muscarinic input, depending on the AChRs expressed by the ganglion cells and by upstream cells.
Electrophysiology and Pharmacology
To test whether the cholinergic circuitry indicated by the IHC data contributed to ganglion cell light responses, we used whole-cell, voltage-clamp recordings to measure the light-evoked currents of physiologically and morphologically identified ganglion cells during pharmacological blockade of nAChRs and mAChRs. The responses of sustained On, transient On, sustained Off, and transient Off ganglion cells of each cell were tested with high-contrast (70 cd/m2) light spots, ranging from 25 to 300 μm in diameter, to identify the cell type and to determine the receptive field center for each cell. The stimulus size that elicited the largest inward current or number of spikes at light onset or offset without the activation of a surround response was defined as the receptive field center. The stimulus that evoked the center response was defined as the optimal spot size for that cell. Stimuli larger than the receptive field center suppressed the center responses or elicited antagonistic excitatory responses opposite of the center response. Responses of cells were then assessed during bath application of muscarinic and nicotinic antagonists. Voltage-clamp mode was used to measure the currents at the cell soma, but due to imperfect voltage control of large cells with complex dendritic fields, unblocked spikes were recorded from some large ganglion cells (see Fig. 8).
Fig. 8.
nAChRs and mAChRs contributed to the sustained component of the light-evoked responses of sustained Off-cells. This sustained Off-cell (image at right; original scale bar, 50 μm) responded to light offset with an initial transient and a high rate of sustained spiking (A, row 1). Blockade of nicotinic receptors with 100 nM MLA and 100 μM HEX significantly decreased the initial inward currents (reduction of 67 pA; 49%) and the AUC (reduction of 9.0 pA·1 s; 32%). The number of spikes was decreased from 98 to 36 (A, row 2). Blockade of muscarinic receptors also significantly decreased the peak inward currents by 87 pA (64%), the AUC by 5.6 nA·1 s (36%) control, and the firing at light offset by 38 spikes compared with control. The light responses recovered partially after wash. For the transient Off-cell (B), nicotinic and muscarinic blockade affected the transient and delayed-sustained components of light-evoked responses. Under control conditions, 200 μm light spots evoked transient inward currents at light onset, followed by spikes with a delay of >500 ms (B, row 1). Gray spikes show the transient components of the Off responses in greater detail. Bath application of HEX/MLA significantly decreased the transient Off responses by 106 pA (46%; gray spikes, row 2), whereas 3 μM ATR significantly enhanced the transient responses by 21 pA (9%; gray spikes, row 3). Both 100 nM MLA/100 μM HEX and 3 μM ATR enhanced the duration of the delayed response and increased the firing rate by 38 and 27 spikes, respectively. Image at right; original scale bar, 50 μm.
On-Cells
Sustained On-cells.
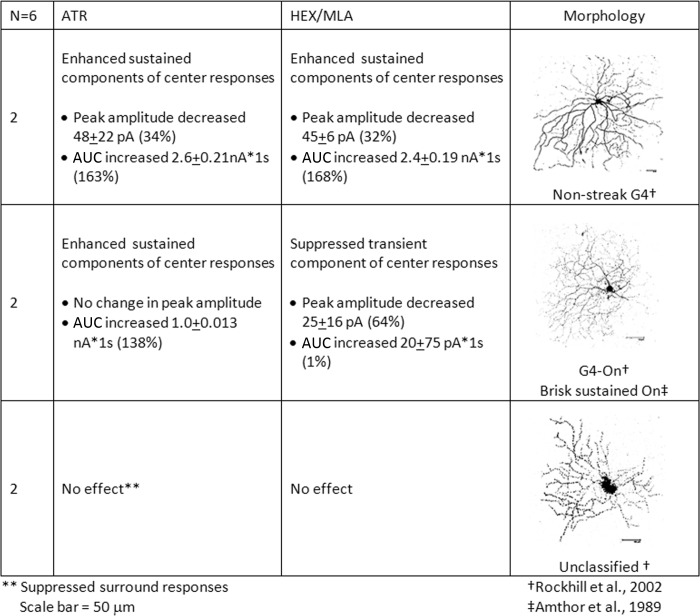
Sustained On-cells responded to light onset with sustained inward currents that lasted for at least 500 ms. Cells with morphologies consistent with brisk-sustained On (Amthor et al. 1989) and visual-streak G4 cells (Rockhill et al. 2002), as well as cells with morphologies similar to nonstreak G4 cells (Rockhill et al. 2002), or unclassified ganglion cells (Rockhill et al. 2002) comprised the sustained On-cells. Peak inward currents (pA) at light onset were measured to identify transient components of the light responses, whereas AUC (average nA·1 s) was calculated to measure sustained components of the light responses. The average changes in peak currents and AUC of sustained On-cells with light responses that were significantly affected by pharmacological blockade are presented in Fig. 4.
Fig. 4.
Sustained On-cells responded to the light onset with sustained inward currents that lasted for at least 500 ms and included cells with morphologies consistent with brisk-sustained On and G4 cells. Peak inward currents (pA) at light onset were measured to identify transient components of the light responses, whereas area under the curve (AUC; average nA·1 s) was calculated to measure sustained components of the light responses. The average changes in peak currents and AUC of sustained On-cells with light responses that were significantly affected by pharmacological blockade are shown by cell type. ATR, atropine; HEX, hexamethonium bromide; MLA, methyllycaconitine.
For sustained On-cells with nonstreak G4 morphology, blockade of mAChRs by bath application of ATR decreased the peak responses by an average of 48 (±22) pA (34%) but also increased the sustained component of the responses by an average of 2.6 (±0.21) nA·1 s (163%). Similarly, nAChR blockade decreased the peak responses by an average of 45 (±6.2) pA (32%) and increased the amplitude of the sustained component of the responses by an average of 2.4 (±0.2) nA·1 s (168%). In contrast, the light-evoked peak inward currents of cells with brisk-sustained/G4 morphology were unaffected by muscarinic blockade and were decreased by an average of 25 (±16) pA (64%) by nicotinic blockade. The sustained components of the light responses were increased by an average of 1.0 (±0.01) nA·1 s (138%) by muscarinic blockade but were unaffected by nicotinic blockade.
