Abstract
We tested the possibility that the trigeminoparabrachial tract (VcPbT), a projection thought to be importantly involved in nociception, might also contribute to sensation of itch. In anesthetized rats, 47 antidromically identified VcPbT neurons with receptive fields involving the cheek were characterized for their responses to graded mechanical and thermal stimuli and intradermal injections of pruritogens (serotonin, chloroquine, and β-alanine), partial pruritogens (histamine and capsaicin), and an algogen (mustard oil). All pruriceptive VcPbT neurons were responsive to mechanical stimuli, and more than half were additionally responsive to thermal stimuli. The majority of VcPbT neurons were activated by injections of serotonin, histamine, capsaicin, and/or mustard oil. A subset of neurons were inhibited by injection of chloroquine. The large majority of VcPbT neurons projected to the ipsilateral and/or contralateral external lateral parabrachial and Kölliker-Fuse nuclei, as evidenced by antidromic mapping techniques. Analyses of mean responses and spike-timing dynamics of VcPbT neurons suggested clear differences in firing rates between responses to noxious and pruritic stimuli. Comparisons between the present data and those previously obtained from trigeminothalamic tract (VcTT) neurons demonstrated several differences in responses to some pruritogens. For example, responses of VcPbT neurons to injection of serotonin often endured for nearly an hour and showed a delayed peak in discharge rate. In contrast, responses of VcTT neurons endured for roughly 20 min and no delayed peak of firing was noted. Thus the longer duration responses to 5-HT and the delay in peak firing of VcPbT neurons better matched behavioral responses to stimulation in awake rats than did those of VcTT neurons. The results indicate that VcPbT neurons may have important roles in the signaling of itch as well as pain.
Keywords: intraparietal sulcus, neuron, neurophysiology, principal sulcus, working memory
itch is defined as an unpleasant sensation that elicits the urge to scratch. A series of findings during the last 25 yr indicate that peripheral and spinal neurons that respond to pruritic stimuli also respond to noxious stimuli (Carstens 1997; Davidson et al. 2007; Han et al. 2013; Jinks and Carstens 2002; Johanek et al. 2008; LaMotte et al. 2014; Ma 2010; Tuckett and Wei 1987; Wei and Tuckett 1991). These findings raise questions about the nature of the coding that underlies the two sensations. Recent studies have shown that pain-inducing stimuli are able to evoke scratching behavior when only pruriceptive primary afferents are activated (Han et al. 2013; Roberson et al. 2013), suggesting that activation of pruriceptive neurons leads to the sensation of itch, even though such neurons are also nociceptive.
Powerful behavioral approaches have been developed that allow investigators to determine whether an agent or manipulation produces pain or itch or both in mice (LaMotte et al. 2011; Shimada and LaMotte 2008). These tests also allow simultaneous determination of the intensity and time course of both sensations. In one method, intradermal injections of pruritogens in the cheek induce scratching with the hindlimb, whereas injections of algogens into the same site induce wiping with the forelimb (LaMotte et al. 2011; Shimada and LaMotte 2008). Klein et al. (2011) used this approach in rats and demonstrated that intradermal injections of chloroquine induce only scratching. Serotonin causes more profound site-directed scratching with very little wiping. In contrast, capsaicin and histamine cause a mixture of these behaviors and are therefore regarded as “partial pruritogens” in this study, as they were previously (Moser and Giesler 2014a). Intradermal injection of mustard oil causes only wiping, indicating that it produces only pain sensation in rats (Klein et al. 2011).
Although significant advances have been made in the understanding of the peripheral mechanisms underlying itch sensation, relatively little is known about the ascending pathways that carry pruriceptive information to the brain. It is known that a population of primate spinothalamic tract (STT) neurons in the lumbar enlargement are activated by both noxious and pruritic stimuli applied to their receptive fields (Davidson et al. 2007, 2009, 2012; Simone et al. 2004). Scratching the receptive field reduced responses of STT neurons to a pruritogen (Davidson et al. 2009). More than half of the examined trigeminothalamic tract (VcTT) neurons in rats were activated by noxious and pruritic stimuli applied to their receptive fields on the face (Moser and Giesler 2013; 2014a). Moreover, centrally administered morphine, an agent that causes severe itch in human subjects when applied spinally (Ballantyne et al. 1988), activates pruriceptive VcTT neurons and potentiates their responses to a pruritic stimulus (Moser and Giesler 2013). These findings support the idea that both STT and VcTT neurons are involved in producing itch.
Lesions of the anterolateral funiculus (ALF) of the human spinal cord block both itch and pain (Bickford 1938; Hyndman and Wolkin 1943), suggesting that pathways that contribute ascending axons to the ALF may contribute to production of both sensations. Axons of STT neurons are an important source of ascending axons in the ALF (Mehler et al. 1960; Nauta and Kuypers 1958). Axons projecting to the parabrachial area (Pb) also ascend within the ALF (Mehler et al. 1960; Nauta and Kuypers 1958) and thus might also contribute to itch. Although neurons contributing to the spinoparabrachial (SPbT) and trigeminoparabrachial tract (VcPbT) have been studied previously with respect to pain, little is known about their potential roles in itch. An anatomical study in which c-fos was used as a measure of activity found that a small percentage of rat SPbT neurons was activated by intradermal injection of serotonin (Merrill et al. 2007). Also, the majority of SPbT and VcPbT neurons are located in lamina I, a region of the spinal cord that is thought to receive a large pruriceptive input (Todd 2010). Therefore, understanding the roles of SPbT and VcPbT neurons in itch signaling is of particular interest.
We studied responses of antidromically identified rat VcPbT neurons to innocuous and noxious mechanical and thermal stimuli within receptive fields on the cheeks. The effects of injections of pruritogens, partial pruritogens, and an algogen were subsequently determined. Additionally, a comparison was made between the responses of VcPbT neurons and VcTT neurons to intradermal injection of pruritogens (Moser and Giesler 2014a). The results suggest that many VcPbT neurons are powerfully activated by itch-producing stimuli and likely contribute to pruriception.
MATERIALS AND METHODS
Surgical preparation.
Male Sprague-Dawley rats (300–350 g) were used according to a protocol approved by the Institutional Animal Care and Use Committee at the University of Minnesota. The animals received an intraperitoneal injection of urethane (1.5 g/kg; Sigma) and acepromazine (7.5 mg/kg; Phoenix), with additional doses as needed to maintain sufficient depth of anesthesia during the experiments. The facial area was closely clipped on both sides, and a tracheotomy was performed. Rats were placed on a feedback-controlled heating pad to maintain a core temperature of 37°C. Rats were positioned in a stereotactic frame, and a laminectomy was performed over the first cervical vertebra to allow insertion of a recording electrode. A craniotomy was performed over Pb to allow access with stimulating electrode(s). The superior sagittal sinus and both transverse sinuses were ligated using silk sutures to avoid bleeding caused by inserting electrodes.
Antidromic identification and mapping of axons of projecting neurons.
Stimulation electrodes were bilaterally positioned using stereotactic coordinates of the external lateral Pb and the adjacent Kölliker-Fuse (KF) nucleus (Paxinos and Watson 1998), nuclei that receive a large projection from VcPbT neurons (Feil and Herbert 1995). Electrodes in Pb delivered 150- to 300-μA amplitude pulses, 200 μs in duration at a frequency of 3 Hz as search stimuli. A stainless steel recording electrode (∼10 MΩ; FHC, Bowdoin, ME) was positioned at the dorsal surface of the lower part of the caudal medulla and C1/C2 segments and moved ventrally. When a time-locked single-unit response was found, the following three established criteria were used to determine antidromic activation: 1) the action potential occurred at a stable latency; 2) it had the ability to follow a ≥300-Hz train; and 3) collision of antidromic and orthodromic action potentials occurred. The stimulation electrode was then repositioned to search for a location where antidromically induced action potentials occurred at a 50% success rate with pulses of ≤30 μA. This location was regarded as the low-threshold point (LTP) and was surrounded in the same rostrocaudal plane by points dorsally, ventrally, medially, and laterally in which greater amplitude currents were required for antidromic activation. In addition, in most of the experiments, the LTP was surrounded rostrally by points at which current pulses ≥300 μA failed to evoke antidromically induced action potentials. Stimulation at a more rostral plane was performed to limit the possibility that the antidromically activated axons passed through the Pb without terminating (Hylden et al. 1985).
Mechanical and thermal characterization.
