Abstract
Both dorsolateral prefrontal and posterior parietal cortex have been implicated in spatial working memory and representation of task information. Prior experiments training animals to recall the first of a sequence of stimuli and examining the effect of subsequent distractors have identified increased ability of the prefrontal cortex to represent remembered stimuli and filter distractors. It is unclear, however, if this prefrontal functional specialization extends to stimuli appearing earlier in a sequence, when subjects are cued to remember subsequent ones. It is also not known how task information interacts with persistent activity representing remembered stimuli and distractors in the two areas. To address these questions, we trained monkeys to remember either the first or second of two stimuli presented in sequence and recorded neuronal activity from the posterior parietal and dorsolateral prefrontal cortex. The prefrontal cortex was better able to represent the actively remembered stimulus, whereas the posterior parietal cortex was more modulated by distractors; however, task effects interfered with this representation. As a result, large proportions of neurons with persistent activity and task effects exhibited a preference for a stimulus when it appeared as a distractor in both areas. Additionally, prefrontal neurons were modulated to a greater extent by task factors during the delay period of the task. The results indicate that the prefrontal cortex is better able than the posterior parietal cortex to differentiate between distractors and actively remembered stimuli and is more modulated by the task; however, this relative preference is highly context dependent and depends on the specific requirements of the task.
Keywords: intraparietal sulcus, working memory, principal sulcus, neuron, neurophysiology
cognitive functions such as working memory and task switching have been traditionally viewed as the domain of the prefrontal cortex (PFC; Goldman-Rakic 1987; Miller and Cohen 2001), although neurons in other areas of the association cortex also exhibit neural correlates of these cognitive operations. The posterior parietal cortex (PPC) is such an area that is activated in a wide range of cognitive tasks (Bisley and Goldberg 2010; Fitzgerald et al. 2012; Rawley and Constantinidis 2009). The relative roles of prefrontal and parietal areas in the execution of cognitive functions are not entirely clear. In principle, cognitive functions may be fully distributed across several brain areas performing these functions in parallel; alternatively, coactivated areas may still be specialized for distinct roles in these functions (Katsuki and Constantinidis 2012). Some specialization appears to exist with respect to spatial working memory. Prefrontal neurons are better able to represent the location of a stimulus actively held in memory and to resist the effect of distractors, while posterior parietal neurons appear to represent the most recent stimulus whether it is a target or distractor (Constantinidis and Steinmetz 1996; di Pellegrino and Wise 1993; Qi et al. 2010; Suzuki and Gottlieb 2013).
The ability of prefrontal neurons to filter distractors has been demonstrated across a variety of behavioral tasks in the studies referenced above and lends credence to the idea of a unique role of the PFC during working memory. However, it is notable that all these experiments involved memory for the first of a series of stimuli and evaluated the effect of subsequent distractors. It is not known if the ability of the PFC to filter distractors extends to stimuli appearing earlier in a sequence, when subjects are cued to remember subsequent ones. Neurons selective for the order of stimulus presentation have been demonstrated in the PFC in tasks requiring memory of multiple objects (Berdyyeva and Olson 2010; Inoue and Mikami 2006), and it is unclear if this task preference is subordinate to filtering of distractors.
The effect of different tasks on neuronal activity is also important. The activity of both prefrontal neurons (Hoshi et al. 1998; Wallis et al. 2001; Wallis and Miller 2003; White and Wise 1999) and posterior parietal neurons (Rawley and Constantinidis 2010; Stoet and Snyder 2004) is modulated by task rules. By some accounts, the PFC is critical for the representation of task information, as evidenced by the profound deficits in the ability to maintain task information in memory and switch between tasks after prefrontal lesions (Buckley et al. 2009; Rossi et al. 2007). However, it is unknown how the representation of stimulus and distractor information in neural activity is influenced by different task rules in the prefrontal compared with the parietal cortex.
We were thus motivated to examine the responses of neurons in the dorsolateral prefrontal (dlPFC) and PPC in tasks that required monkeys to remember either the first or second of two stimuli appearing in sequence. Our experiment addressed whether the PFC is better able to filter distractors appearing either before or after the actively remembered stimulus, which was used to guide an eye movement, and how task information influenced this representation in the two areas.
METHODS
Two male, rhesus monkeys (Macaca mulatta) weighing 7–9 kg were used in this study. All surgical and animal use procedures in this study followed guidelines by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals and the National Research Council's Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Wake Forest University Institutional Animal Care and Use Committee.
Surgery and neurophysiology.
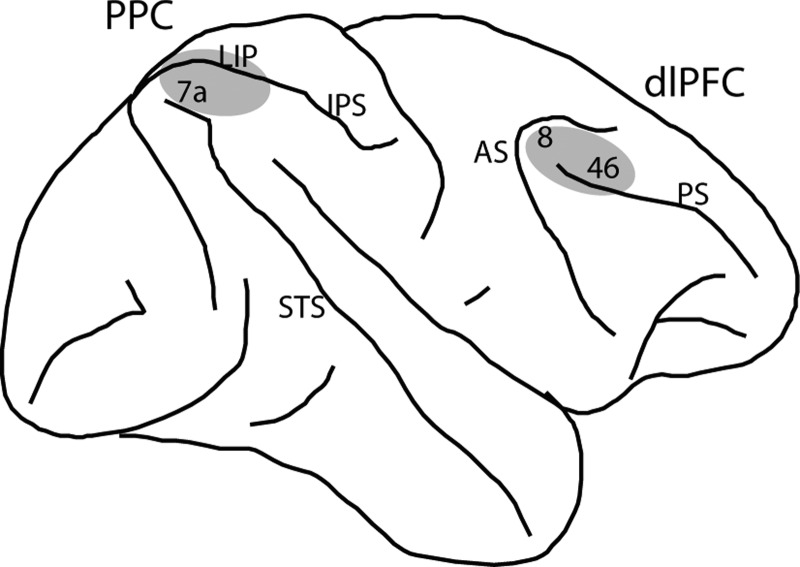
Two 20-mm diameter recording cylinders were implanted over the dlPFC and the PPC of the same hemisphere in each monkey (Fig. 1A). Extracellular activity of single units was recorded using arrays of two to eight microelectrodes in each cylinder, either with glass-coated, tungsten microelectrodes (250-μm diameter, impedance of 1 MΩ at 1 kHz; Alpha-Omega Engineering, Nazareth, Israel) or epoxylite-coated tungsten microelectrodes (125- or 250-μm diameter; impedance of 4 MΩ at 1 KHz; FHC, Bowdoin, ME). Electrodes were advanced individually into the cortex with a microdrive system (EPS drive; Alpha-Omega Engineering). The electrical signal from each electrode was amplified, band-pass filtered between 500 and 8 kHz, and recorded with a modular data acquisition system at a 25-μs resolution (APM system; FHC). The anatomical location of electrode penetration was determined based on MR imaging of the brain. Neuronal data were collected from areas 8a and 46 of dlPFC including both banks of the principal sulcus and areas 7a and the lateral intraparietal area of the PPC in the lateral bank of the intraparietal sulcus.
Fig. 1.
A: schematic diagram of the monkey brain, highlighting areas where recordings were performed. Recordings in posterior parietal cortex (PPC) sampled areas 7a and lateral intraparietal area (LIP). Recordings in dorsolateral prefrontal cortex (dlPFC) sampled areas 8 and 46. IPS, intraparietal sulcus; STS, superior temporal sulcus; AS, arcuate sulcus; PS, principal sulcus.
Behavioral tasks.
The monkeys faced a computer monitor 60 cm away in a dark room with their head fixed. Eye position was sampled at 240 Hz, digitized, and recorded with an infrared eye position tracking system (model RK-716; ISCAN, Burlington, MA). The visual stimulus presentation and behavior monitoring were controlled by in-house software (Meyer and Constantinidis 2005) implemented in the MATLAB computational environment (Mathworks, Natick, MA), using the Psychophysics Toolbox (Brainard 1997).