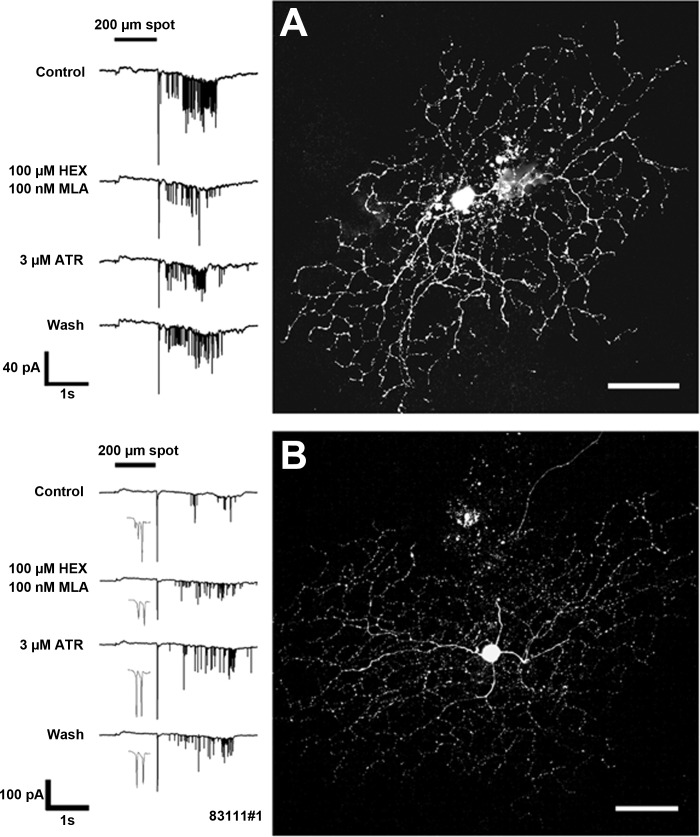
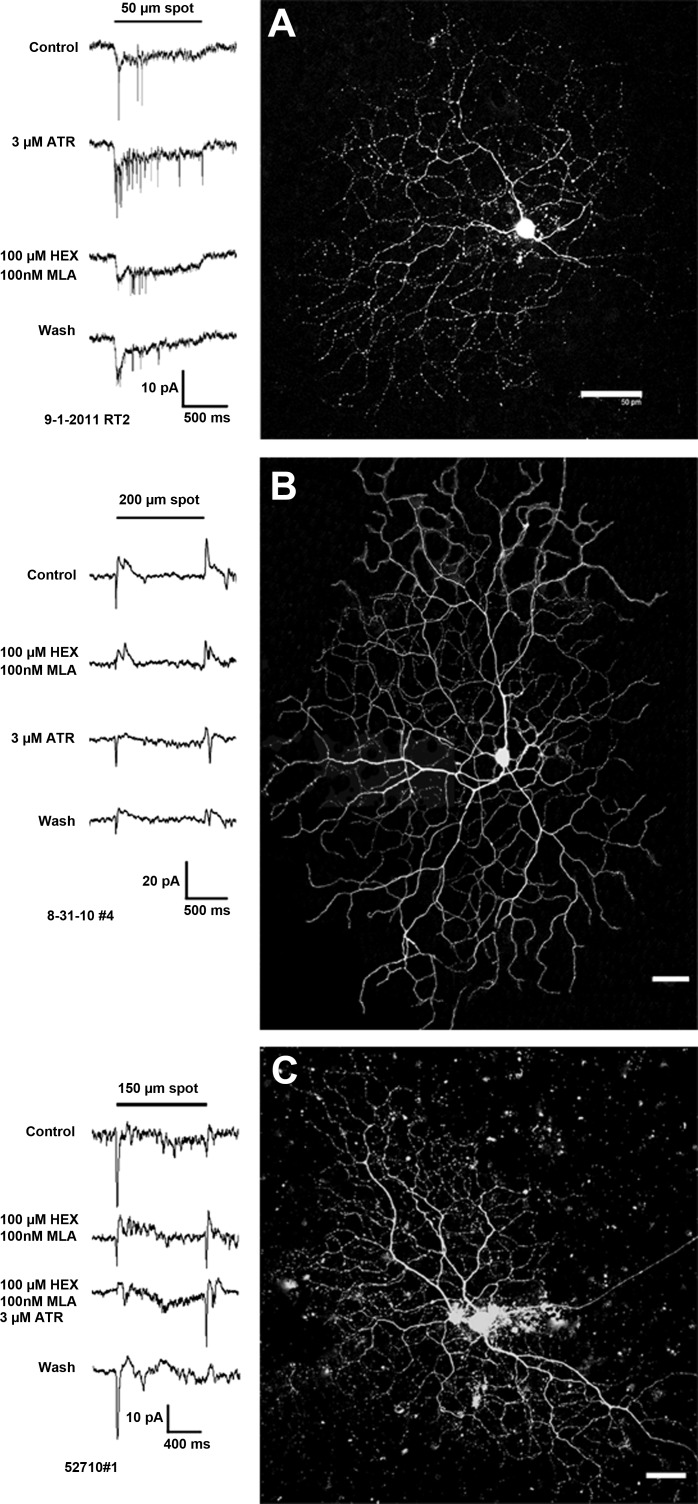
Figure 5A is an example of a brisk-sustained/G4 On ganglion cell, for which muscarinic receptor blockade by bath application of 3 μM ATR resulted in significant enhancement of light-evoked inward currents. Whereas the peak amplitude (18 pA) of the currents did not increase significantly, firing increased from three spikes to 22 spikes, and the sustained inward currents (AUC) were increased by 1.2 nA·1 s (50%). This suggests that the cell received tonic m2/m4 muscarinic inhibition under control conditions. Blockade of nAChRs with 100 nM MLA and 100 μM HEX significantly suppressed the peak transient component of the inward currents at light onset by 9 pA (50%), an observation consistent with blockade of nAChRs expressed by the ganglion cell or upstream bipolar cells. nAChR blockade also slightly enhanced the sustained component of the inward currents (115 pA·1 s; 5%) and enhanced the firing (11 spikes) compared with control, possibly due to a reduction of inhibitory input via blockade of nAChRs expressed by GABA and/or glycinergic amacrine cells. Neither nicotinic nor muscarinic blockade affected the center responses of the sustained On-cells with unclassified morphologies (n = 2), but ATR suppressed the surround responses of these cells (data not shown).
Fig. 5.
