Abstract
The Xenopus tadpole optic tectum is a multisensory processing center that receives direct visual input as well as nonvisual mechanosensory input. The tectal neurons that comprise the optic tectum are organized into layers. These neurons project their dendrites laterally into the neuropil where visual inputs target the distal region of the dendrite and nonvisual inputs target the proximal region of the same dendrite. The Xenopus tadpole tectum is a popular model to study the development of sensory circuits. However, whole cell patch-clamp electrophysiological studies of the tadpole tectum (using the whole brain or in vivo preparations) have focused solely on the deep-layer tectal neurons because only neurons of the deep layer are visible and accessible for whole cell electrophysiological recordings. As a result, whereas the development and plasticity of these deep-layer neurons has been well-studied, essentially nothing has been reported about the electrophysiology of neurons residing beyond this layer. Hence, there exists a large gap in our understanding about the functional development of the amphibian tectum as a whole. To remedy this, we developed a novel isolated brain preparation that allows visualizing and recording from all layers of the tectum. We refer to this preparation as the “horizontal brain slice preparation.” Here, we describe the preparation method and illustrate how it can be used to characterize the electrophysiology of neurons across all of the layers of the tectum as well as the spatial pattern of synaptic input from the different sensory modalities.
Keywords: development, electrophysiology, multisensory integration, tadpole, tectum
the amphibian optic tectum, homologous to the mammalian superior colliculus, is a multisensory processing center that receives visual input from the retinal ganglion cells (RGCs) in the eye as well as from nonoptic mechanosensory inputs originating from the somatosensory, lateral line, auditory, and vestibular systems (Behrend et al. 2006; Lowe 1986; Munoz et al. 1995). The neurons that comprise the optic tectum, collectively referred to as tectal neurons, organize into somatic layers with the deepest layer being the most periventricular and most medial. In the developing tadpole tectum, the dendrites of these tectal neurons project laterally from the somatic layer into a single vast neuropil where they receive strong direct synaptic visual input from RGCs of the contralateral eye and mechanosensory inputs that arrive at the tectum via the hindbrain (HB). Ultimately, the tectum of the adult Xenopus frog will organize into a total of nine layers: cellular layers interleaved with layers of neuropil or where efferent outputs exit the tectum (Lazar 1973). Based on receptive field properties, seven classes of tectal neurons have been identified in the adult frog (Grusser and Grusser-Cornehls 1976). The immature tadpole tectum is considerably less complex compared with the adult but rapidly developing: by developmental stage 45, which is approximately 7–10 days postfertilization (dpf), the somatic layer of the tectum appears to be 5–10 somata deep, although at this early stage of development, defined layers have not been described. Also by this stage in development, both visual and mechanosensory inputs have entered the tectum and have begun forming synapses (Hiramoto and Cline 2009). By developmental stage 49 (∼16 dpf), the tectal neuron somata have organized into six compact somatic layers and have begun to display a range of different morphologies (Lazar 1973). Furthermore, a detailed immunohistochemical study indicates that even before developmental stage 49, the neurons across the tectum can be categorized as expressing either GABA or CaMKII and that the spatial pattern of GABA-expressing neurons has redistributed from clustered to being more evenly dispersed throughout the somatic layers (Miraucourt et al. 2012). Although these morphological and immunohistochemical studies indicate that, even at relatively early developmental stages, tectal neurons are differentiating and reorganizing, the electrophysiology of the neurons beyond the deepest, most periventricular somatic layer, henceforth referred to as the “deep-layer” neurons, has not been described. Whole cell electrophysiological studies of the tadpole tectum, whether using the in vivo preparation or the isolated, whole brain preparation, have focused solely on the deep-layer neurons. This is because to record from tectal neurons using either the in vivo or whole brain preparation, the ventricular membrane is peeled away, making accessible the deep-layer neurons that line the ventricle. This approach does not permit recording from neurons of outer layers since there is no obvious way to circumvent the deep-layer neurons. As a result, whereas the development and plasticity of these deep-layer neurons has been well-studied, essentially nothing has been reported about the electrophysiology of neurons residing beyond this layer. Hence, there exists a large gap in our understanding about the functional development of the amphibian tectum as a whole. To remedy this, we have developed a novel isolated brain preparation that allows for the visualization and whole cell recording of individual neurons across the entire medial-lateral axis of the tectum. This preparation, henceforth referred to as the “horizontal brain slice preparation,” provides a means to study readily the physiology of a large population of tectal neurons that, until now, could not be studied. Also, as with the original whole brain preparation, the horizontal brain slice preparation retains the sensory inputs from the RGCs in the eye and the mechanosensory inputs from the HB. Therefore, the pattern and strength of the different inputs received by outer-layer neurons can be explored. Also, this preparation makes it possible to study the development of local tectal-tectal connectivity between the different layers. Although it is evident that there exists extensive local connectivity between tectal neurons (Pratt et al. 2008), there are no reports, to date, of connections between individual tectal neurons, probably because the connections exist across layers and so could not be identified using the whole brain or in vivo preparation.