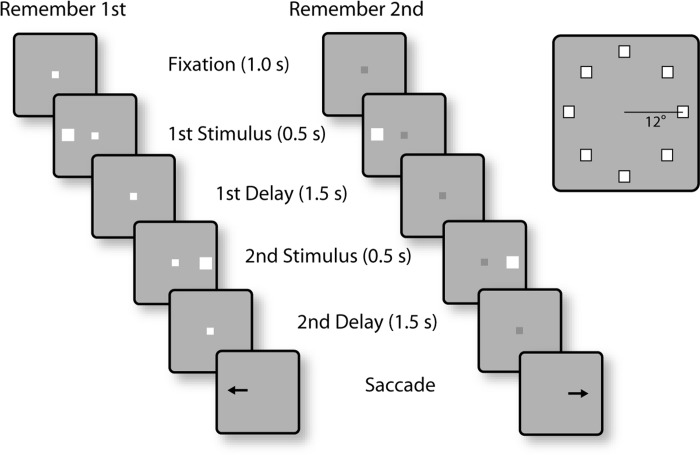
The tasks used in the present study were variations of the oculomotor delayed response task (Funahashi et al. 1989) but involved two stimuli appearing in sequence, requiring the monkey to remember and make an eye movement to either the first or the second stimulus (see Figs. 2 and 6). The monkeys were trained to saccade to the location of the remembered stimulus according to the color of fixation point (Fig. 2). After the animals fixated at a white/blue square (0.2° in size) located at the center of the monitor for 1 s, two white squares (1.5° in size) were displayed sequentially for 0.5 s, with a 1.5-s intervening delay period (D1). The first stimulus (S1) was displayed pseudorandomly at one of eight locations arranged along a circular ring of 12° eccentricity, with a 45° angular separation between neighboring stimuli. The second stimulus (S2) always appeared at the diametric location relative to the first stimulus. After a second delay period of 1.5 s (D2), the monkeys were required to saccade to the location of the first stimulus if the fixation point was white in color (remember-first condition) and to the location of the second stimulus if the fixation point was blue (remember-second condition). To minimize the uncertainty about the stimulus to be remembered, the remember-first and remember-second conditions were presented in blocks of trials. The animal was required to perform eight correct trials of the remember-first task, involving presentation of the first stimulus in each of the eight locations in the ring, before the task alternated to the remember-second condition. The monkeys were rewarded with fruit juice after making a correct saccade. Breaking fixation exceeding a 2° window led to the immediate termination of the trial without reward.
Fig. 2.
Successive frames illustrate the sequence of stimulus presentations in the behavioral tasks. In the remember-1st task (left), the white color of the fixation point instructed the monkeys that the 1st stimulus should be remembered. After the 2 stimulus presentations, with delay periods intervening between them, the fixation point turned off and that cued the animal to make an eye movement towards the direction of the 1st stimulus. In the remember-2nd task (middle), the blue fixation point (dark gray) instructed them to remember and saccade to the location of the 2nd stimulus. The 1st stimulus in either task could appear at 1 of 8 locations arranged on a ring of 12° eccentricity (right). The 2nd stimulus appeared always diametric to the 1st. Blocks of remember-fist and remember-2nd trials alternated during recordings.
Fig. 6.
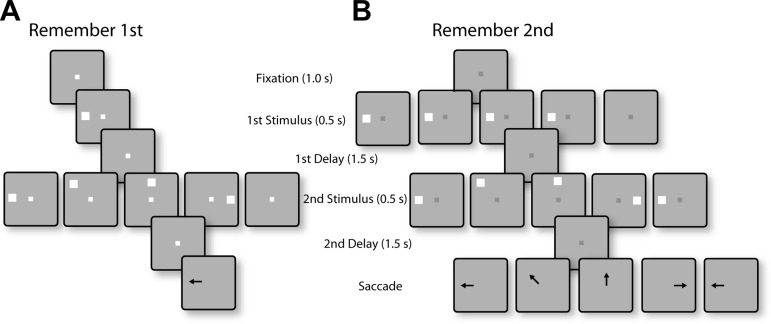
Successive frames illustrate the sequence of stimulus presentations in the randomized distractor tasks. A: in the remember-1st task, the 1st stimulus could appear at 1 of 2 locations; one typically in the receptive field of the neuron under study (shown as left location) and its diametric (not shown). Each 1st stimulus could be followed by 5 randomly interleaved conditions: a 2nd stimulus at the same location as the 1st, at a location offset by 45, 90, or 180°, or no 2nd stimulus (null condition). The monkey was required to make an eye movement to the 1st stimulus, regardless of the location of the distractor. B: in the remember-2nd task, the 1st stimulus (which was now a distractor) could again appear at 1 of the 2 locations, followed by a 2nd stimulus at the same possible locations relative to the 1st, as in the remember-1st task. The null condition in the remember-2nd task involved no stimulus presentation during the 1st interval. The monkey was required to make an eye movement to the location of the 2nd stimulus.
The two animals were also tested with a variation of the task, using stimulus sets where the second stimulus was randomized with respect to the first (see Fig. 6). The basic rules of the task and the set of possible stimulus locations remained the same, requiring the monkeys to make a saccade to the location of first stimuli when the fixation point was white and to the location of the second stimulus when it was blue. However, the location of the second stimulus varied with respect to the first stimulus and was either the same as S1 or offset (either clockwise or counterclockwise in different sets) by 45, 90, or 180°; a final condition involved no second stimulus presentation (see Fig. 6). The stimulus sets we used involved presentation of the first stimulus at only one of two possible locations: in the receptive field and diametric to it. These were followed by five possible second stimulus conditions as described above. Trials with white and blue fixation points were presented in blocks, also as described above. When these randomized-stimulus sessions were run, the monkey was first tested with a single-stimulus oculomotor delayed response task to map the receptive field of neurons recorded, so as to guide selection of the location of stimuli. The single-stimulus oculomotor delayed response task was not used in the diametric-distractor sets, as these sets involved presentation of the first and second stimulus in all eight locations, and mapping of the receptive field was not necessary ahead of time.
Behavioral performance.
We calculated behavioral performance as the percentage of trials that resulted in correct saccades into the target window. Trials that were aborted before end of the second delay period (due to premature saccades or blinks) were not included in this analysis. The target window was a 7° circle around the center of the stimulus. As the distance between adjacent targets was 9.2°, some saccades that landed closer to an adjacent location than the target itself were still rewarded. In this sense, our finding of lower performance for sequences of targets appearing at adjacent locations (see Fig. 3B) was conservative.
Fig. 3.
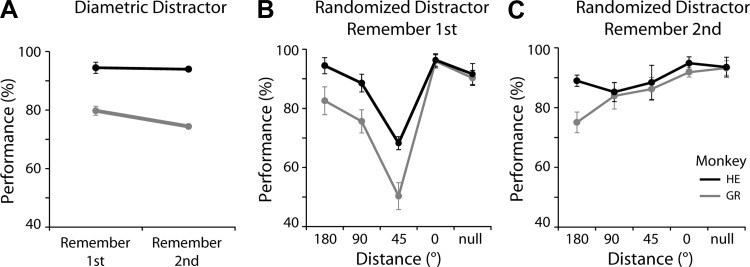
A: behavioral performance (percentage of trials that ended in correct responses) in the task using diametric distractors, during recording sessions. Black (monkey HE) and gray (monkey GR) represent the 2 animals in the remember-1st and remember-2nd conditions (n = 28, 31 sessions for monkeys GR and HE, respectively). Error bars represent means ± SE across daily sessions. B: behavioral performance in the remember-1st task, with randomized distractors. Different 2nd-stimulus conditions are depicted in the abscissa based on their angular distance from the 1st stimulus. In the null condition, no 2nd stimulus was presented. C: behavioral performance in the remember-2nd task, with randomized distractors. In the null condition, no 1st stimulus was presented.
Neural data analysis.
Recorded spike waveforms were sorted into separate units using an automated cluster analysis method referred to as the KlustaKwik algorithm (Harris et al. 2000), which applied principal component analysis of the waveforms. Neurons with significant elevation of firing rate during the presentation of delay periods were identified by comparing the firing rate in the 1.5-s interval of the delay period with the 1-s interval of fixation (paired t-test, P < 0.05). Data from correct trials only are presented here. Most analysis was performed on neurons with significantly elevated responses during the first delay period. The stimulus location that elicited the best response in this period was identified, and this location was used for comparison between tasks.
To quantify the relative selectivity for remembered stimuli and distractors, we calculated three selectivity indexes (SI1–3). In each case, the selectivity index compares the mean discharge rate following different stimuli in the same delay period, as follows:
1) SI1 = (distractor in − distractor out)/(distractor in + distractor out), for the second delay period of the remember-first task.
2) SI2 = (distractor in − distractor out)/(distractor in + distractor out), for the second delay period of the remember-second task.
3) SI3 = (remembered − distractor)/(remembered + distractor), for the first delay period, remembered stimulus from the remember-first task, and distractor from the remember-second task.
RESULTS
Neurophysiological data were collected from areas 8a and 46 (Fig. 1) of the dlPFC and areas 7a and lateral intraparietal area of the PPC of two monkeys as they performed working memory tasks. The monkeys were trained to remember one of two stimuli presented in sequence depending on the color of the fixation point and make a saccade towards it (Fig. 2). A white fixation point instructed them to remember the location of the first stimulus; we refer to this as the “remember-first” condition. A blue fixation point indicated that the monkeys needed to saccade towards the second stimulus (“remember-second” condition). The monkeys received liquid rewards for correct responses. To reduce the ambiguity of the task, we ran the two conditions in blocks of trials rather than completely randomized. The monkeys needed to complete 8–10 correct trials in the remember-first condition (1 of each trial type) before switching to the remember-second condition. For both conditions, the stimuli appeared pseudorandomly in one of eight locations, arranged on a ring of 12° eccentricity (Fig. 2, right).