Muscarinic and nicotinic blockade affects sustained and transient On-cell light responses. High-contrast spot stimuli were used to test for interactions of nAChR and mAChR activation in the modulation of ganglion cell light responses in whole-cell, voltage-clamp mode. The application of 3 μM ATR (A, row 2) significantly increased the sustained inward currents by 1.2 nA·1 s (50%) and the number of spikes from 3 to 22 during light stimulation of this brisk-sustained/G4 On-cell (image at right; original scale bar, 50 μm). Blockade of nAChRs with 100 nM MLA and 100 μM HEX (A, row 3) significantly suppressed the peak transient component of the inward currents at light onset by 9 pA (50%). nAChR blockade slightly enhanced the component of the inward currents (115 pA·1 s; 5%) and enhanced the firing (11 spikes) compared with control. Light responses recovered partially after wash (A, row 4). Nicotinic and muscarinic blockade differentially affected the center and surround responses of a subset of transient On center ganglion cells with G11 morphology (B and C). (B) For the cell, blockade of nAChRs with 100 nM MLA and 100 μM HEX (row 2) significantly reduced peak inward currents at light onset from 18 to 3 pA and significantly reduced the peak outward currents at light offset (AUC) from 19 to 10 pA. (C) For the cell, nicotinic blockade (row 2) significantly reduced peak inward currents at light onset from 38 to 16 pA and significantly increased inward currents at light offset from 7 to 12 pA. Blockade of mAChRs with 3 μM ATR also significantly reduced (B, row 3; 8 pA reduction) or eliminated (C, row 3) light-evoked peak inward currents of both cells and revealed inward currents at light offset, i.e., antagonistic surround responses (B, row 3). Full cholinergic blockade, during coapplication of 100 nM MLA and 100 μM HEX with 3 μM ATR, further enhanced inward currents at light offset that were revealed by nicotinic blockade alone, resulting in the complete reversal of the center On response to an Off surround response (C, row 3). Images at right; original scale bars, 50 μm.
Transient On-cells.
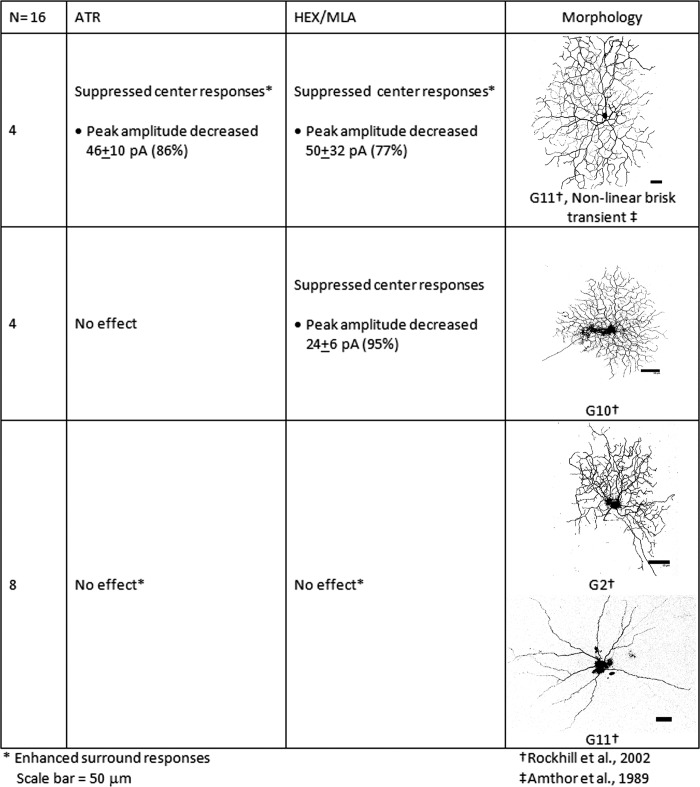
Transient On-cells were defined by inward currents at light onset that lasted <500 ms. The 16 transient On-cells that we studied included cells with morphologies consistent with nonlinear brisk-transient ganglion cells (Amthor et al. 1989) or G11 ganglion cells (Rockhill et al. 2002) and others with morphologies consistent with the G2 or G10 subtypes (Rockhill et al. 2002). The average changes in peak currents of transient On-cells with light responses that were significantly affected by pharmacological blockade are presented in Fig. 6. Muscarinic and nicotinic blockade each enhanced or revealed surround responses in the vast majority of G11 cells, as reflected by reduced inhibition at light onset or increased excitation at light offset. However, nAChR and mAChR blockade affected the center light responses of only one-third of these cells. In these cases, muscarinic blockade resulted in suppression of the light-evoked peak inward currents by an average of 46 (±10) pA (86%), whereas nicotinic blockade suppressed the light-evoked peak inward currents by an average of 50 (±32) pA (77%). Thus only a subset of G11 cells had excitatory ACh-mediated input to the receptive field centers, but the majority of G11 cells tested had inhibitory ACh-mediated input to the surrounds (data not shown).
Fig. 6.
Transient On-cells were defined by inward currents at light onset that lasted <500 ms. The 16 transient On-cells included cells with morphologies consistent with nonlinear brisk-transient, G11 ganglion cells, and cells with morphologies consistent with the G2 or G10 subtypes. The average changes in peak currents of transient On-cells with light responses that were significantly affected by pharmacological blockade are shown by cell type.
In contrast, nicotinic receptor blockade, but not muscarinic receptor blockade, decreased the peak inward currents of the cells with G10 morphology by an average of 24 (±6) pA (95%). Neither blockade of nicotinic receptors nor muscarinic receptors affected the surround responses of G10 cells. The data indicate that nAChR activation contributed to the center responses but not the surrounds of G10 cells.
The transient On-cells shown in Fig. 5, B and C, are examples of G11 cells for which blockade of nAChRs with 100 nM MLA and 100 μM HEX suppressed the transient On responses and also reduced inhibition at light offset. For the first cell (Fig. 5B), application of 100 nM MLA and 100 μM HEX significantly reduced peak inward currents at light onset from 18 to 3 pA and reduced the peak outward currents at light offset (AUC) from 19 to 10 pA. For the other cell (Fig. 5C), nicotinic blockade also significantly reduced peak inward currents at light onset from 38 to 16 pA but significantly increased inward currents at light offset from 7 pA to 12 pA. These data indicate that blockade of nAChRs decreased excitatory input to the receptive field center, possibly via blockade of nAChRs expressed on upstream bipolar cells or on the ganglion cells themselves. Blockade of nAChRs also reduced inhibitory input to the receptive field surround, probably via blockade of nAChRs expressed on amacrine cells. Blockade of mAChRs with 3 μM ATR also significantly reduced (8 pA) or eliminated the light-evoked peak inward currents of both cells and revealed inward currents at light offset, i.e., antagonistic surround responses (Fig. 5B). Full cholinergic blockade, during coapplication of 100 nM MLA and 100 μM HEX with 3 μM ATR further enhanced inward currents at light offset that were revealed by nicotinic blockade alone, resulting in the complete reversal of the center On response to an Off surround response (Fig. 5C). These data indicate that nAChR and mAChR activation contributes to enhancement of the center responses and suppression of surround responses of G11 cells under control conditions.