After a LTP was established for an isolated neuron, the cutaneous receptive field was mapped using a soft-bristled brush and pinches using blunt forceps. Noxious stimuli are known to inhibit pruriceptive responses. Therefore, efforts were made to use the minimum number and intensity of pinches. Only units with receptive fields that included the cheek were examined further. The neuron was then mechanically classified using the brush, a weak arterial clip (2.95 N; pressure), and a stronger arterial clip (6.37 N; noxious pinch). Low-threshold (LT) neurons responded to innocuous brushing with an equal or greater discharge rate than to pressure or pinch stimuli; wide dynamic range (WDR) neurons responded to all mechanical stimuli, with a higher discharge rate during pressure/pinch; and high-threshold (HT) neurons responded only to pressure/pinch. Subsequently, neurons were tested for responses to thermal stimuli using a Peltier thermode (contact surface = 3 mm2; Yale Instruments) applied centrally in the receptive field. From a baseline of 32°C, temperatures were increased to 40, 45, and 50°C and lowered to 25, 20, 15, and 10°C, each for 5 s with 60 s of baseline between subsequent temperatures. Transcutaneous electrical stimulation (≤5 mA) delivered through a pair of needle electrodes (separated by 3 mm) repeatedly inserted into the superficial skin in multiple areas of the cheek and adjacent body was used to establish responsive areas for units that did not respond to mechanical stimulation.
Characterization using pruritic and algogenic agents.
The following agents were injected in the receptive field: 5-hydroxytryptamine hydrochloric acid (5-HT; 1%, 47 mM, in saline; Sigma), histamine dihydrochloride (10%, 900 mM, in saline; Sigma), chloroquine diphosphate salt (5%, 100 mM, in saline; Sigma), β-alanine (4.5%, 50 mM, in saline; Sigma), and capsaicin (0.1%, 3.3 mM, in 7% Tween 80 in saline; Sigma). These agents cause significant scratching behavior in rats when injected in the cheek (Klein et al. 2011; Moser and Giesler 2014b). Mustard oil (10%, 1000 mM, in 7% Tween 80 in saline; Sigma) was also injected intradermally. It causes only wiping behavior (Klein et al. 2011). β-Alanine causes scratching behavior in mice but has not been studied in rats to our knowledge (Liu et al. 2012). Agents were injected intradermally with a 28-gauge needle in a 10-μl volume, causing formation of a small bleb in the skin. Injections of active agents were preceded by an injection of vehicle [7% Tween 80 in saline for capsaicin and mustard, saline for the other agents] and applied in a randomized order, with injections of capsaicin and mustard oil following the other agents. Subsequent injections were separated by at least 5 min and efforts were made to separate injections sites by ≥5 mm.
Identification of stimulation and recording sites.
Electrolytic lesions were made at the antidromic LT point(s) and recording point at the end of each experiment. Subsequently, the rats were transcardially perfused with 0.9% saline followed by a solution of 10% formalin and 1% ferrocyanide in water to produce a Prussian blue reaction product at the site of the lesion. The brain and upper cervical spinal cord were then removed, postfixed in the same solution for at least 12 h at 4°C, and cut on a freezing microtome in 75 μm (brainstem) or 50 μm (lower medulla and upper cervical cord) thick sections. Sections were air-dried on glass slides, stained with neutral red, and coverslipped.
Data analysis.
Action potentials were amplified and filtered (WPI model DAM80) and then digitized and wave-form discriminated using DAPSYS data acquisition software (http://www.dapsys.net). We wished to limit the effects of manipulation of the skin and needle insertion on the analyses of responses. Therefore, the period from 5 s before to 30 s following needle insertion was not included in the analyses. A neuron was considered responsive if 1) the discharge rate was ≥1.5 times the mean discharge rate during baseline (the period from 65 to 5 s before injection); 2) the discharge rate was ≥1.5 times the mean discharge rate following injection of vehicle; and 3) the response persisted for at least 60 s. We defined firing rates ≤50% of baseline and responses to vehicle as inhibitory responses. Individual responses of neurons are reported in 1-s bins. To determine mean responses, baseline firing rates were subtracted from firing rates following each stimulus. Mean responses are reported in 15- or 30-s bins (specified in text). For spike-timing analyses, spike trains of a 5-min duration starting 30 s after injection were analyzed. Analyses of 5-HT-responsive neurons involved spike trains of a 10-s duration starting 5 min after injection of 5-HT and spike trains during 5 s (heat) to 10 s (pinch) of lasting noxious stimuli. The coefficient of variation (CV) of the spike trains was calculated by dividing the standard deviation of the interspike intervals (ISIs) by the mean length of the ISIs. ISI distribution histograms were made by counting the number of ISIs in a certain range, expressed in a percentage of the total amount of ISIs. These data were plotted on two different x-axes labeled with powers of 10: one with logarithmic spacing and one with linear spacing between tick marks. The latter approach more effectively revealed differences in several firing frequencies (e.g., 10–50 and 1,000–2,500 ms ISIs; Moser and Giesler 2014a). Results of different subpopulations of neurons or different stimuli were compared using Kruskal-Wallis ANOVA with Mann Whitney U-tests as post hoc tests for multiple comparisons and the Mann-Whitney U-test or Wilcoxon signed ranks test when one comparison was made. Proportional data were analyzed using the Pearson's χ2-test or Fisher's exact test. A P value < 0.05 was considered significant.
RESULTS
Extracellular single-unit recordings were made from 47 antidromically identified neurons in 35 rats. Each neuron was antidromically activated from a LTP in the Pb. All studied neurons were mechanically sensitive and had a facial cutaneous receptive field including part of the cheek. All were tested for their responses to at least one pruritogen. Thirty-three neurons (70%) were tested for their responses to all pruritogens and partial pruritogens used in this study. Thirty-eight VcPbT neurons (81%) were located in the superficial dorsal horn (sDH), and nine (19%) were located in the deep dorsal horn (dDH). Nineteen of the examined VcPbT neurons (40%) were classified as HT, 21 neurons as WDR (45%), and 7 neurons (15%) as LT. Characterization with graded heat and cold stimuli revealed that 24 neurons (51%) were responsive to heat (1 neuron was inhibited by heat), and 19 neurons (40%) were responsive to cold (1 neuron was inhibited by cold). Twelve (26%) were responsive to both heat and cold. Occasionally, the search strategy led to antidromically activated single units that were insensitive to mechanical or thermal stimulation of the facial area or other area of the adjacent body including intraoral and corneal surfaces. None of these neurons responded to transcutaneous electrical stimulation of facial cutaneous areas. All were encountered at a considerable distance from the surface of the dorsal horn (700–1,200 μm), presumably in the deep dorsal horn or lateral spinal or cervical nuclei.
Pruriceptive VcPbT neurons.
Thirty-three VcPbT neurons (70%) responded to one or more pruritogens or partial pruritogens and were therefore defined as pruriceptive. Of these, 13 (39%) were classified HT, 15 (45%) as WDR, and 5 (15%) as LT. None of the neurons tested were activated by intradermal injection of chloroquine. However, injection of chloroquine reduced firing in a subset of VcPbT neurons. Each of these neurons was activated by mechanical stimuli and by injection of other pruritogens or mustard oil.