Database.
We collected a total of 552 neurons from the PPC (300 and 252 from monkeys GR and HE, respectively) and 426 neurons from the dlPFC (158 and 268 from monkeys GR and HE), across all experiments and task conditions. We initially used a stimulus set in which the second stimulus always appeared at a location diametric to the first, an arrangement that allowed us to test the effects of remembered stimulus and distractor (nonremembered stimulus) presentation in and out of the receptive field and to compare our results with those of previous studies using the identical remember-first task (Qi et al. 2010). A total of 220 neurons from dlPFC and 314 from PPC were recorded during this experiment. Both animals performed the remember-first task better (Fig. 3A), although the difference in performance was significant for only one monkey (paired t-test, t27 = 2.28, P < 0.05; t30 = 0.52, P > 0.6, for the 2 animals, respectively). Performance varied considerably when we used an alternative task, varying the location of the second stimulus (Fig. 3, B and C, discussed below). A total of 205 neurons from dlPFC and 235 from PPC were recorded with this randomized stimulus set.
First and second delay period activity.
In order for the monkeys to perform the task, they must be able to represent the relevant stimulus in neuronal activity. We focused particularly on persistent activity following the presentation of each stimulus, which provides a neural correlate of spatial working memory (Funahashi et al. 1989). Responses of a representative neuron from dlPFC are shown in Figure 4, with histograms arranged so as to indicate the location of the first stimulus presented in each trial. Based on the first stimulus presentation in the remember-first task, the neuron exhibited a large, bilateral receptive field, with strongest responses in the lower hemifield (Fig. 4A). Appearance of the second stimulus in the receptive field also elicited responses (e.g., S2 stimulus in top right histogram in Fig. 4A). Neuronal activity persisted after stimulus presentation in the first (D1) and second delay periods (D2). Importantly, switching between tasks produced systematic changes in neuronal activity. During the remember-second task (Fig. 4B), neuronal responses were generally decreased. This was most obvious for the activity in the first delay period (Fig. 4C). Analysis of such responses in the different epochs and conditions of the task allowed us to address how the neuronal activity differed for the remembered stimulus and the distractor, how activity changed when the animals were cued to remember the first or the second stimulus, and how activity differed between the two cortical areas we recorded from.
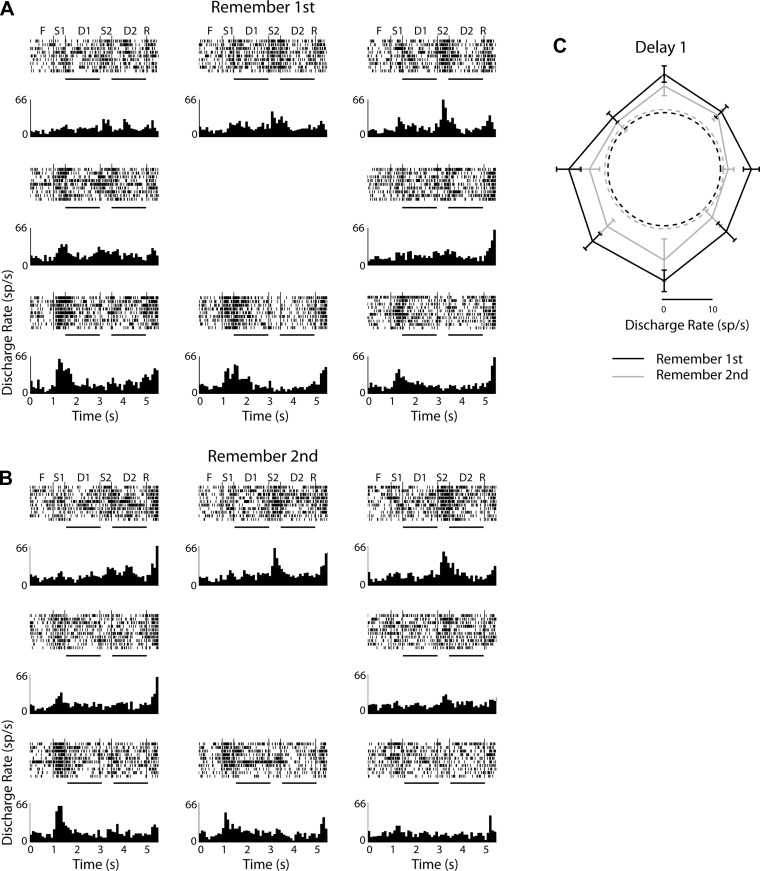
Fig. 4.
Rasters and histograms from 1 representative neuron recorded in the dlPFC with the diametric distractor set. A: responses in the remember-1st task. Histograms are arranged in so as to indicate the location of the 1st stimulus location in the screen. Labels over the rasters indicate fixation epoch (F), 1st stimulus (S1), 1st delay (D1), 2nd stimulus (S2), 2nd delay (D2), and response (R). Horizontal lines under rasters indicate the 1st and 2nd delay periods, which were used for analysis. B: responses in the remember-2nd task. C: polar plots depict responses during the 1st delay period in the remember-1st (black) and remember-2nd (gray) tasks. Dotted circles represent firing rate during the fixation period in the 2 tasks, averaged across all trials; sp/s, spikes per second.
We identified neurons with significantly elevated responses in the first delay period, compared with the fixation period (paired t-test, P < 0.05). A total of 107 such neurons were identified in the dlPFC (10 and 97 in monkeys GR and HE, respectively) and 95 in the PPC (22 and 73 in monkeys GR and HE, respectively). We then examined whether responses in the first delay period were greater overall in the population of neurons when the monkey was cued to remember the first stimulus. If the PFC selectively represents relevant stimulus information, we reasoned, neurons that selectively filter distractor activation should provide a neural correlate for this functional specialization. We performed a two-way ANOVA for the responses of each neuron, using spatial location and task (remember-first or remember-second) as factors, and identified neurons with significant task effects. In the first delay period, a total of 10% (11/107) neurons had a significant task effect (P < 0.05), with a preference for the remember-first task (Fig. 5A). In other words, these neurons had a higher overall preference to the remember-first condition over the remember-second condition for identical stimuli, presented at the best receptive field location, as would be expected by neurons representing actively remembered stimuli and filtering distractors. However, a twice as large population of neurons (23/107, 21%) exhibited the opposite preference: a significant task effect with an overall preference for the remember-second task, meaning higher responses to the first stimulus in the receptive field when the monkey was cued that it is the second stimulus to be remembered (Fig. 5B). In the second delay period, 16% (17/107) of the neurons had a significant task effect, 2% (2/107, less than the expected 2.5% false positive rate of the test) with a preference for the remember-first task, and 14% (15/107) with a preference for the remember-second task.
Fig. 5.
A: population peristimulus time histograms (PSTH) averaging activity from dlPFC neurons with significantly elevated activity in the 1st delay period and a significant task effect, with a preference for the remember-1st task (black) over the remember-2nd task (blue) in this interval (n = 11). Data are plotted for conditions with the 1st stimulus appearing in the receptive field (solid lines) and out of the receptive field (dotted lines). B: population PSTH of dlPFC neurons with elevated activity in the 1st delay period and an overall preference for the remember-2nd task (n = 23). C: population PSTH of PPC neurons with significantly elevated activity in the 1st delay period and significant task effect, with a preference for the remember-1st task (n = 7). D: population PSTH of PPC neurons with a preference for the remember-2nd task (n = 7). Insets next to the PSTH represent schematically the presentation of the 1st and 2nd stimulus relative to the receptive field (arc); the location of the receptive field and stimulus differed for each neuron.
Distractor-preferring neurons were not an exclusive property of the dlPFC either. The PPC also exhibited neurons that responded best to a stimulus when it appeared as a distractor (Fig. 5, C and D). Using the same two-way ANOVA test with spatial location and remember-first/remember-second task as factors, we found that in the first delay period 15% (14/95) of the neurons with elevated delayed period activity had a significant task effect (P < 0.05), with equal numbers preferring the remember-first (7/95) and the remember-second task (7/95). In the second delay period, 6% (6/95, close to the expected 5% false positive rate of the test) neurons had a significant task effect, again with comparable numbers of neurons preferring the remember-first task (3/95) and the remember-second task (3/95).
Randomized distractor.