Off-Cells
Nicotinic and muscarinic receptor activation also contributed to the light responses of sustained and transient Off ganglion cells. Sustained Off-cells responded to the light offset with sustained inward currents that lasted for at least 500 ms. Cells with inward currents at light offset that lasted <500 ms were defined as transient Off-cells. The peak inward currents (pA) and AUC (average nA·1 s) at light offset were measured to identify the transient and sustained components of the light responses, respectively.
Sustained Off-cells.
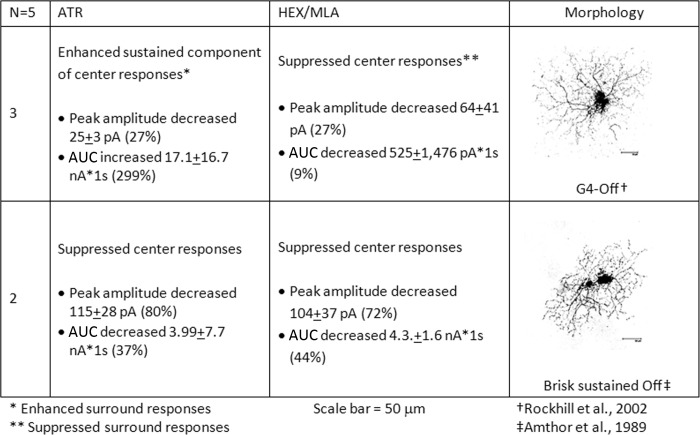
The center light responses of sustained Off-cells were affected by bath application of nicotinic and muscarinic antagonists. The average changes in peak currents and AUC of sustained Off-cells with light responses that were significantly affected by pharmacological blockade are presented in Fig. 7. The morphologies of sustained Off-cells were consistent with either brisk-sustained Off-cells (Amthor et al. 1989) or the G4 Off subtype (Rockhill et al. 2002). mAChR blockade suppressed the light-evoked firing at light offset of cells with the brisk-sustained Off morphology by significantly decreasing the peak inward currents by an average of 115 (±28) pA (80%) and the AUC by an average of 3.9 (±7.7) nA·1 s (37%). nAChR blockade also significantly suppressed the light-evoked center responses of these cells by suppressing the peak inward currents by an average of 104 (±37) pA (72%) and decreasing the AUC by an average of 4.3 (±1.6) nA·1 s (44%).
Fig. 7.
Sustained Off-cells were identified by responses of longer than 500 ms to light decrements. The morphologies were consistent with either brisk-sustained Off-cells or the G4 Off subtype. The average changes in peak currents and AUC of sustained Off-cells with light responses that were significantly affected by pharmacological blockade are shown by cell type.
Figure 8 provides evidence that under control conditions, both muscarinic and nicotinic receptor activation contributed to the enhancement of the center responses at light offset of cells with brisk Off morphology. Under nicotinic blockade, the inward currents of the sustained Off-cell, shown in Fig. 8A, were reduced significantly. The initial peak response was reduced by 67 pA (49%), and the AUC was decreased by 9.0 nA·1 s (32%). These changes were accompanied by decreases in the number of spikes and duration of firing. Blockade of muscarinic receptors also significantly decreased the peak inward currents by 87 pA compared with control (64%), the AUC by 5.6 nA·1 s (36%), and the firing by 38 spikes.
mAChR blockade significantly enhanced the light-evoked center responses of cells with G4 morphology (Fig. 7) by increasing the sustained depolarization and firing at light offset [average AUC increase of 17.1 (±16.7) nA·1 s; 299%], although the peak inward currents were reduced in some cases. nAChR blockade also significantly suppressed the peak inward currents of G4 Off-cells by an average of 64 (±41) pA (27%) and suppressed the antagonistic surround (On) responses (data not shown) without affecting the sustained components of the Off responses.
These data indicate that under control conditions, G4 Off-cells and brisk-sustained Off-cells received excitatory nicotinic input that contributed to the receptive field center response but that G4 Off-cells received inhibitory muscarinic inputs to the receptive field center, whereas brisk-sustained Off-cells received excitatory muscarinic inputs to the receptive field center.
Transient Off-cells.
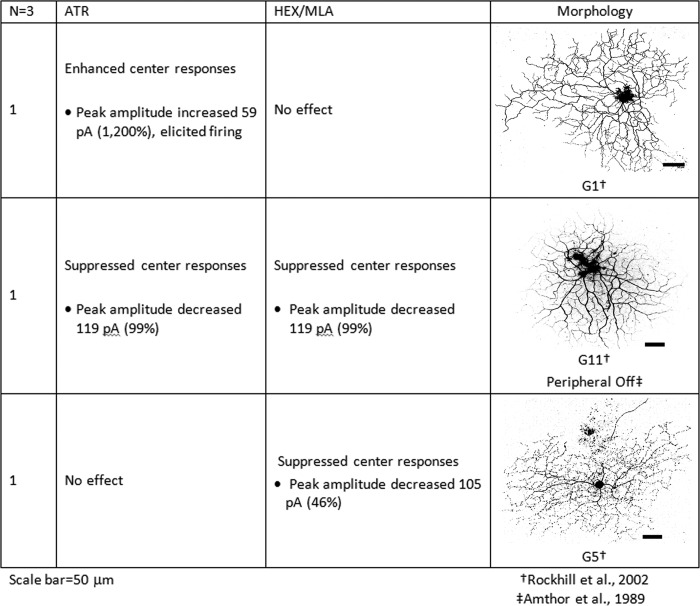
Transient Off-cells were defined by transient (<500 ms) inward currents in response to the offset of optimally sized light stimuli. Cells were identified according to previously published morphological criteria (Amthor et al. 1989; Rockhill et al. 2002). Each of the three transient Off-cells included in this study had different morphologies and displayed different responses to mAChR blockade. The average changes in peak currents of transient Off-cells with light responses that were significantly affected by pharmacological blockade are presented in Fig. 9. mAChR blockade increased the firing and enhanced the peak inward currents of a transient Off ganglion cell with G1 morphology by 59 pA. The responses of this cell were unaffected by nicotinic blockade. The 119-pA inward currents at light offset of the cell with peripheral Off center ganglion cell morphology (Amthor et al. 1989) were eliminated by mAChR and by nAChR blockade. Another transient Off-cell had inward currents <500 ms duration at light offset that were followed by delayed, sustained spiking (Fig. 8B). This cell had morphology similar to the G5 cell (Rockhill et al. 2002) and was identified as transient, because the latency of the sustained component of the light responses was >500 ms after light offset. Bath application of HEX/MLA significantly decreased the transient Off responses of this cell by 106 pA (46%; Fig. 8B), whereas 3 μM ATR enhanced the transient responses by 21 pA (9%; Fig. 8B). Both 100 nM MLA/100 μM HEX and 3 μM ATR enhanced the duration of the delayed responses and the firing rate by 38 and 27 spikes, respectively.