In addition to being able to record from neurons across layers, this horizontal brain slice preparation also provides direct visualization and access to the neuropil where the dendrites of tectal neurons receive synaptic input from the different sensory modalities. With access to the medial-lateral axis of the neuropil, combined with the ability to activate the two distinct sensory inputs, this preparation provides a straightforward method to assess the spatial pattern of functional synapses formed onto tectal cell dendrites by the axons of different sensory modalities. It has previously been established that the axons of the different sensory modalities segregate to specific regions of the dendrite: RGC (visual) input targets the most distal region of the dendrite, whereas the nonvisual mechanosensory targets the proximal region of the same dendrite (Deeg et al. 2009; Hiramoto and Cline 2009). This suggests that the functional synapses associated with each modality are spatially distinct. Using the horizontal brain slice preparation, the spatial pattern of synaptic input can be measured directly by recording field potentials (FPs) at equidistant points across the medial-lateral axis of the neuropil, which corresponds to the proximal-to-distal axis of the dendrites, in response to activation of one, and then the other, sensory modality. This method of studying the spatial pattern of synaptic input by recording FPs in response to sensory stimulation has been used extensively to characterize organization of retinal (Chung et al. 1974a; Nakagawa et al. 1997; Nakagawa and Matsumoto 1998, 2000) and somatosensory (Tsurudome et al. 2005) inputs, in vivo, in the adult frog tectum but has been applied considerably less to study the juvenile tadpole due to technical difficulties associated with advancing the recording pipette down through the very thin tadpole tectum as discussed in a FP study using stage 55 Xenopus tadpoles (Chung et al. 1974b). The horizontal brain slice preparation avoids these technical difficulties because the recording pipette is moved along the horizontal axis across the slice surface and never down vertically through the tissue (see materials and methods). Therefore, this new preparation provides a means to study the earliest stages of the development of the spatial pattern of synaptic inputs, how this pattern may change over development, and the mechanisms and functional relevance in the context of sensory integration.
In summary, because the horizontal brain slice preparation allows for the visualization and access across the entire medial-lateral axis of the tectum, the experimental attributes of this horizontal brain slice preparation are twofold: 1) it allows, for the first time, the systematic characterization of neurons of different layers of the developing tectum, including the specific pattern and strength of their sensory inputs; and 2) it provides a platform to study how the synaptic connections made by the different sensory afferents are targeted to specific regions of the dendrite as well as the functional consequence of this subcellular level of organization in the context of sensory integration. Here, we illustrate these two experimental attributes.