An important caveat for the interpretation of these results is that the location of the second stimulus was not randomized with respect to the first. Therefore, the first stimulus was informative about the location of the impending saccade even when the animals were cued with the blue fixation point to remember the second stimulus. It is possible that the preference for the distractor stimulus we obtained in some neurons was only a consequence of the predictability of stimulus location in the task. This may have also been the reason that we failed to detect a greater ability of dlPFC than PPC to filter irrelevant stimuli (e.g., the absence of responses after the first stimulus in the remember-second task), since the location of the “distractor” stimulus could have been used by the monkeys to perform the task. For these reasons, we tested the same animals with a different stimulus set, which randomized the location of the second stimulus with respect to the first (Fig. 6).
As in the diametric-distractor set, the monkeys were cued by the color of the fixation point to remember either the first or second stimulus, and trials were presented in blocks, requiring the monkeys to correctly complete all conditions of each stimulus set (10 correct trials in this case) before moving to a block of trials with a different fixation color. In the remember-first task, the first stimulus could appear in one of two locations, one typically at the best location of the receptive field of the neuron under study (e.g., to the left of fixation as depicted in Fig. 6A) and its diametric location (not shown in Fig. 6A). Each of the first stimulus presentations was followed by 1 of 5 possible conditions involving the second stimulus (Fig. 6A), making up a total of 10 stimulus sequences. The second stimulus could appear at the same location as the first, at a location offset by 45°, at a location orthogonal to the first stimulus, and at a location diametric to it, or the second stimulus might not be presented at all (null condition). In the remember-second task, nearly identical stimulus sequences were used, with the only difference being that the null condition now involved no presentation of the first rather than the second stimulus (Fig. 6B). Additionally, the rewarded response saccade direction for each condition differed for each sequence, since the location of the second stimulus varied, and this was the stimulus that needed to be remembered.
The randomized stimulus sets were generally more challenging for the monkeys, as evidenced by lower performance, particularly for some of the conditions of the remember-first task. The lowest performance for both animals was observed when the second stimulus appeared at a location offset by 45° from the initial stimulus, which were to be remembered (Fig. 3B). Such pattern of behavior is predicted by models of working memory maintenance (Compte et al. 2000) and is similar to results from human studies (Herwig et al. 2010). Interestingly, performance in the remember-second task was markedly higher. It appears that the randomized stimulus location did not produce a behavioral challenge in the context of this task, even when the first stimulus was offset by 45° from the second one (Fig. 3C).
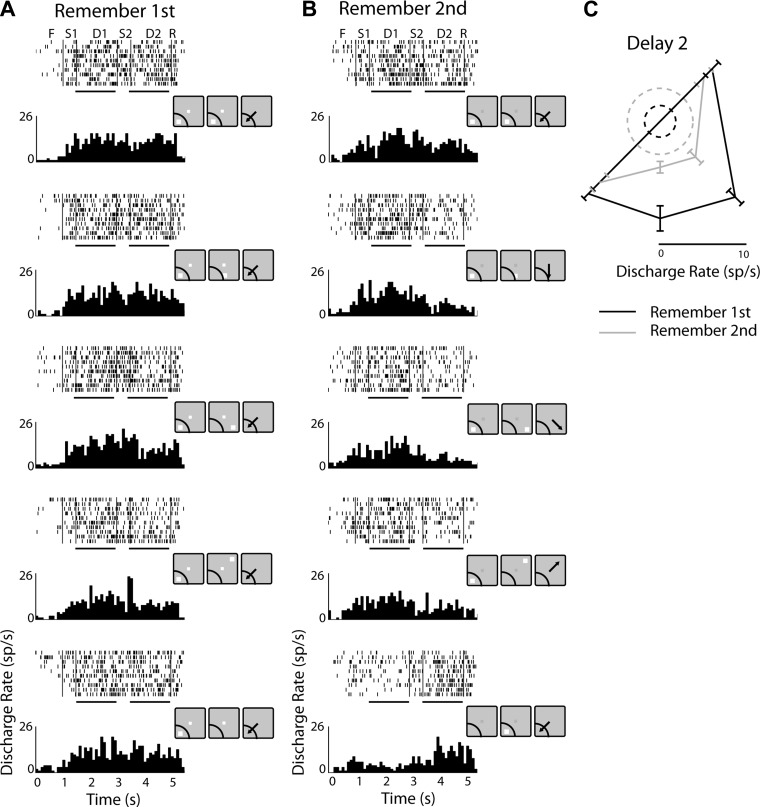
Using this stimulus set, we recorded neuronal responses from the dlPFC and PPC, as in the first phase of the experiments. Responses of one representative neuron from the dlPFC are shown in Fig. 7. In the remember-first task, the neuron exhibited sustained activity in the first delay period after the first stimulus presentation in the receptive field (Fig. 7A). This was modulated transiently during the presentation of the second stimulus, depending on its location, but generally persisted in the second delay period (D2) as well (see black trace in polar plot of Fig. 7C for the 4 available locations of the second stimulus). As was the case with the basic stimulus set, many neurons exhibited task effects and responded differently to identical stimulus sequences when the animal was cued to remember the first or second stimulus. The neuron depicted in Fig. 7 had markedly reduced activity in the second delay period of the remember-second task (Fig. 7, B and C). This change was partly explained by which stimulus needed to be remembered. For example, when the first stimulus needed to be remembered and that was in the receptive field, activity in the second delay period was higher compared with when the identical sequence required the second stimulus to be remembered, and that was not in the receptive field (compare 3rd and 4th rows of Fig. 7A with 3rd and 4th rows of Fig. 7B). However, changes were observed in many neurons also for conditions that involved presentation of the first and second stimulus in the same location (Fig. 7, A and B, top rows).
Fig. 7.
A: rasters and histograms from 1 representative neuron recorded in the dlPFC, tested with the randomized distractor stimulus set, in the remember-1st task. Insets next to each histogram represent the actual stimulus and receptive field location for this neuron. Horizontal lines under rasters indicate the 1st and 2nd delay periods, which were used for analysis B: responses of the same neuron in the remember-2nd task. C: polar plot depicting responses in the tested locations for the 2nd stimulus with the remember-1st (black) and remember-2nd task (gray). Dotted circles represent firing rate during the fixation period in the two tasks, averaged across all trials.
We proceeded to analyze responses across the population of neurons recorded in the dlPFC and PPC, again focusing on neurons with significantly elevated activity after a stimulus presentation. We recorded 75 neurons with persistent activity in the delay period after the first stimulus presentation in the dlPFC (47 and 28, from the monkeys GR and HE, respectively) and 85 such neurons in the PPC (21 and 64, from monkeys GR and HE, respectively). We were particularly interested to examine whether higher responses for a stimulus when it appeared as a distractor that we observed in the first phase of experiments were abolished for the stimulus set that randomized the stimulus location. Using a two-way ANOVA analysis of discharge rates in the first delay period, with factors spatial location and remember-first/remember-second task, revealed that this was not the case. A total of 23% (17/75) of dlPFC neurons had a significant task effect (P < 0.05), with the majority of those (11/75, 15%) having a preference for the remember-second task compared with 8% (6/75) that preferred the remember-first task. In the PPC, 12% (10/85) of neurons exhibited task effects during the first delay period, with 9% (8/85) exhibiting a preference for the remember-first task and 2% (2/85, less than the expected 2.5% false positive rate of the test) having a preference for the remember-second task.
Remembered stimulus vs. distractor.