Fig. 9.
Transient Off-cells were defined by transient (<500 ms) inward currents in response to the offset of optimally sized light stimuli. Cells were identified according to previously published morphological criteria (Amthor et al. 1989; Rockhill et al. 2002). Each of the 3 transient Off-cells included in this study had different morphologies and displayed different responses to mAChR blockade. The average changes in peak currents of transient Off-cells with light responses that were significantly affected by pharmacological blockade are shown for each cell.
DISCUSSION
We have extended previous studies by performing double-label IHC to identify the expression patterns of nicotinic and muscarinic receptors relative to each other and begun to assess the contributions of muscarinic and nicotinic receptors to ganglion cell response properties by examining the changes in light-evoked currents resulting from the application of nicotinic and muscarinic antagonists.
Immunohistochemistry
IHC results showed combinatorial patterns of nAChR and mAChR expression in the inner retina. To summarize, m1 and m4 muscarinic receptors were expressed together with both non-α7 and α7 nicotinic receptors in presumptive amacrine and ganglion cells. m5 muscarinic receptors and non-α7 nicotinic receptors were also coexpressed by subpopulations of amacrine and ganglion cells. Expression of nAChRs and mAChRs by GABAergic and glycinergic amacrine cells has been reported previously (Dmitrieva et al. 2001, 2003, 2007; Strang et al. 2010). However, this is the first report demonstrating that there is coexpression of both AChR subtypes by amacrine cells. The αBgt data suggest that a small proportion of the cells that express both nicotinic α7 nAChRs and mAChRs is cholinergic amacrine cells. Expression of both mAChRs and nAChRs on many presumptive amacrine cells suggests that ACh has an important role in controlling the speed and duration of release of inhibitory neurotransmitters. Our immunohisotochemical data are also consistent with previous synaptic blockade studies showing that ganglion cells directly express AChRs (Strang et al. 2003, 2005, 2010).
The observation of m1 expression by bipolar cells in rabbit retina is a new finding. Whereas a previous study (Strang et al. 2010) has demonstrated m1 IR in the OPL, the bipolar cell bodies were not labeled. The antigen-retrieval step necessary for the use of antibodies against α7 nAChR subunits (Dmitrieva et al. 2007) may have enhanced the detection of m1 by the antibodies that we used. There are four known cell populations that may mediate cholinergic inputs to ganglion cell via bipolar cell activation. These include α7 nAChR-positive populations that are calbindin positive, glycine positive, and a third unidentified bipolar cell population (Dmitrieva et al. 2007), as well as m3 mAChR-positive bipolar cells with type 4 cone bipolar morphology (Strang et al. 2010).
These data suggest multiple mechanisms by which mAChRs and α7 and non-α7 AChRs may affect ganglion cell responses. For example, the coexpression of excitatory mAChRs with nAChRs by bipolar cells and ganglion cells would result in ACh-mediated increases in cell excitability, whereas the correlation of inhibitory mAChRs with nAChR subtypes suggests a mechanism by which light-evoked ACh release would elicit fast nicotinic excitation, followed by slower muscarinic inhibition. Based on these results and the corresponding results from physiologically and morphologically identified ganglion cells, we suggest that many cell types in the retina have unique signatures based on the pattern of AChR expression.
Physiology and Morphology
Sustained On- and Off-cells.
Our physiological data showed that populations of ganglion cells were differentially affected by nAChR and mAChR blockade. For example, the center responses of G4 On-, nonstreak G4 On-, and G4 Off-cells were enhanced by mAChR blockade, predominantly via an increase in sustained currents, suggesting that under control conditions, mAChR activation suppresses the center responses of these cells, an effect that could be mediated by m2 or m4 mAChRs expressed directly on the ganglion cells. In contrast, brisk-sustained Off-cells received excitatory muscarinic inputs to the receptive field center, an effect consistent with expression of m1 or m3 receptors on the ganglion cells themselves or on upstream bipolar cells.
nAChR blockade suppressed the peak inward currents of all sustained cells, indicating the expression of nicotinic receptors directly on the ganglion cells. In contrast, nAChR blockade enhanced the sustained responses only of nonstreak G4 On-cells, an effect consistent with reduced ACh-mediated excitation of inhibitory amacrine cells. nAChR blockade suppressed the sustained responses of both G4 and brisk-sustained Off-cells. Nicotinic effects on peak responses were consistent with mediation by rapidly desensitizing α7 nAChRs, whereas nicotinic effects on the sustained components of the light responses may have been mediated by the more slowly desensitizing non-α7 nAChR subtypes.
Transient On-cells.
The center light responses of one-third of the brisk-transient/G11 On-cells were suppressed by both muscarinic and nicotinic blockade, suggesting that the center light responses are also shaped by excitation mediated by both mAChRs and nAChRs. Whereas a subset of G11 cells had excitatory nicotinic and muscarinic input to the center, all G11 cells had inhibitory nicotinic and muscarinic input to the surrounds. The lack of effects of nicotinic and muscarinic blockade on the center light responses of the remaining brisk-transient/G11 On-cells suggests that there are subcategories within this morphological type.
The data indicate that nicotinic activation alone contributes to the center responses of G10 cells. Nicotinic blockade suppressed the center responses of G10 cells but not G2 cells. Muscarinic blockade had no effect on the center responses of cells with the G2 or G10 morphologies. G2 and G10 ganglion cells have been physiologically identified as sluggish and On DS ganglion cells, respectively (Rockhill et al. 2002). Because we did not test for directional responses, we retained these cells within the transient On category.
Sources of Variability
Friedman's nonparametric repeated-measures ANOVA was performed for each cell to test the statistical significance of changes in response to mAChR and nAChR blockade. Only those cells with statistically significant responses were included in the reported cell-type averages. However, whereas the variability within cells was small, there was a large degree of variability in the amplitude of responses between cells of the same physiological and morphological types. This variability was only in the amplitude and not the direction of responses. That is, the effects of mAChR or nAChR blockade were consistent for the cells of a given type, although the amplitude of those effects varied.
There were several potential sources of between-cell variability. The first potential source of variability was differences between cells in the efficacy of space clamp. Ganglion cell dendritic fields are notoriously difficult to space clamp, due to the intricacies of the dendritic arbors, and the delayed firing observed for several transient cells may have resulted from dendritic spikes in cells that were poorly clamped. However, input resistance for all included cells remained steady throughout the experiment, and variability due to space clamp would affect the amplitude but not the direction of the responses, which were consistent within morphologically defined groups; only cells that exhibited recovery after washout of the drugs were included in this analysis. Thus any differences in space clamp do not negate the reported effects of AChR blockade.