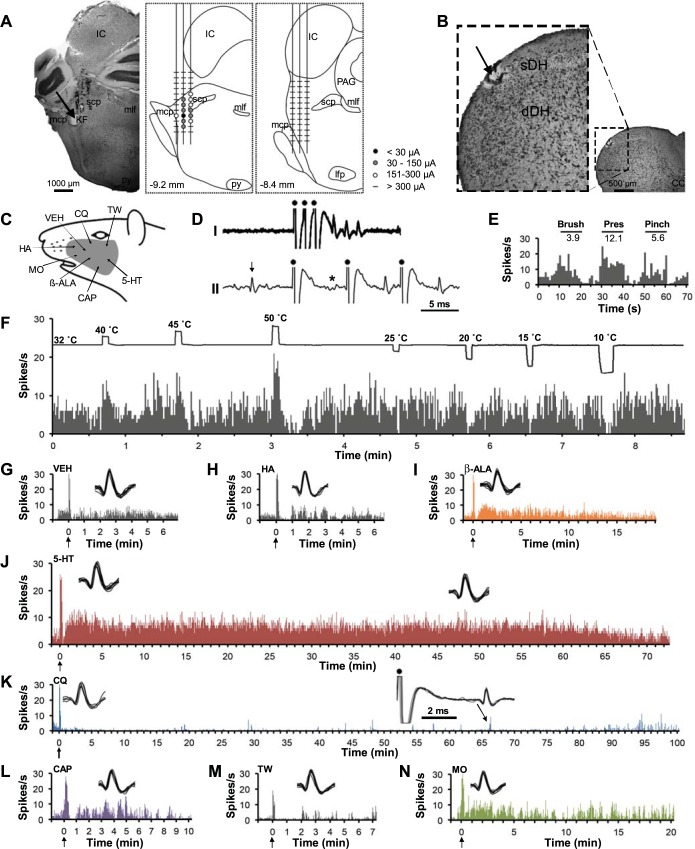
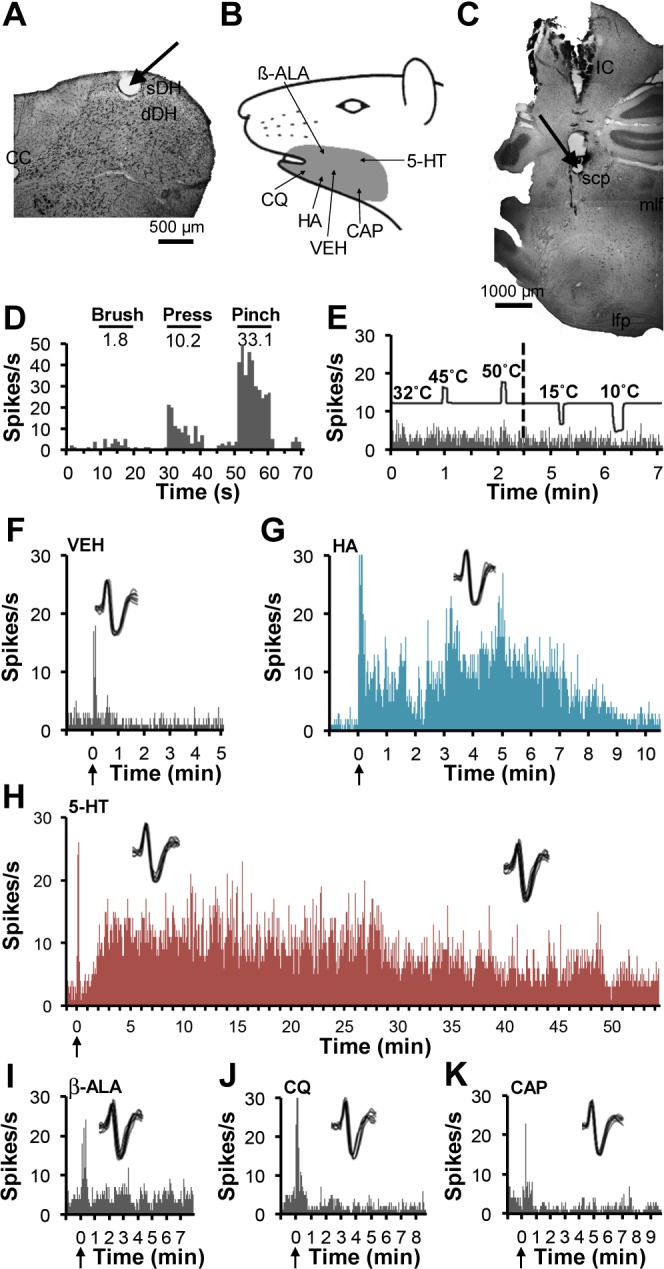
Figure 1 illustrates an example of a pruriceptive VcPbT neuron. The unit met the criteria for antidromic activation (Fig. 1D). The lesion at the LTP was located in the ipsilateral KF nucleus (Fig. 1A). The recording point was located in the sDH at the junction of C1/C2 segments (Fig. 1B). The neuron was classified as a WDR neuron (Fig. 1E). It was activated by warming (40°C) and noxious heat (50°C) and inhibited by cold stimuli (20, 15, and 10°C; Fig. 1F). Firing levels returned rapidly to baseline after an intradermal injection of vehicle (Fig. 1G). A prolonged increase of firing followed intradermal injection of β-alanine (∼ 15 min), 5-HT (∼70 min), capsaicin (∼10 min), and mustard oil (∼20 min) (Fig. 1, I, J, L, and N). Firing was reduced for ∼100 min following an injection of chloroquine (Fig. 1K). Antidromic responses were elicited during this period (Fig. 1K, inset), indicating that recording of the neuron was stable. The neuron did not respond to injections of histamine or Tween (Fig. 1, H and M).
Fig. 1.
A trigeminoparabrachial tract (VcPbT) neuron responsive to β-alanine (β-ALA), 5-HT, capsaicin (CAP), and mustard oil (MO) and inhibited by chloroquine. A: lesion (arrow) made at the low-threshold point (LTP) in the Kölliker-Fuse nucleus ipsilateral to the recording point (left). Amplitudes of current pulses necessary to elicit antidromic activation were greater medially, laterally, dorsally and ventrally as illustrated (middle), whereas current pulses >300 μA were ineffective at more rostral positions (right). B: lesion (arrow) at the recording point in the superficial dorsal horn at the junction of C1 and C2. C: receptive field, including the cheek. Arrows indicate the site of intradermal injection of the various agents. Mechanical and thermal stimuli were applied to the center of the receptive field. D: the neuron met the criteria for antidromic activation: I: an overlay of 30 spike trains following a high-frequency train of current pulses (dots) with invariant latency; II: collision of a putative antidromic spike (indicated by an asterisk) with an orthodromic spike (indicated by an arrow). E: mechanical characterization, black bars indicate the duration of the mechanical stimulus, and numbers indicate firing frequency (Hz) during stimulus application after subtraction of the baseline. F: responses to thermal stimuli. G–H: intradermal injections of vehicle and histamine did not result in a sustained response. I–J: the neuron responded to injections of β-alanine and 5-HT. K: intradermal injection of chloroquine reduced firing compared with prestimulus baseline and the preceding injection of vehicle. Antidromic spikes could still be generated during period of low activity (right inset). L–N: the neuron responded to injections of capsaicin and mustard oil. Arrows indicate the timing of injection. Agents were injected in the same order as illustrated. Insets: overlay of 10 consecutive spikes randomly selected from the spike train at time points of high discharge rate, roughly matching the location of the inset. CC, central canal; dDH, deep dorsal horn; IC, inferior colliculus; KF, Kölliker-Fuse nucleus; lfp, longitudinal fasciculus pontis; mlf, medial longitunal fasciculus; mcp, medial cerebellar peduncle; PAG, periaqueductal gray; py, pyramidal tract; scp, superior cerebellar peduncle; sDH, superficial dorsal horn; HA, histamine dihydrochloride; TW, 7% Tween 80 in saline; VEH, saline.
Figure 2 illustrates a pruriceptive VcPbT neuron that projected to the contralateral Pb, likely within the rostral part of the external lateral Pb (Fig. 2C). The recording point was located in the sDH at the junction of C1/C2 (Fig. 2A). This WDR neuron (Fig. 2D) was unresponsive to heat and cold stimuli (Fig. 2E). Clear increases in firing were evoked by intradermal injections of histamine (∼10 min) and 5-HT (∼60 min) (Fig. 2, G and H). The neuron did not respond to injections of β-alanine, chloroquine, or capsaicin (Fig. 2, I–K). The effects of mustard oil were not determined.
Fig. 2.
A VcPbT neuron responsive to histamine and 5-HT. A: lesion (arrow) at the recording point in the superficial dorsal horn at the junction of C1 and C2. B: receptive field. C: lesion (arrow) made at the LTP in the contralateral Pb. D: mechanical characterization. E: effects of thermal stimuli applied to the receptive field, only the 2 highest and lowest tested temperatures are shown. F: effects of intradermal injection of vehicle. G–H: sustained responses were observed after intradermal injections of histamine and 5-HT. I–K: the neuron did not respond to intradermal injections of β-alanine, chloroquine, or capsaicin. Agents were injected in the same order as illustrated.
Nonpruriceptive VcPbT neurons.
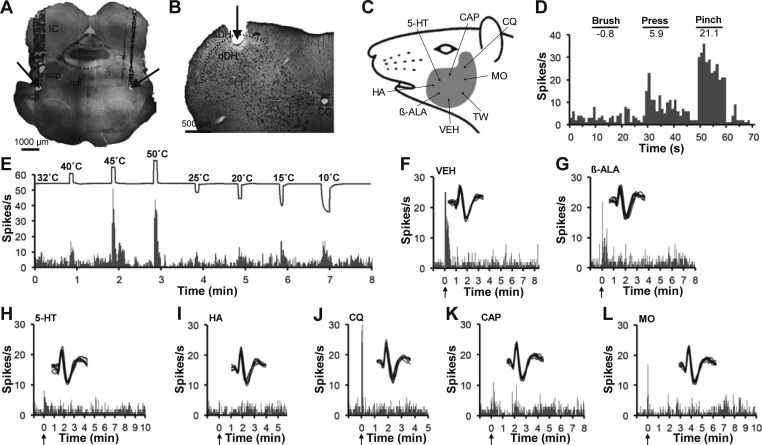
Thirteen neurons (28%) did not respond to any of the pruritogens or partial pruritogens tested. None of the five nonpruriceptive neurons tested with mustard oil were responsive to this agent. One WDR neuron was responsive to mustard oil but was not tested with 5-HT and histamine and therefore could not be identified with certainty as nonpruriceptive. Six nonpruriceptive neurons (43%) were classified as HT, five (43%) were classified as WDR, and two (14%) were classified as LT. Figure 3 illustrates an example of a VcPbT neuron that did not respond to any of the intradermally injected pruritic or algogenic agents tested (Fig. 3, G–L). The neuron was antidromically activated from both the ipsilateral and contralateral KF nuclei (Fig. 1A), recorded in the dDH (Fig. 3B), classified as HT (Fig. 3D), and responded to both heat and cold stimuli (Fig. 3E).