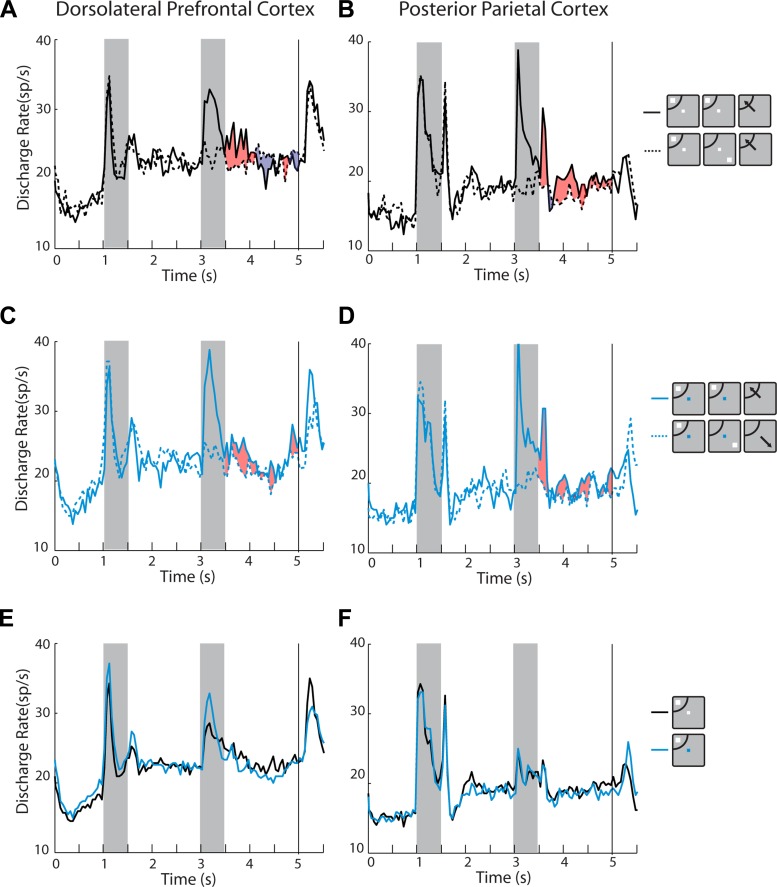
We next sought to determine whether systematic differences exist between the two brain areas in their ability to represent the remembered stimulus and to resist distractors, using the data from the randomized distractor set. We plotted activity from neurons with significantly elevated delay period activity and computed the difference between conditions. In the remember-first task, only a minor difference in dlPFC activity was observed when the second stimulus (distractor) appeared in the receptive field, or opposite to it (net positive difference is represented as red shading in Fig. 8A; net negative difference as blue shading). The difference between conditions was more pronounced for the PPC, indicating that neuronal activity diminished to a greater extent after the appearance of a distractor out of the receptive field, even though the monkey was required to remember the initial stimulus (Fig. 8B). The average value of the selectivity index SI1, which computes the difference in the second delay period of the remember-first task as follows, SI1 = (distractor in − distractor out)/(distractor in + distractor out) was −0.002 for the dlPFC and 0.096 for PPC, which was a significant difference (t-test, P < 0.01). Also, only the mean SI1 value for the PPC was significantly different from zero (one-sample t-test, P < 0.005 for PPC, P > 0.8 for dlPFC). It was notable that most of this difference between areas emerged late in the delay period (shaded areas, Fig. 8, A and B).
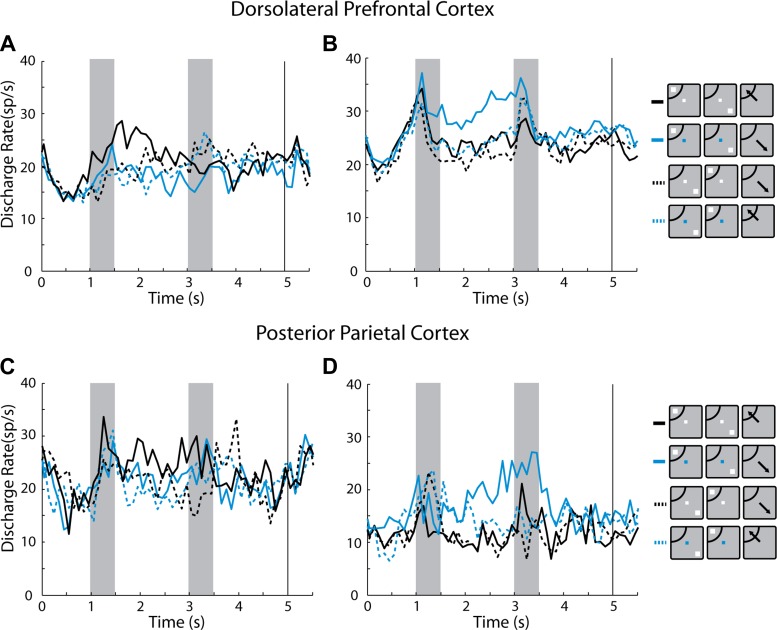
Fig. 8.
A: population PSTH averaging activity from dlPFC neurons with significantly elevated activity in the 1st delay period, with the randomized distractor set, in the remember-1st task (n = 75). Traces with solid and dotted lines differ in the location of the 2nd stimulus (distractor), in the receptive field or opposite to it, respectively. Red shaded area illustrates the positive difference between the two conditions; blue shaded area illustrates the negative difference (this is not an indication of statistical significance). B: same as in A for PPC neurons (n = 85). C: population PSTH of dlPFC neurons with elevated activity in the 1st delay period in the remember-2nd task (n = 75). Traces with solid and dotted lines differ in the location of the 1st stimulus (distractor) in the receptive field and opposite to it, respectively. Shaded area illustrates the (positive) difference between the 2 conditions (this is not an indication of statistical significance). D: same as in C, for PPC neurons (n = 85). E: population PSTH of neurons with elevated activity in the 1st delay period. Blue and black lines represent the remember-1st and remember-2nd conditions, respectively (n = 75). F: same as in E for PPC neurons (n = 85). Insets next to the PSTH represent schematically the presentation of the 1st and 2nd stimulus relative to the receptive field (arc), which differed for each neuron and so did the actual location of the stimulus.
To address more directly how well individual neurons in the remember-first task represented the remembered stimulus and distractors, we performed a two-way ANOVA on the mean firing rate of neurons in the second delay period, using as factors the location of the first stimulus and the location of the second stimulus. The ANOVA revealed that in the dlPFC 25% of neurons exhibited a significant (P < 0.05) main effect of the first (remembered) stimulus and only 12% of the second (distractor). In the PPC, the preference was reversed: 15% of the neurons exhibited a significant (P < 0.05) main effect of the first stimulus location, whereas 26% of the neurons exhibited a main effect for the second stimulus. Collectively, these analyses of the remember-first task demonstrated that prefrontal activity was better able to represent the first stimulus location and resist the effect of a distractor, a result replicating the effect of prior studies (Qi et al. 2010).
In the remember-second task, the effect of a distractor appearing out of the receptive field was more similar for the two areas (Fig. 8, C and D). Over the second delay period, both areas represented equally effectively the second, most recent stimulus, which was to be remembered, even when the first stimulus had appeared out of the receptive field. The average value of the selectivity index SI2 = (distractor in − distractor out)/(distractor in + distractor out) was 0.053 for the dlPFC and 0.095 for PPC that were not significantly different from each other (t-test, P > 0.05). Both values were significantly different from zero (one-sample t-test, P < 0.005 for both areas). A two-way ANOVA on the mean firing rate of neurons in the second delay period, using as factors the location of the first stimulus and the location of the second stimulus, revealed predominance of the second stimulus representation in both dlPFC and PPC. A total of 29% of PPC neurons exhibited a significant main effect for the second stimulus (P < 0.05), compared with 5% of neurons with a significant effect for the first stimulus (equal to the expected false positive rate of the test). In the dlPFC, the equivalent percentages were 16% and 15%, respectively.
Pooling data of the second delay period from both the remember-first and remember-second tasks and performing a three-way ANOVA with factors first stimulus location, second stimulus location, and task confirmed the dominance of second stimulus information in the PPC across tasks. A total of 39 (46%) of PPC neurons exhibited a significant (P < 0.05) main effect of the second stimulus location, whereas only 14 (16%) exhibited an effect of the first stimulus. In contrast, first stimulus information predominated in the dlPFC: 23 (31%) of neurons had a significant effect of first stimulus location and 17 (23%) of the second.
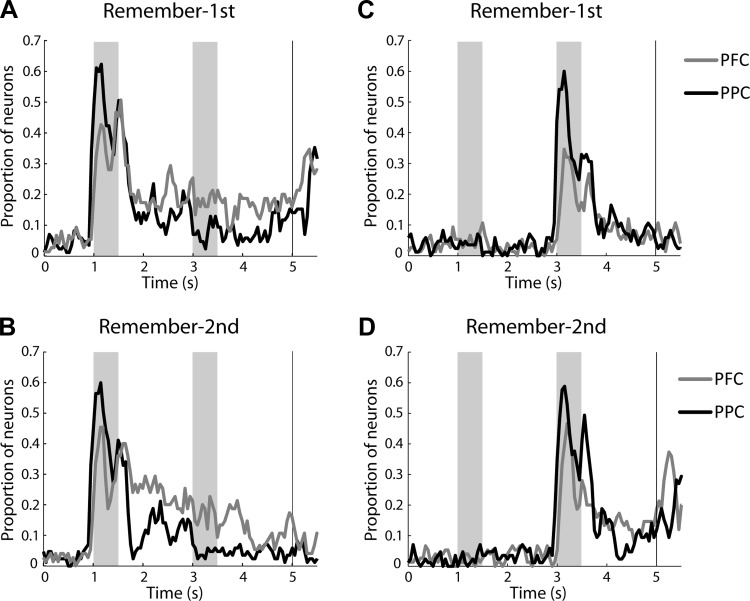
We also wished to compare the relative timing of stimulus representation in the two areas. We thus performed a two-way ANOVA using as factors the location of the first stimulus and the location of the second stimulus in sliding 250-ms windows spanning the entire trial. This allowed us to determine the percentage of neurons with a significant main effect of first stimulus location at each time point, plotted at the center of each 250-ms window used, for the two tasks and areas (Fig. 9). As already noted, information about the first stimulus persisted in the second delay period to a greater extent for the dlPFC. The present analysis revealed that in the remember-first task both areas encoded the location of the stimulus robustly while it was present on the screen. The relative superiority of the dlPFC to represent the remembered stimulus appeared shortly after the end of the stimulus presentation and remained fairly stable through the remainder of the trial (Fig. 9A). In the remember second task, the representation of the initial stimulus declined quickly in the first delay period, although a residual percentage of dlPFC neurons continued to represent the initial stimulus throughout the trial (Fig. 9B). Representation of the second stimulus only emerged late in trial, and it was greatly diminished in the dlPFC compared with the PPC when the monkey was required to remember the first stimulus (Fig. 9C). Similar representations in the two areas for the second stimulus were observed in the remember-second task (Fig. 9D).