Differences in the intrinsic light responses of each cell were a second source of variability. The responses of cells with higher-amplitude light responses in control conditions were proportionally more affected by the pharmacological agents than cells with lower-amplitude responses. Thus a single cell with very strong light responses could increase the standard error for all of the cells of a given subtype.
The final source of variability may have arisen from differences in the levels and expression patterns of receptor expression. Whereas our immunohistochemical data demonstrate that all inner retina cell types express one or more AChR subtypes, the data do not provide an estimate of the number of receptors expressed by a given cell nor do they provide direct information about the specific upstream inputs or the effects of AChR blockade on the responses of upstream cells that, in turn, shape the responses of the ganglion cells. Because the properties of nAChRs and mAChRs have been well described, it is possible to infer some of the effects resulting from blockade of AChRs expressed by upstream cells.
Retinal Circuitry
Application of the cholinergic antagonists MLA, HEX, and ATR helped to separate the effects of the nicotinic and muscarinic cholinergic systems. MLA is an antagonist for α7 and α6-containing nAChRs (Alkondon et al. 1992; Mogg et al. 2002), HEX is a nonspecific antagonist for nAChRs, and together, they provide complete nicotinic blockade. However, because α7 nAChRs rapidly desensitize in high concentrations of agonist, effects on early responses were likely to be mediated via the α7 subtype, whereas nicotinic effects on the AUC were more likely to have been mediated by the more slowly desensitizing non-α7 nAChR subtypes. ATR is a nonspecific antagonist of mAChRs. Although ATR has been reported to block α3β4 nAChRs in the medial habenula with an EC50 of 4 μM (Parker et al. 2003), biochemical studies show that ∼80% of α3-containing nAChRs in rabbit retina is associated with the β2 subunit (Keyser et al. 2000), and the α3β2 nAChR subtype has not been reported to be ATR sensitive. The predominance of α3β2 nAChRs, combined with the differential effects of MLA/HEX and ATR application, builds confidence that the data we report provide an accurate overview of the combined effects of nicotinic and muscarinic activation of ganglion cell response properties.
AChR activation may affect center responses through direct excitation or in the case of m2 and m4 mAChRs, direct inhibition mediated by receptors expressed on ganglion cell dendritic membranes. Ganglion cell center responses may also be enhanced by indirect excitation mediated by m1, m3, and m5 mAChRs and/or α7 nAChRs expressed by bipolar cells or by disinhibition mediated by m2 and m4 mAChRs expressed by amacrine cells. The activation of m1, m3, and m5 mAChRs or nAChRs expressed on GABA and/or glycinergic amacrine cells provides an additional mechanism for inhibition of ganglion cell center responses.
Direct activation of receptors expressed by the ganglion cells, together with effects on upstream cells, may contribute to the tuning of ganglion cell responses to complex visual stimuli. For example, differences in the time course of the activation of ionotropic and GPCRs can affect the time course of ganglion cell responses. A ganglion cell that expresses both non-α7 nAChRs and m4 mAChRs might respond to light-evoked ACh release with an initial depolarization, due to nAChR activation. The direct nicotinic depolarization would then be truncated by a delayed hyperpolarization mediated by muscarinic activation, and activation of m3 mAChRs or α7 nAChRs expressed by bipolar cells would increase glutamate release onto downstream ganglion cells and result in additional sustained or transient ganglion cell depolarization.
Ganglion cell responses may also be affected by the activation of α7 and mAChRs by extracellular choline that results from the hydrolysis of ACh by AChE. Although ACh is the primary endogenous agonist for all nAChRs and mAChRs, choline can act as an endogenous ligand for α7 nAChRs and for all muscarinic subtypes (Albuquerque et al. 1997; Carriere and El-Fakahany 2000; Costa et al. 1986; Costa and Murphy 1984; Papke et al. 2000), albeit with a lower rank-order efficacy than ACh (Papke and Porter 2002). Indeed, extracellular choline concentrations, sufficient to activate α7 nAChRs, have been reported in other parts of the brain (Alkondon et al. 1999).
Implications
Because the affinity of mAChRs for ACh is in the nanomolar range (Kellar et al. 1985; Kubo et al. 1986; Rama Sastry and Cheng 1972), and the affinity of nAChRs is in the micromolar range (Bertrand et al. 1992; Brioni et al. 1997; Chavez-Noriega et al. 1997; Wang et al. 1996), tonic or low-level release of ACh in the retina may be sufficient to activate muscarinic receptors, whereas nicotinic activation requires ACh concentrations, at least one order of magnitude greater. Thus the light responses of ganglion cells are likely affected, not only by ACh but also by relative differences in the concentration of ACh and choline and the differential activation of nAChRs and mAChRs expressed by ganglion cells, amacrine cells, and/or bipolar cells.
The balance of nicotinic and muscarinic activation is likely to be tightly regulated. mAChRs, expressed by starburst amacrine cells, provide an auto-feedback mechanism to reduce further ACh release (Cunningham et al. 1983; Strang et al. 2010). Desensitization and internalization of nicotinic and muscarinic receptors can be regulated by neurotransmitter levels and synaptic activity (Alkondon et al. 1997; van Koppen and Kaiser 2003; Willets et al. 2007). Thus the interplay among ACh release, ACh hydrolysis, receptor regulation, and the diverse kinetics of cholinergic receptors provides for precise and complex regulation of retinal processing.
Conclusions
This report contains the first comprehensive evaluation of the expression patterns of nAChRs and mAChRs relative to one another in mammalian retina. Combined with patch-clamp analysis of ganglion cell light responses, these data provide insight into the activation patterns of AChR subtypes that shape ganglion cell response properties and provide evidence that specific ganglion types have unique signatures shaped by the pattern of AChR expression and cholinergic inputs.
The understanding of the mechanisms of endogenous cholinergic signaling is important for knowledge of visual processing in the healthy retina but also has implications for insight into visual defects in disease states that result from decreased ACh availability, such as the changes in visual-evoked potentials, visual-field defects, and blurred vision associated with Parkinson's and Alzheimer's diseases (Daniels et al. 1994; Strenn et al. 1991). AChE inhibitors, such as those used in the treatment of glaucoma and myopia, as well as Parkinson's and Alzheimer's diseases (Chua et al. 2006), may disrupt the endogenous balance of ACh and choline in the retina by prolonging the availability of ACh, while reducing the amount of choline at synapses. These drugs have as much potential to exacerbate as to rescue cholinergic visual dysfunction.
GRANTS
Financial support for the conduct of the research was provided by The Eyesight Foundation of Alabama and National Eye Institute Grants P30EYE3039 and R01 EY07845.