Fig. 3.
A VcPbT neuron responsive to noxious mechanical and thermal stimuli but unresponsive to pruritic stimuli. A: bilateral lesions (arrows) made at LTPs in both the ipsilateral and contralateral Kölliker-Fuse nucleus. B: lesion (arrow) in the deep dorsal horn. C: receptive field. D: mechanical characterization. E: effects of thermal stimuli. F–L: the neuron was unresponsive to intradermal injections of vehicle, β-alanine, 5-HT, histamine, chloroquine, capsaicin, and mustard oil. Agents were injected in the same order as illustrated.
Responses to pruritic and algogenic agents.
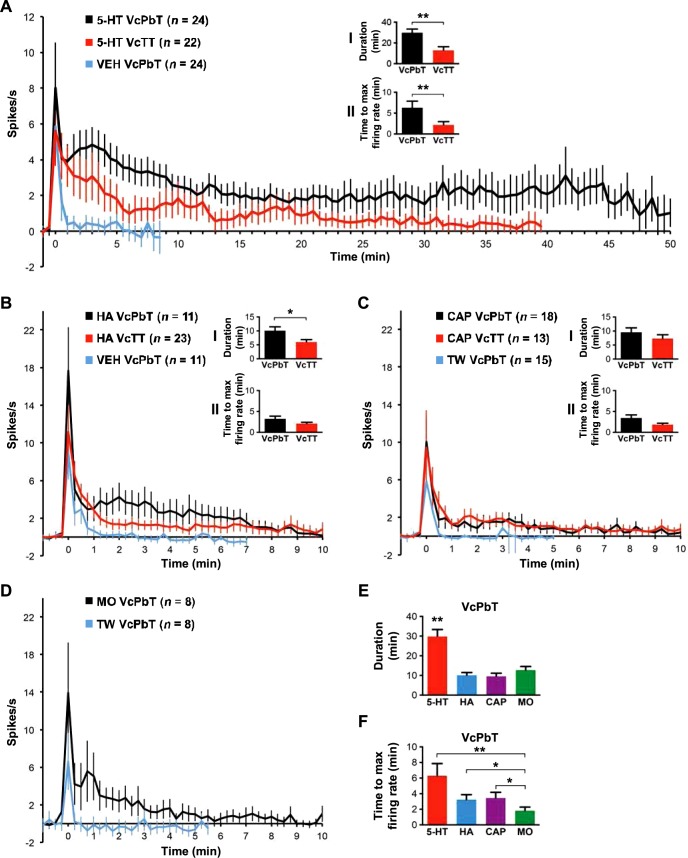
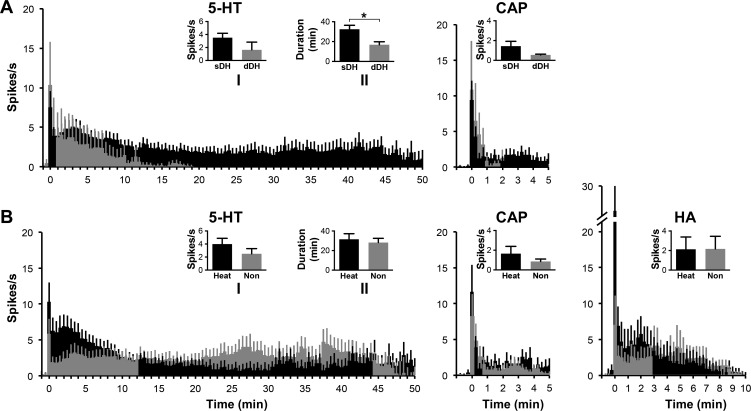
Eighty-eight percent (28 out of 32) of pruriceptive VcPbT neurons were activated by injection of 5-HT, 4% (1 out of 25) by injection of β-alanine, 42% (11 out of 26) by histamine, 67% (18 out of 27) by capsaicin, and 37% (7 out of 20) by mustard oil. In Fig. 4, responses of VcPbT neurons activated by 5-HT, histamine, capsaicin, and mustard oil are compared with the responses of VcTT neurons obtained in our previous study (Moser and Giesler 2014a). Mean responses to injection of 5-HT showed that VcPbT neurons responded longer and at higher firing frequencies than did VcTT neurons (Fig. 4 A, inset I). Moreover, the mean latency at which the responses of individual neurons reached peak discharge rates was significantly greater for VcPbT neurons; little delay was seen in the responses of VcTT neurons (Fig. 4A, inset II). A significantly longer duration of the mean response to histamine was also observed for VcPbT neurons (Fig. 4B, inset I). No differences were noted between the capsaicin-responsive populations (Fig. 4C). Analyses of proportions of VcPbT and VcTT neurons responsive to pruritic agents demonstrated some notable differences: the percentage VcPbT neurons activated by injection of 5-HT (62%) was significantly larger than the percentage of VcTT neurons activated by 5-HT (27%; P < 0.001, Pearson's χ2-test). The percentage of VcPbT neurons activated by injection of capsaicin (50%) was also greater than the percentage of VcTT neurons activated by the agent (27%; P = 0.026, Pearson's χ2-test). No differences were noted in the proportions responsive to histamine (30 and 28% respectively). The responses of VcPbT neurons to injections of 5-HT were significantly longer than the responses to injections of other pruritogens (Fig. 4E). Injections of the algogen mustard oil induced a response that endured for roughly 9 min (Fig. 4D). The time to peak discharge rate for mustard oil was significantly shorter than for any of the tested pruritogens (Fig. 4F).
Fig. 4.
Mean responses to 5-HT, histamine, capsaicin, and mustard oil. A: mean response curves (±SE) of 5-HT-responsive VcPbT neurons to 5-HT (black) and vehicle (blue) and 5-HT-responsive trigeminothalamic tract (VcTT) neurons to 5-HT (red); inset I: mean response duration calculated by determining the time point in a 60-s bin histogram when the firing rate dropped <1.5 times the prestimulus baseline of 60 s for a period of ≥2 min; **P < 0.005; inset II: mean time from injection for the response to reach the maximal discharge rate, calculated by determining the time point in a 60-s bin-histogram when the firing rate peaked; **P < 0.005. B: mean response curves of histamine-responsive VcPbT or VcTT neurons to histamine and vehicle (only VcPbT neurons); insets I and II: mean response duration and mean time to maximal discharge rate; *P < 0.05. C: mean response curves of capsaicin-responsive VcPbT and VcTT neurons to capsaicin and Tween (only VcPbT neurons); insets I and II: mean response duration and mean time to maximal discharge rate. D: mean response curves of mustard oil-responsive VcPbT neurons to mustard oil and Tween. E: mean response duration of VcPbT neurons to the various agents tested. **P < 0.005. F: mean time to maximal discharge rate of VcPbT neurons to the various agents tested. *P < 0.05, **P < 0.005. For A–D, every data point in the response curves represents a 30-s bin (for A) or 15-s bin (for B–D). Data were only included when the receptive field was not manipulated during the response to the agent. Response curves were corrected for baseline activity by subtracting activity spanning 65 to 5-s preceding injection of the agent. Error bars indicate means ± SE. Agents were injected at time point zero. Statistical tests: Mann-Whitney U-test (in A and B) and Kruskal-Wallis ANOVA with Mann-Whitney U-tests as post hoc tests (in E and F). Since only one neuron was responsive to β-alanine, no analyses were performed.