Fig. 9.
A: proportion of neurons with significantly elevated delay period activity and a significant main effect of 1st stimulus location during the remember-1st task. This was computed in a time-resolved fashion, in sliding windows of 250-ms bin size, for the dlPFC (gray line, total n = 75) and PPC cortex (black line, total n = 85). B: data for the same neurons plotted as in A for the remember-2nd task. C: proportion of neurons with significantly elevated delay period activity and a significant main effect of the 2nd stimulus location during the remember-1st task. D: data for the same neurons plotted as in C for the remember-2nd task.
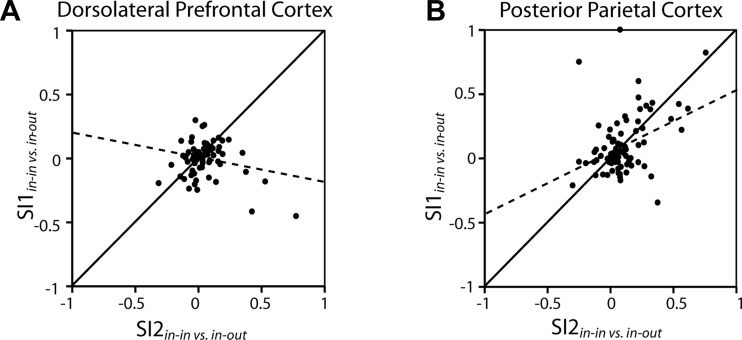
We went on to compare the remember-first and remember-second tasks in terms of neurons' ability to resist a distractor whether it appeared as first (in the remember-second task) or second in the sequence (in the remember-first task). A linear regression of SI2 on SI1 revealed that only PPC neurons exhibited similar distractor selectivity in the two tasks, as evidenced by a positive and significant slope of the regression line of Fig. 10 for the PPC (P < 0.0005). The dlPFC exhibited a negative slope of SI2 regressed on SI1, which was significantly different from the slopes of the PPC neurons (F-test, P < 0.001). This result also demonstrates the unique ability of the PFC to resist distractors, once the first stimulus was held in memory.
Fig. 10.
A: each dot represents a single dlPFC neuron with significantly elevated activity in the delay period (n = 75). Values depicted in the ordinate correspond to contrast ratio SI1, obtained from the mean firing rate of the 2nd delay period, in the remember-1st task, when the 1st stimulus appeared in the receptive field. SI1 represents the difference in mean rate in the 2nd delay period of the remember-1st task between trials where the 2nd stimulus appeared in the receptive field vs. trials where the 2nd stimulus appeared out of the receptive field, divided by their sum. The abscissa represents SI2, involving the same firing rate contrast as SI1 but obtained from the remember-2nd task. B: same as in A for PPC neurons (n = 85). Solid lines indicate the diagonal where SI1 = SI2. Dotted lines represent linear regression lines through the data.
A corollary of the substantial percentage of neurons with higher responses after distractors described in the previous section was that there was no overwhelming preference for a remembered stimulus over a distractor across the entire population. Examining the first delay period (Fig. 8, E and F) revealed essentially identical responses for a remembered stimulus (in the remember-first task) and the same stimulus appearing as a distractor (in the remember-second task). This was true for both areas. Average values for a selectivity index comparing activity in the first delay period SI3 = (remembered − distractor)/(remembered + distractor) were −0.013 for dlPFC and 0.004 for PPC. There was no difference between areas (t-test, P > 0.2), and neither value was significantly different from zero (one-sample test, P > 0.3 for both areas).
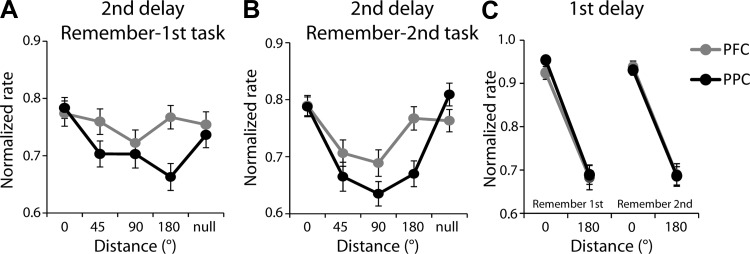
Since the main condition differentiating dlPFC and PPC was the better ability of dlPFC to resist a distractor in the remember-first task, we further explored the effect of distractors (second stimulus) in this task appearing at different locations, following presentation of the first stimulus in the receptive field. There was little influence of the location of the distractor on the activity of dlPFC neurons in the second delay period (Fig. 11A) and no significant difference in firing rate, depending on the location of the distractor (ANOVA, F4,370 = 0.82, P > 0.5). In contrast, PPC activity varied significantly depending on the location of the distractor (ANOVA, F4,420 = 4.06, P < 0.005). In the remember-second task, activity in the second delay period (which now followed the actively remembered stimulus) varied depending on the location of the second stimulus in both areas (Fig. 11B). There was little difference between areas in their overall spatial selectivity, as measured by the difference in responses between the best and diametric location in the first delay period (Fig. 11C). No significant effect of area was detected (three-way ANOVA, with factors area, task, and location, P > 0.2).
Fig. 11.
A: averaged, normalized discharge rate in the 2nd delay period of the remember-1st task is depicted as a function of the distance of the 2nd stimulus relative to the 1st for dlPFC (gray, n = 75) and PPC (black, n = 85). Firing rates have been normalized relative to the stimulus condition that elicits the best response in the delay period across all conditions, for each neuron. Error bars represent means ± SE across neurons. B: mean normalized firing rates in the 2nd delay period of the remember-2nd task. C: averaged, normalized firing rate in the 1st delay period, using the same normalization procedure, for the same neurons used in A and B.
Task effects.
While our primary goal was to determine the relative responsiveness of dlPFC and PPC to the first and second stimulus when they were being actively remembered, our study also allowed us to compare the modulation of neurons by task rules in the two areas. Profound impairments in the representation of task rules in working memory and in switching between rules have been reported specifically after lesions of the PFC (Buckley et al. 2009; Rossi et al. 2007), suggesting that task representation may be more dominant in the prefrontal than the parietal cortex. We sought to test therefore the relative proportion of neurons that are modulated by task rules.
As already described above, the remember-first and remember-second tasks modulated neuronal responses to the same stimulus sequences depending on the task executed, so we identified neurons with a significant main effect of task involving identical first and second stimulus locations, using a two-way ANOVA. In the experimental condition where the second stimulus was always diametric to the first, 32% (34/107) neurons in the dlPFC exhibited a significant task effect (P < 0.05) during the first delay period. In contrast only 15% (14/95) PPC neurons exhibited the same type of effect, a significant difference between areas (χ2-test, P < 0.005). Comparable percentages of neurons exhibited significant interaction (P < 0.05) between the task and location factors (10/107 in dlPFC, and 12/96 in PPC). For the second delay period, 16% (17/107) of dlPFC neurons exhibited a task effect, contrasted with 6% (6/95, comparable to the 5% false positive rate of the test) of PPC neurons, which again represented a significant difference (χ2-test, P < 0.05). A total of 11% (12/107) neurons in dlPFC and 4% (4/96, comparable to the expected 5% false positive rate of the test) neurons in PPC exhibited significant interaction during the second delay period. These results confirmed that more dlPFC than PPC neurons are modulated by task rules during the delay periods of the task. A similar trend was observed for the experimental condition where the location of the second stimulus was randomized relative to the first: a total of 23% (17/75) neurons in the dlPFC exhibited a significant task effect during the first delay period whereas 12% (10/85) PPC neurons exhibited the same type of effect, although the difference between areas did not reach statistical significance (χ2-test, P = 0.058). A total of 12% (9/75) neurons in dlPFC and 4% (3/85, less than the expected 5% false positive rate of the test) neurons in PPC exhibited significant interaction in this epoch. For the second delay period, 8% of dlPFC neurons exhibited a task effect, contrasted with 6% of PPC neurons (χ2-test, P > 0.5).