DISCLOSURES
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
Author contributions: C.E.S., Y.L., F.R.A., and K.T.K. conception and design of research; C.E.S., Y.L., and K.E.G. performed experiments; C.E.S., Y.L., K.E.G., and K.T.K. analyzed data; C.E.S., Y.L., K.E.G., F.R.A., and K.T.K. interpreted results of experiments; C.E.S. and Y.L. prepared figures; C.E.S. and Y.L. drafted manuscript; C.E.S., F.R.A., and K.T.K. edited and revised manuscript; C.E.S., Y.L., K.E.G., F.R.A., and K.T.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Alexander Zotov for programming assistance.
REFERENCES
- Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther 280: 1117–1136, 1997. [PubMed] [Google Scholar]
- Alhomida AS, Al-Rajhi AA, Kamal MA, Al-Jafari AA. Kinetic analysis of the toxicological effect of tacrine (Cognex) on human retinal acetylcholinesterase activity. Toxicology 147: 33–39, 2000. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 9: 2734–2742, 1997. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci 19: 2693–2705, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol 41: 802–808, 1992. [PubMed] [Google Scholar]
- Ames A, Nesbett FB. In vitro retina as an experimental model of the central nervous system. J Neurochem 37: 867–877, 1981. [DOI] [PubMed] [Google Scholar]
- Amthor FR, Takahashi ES, Oyster CW. Morphologies of rabbit retinal ganglion cells with concentric receptive fields. J Comp Neurol 280: 72–96, 1989. [DOI] [PubMed] [Google Scholar]
- Ariel M, Daw NW. Effects of cholinergic drugs on receptive field properties of rabbit retinal ganglion cells. J Physiol 324: 135–160, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge WH. Optical recordings of the effects of cholinergic ligands on neurons in the ganglion cell layer of mammalian retina. J Neurosci 16: 5060–5072, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Bertrand S, Ballivet M. Pharmacological properties of the homomeric alpha 7 receptor. Neurosci Lett 146: 87–90, 1992. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Decker MW, Sullivan JP, Arneric SP. The pharmacology of (-)-nicotine and novel cholinergic channel modulators. Adv Pharmacol 37: 153–214, 1997. [DOI] [PubMed] [Google Scholar]
- Brown DA. Muscarinic acetylcholine receptors (mAChRs) in the nervous system: some functions and mechanisms. J Mol Neurosci 41: 340–346, 2010. [DOI] [PubMed] [Google Scholar]
- Carmine AA, Brogden Pirenzepine RN. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in peptic ulcer disease and other allied diseases. Drugs 30: 85–126, 1985. [DOI] [PubMed] [Google Scholar]
- Carriere JL, El-Fakahany EE. Choline is a full agonist in inducing activation of neuronal nitric oxide synthase via the muscarinic m1 receptor. Pharmacology 60: 82–89, 2000. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther 280: 346–356, 1997. [PubMed] [Google Scholar]
- Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, Tan D. Atropine for the treatment of childhood myopia. Ophthalmology 113: 2285–2291, 2006. [DOI] [PubMed] [Google Scholar]
- Costa LG, Kaylor G, Murphy SD. Interaction of choline with muscarine receptor-stimulated phosphoinositide metabolism in the rat brain. Naunyn Schmiedebergs Arch Pharmacol 334: 536–539, 1986. [DOI] [PubMed] [Google Scholar]
- Costa LG, Murphy SD. Interaction of choline with nicotinic and muscarinic cholinergic receptors in the rat brain in vitro. Clin Exp Pharmacol Physiol 11: 649–654, 1984. [DOI] [PubMed] [Google Scholar]
- Cunningham JR, Dawson C, Neal MJ. Evidence for a cholinergic inhibitory feed-back mechanism in the rabbit retina. J Physiol 340: 455–468, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R, Harding GF, Anderson SJ. Effect of dopamine and acetylcholine on the visual evoked potential. Int J Psychophysiol 16: 251–261, 1994. [DOI] [PubMed] [Google Scholar]
- Dmitrieva NA, Lindstrom JM, Keyser KT. The relationship between GABA-containing cells and the cholinergic circuitry in the rabbit retina. Vis Neurosci 18: 93–100, 2001. [DOI] [PubMed] [Google Scholar]
- Dmitrieva NA, Pow DV, Lindstrom JM, Keyser KT. Identification of cholinoceptive glycinergic neurons in the mammalian retina. J Comp Neurol 456: 167–175, 2003. [DOI] [PubMed] [Google Scholar]
- Dmitrieva NA, Strang CE, Keyser KT. Expression of alpha 7 nicotinic acetylcholine receptors by bipolar, amacrine, and ganglion cells of the rabbit retina. J Histochem Cytochem 55: 461–476, 2007. [DOI] [PubMed] [Google Scholar]
- Dorje F, Levey AI, Brann MR. Immunological detection of muscarinic receptor subtype proteins (m1-m5) in rabbit peripheral tissues. Mol Pharmacol 40: 459–462, 1991. [PubMed] [Google Scholar]
- Firth SI, Li W, Massey SC, Marshak DW. AMPA receptors mediate acetylcholine release from starburst amacrine cells in the rabbit retina. J Comp Neurol 466: 80–90, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci 14: 3351–3363, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Hashimoto M, Kaneko A. Neuronal nicotinic acetylcholine receptors of ganglion cells in the cat retina. Jpn J Physiol 45: 491–508, 1995. [DOI] [PubMed] [Google Scholar]
- Kellar KJ, Martino AM, Hall DP Jr, Schwartz RD, Taylor RL. High-affinity binding of [3H]acetylcholine to muscarinic cholinergic receptors 1. J Neurosci 5: 1577–1582, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser KT, MacNeil MA, Dmitrieva N, Wang F, Masland RH, Lindstrom JM. Amacrine, ganglion and displaced amacrine cells in the rabbit retina express nicotinic acetylcholine receptors. Vis Neurosci 17: 743–752, 2000. [DOI] [PubMed] [Google Scholar]
- Kittila CA, Massey SC. Pharmacology of directionally selective ganglion cells in the rabbit retina. J Neurophysiol 77: 675–689, 1997. [DOI] [PubMed] [Google Scholar]
- Kubo T, Fukuda K, Mikami A, Maeda A, Takahashi H, Mishina M, Haga T, Haga K, Ichiyama A, Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor 18. Nature 323: 411–416, 1986. [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Hersch SM, Wiley RG, Heilman CJ. Light and electron microscopic study of m2 muscarinic acetylcholine receptor in the basal forebrain of the rat. J Comp Neurol 351: 339–356, 1995. [DOI] [PubMed] [Google Scholar]