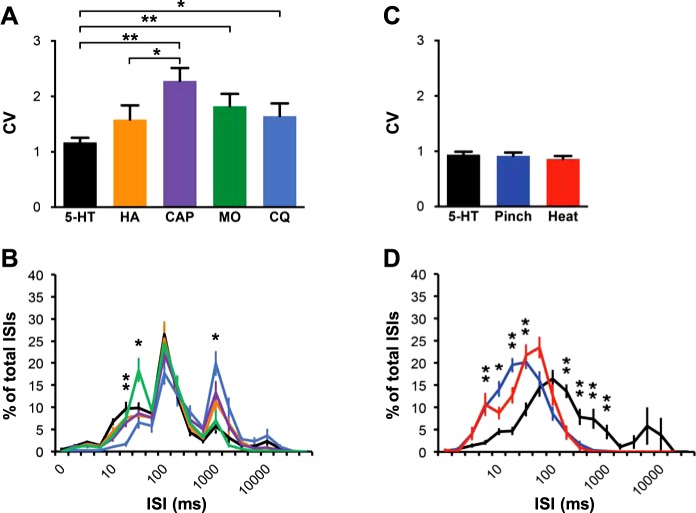
The spike-timing dynamics of responses to the different agents used in this study were compared. The CV is a measure of the variability in the period between two individual spikes within a spike train, the ISI. Significant differences in CV were found between spike trains after injection of 5-HT compared with those of capsaicin, chloroquine and mustard oil, indicating less variation in ISIs in responses to 5-HT. The distribution of ISIs was analyzed both on a logarithmic scale and a logarithmic scale with linear spaced tick marks between log steps on the x-axis, as was done previously (Moser and Giesler 2014a). No significant differences in ISI distribution were observed between the various agents on a logarithmic scale (data not shown). However, when analyzed on a scale with linear spacing, the distribution of ISIs for chloroquine was shifted to the right compared with that of other agents, indicating low-frequency firing, which is consistent with the inhibitory responses of VcPbT neurons to this agent. Interestingly, injections of the algogen mustard oil produced a significantly greater peak in the ISI distribution at 50–75 ms than did injections of pruritogens or partial pruritogens (Fig. 5B). The CV and ISI distributions of responses to the noxious stimuli pinch and heat were separately analyzed in the population of 5-HT-responsive VcPbT neurons (Fig. 5, C and D). No differences were noted in CV (Fig. 5C), whereas ISI distributions for responses to the three stimuli on a logarithmic scale show a clear shift to the left, i.e., shorter ISIs, for pinch and heat (Fig. 5D). The relatively lower firing frequencies in responses to pruritic stimuli (Fig. 5, B and D) are in accordance with findings in previous studies of VcTT (Moser and Giesler 2014a) and spinothalamic neurons (Davidson et al. 2012).
Fig. 5.
Spike-timing dynamics of responses to 5-HT, histamine, capsaicin, mustard oil, and chloroquine. A: coefficient of variation (CV) of interspike intervals (ISIs) during responses to the different agents. *P < 0.05, **P < 0.005. B: ISI distribution of the agents tested, represented by their corresponding colors (in A). From left to right: **P < 0.005 (CQ vs. other agents), *P < 0.05 (MO vs. other agents), *P < 0.05 (CQ vs. other agents). C: CV of ISIs within the responses to 5-HT, pinch, and heat (50°C) in 5-HT-responsive VcPbT neurons. D: ISI distribution of responses to 5-HT, pinch and heat in 5-HT-responsive neurons, represented by their corresponding colors (in C). *P < 0.05, **P < 0.005 (5-HT vs. pinch and heat; differences in ISI distribution for pinch vs. heat were not studied). For B, linear spacing between x-axis tick marks. For D, logarithmic spacing between x-axis labels. For these analyses, the same sample of responsive VcPbT neurons was used as described in Figs. 4 and 7. Error bars indicate standard error of the mean. Statistical tests: Kruskal-Wallis ANOVA with Mann-Whitney U-tests as post hoc tests.
Twenty-two (67%) of the 33 VcPbT neurons tested with all pruritogens and partial pruritogens were responsive to at least one of these agents. Table 1 summarizes the responses of VcPbT neurons to pruritic and algogenic agents. The majority of pruriceptive neurons were classified as HT or WDR. A few neurons responsive to 5-HT, histamine, chloroquine, and/or capsaicin were classified as LT. Twenty (61%) of the total population of 33 pruriceptive VcPbT neurons were excited by heat, which was significantly more than the proportion of heat-excited nonpruriceptive neurons [3 out of 13 neurons (23%); P = 0.022, Pearson χ2-test]. No differences were noted in the responsiveness to cold: 12 pruriceptive neurons (36%) and 5 nonpruriceptive neurons (38%) were excited by cold.
Table 1.
Classification of VcPbT neurons responsive to various agents
| Mechanical Classification |
Thermal Classification |
Recording Point |
||||||
|---|---|---|---|---|---|---|---|---|
| Agent | Responsive | HT | WDR | LT | Heat | Cold | sDH | dDH |
| 5-HT | 28 of 45 (62%) | 12 (43%) | 12 (43%) | 4 (14%) | 16 (57%) | 11 (39%) | 24 (86%) | 4 (14%) |
| β-ALA | 1 of 37 (3%) | 0 | 1 (100%) | 0 | 1 (100%) | 0 | 1 (100%) | 0 |
| HA | 11 of 37 (30%) | 4 (36%) | 5 (45%) | 2 (18%) | 6 (55%) | 3 (27%) | 9 (82%) | 2 (18%) |
| CQ | 13 of 41 (32%)* | 3 (23%) | 9 (69%) | 1 (8%) | 10 (77%) | 7 (54%) | 13 (100%) | 0 |
| CAP | 18 of 36 (50%) | 7 (39%) | 7 (39%) | 4 (22%) | 10 (56%) | 6 (33%) | 15 (83%) | 3 (17%) |
| MO | 8 of 23 (35%) | 3 (38%) | 5 (63%) | 0 | 8 (100%) | 5 (63%) | 8 (100%) | 0 |
Percentages represent proportions of the total population of neurons responsive to the agent. VcPbT, trigeminoparabrachial tract; HT, high threshold; LT, low threshold; WDR, wide dynamic range; sDH, superficial dorsal horn; dDH, deep dorsal horn; β-ALA, β-alanine; HA, histamine dihydrochloride; CQ, chloroquine diphosphate salt; CAP, capsaicin; MO, mustard oil.
Responsive neurons showed a decrease in firing rate following injection that met the criteria for an inhibitory response.
Figure 6 shows responses of different classes of pruriceptive neurons based on the location of the recording point (Fig. 6 A) and on their responsiveness to heat (Fig. 6 B). VcPbT neurons in the sDH responded to 5-HT significantly longer than did neurons in the dDH (Fig. 6A, inset). No significant differences were noted between neurons responsive and unresponsive to heat (Fig. 6B).
Fig. 6.
Mean responses to 5-HT, histamine, and capsaicin of VcPbT neurons. A: mean response histograms of subpopulations of 5-HT and capsaicin-responsive VcPbT neurons based on location in the superficial (black) or deep (gray) dorsal horn. Insets: mean firing rate (5-HT inset I: firing rate during first 20 min after injection; CAP inset: firing rate during first 5 min after injection) and mean duration of response (5-HT inset II: calculated as in Fig. 4; *P < 0.05). B: responses of subpopulations of 5-HT, histamine, and capsaicin-responsive VcPbT neurons based on their response to heat: increase in firing rate (black) or inhibited/unresponsive (gray). Insets: mean firing rate (5-HT inset I and HA and CAP insets: during time period of the illustrated response histogram) and mean duration of response (5-HT inset II). Data reported in 30-s bins (5-HT) and 15-s bins (HA and CAP). Same sample of responsive VcPbT neurons was used as described in Fig. 4. Data was only included when the sample of the subpopulation was greater than or equal to 3 neurons. Error bars indicate means ± SE. Agents were injected at time point zero. Statistical tests: Mann-Whitney U-test.
Inhibitory effect of chloroquine on VcPbT neurons.
None of the examined VcPbT neurons were activated by chloroquine. Thirteen of 41 neurons (32%) were inhibited by chloroquine, and all were located in the sDH (Table 1). Twelve (92%) of these neurons were identified as pruriceptive. The mean response to injection of chloroquine is shown in Fig. 7. In several cases, inhibition lasted for more than 60 min and often did not return to prestimulus baseline without manipulation of the receptive field. We also examined the inhibitory effect of chloroquine on the responses of VcPbT neurons to innocuous and noxious mechanical stimuli. In seven neurons, baseline responses to brush and pinch stimuli were compared with responses to the same stimuli applied 20–50 min after injection of chloroquine. Responses to both stimuli were reduced by roughly two-thirds after injection of chloroquine (Fig. 7, insets I–IV).
Fig. 7.