We also wished to determine if there were neurons with preference for one task over the other, even before the appearance of any stimuli. We thus identified 49 neurons in the dlPFC (22%) out of a total 220 neurons recorded in the experimental condition where the second stimulus was always diametric to the first and 12 neurons in the PPC (4%, less than the expected 5% false positive rate of the test) out of 314 total neurons, with significantly different responses during the fixation period between task conditions (t-test, P < 0.05). The difference in the percentage neurons with task effects in the two areas was highly significant (χ2-test, P < 10−5). We found virtually identical results in the experimental condition with the randomized distractor location: 42/205 (20%) of dlPFC neurons and 13/235 (6%, comparable with the expected 5% false positive rate of the test) of PPC neurons exhibited significant task effects in the fixation period (χ2-test, P < 10−5).
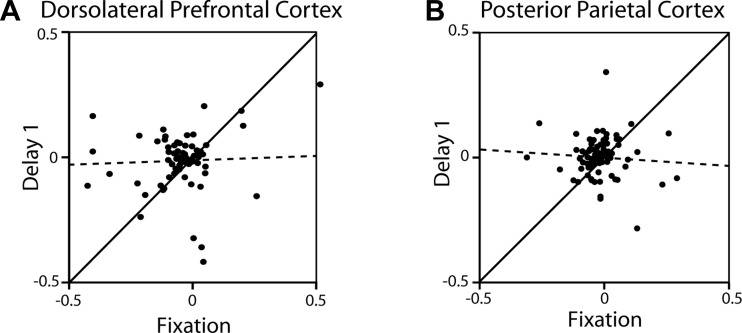
We lastly examined whether task preference during the delay period of the task was the result of task preference already present during the fixation period and whether the difference between areas in task preference could be accounted for by responses in the delay period. This was not the case. We found no significant correlation between preference for the remember-first over the remember-second task during the fixation interval and the first delay interval (where we saw the greatest task effects). This was the case for both the dlPFC (r = 0.04, P > 0.7; Fig. 12A) and the PPC (r = 0.07, P > 0.5; Fig. 12B).
Fig. 12.
A: each dot represents one dlPFC neuron with significant delay period activity (n = 75). Values depicted in the ordinate correspond to difference in firing rate between the remember-1st and remember-2nd task in the 1st delay period, divided by the sum of the 2 values. Values depicted in the abscissa represent difference between the remember-1st and remember-2nd task during the fixation period, divided by the sum of the 2 values. B: same as A for PPC neurons (n = 85). Solid lines indicate the diagonal; dotted lines represent linear regression lines through the data.
DISCUSSION
Our study compared neuronal activity in the PPC and dlPFC, two cortical areas implicated in the maintenance of spatial working memory and planning of future actions (Constantinidis and Procyk 2004), using tasks requiring recall of either the initial or final stimulus in a sequence. This paradigm allowed us to determine neural activity representing identical stimuli when they have to be remembered or when they are presented as distractors. Our results revealed differences between areas, as PPC activity was affected by the most recent stimulus, and dlPFC was better able to better represent the actively remembered stimulus, resisting the effects of distractors. Additionally, we found that more neurons in the dlPFC than PPC were modulated by the task, as evidenced by different responses to identical stimuli, depending on whether the monkey performed the remember-first or remember-second task. Unexpectedly, we also observed a large proportion of neurons active during the delay period exhibiting paradoxical activity, with stronger representation of a stimulus when it appeared as a distractor than when it was required to be remembered, in both areas, presumably as a result of activity modulation by task rules. Our results are revealing about the specialization of cortical areas and about how the representation of task rules is interfaced with spatial working memory.
Behavioral performance.
The two monkeys exhibited similar patterns of behavioral performance in our task. When tested with distractors that always appeared at a diametric location to the remembered stimulus, performance was slightly worse in the remember-second than in the remember-first condition (Fig. 3A). We replicated this finding for the diametric distractor in the randomized set (compare 180° data point in Fig. 3, B vs. C). The results suggest that the initial stimulus is easier to remember and that recall of the second stimulus is worse, although the difference in performance between conditions was modest. We should note, however, that the monkeys were first trained to remember the first stimulus and it is possible that they exhibited an overall preference for this task throughout experiments.
Behavioral performance degraded considerably when distractors were placed at locations closer to the stimulus to be remembered (Fig. 3, B and C). The most prominent effect in both monkeys was observed when the monkey needed to remember the first stimulus and a distractor followed it at a 45° angular displacement (Fig. 3B). The ability of a distractor to disrupt persistent activity when appearing at a nearby location relative to the remembered stimulus has been predicted by computational models as a property of network connections between populations of neurons with overlapping receptive fields (Compte et al. 2000; Wimmer et al. 2014). Similar interference by a nearby distractor has also been described in humans (Herwig et al. 2010). In contrast, when the monkeys knew that the initial stimulus was to be ignored, they were able to successfully represent the second stimulus, even when the two stimuli appeared at the closest, 45° distance (Fig. 3C), suggesting behavioral performance depending on stimulus-specific interactions.
Comparison of prefrontal and parietal cortex.
Analysis of neural activity in the remember-first task replicated a number of prior findings from the PFC and PPC. We confirmed that both prefrontal and posterior parietal neurons exhibited persistent responses during the delay period of the task (Chafee and Goldman-Rakic 1998; Constantinidis and Steinmetz 1996; Quintana and Fuster 1992). We also found that prefrontal activity representing the initial, remembered stimulus was less affected by distractors (Figs. 8, A and B, 9A, and 11), as also previously described (Qi et al. 2010; Suzuki and Gottlieb 2013). Consistent with this finding, we saw that when we varied the location of the second stimulus relative to the first, activity in the second delay period was representing the location of the initial stimulus for more dlPFC neurons, whereas PPC neurons were modulated to a greater extent by the location of the second (distractor) stimulus (Fig. 11A). A number of anatomical and physiological specializations between these two areas have been identified in terms of dopaminergic innervation, neurotransmitter receptor types, the spatial distribution of functional connectivity within each area, and the properties of putative interneuron types that could be responsible for differences in their functional properties (Katsuki and Constantinidis 2012; Katsuki et al. 2013; Zhou et al. 2012).
The remember-second task allowed us to address the contributions of these areas in a task requiring spatial memory of a stimulus other than the first in the sequence and planning of a saccade towards it. We reasoned that if the PFC is representing the actively remembered stimulus and PPC the most recent one, then robust activity representing the second stimulus would be present in both areas following the second stimulus in the remember-second condition. Our results were indeed consistent with this interpretation. We found that persistent activity over the delay periods of the task in both areas was equally able to represent the second, remembered stimulus, regardless of the location of the first (Fig. 8, C and D, 11). One might also expect that execution of the remember-second task would suppress responses following presentation of the initial stimulus in the receptive field, at least in the PFC. However, there was little evidence of such reduction of responses compared with the remember-first task (compare Fig. 8, C with A). As a consequence, the remember-second task did not differentiate well between prefrontal and parietal activity, since the most recent stimulus was the one to be remembered.
Task effects.
Performing either the remember-first or remember-second task requires remembering the stimulus location as well as the task that is being rewarded. Modulation of neuronal activity elicited by identical stimuli when the monkey performs a different task has been previously demonstrated both in the PFC (Hoshi et al. 1998; Wallis et al. 2001; Wallis and Miller 2003; White and Wise 1999) and PPC (Rawley and Constantinidis 2010; Stoet and Snyder 2004). Neurons have also been described that respond selectively to stimuli depending on their ordinal position in a sequence (Berdyyeva and Olson 2010; Inoue and Mikami 2006). A direct comparison between prefrontal and parietal cortex has not been available in the context of spatial working memory although lesion studies in nonhuman primates reveal profound deficits in the ability to remember and switch between tasks, specifically after prefrontal lesions (Buckley et al. 2009; Rossi et al. 2007). Recent physiological studies have also shown stronger rule-based modulation of prefrontal responses compared with parietal ones in categorization paradigms (Crowe et al. 2013; Goodwin et al. 2012), although other studies have reached opposite conclusions (Swaminathan and Freedman 2012). We sought therefore to test if a larger proportion of prefrontal neurons represent and are modulated by the task information in the context of the working memory task.