- Levey AI, Stormann TM, Brann MR. Bacterial expression of human muscarinic receptor fusion proteins and generation of subtype-specific antisera. FEBS Lett 275: 65–69, 1990. [DOI] [PubMed] [Google Scholar]
- Liu J, McGlinn AM, Fernandes A, Milam AH, Strang CE, Andison ME, Lindstrom JM, Keyser KT, Stone RA. Nicotinic acetylcholine receptor subunits in rhesus monkey retina. Invest Ophthalmol Vis Sci 50: 1408–1415, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH, Livingstone CJ. Effect of stimulation with light on synthesis and release of acetylcholine by an isolated mammalian retina. J Neurophysiol 39: 1210–1219, 1976. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Jobling AI, Truong HT, Cottriall CL, Gentle A. Expression of muscarinic receptor subtypes in tree shrew ocular tissues and their regulation during the development of myopia. Mol Vis 15: 464–475, 2009. [PMC free article] [PubMed] [Google Scholar]
- Mogg AJ, Whiteaker P, McIntosh JM, Marks M, Collins AC, Wonnacott S. Methyllycaconitine is a potent antagonist of alpha-conotoxin-MII-sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. J Pharmacol Exp Ther 302: 197–204, 2002. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press, 2011. [Google Scholar]
- Ndoye A, Buchli R, Greenberg B, Nguyen VT, Zia S, Rodriguez JG, Webber RJ, Lawry MA, Grando SA. Identification and mapping of keratinocyte muscarinic acetylcholine receptor subtypes in human epidermis. J Invest Dermatol 111: 410–416, 1998. [DOI] [PubMed] [Google Scholar]
- Papke RL, Meyer E, Uteshev VV. α7 Receptor-selective agonists and modes of α7 receptor activation. Eur J Pharmacol 393: 179–195, 2000. [DOI] [PubMed] [Google Scholar]
- Papke RL, Porter PJ. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol 137: 49–61, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JC, Sarkar D, Quick MW, Lester RA. Interactions of atropine with heterologously expressed and native alpha 3 subunit-containing nicotinic acetylcholine receptors. Br J Pharmacol 138: 801–810, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BN, Masland RH. Responses to light of starburst amacrine cells. J Neurophysiol 75: 469–480, 1996. [DOI] [PubMed] [Google Scholar]
- Pourcho RG. Localization of cholinergic synapses in mammalian retina with peroxidase-conjugated alpha-bungarotoxin. Vision Res 19: 287–292, 1979. [DOI] [PubMed] [Google Scholar]
- Rama Sastry BV, Cheng HC. Dissociation constants of D- and L-lactoylcholines and related compounds at cholinergic receptors 26. J Pharmacol Exp Ther 180: 326–339, 1972. [PubMed] [Google Scholar]
- Renna JM, Strang CE, Amthor FR, Keyser KT. Strychnine, but not PMBA, inhibits neuronal nicotinic acetylcholine receptors expressed by rabbit retinal ganglion cells. Vis Neurosci 24: 503–511, 2007. [DOI] [PubMed] [Google Scholar]
- Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. J Neurosci 22: 3831–3843, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry BV. Cholinergic systems and multiple cholinergic receptors in ocular tissues. J Ocul Pharmacol 1: 201–226, 1985. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Humphrey MF, Wassle H. Action and localization of acetylcholine in the cat retina. J Neurophysiol 58: 997–1015, 1987. [DOI] [PubMed] [Google Scholar]
- Strang CE, Amthor FR, Keyser KT. Rabbit retinal ganglion cell responses to nicotine can be mediated by β2-containing nicotinic acetylcholine receptors. Vis Neurosci 20: 651–662, 2003. [DOI] [PubMed] [Google Scholar]
- Strang CE, Andison ME, Amthor FR, Keyser KT. Rabbit retinal ganglion cells express functional {alpha}7 nAChRs. Am J Physiol Cell Physiol 289: C644–C655, 2005. [DOI] [PubMed] [Google Scholar]
- Strang CE, Renna JM, Amthor FR, Keyser KT. Muscarinic acetylcholine receptor localization and activation effects on ganglion response properties. Invest Ophthalmol Vis Sci 51: 2778–2789, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang CE, Renna JM, Amthor FR, Keyser KT. Nicotinic acetylcholine receptor expression by directionally selective ganglion cells. Vis Neurosci 24: 523–533, 2007. [DOI] [PubMed] [Google Scholar]
- Strenn K, Dal-Bianco P, Weghaupt H, Koch G, Vass C, Gottlob I. Pattern electroretinogram and luminance electroretinogram in Alzheimer's disease. J Neural Transm Suppl 33: 73–80, 1991. [DOI] [PubMed] [Google Scholar]
- van Koppen CJ, Kaiser B. Regulation of muscarinic acetylcholine receptor signaling 1. Pharmacol Ther 98: 197–220, 2003. [DOI] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells G, Anand R, Peng X, Keyser KT, Lindstrom J. Assembly of human neuronal nicotinic receptor α5 subunits with α3, β2 and β4 subunits. J Biol Chem 271: 17656–17665, 1996. [DOI] [PubMed] [Google Scholar]
- Wang H, Han H, Zhang L, Shi H, Schram G, Nattel S, Wang Z. Expression of multiple subtypes of muscarinic receptors and cellular distribution in the human heart. Mol Pharmacol 59: 1029–1036, 2001. [DOI] [PubMed] [Google Scholar]
- Wasselius J, Johansson K, Bruun A, Zucker C, Ehinger B. Correlations between cholinergic neurons and muscarinic m2 receptors in the rat retina. Neuroreport 9: 1799–1802, 1998. [DOI] [PubMed] [Google Scholar]
- Wess J, Liu J, Blin N, Yun J, Lerche C, Kostenis E. Structural basis of receptor/G protein coupling selectivity studied with muscarinic receptors as model systems. Life Sci 60: 1007–1014, 1997. [DOI] [PubMed] [Google Scholar]
- Willets JM, Nelson CP, Nahorski SR, Challiss RA. The regulation of m1 muscarinic acetylcholine receptor desensitization by synaptic activity in cultured hippocampal neurons 10. J Neurochem 103: 2268–2280, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada ES, Dmitrieva N, Keyser KT, Lindstrom JM, Hersh LB, Marshak DW. Synaptic connections of starburst amacrine cells and localization of acetylcholine receptors in primate retinas. J Comp Neurol 461: 76–90, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker CL, Ehinger B. Gamma-aminobutyric acid A receptors on a bistratified amacrine cell type in the rabbit retina. J Comp Neurol 393: 309–319, 1998. [DOI] [PubMed] [Google Scholar]