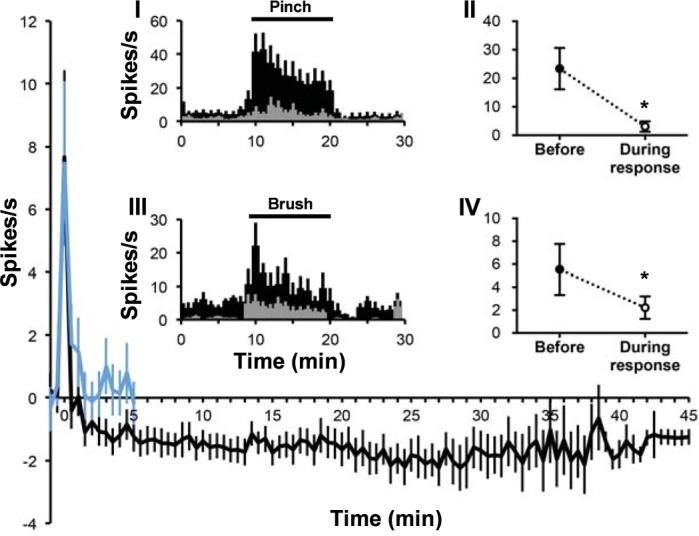
Mean response to chloroquine. Mean response curve of chloroquine-responsive VcPbT neurons (n = 11) to chloroquine (black) and vehicle (blue). Insets I and III: mean response histograms of a subset of chloroquine-inhibited neurons to pinch (I; n = 7) and brush (III; n = 5) before (in black) and during the response to chloroquine (in gray; 20–50 min after injection). Insets II and IV: mean firing frequency during pinch (II) and brush (IV) before and during the response to chloroquine. *P = 0.018 (II) and *P = 0.043 (IV). Every data point in response curve represents a 30-s bin. For insets II and IV, data were corrected for baseline activity by subtracting mean firing rate in 10 s preceding the stimulus. Statistical tests: Wilcoxon signed ranks test.
Recording points and projection targets.
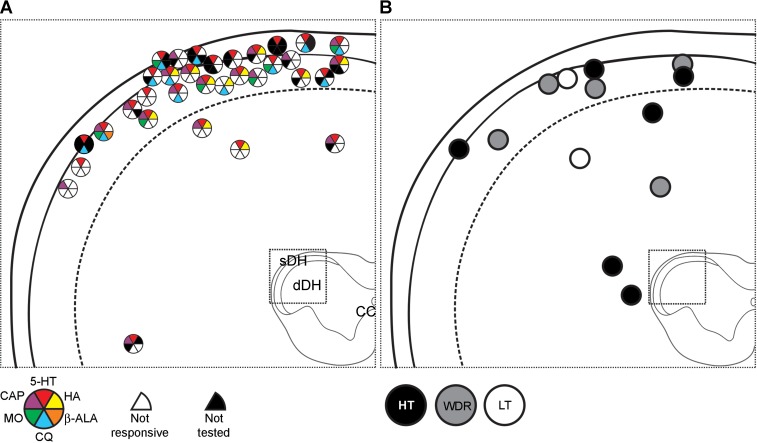
Lesions at recording points were recovered for all VcPbT neurons and were located in the medullary dorsal horn or in C1 or C2 (Fig. 8). One neuron was excluded from the figure as it was not tested with most pruritogens and therefore could not be identified as pruriceptive or nonpruriceptive. The majority (29 of 33, 88%) of pruriceptive VcPbT neurons were recorded in the sDH. However, the proportions of pruriceptive VcPbT neurons recorded within the sDH (29 of 37, 78%) and dDH (4 of 9, 44%) did not differ significantly. All VcPbT neurons responsive to mustard oil were located in the sDH, as well as all neurons inhibited by chloroquine. No apparent clustering of subpopulations of pruriceptive neurons was noted, other than the aforementioned absence of neurons responsive to chloroquine or mustard oil in the dDH (Fig. 8A). No site in dorsal horn could be identified in which nonpruriceptive neurons were concentrated (Fig. 8B).
Fig. 8.
Recording points of all VcPbT neurons (n = 46). A: recording points of pruriceptive neurons at the level of C1. Responsiveness to the different agents tested is indicated by colored wedges and their position in the pie chart. A white wedge at the position of the respective agent indicates that the neuron was unresponsive to it; a black wedge indicates that the neuron was not tested for this agent. B: recording points of nonpruriceptive neurons and their mechanical classification. All recording point are illustrated at the level of C1, as all observed lesions were located in this segment or at the junction of this segment with the lower medulla or C2. HT, high threshold; LT, low threshold; WDR, wide dynamic range.
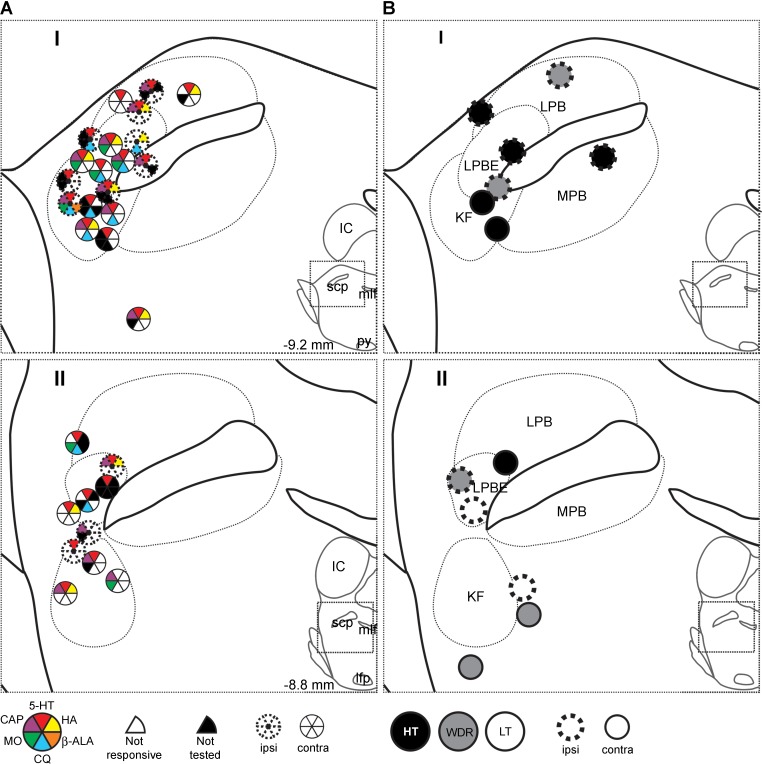
Lesions in the ipsilateral or contralateral Pb and KF nuclei at antidromic LTPs were recovered for all neurons: 25 (53%) were antidromically activated from the contralateral Pb, 19 (40%) were activated from the ipsilateral Pb, and 3 (6%) were bilaterally activated from the Pb. Forty-three neurons (91%) were surrounded rostrally by current pulses >300 μA. Lesions at LTPs were for the large majority distributed over an area involving the KF nucleus and the external lateral Pb (Fig. 9). No association was noted between the location of the LTP and the responsiveness to a specific agent (Fig. 9A) or the mechanical classification of the neuron (Fig. 9B).
Fig. 9.
LTPs of all rostrally surrounded VcPbT neurons (n = 42). A: LTPs of pruriceptive neurons in or close to the Pb and Kölliker-Fuse nucleus at a caudal (I) and more rostral (II) level. Color coding is similar as described for Fig. 9. B: LTPs of nonpruriceptive neurons and their mechanical classification. Ipsilateral projections are represented by dotted circles, contralateral projections are represented by solid circles. For all bilateral projecting neurons, only one LTP is illustrated.
The mean conduction velocity (±SD) of VcPbT neurons was 4.6 ± 2.0 m/s with a range of 2.4–11.7 m/s. These velocities indicate that the axons of VcPbT neurons are thinly myelinated. The conduction velocity for VcPbT neurons located in the sDH (4.0 ± 1.0; n = 38) was significantly lower than for those in the dDH (7.0 ± 2.9; n = 9; P = 0.008, Student's t-test).
DISCUSSION
We studied the responses of neurons located in the spinal trigeminal nucleus and the first and second cervical segments that projected to the ipsilateral and/or contralateral Pb. Responses were determined to innocuous and noxious mechanical and thermal stimulation of receptive fields located on the cheek. Responses of the same units to intradermal injection of pruritogens, to partial pruritogens, and to the algogen mustard oil were also examined. All characterized VcPbT neurons were responsive to mechanical stimuli and had a receptive field that included at least part of the cheek. The large majority of pruriceptive neurons were nociceptive, classified as HT or WDR. A small population of pruriceptive neurons were classified as LT.