In our study, the representation of task information in neuronal activity manifested itself in the form of preferential representation of one over the other task. This task preference was presumably responsible for the paradoxical preference for distractor stimuli that we observed, as well. Large proportions of neurons with delay period activity and task effects in both the dlPFC and PPC exhibited higher activity during the first delay period of the remember-second task compared with the remember-first task, even though the first stimulus was a distractor and the monkey correctly recalled the second stimulus. Preference for a second stimulus appearing as a distractor was also present among many neurons. We initially relied on a stimulus set where the distractor location was always diametric to the cue. It was conceivable therefore that the monkey planned a response to the second stimulus during the initial distractor presentation (in the remember-second task) or reinforced his plan for a movement to the initial, remembered stimulus during the presentation of the distractor (in the remember-first task). However, even when we randomized the second stimulus presentation, large proportions of neurons with delay period activity in both areas continued to exhibit this paradoxical distractor preference. As a consequence, we saw a negligible difference in activity in the first delay period following either presentation of a stimulus to be remembered or a distractor (Fig. 8, E and F). This selectivity for the distractor was robust and persisted until the end of the trial (Fig. 9B). A somewhat analogous effect of strong representation of a stimulus that is not to be the target of the saccade has been previously reported in the PFC (Hasegawa et al. 2004). It is possible that the paradoxical representation of the distractor stimulus is mediated by the same population of neurons that are activated by stimuli that represent inappropriate targets of a saccade.
Since the task rule was conveyed in our paradigm based on the color of the fixation point, the task differences we identified might also be attributed to some extent to differential representation of the color of the fixation point that persisted throughout the trial. Analysis of the time course of the task effect revealed dlPFC and PPC neurons exhibiting preference for one of the two fixation point colors; this, however, did not persist in the delay period (Fig. 12). We cannot rule out an interaction between fixation point color and delay period activity, which appears only after a distractor presentation. Whether the effect was attributable specifically to the task rule or to an interaction between color and stimulus presentation, our conclusion remains the same, that representation of a remembered stimulus over distractors is context dependent and vulnerable to task attributes. For example, during the first delay period, a larger percentage of PFC than PPC neurons was more responsive to distractors than the remembered stimuli. A recent study comparing the PFC with the ventral intraparietal area of the PPC in the context of a numerosity matching task also found a stronger encoding of distractors in the PFC (Jacob and Nieder 2014).
Taken together, our results demonstrate that persistent activity in the PFC better represents actively remembered stimuli and is modulated by task encoding to a greater extent, whereas the PPC encodes a more faithful representation of the physical world, regardless of task. On the other hand, we find that the difference with the PPC is quantitative rather than qualitative and that the ability to represent remembered stimuli is itself context dependent, so that a population of dlPFC neurons may respond strongly to distractors in animals trained to alternate between tasks.
GRANTS
The research reported in this article was supported by the National Institutes of Health Grants R01-EY-16773 and R25-GM-064249 and by the Tab Williams Family Endowment and Harry O'Parker Neurosciences Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.-L.Q. and C.C. conception and design of research; X.-L.Q., A.C.E., and B.C.L. performed experiments; X.-L.Q. and C.C. analyzed data; X.-L.Q. and C.C. interpreted results of experiments; X.-L.Q. and C.C. prepared figures; X.-L.Q. and C.C. drafted manuscript; X.-L.Q., A.C.E., B.C.L., and C.C. approved final version of manuscript; A.C.E. and B.C.L. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Kathini Palaninathan for technical help.
REFERENCES
- Berdyyeva TK, Olson CR. Rank signals in four areas of macaque frontal cortex during selection of actions and objects in serial order. J Neurophysiol 104: 141–159, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33: 1–21, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Buckley MJ, Mansouri FA, Hoda H, Mahboubi M, Browning PG, Kwok SC, Phillips A, Tanaka K. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science 325: 52–58, 2009. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol 79: 2919–2940, 1998. [DOI] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex 10: 910–923, 2000. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Procyk E. The primate working memory networks. Cogn Affect Behav Neurosci 4: 444–465, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Neuronal activity in posterior parietal area 7a during the delay periods of a spatial memory task. J Neurophysiol 76: 1352–1355, 1996. [DOI] [PubMed] [Google Scholar]
- Crowe DA, Goodwin SJ, Blackman RK, Sakellaridi S, Sponheim SR, MacDonald AW 3rd, Chafee MV. Prefrontal neurons transmit signals to parietal neurons that reflect executive control of cognition. Nat Neurosci 16: 1484–1491, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Wise SP. Effects of attention on visuomotor activity in the premotor and prefrontal cortex of a primate. Somatosens Mot Res 10: 245–262, 1993. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JK, Swaminathan SK, Freedman DJ. Visual categorization and the parietal cortex. Front Integr Neurosci 6: 18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349, 1989. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. In: Handbook of Physiology. The Nervous System. Functions of the Brain, edited by Plum F, Mountcastle VB. Bethesda, MD: Am Physiol Soc, 1987, vol. 5, p. 373–417. [Google Scholar]
- Goodwin SJ, Blackman RK, Sakellaridi S, Chafee MV. Executive control over cognition: stronger and earlier rule-based modulation of spatial category signals in prefrontal cortex relative to parietal cortex. J Neurosci 32: 3499–3515, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84: 401–414, 2000. [DOI] [PubMed] [Google Scholar]
- Hasegawa RP, Peterson BW, Goldberg ME. Prefrontal neurons coding suppression of specific saccades. Neuron 43: 415–425, 2004. [DOI] [PubMed] [Google Scholar]
- Herwig A, Beisert M, Schneider WX. On the spatial interaction of visual working memory and attention: evidence for a global effect from memory-guided saccades. J Vis 10: 8, 2010. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Shima K, Tanji J. Task-dependent selectivity of movement-related neuronal activity in the primate prefrontal cortex. J Neurophysiol 80: 3392–3397, 1998. [DOI] [PubMed] [Google Scholar]
- Inoue M, Mikami A. Prefrontal activity during serial probe reproduction task: encoding, mnemonic, and retrieval processes. J Neurophysiol 95: 1008–1041, 2006. [DOI] [PubMed] [Google Scholar]
- Jacob SN, Nieder A. Complementary roles for primate frontal and parietal cortex in guarding working memory from distractor stimuli. Neuron 83: 226–237, 2014. [DOI] [PubMed] [Google Scholar]
- Katsuki F, Constantinidis C. Unique and shared roles of the posterior parietal and dorsolateral prefrontal cortex in cognitive functions. Front Int Neurosci 6: 17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki F, Qi XL, Meyer T, Kostelic PM, Salinas E, Constantinidis C. Differences in intrinsic functional organization between dorsolateral prefrontal and posterior parietal cortex. Cereb Cortex 24: 2334–2349, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Constantinidis C. A software solution for the control of visual behavioral experimentation. J Neurosci Methods 142: 27–34, 2005. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202, 2001. [DOI] [PubMed] [Google Scholar]
- Qi XL, Katsuki F, Meyer T, Rawley JB, Zhou X, Douglas KL, Constantinidis C. Comparison of neural activity related to working memory in primate dorsolateral prefrontal and posterior parietal cortex. Front Syst Neurosci 4: 12, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana J, Fuster JM. Mnemonic and predictive functions of cortical neurons in a memory task. Neuroreport 3: 721–724, 1992. [DOI] [PubMed] [Google Scholar]
- Rawley JB, Constantinidis C. Effects of task and coordinate frame of attention in area 7a of the primate posterior parietal cortex. J Vis 10: 1–16, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawley JB, Constantinidis C. Neural correlates of learning and working memory in the primate posterior parietal cortex. Neurobiol Learn Mem 91: 129–138, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AF, Bichot NP, Desimone R, Ungerleider LG. Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. J Neurosci 27: 11306–11314, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoet G, Snyder LH. Single neurons in posterior parietal cortex of monkeys encode cognitive set. Neuron 42: 1003–1012, 2004. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Gottlieb J. Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat Neurosci 16: 98–104, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan SK, Freedman DJ. Preferential encoding of visual categories in parietal cortex compared with prefrontal cortex. Nat Neurosci 15: 315–320, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature 411: 953–956, 2001. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol 90: 1790–1806, 2003. [DOI] [PubMed] [Google Scholar]
- White IM, Wise SP. Rule-dependent neuronal activity in the prefrontal cortex. Exp Brain Res 126: 315–335, 1999. [DOI] [PubMed] [Google Scholar]
- Wimmer K, Nykamp DQ, Constantinidis C, Compte A. Bump attractor dynamics in prefrontal cortex explains behavioral precision in spatial working memory. Nat Neurosci 17: 431–439, 2014. [DOI] [PubMed] [Google Scholar]
- Zhou X, Katsuki F, Qi XL, Constantinidis C. Neurons with inverted tuning during the delay periods of working memory tasks in the dorsal prefrontal and posterior parietal cortex. J Neurophysiol 108: 31–38, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]