Bester et al. (2000) found that 75% of SPbT neurons located in lamina I were classified as HT and 25% as WDR; no LT neurons were encountered. The vast majority of SPbT neurons in cats were also activated specifically by noxious stimuli in early studies (Hylden et al. 1986; Light et al. 1993). None of the examined neurons in these previous studies were examined for potential responses to pruritogens. We found that only 40% of VcPbT neurons were classified as HT, 44% WDR, and 15% as LT. These findings indicate that the overwhelming majority of both SPbT and VcPbT neurons are nociceptive and that a considerably higher percentage of VcPbT neurons (i.e., WDR and LT neurons) are capable of conveying information related to innocuous tactile stimulation (related to the face) to the lateral Pb.
Twenty-two neurons (67%) tested with all five pruritogens and partial pruritogens responded to at least one agent. The majority of these neurons responded to multiple pruritogens. Subsequent comparisons of recordings obtained from VcPbT neurons and data reported in a previous study of VcTT neurons (Moser and Giesler 2014a) suggested several clear differences between these two populations of neurons. First, the proportion of neurons responsive to injection of 5-HT was higher in VcPbT neurons. Sixty-two percent of the VcPbT neurons were activated by an intradermal injection of 5-HT, whereas only 27% of VcTT neurons tested were responsive to the same dose of 5-HT (Moser and Giesler 2014a). Second, the mean duration of the responses of VcPbT neurons to 5-HT was significantly longer (Fig. 4A), often enduring 60 min or more. Third, injection of 5-HT caused a significantly later peak in firing in VcPbT neurons than it did in VcTT neurons (Fig. 4A). Injection of 5-HT in the dose used in these experiments caused scratching that lasted for approximately 1 h and maximum levels of scratching occurred roughly 10 min after injection (Hachisuka et al. 2010; Klein et al. 2011; Moser and Giesler 2014b). Therefore, both the delayed peak of firing and the sustained duration of the responses of VcPbT neurons to injection of 5-HT better matched behavioral responses to injections of 5-HT. Fourth, VcPbT neurons that produced long duration responses to 5-HT were located in the sDH (Fig. 6A) whereas VcTT neurons that produced longer duration responses to 5-HT were located in the dDH (Moser and Giesler 2014a). Fifth, we found a small population of VcPbT neurons (n = 5), mechanically classified as LT, that responded to one or more pruritogens and/or partial pruritogens. None of the VcTT neurons classified as LT responded to pruritic agents (Davidson et al. 2012; Moser and Giesler 2014a). Additionally, all pruriceptive STT neurons recorded in monkeys were also responsive to noxious mechanical, thermal, and/or chemical stimuli (Davidson et al. 2007, 2009, 2012; Simone et al. 2004). Therefore, the ability of LT neurons to respond to pruritogens appears to be an unusual feature of the VcPbT. It should be noted that a small percentage of axons in the ALF of cats that responded to application of the pruritogen cowhage were classified as LT (Wei and Tuckett 1991). The brain areas to which the ALF axons projected were not determined in this previous study. It is possible that some of the pruriceptive LT axons recorded by Wei and Tuckett (1991) ascended to the Pb.
Intradermal injection of the algogen mustard oil induced a specific pattern of discharges in a relatively high-frequency range compared with discharges resulting from any of the pruritogens or partial pruritogens (Fig. 5B). Moreover, noxious heat and pinch induced higher frequency discharges than did 5-HT in the same neurons (Fig. 5D). These findings are in accordance with similar analyses in VcTT neurons (Moser and Giesler 2014a) and differences observed in responses to histamine and capsaicin, a painful agent in monkeys, in monkey STT neurons (Davidson et al. 2012; Moser and Giesler 2014a). This indicates that these differing populations of projection neurons in different species often respond to pruritic stimuli with lower frequency discharges and respond to noxious stimuli with relatively higher frequency discharges, an observation that is consistent with the intensity theory of coding of itch and pain, in which low levels of firing have been suggested to produce itch, whereas higher levels are suggested to produce pain (described in McMahon and Koltzenburg 1992; Davidson and Giesler 2010).1
Intradermal injection of histamine or capsaicin in the concentrations used in this study induces both scratching and wiping, indicating that both agents cause pain and itch in awake rats (Klein et al. 2011). Mean responses of VcPbT neurons to histamine and capsaicin match the time course of scratching after cheek injections in rats, as both mean behavioral and electrophysiological responses peaked within 5 min and lasted for <20 min (Fig. 4; Moser HR, Giesler GJ, unpublished observations). These findings suggest that VcPbT neurons might also contribute to itch produced by injection of histamine or capsaicin in rats.
An interesting finding in the present study is the profound inhibitory effect of chloroquine on a subset of VcPbT neurons, an effect not previously observed in rat VcTT neurons (Moser and Giesler 2014a) or in mouse sDH neurons (for which axonal projections were not determined) (Akiyama et al. 2012b, 2014). No other pruritogen or partial pruritogen caused inhibition of firing of VcPbT, VcTT (Moser and Giesler 2014a), or primate STT neurons (Davidson et al. 2012). Chloroquine-induced inhibition was only seen in VcPbT neurons recorded in the sDH. The majority of these neurons were activated by injection of 5-HT and some additionally by histamine and/or capsaicin. Thus there appears to be a fundamental difference in the responses of VcPbT neurons to injections of chloroquine vs. those induced with 5-HT, histamine, or capsaicin. Differences between chloroquine and other pruritogens have been noted previously. In a behavioral study on the potential of different pruritogens to produce touch-evoked itch (alloknesis) in mice, in contrast to histamine and 5-HT, chloroquine was found not to cause alloknesis (Akiyama et al. 2012a). Furthermore, in mice, activity-dependent peripheral blockade of chloroquine-induced itch did not affect histamine-induced itch and vice versa, suggesting that different sets of primary afferent neurons are required for itch sensation resulting from the two pruritogens (Roberson et al. 2013). A subset of primary afferent neurons expresses the receptor for chloroq MrgprA3 (Liu et al. 2009). It might be important in future studies to determine whether the inhibitory responses of VcPbT neurons are mediated through MrgprA3 receptors. It is possible that MrgprA3-expressing dorsal root ganglion neurons in rats activate inhibitory interneurons in the dorsal horn that project to VcPbT neurons, a possibility that could also be evaluated in future studies. It should be noted that it is unclear at present whether the inhibitory responses of VcPbT neurons contribute to chloroquine-induced itch in rats.
Anatomical studies indicate that the majority of VcPbT neurons project to the ipsilateral Pb. Lesser numbers project to the contralateral Pb (Cechetto et al. 1985; Feil and Herbert 1995). It has also been reported that ∼20% of the total population of lamina I neurons project to the Pb bilaterally (Li and Li 2000; Yamada and Kitamura 1992). Roughly half of the VcPbT neurons examined in the present study projected to the contralateral Pb, and a slightly smaller number projected to the ipsilateral Pb. In addition, three neurons (6%) were antidromically activated bilaterally from LTPs in the Pb. It should be pointed out that our methods were not ideal for detection of bilaterally projecting VcPbT neurons. We could not antidromically activate VcPbT neurons using high amplitude pulses delivered in a large number of points at levels rostral to the large majority of LTPs, indicating that the examined axons or axonal branches terminated within the Pb or KF. It is likely that information carried by these axons reached an area in close proximity of the LTP. It is possible that additional axonal branches from the axons of the examined neurons terminated in other targets in the brain.
Bester et al. (2000) used similar antidromic activation techniques to determine the areas of the Pb to which SPbT neurons project in rats. The LTPs in their study were distributed throughout the lateral Pb, but few were located in KF. In the present study, LTPs were concentrated farther ventrally within or close to the external lateral PB and KF, in accordance with anatomical data on termination zones of trigeminal projecting neurons (Cechetto et al. 1985; Feil and Herbert 1995). It has been reported that the dendritic trees of Pb neurons responsive to mechanical stimulation of the facial area are located primarily in this area (Bourgeais et al. 2003). Our results suggest that VcPbT neurons provide sensory input to these neurons.
Anterograde tracing techniques have shown that neurons located in the lateral Pb nucleus project densely to the hypothalamus and amygdala in rats (Bernard et al. 1993; Bester et al. 1997). A smaller number of lateral Pb neurons project to the periaqueductal grey, ventrolateral medulla, and nucleus of the solitary tract (Gauriau and Bernard 2002; Herbert et al. 1990; Saper and Loewy 1980). The more medially located internal lateral Pb nucleus contains large numbers of neurons projecting to various medial thalamic nuclei (Bester et al. 1999; Fulwiler and Saper 1984). These various projection targets of Pb neurons have been hypothesized to be implicated in behavioral, autonomic, and emotional responses to noxious stimuli (Gauriau and Bernard 2002). The present results suggest that these projections might additionally be involved in affective and autonomic responses to itch. It might be valuable in future studies to determine whether the comparable projections play similar roles in primates.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants P01-NS-047399 (to G. J. Giesler) and NS-062158 (core funding).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.A.J. and G.J.G.J. conception and design of research; N.A.J. performed experiments; N.A.J. analyzed data; N.A.J. and G.J.G.J. interpreted results of experiments; N.A.J. and G.J.G.J. prepared figures; N.A.J. and G.J.G.J. drafted manuscript; N.A.J. and G.J.G.J. edited and revised manuscript; N.A.J. and G.J.G.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank H. Truong, Dr. X. Zhang, and B. Lipshetz for valuable technical assistance and R. Speltz and Drs. C. Honda and D. Simone for critically reading an early version of the manuscript.
Footnotes
The potential for the intensity theory as a foundation for understanding pruriception has been downplayed (McMahon and Kotzburg 1992; Akiyama and Carstens 2013), based principally on a report that electrical stimulation of skin can induce itch and that increasing the frequency of stimulation increased itch but did not induce pain, as the intensity theory would suggest (Tuckett 1982). However, it has not been shown that activation of pruriceptors with electric stimulation is capable of driving spinal neurons to high levels of firing. It is clear that activation of pruriceptors using doses of pruritogens that produce intense scratching only induces low-frequency discharge in the hundreds of spinal neurons that have been examined to date; all studies of pruriceptive responses of spinal neurons in mice, rats and monkeys have shown that, regardless of the pruritogen employed, itch-producing stimuli induce lower frequency (almost always <10 spikes/s) discharges (Akiyama et al. 2009, 2012b, 2014; Davidson et al. 2007, 2009, 2012; Moser and Giesler 2014a). In contrast, in these same neurons, noxious stimuli frequently induce higher levels of firing. Therefore, it is possible that electrical activation of pruriceptors by Tuckett (1982) induced low levels of firing in dorsal horn neurons. If so, that study was not a valid test of intensity theory, at least as it applies to transmission of information related to pain and itch by spinal cord neurons.
REFERENCES
- Akiyama T, Carstens E. Neural processing of itch. Neuroscience 250: 697–714, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis). J Invest Dermatol 132: 1886–1891, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Carstens MI, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J Neurosci 29: 6691–6699, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Carstens MI, Carstens EE. Site-dependent and state-dependent inhibition of pruritogen-responsive spinal neurons by scratching. Eur J Neurosci 36: 2311–2316, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Tominaga M, Carstens MI, Takamori K, Carstens E. Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain 155: 80–92, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain 33: 149–160, 1988. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol 329: 201–229, 1993. [DOI] [PubMed] [Google Scholar]
- Bester H, Besson JM, Bernard JF. Organization of efferent projections from the parabrachial area to the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 383: 245–281, 1997. [DOI] [PubMed] [Google Scholar]
- Bester H, Bourgeais L, Villanueva L, Besson JM, Bernard JF. Differential projections to the intralaminar and gustatory thalamus from the parabrachial area: a PHA-L study in the rat. J Comp Neurol 405: 421–449, 1999. [PubMed] [Google Scholar]
- Bester H, Chapman V, Besson JM, Bernard JF. Physiological properties of the lamina I spinoparabrachial neurons in the rat. J Neurophysiol 83: 2239–2259, 2000. [DOI] [PubMed] [Google Scholar]
- Bickford RG. Experiments relating to the itch sensation, its peripheral mechanism, and central pathways. Clin Sci 3: 377–386, 1938. [Google Scholar]
- Bourgeais L, Gauriau C, Monconduit L, Villanueva L, Bernard JF. Dendritic domains of nociceptive-responsive parabrachial neurons match terminal fields of lamina I neurons in the rat. J Comp Neurol 464: 238–256, 2003. [DOI] [PubMed] [Google Scholar]
- Carstens E. Responses of rat spinal dorsal horn neurons to intracutaneous microinjection of histamine, capsaicin, and other irritants. J Neurophysiol 77: 2499–2514, 1997. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol 240: 153–160, 1985. [DOI] [PubMed] [Google Scholar]
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci 33: 550–558, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ Jr. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol 108: 1711–1723, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ Jr. Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci 12: 544–546, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ Jr. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci 27: 10007–10014, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil K, Herbert H. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kolliker-Fuse nuclei. J Comp Neurol 353: 506–528, 1995. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res Rev 7: 229–259, 1984. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol 87: 251–258, 2002. [DOI] [PubMed] [Google Scholar]
- Hachisuka J, Furue H, Furue M, Yoshimura M. Responsiveness of C neurons in rat dorsal root ganglion to 5-hydroxytryptamine-induced pruritic stimuli in vivo. J Neurophsiol 104: 271–279, 2010, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, Lamotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16: 174–182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990. [DOI] [PubMed] [Google Scholar]
- Hylden JLK, Hayashi H, Bennett GJ, Dubner R. Spinal lamina I neurons projecting to the parabrachial area of the cat midbrain. Brain Res 336: 195–198, 1985. [DOI] [PubMed] [Google Scholar]
- Hylden JLK, Hayashi H, Dubner R, Bennett GJ. Physiology and morphology of the laminaI spinomesencephalic projection. J Comp Neurol 247: 505–515, 1986. [DOI] [PubMed] [Google Scholar]
- Hyndman OR, Wolkin J. Anterior chordotomy: further observations on physiologic results and optimum manner of performance. Arch Neurol Psychiatry 50: 129, 1943. [Google Scholar]
- Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol 87: 1280–1289, 2002. [DOI] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci 28: 7659–7669, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol 106: 1078–1088, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci 15: 19–31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Shimada SG, Sikand P. Mouse models of acute, chemical itch and pain in humans. Exp Dermatol 20: 778–782, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li YQ. Collateral projection of substance P receptor expressing neurons in the medullary dorsal horn to bilateral parabrachial nuclei of the rat. Brain Res Bull 53: 163–169, 2000. [DOI] [PubMed] [Google Scholar]
- Light AR, Sedivec MJ, Casale EJ, Jones SL. Physiological and morphological characteristics of spinal neurons projecting to the parabrachial region of the cat. Somatosen Mot Res 10: 309–325, 1993. [DOI] [PubMed] [Google Scholar]
- Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X. Mechanisms of itch evoked by beta-alanine. J Neurosci 32: 14532–14537, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139: 1353–1365, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest 120: 3773–3778, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci 15: 497–501, 1992. [DOI] [PubMed] [Google Scholar]
- Mehler WR, Feferman ME, Nauta WJ. Ascending axon degeneration following anterolateral cordotomy. An experimental study in the monkey. Brain 83: 718–750, 1960. [DOI] [PubMed] [Google Scholar]
- Merrill AW, Carstens MI, Carstens E. Low percentage of potential itch-signaling superficial spinal neurons project in spinothalamic or spinoparabrachial pathways in rat. Acta Derm Venereol 87: 475, 2007. [Google Scholar]
- Moser HR, Giesler GJ Jr. Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons. J Neurosci 33: 6093–6101, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser HR, Giesler GJ Jr. Characterization of pruriceptive trigeminothalamic tract neurons in rats. J Neurophysiol 111: 1574–1589, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser HR, Giesler GJ Jr. Itch elicited by intradermal injection of serotonin, intracisternal injection of morphine, and their synergistic interactions in rats. Neuroscience 274: 119–127, 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJ, Kuypers HG. Some ascending pathways in the brain stem reticular formation. In: Reticular Formation of the Brain, edited by Jasper HH, Proctor LD. Boston, MA: Little, Brown, 1958, p. 3–30, [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed) New York: Academic, 1998. [DOI] [PubMed] [Google Scholar]
- Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, Binshtok AM, Woolf CJ. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci 16: 910–918, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res 197: 291–317, 1980. [DOI] [PubMed] [Google Scholar]
- Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain 139: 681–687, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ Jr. Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol 91: 213–222, 2004. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11: 823–836, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckett RP. Itch evoked by electrical stimulation of the skin. J Invest Dermatol 79: 368–373, 1982. [DOI] [PubMed] [Google Scholar]
- Tuckett RP, Wei JY. Response to an itch-producing substance in cat. II. Cutaneous receptor populations with unmyelinated axons. Brain Res 413: 95–103, 1987. [DOI] [PubMed] [Google Scholar]
- Wei JY, Tuckett RP. Response of cat ventrolateral spinal axons to an itch-producing stimulus (cowhage). Somatosen Mot Res 8: 227–239, 1991. [DOI] [PubMed] [Google Scholar]
- Yamada J, Kitamura T. Spinal cord cells innervating the bilateral parabrachial nuclei in the rat A retrograde fluorescent double-labeling study. Neurosci Res 15: 273–280, 1992. [DOI] [PubMed] [Google Scholar]