MATERIALS AND METHODS
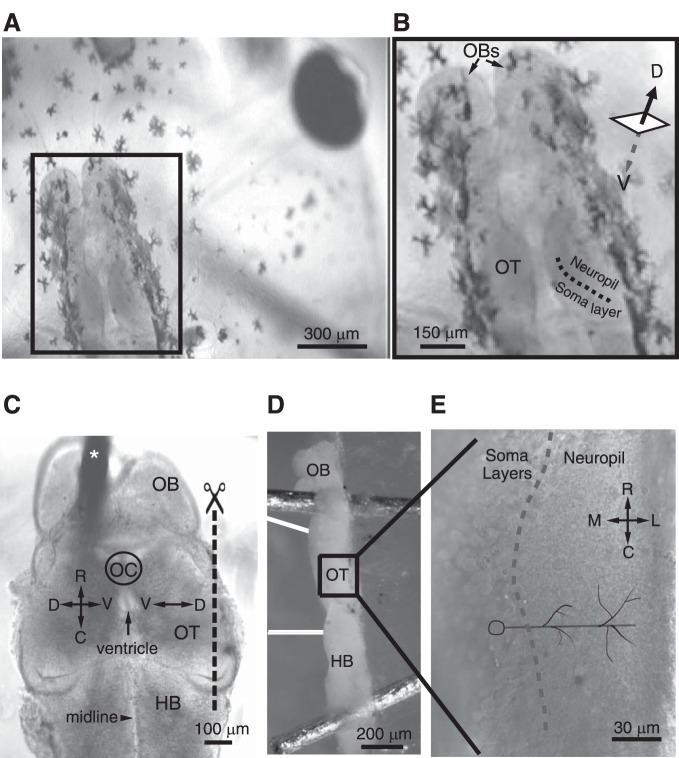
All experimental protocols have been approved by the University of Wyoming's Institutional Animal Care and Use Committee (IACUC). Xenopus laevis tadpoles were reared in Steinberg's solution at 25°C on a 12:12-h light-dark schedule. Tadpoles were staged according to the developmental table described by Nieuwkoop and Faber (1994). The first step for preparing the horizontal brain slice brain preparation is equivalent to the whole brain preparation described by Wu et al. (1996) and Pratt and Aizenman (2007). For this, tadpoles are anesthetized in Steinberg's solution containing 0.02% MS-222, moved to the recording dish, and pinned to a block of Sylgard silicone elastomer submerged in external recording solution (in mM: 115 NaCl, 2 KCl, 3 CaCl2, 3 MgCl2, 5 HEPES, and 10 glucose, pH 7.25, osmolarity 255 mosM). Next, using a sterile 26-gauge needle, the skin overlying the brain is peeled away (Fig. 1, A and B) and the brain and brain stem filleted by making a shallow incision along the midline such that the dorsal postoptic commissure is severed, whereas the floor plate is left intact. The filleted brain and brain stem are then dissected out and secured to the Sylgard block by placing one insect pin in either olfactory bulb and one in the brain stem (Fig. 1C). This yields the whole brain preparation that is commonly used for recording from the tectum. In this configuration, the somata of the deep-layer neurons are immediately visible and readily accessible for recording, and their dendrites are pointing down (parallel with the z-axis) toward the piece of Sylgard. The second step in this new preparation involves making a cut parallel to the rostral-caudal plane such that the most lateral one-fourth (approximately) of the tectum, which in the intact tectum corresponds to making a cut in the horizontal plane such that the most dorsal one-fourth of the tectum is excised and the remaining three-fourths is kept for recording (Fig. 1C). This cut is made manually using a razor blade. The removal of this small portion of the tectum permits access to the most extensive somatic and neuropil layers (i.e., where the somatic and neuropil layers are widest). Importantly, slicing away this small portion of the tectum leaves intact the RGC and mechanosensory (HB) inputs and hence preserves the capability to activate these inputs at different stimulation intensities and record the resulting responses from individual tectal neurons, the major experimental advantage of the traditional whole brain preparation over the in vivo configuration. Next, the preparation is pinned onto the side of the aforementioned Sylgard block such that the sliced side is facing up and the ventricle side is facing away from the Sylgard block (Figs. 1D and 2). This inside-facing-out orientation allows for a bipolar stimulating electrode to be placed onto the optic chiasm and the HB so that RGC and mechanosensory inputs, respectively, can be activated (Figs. 1D and 2). Figure 1E shows a portion of the tectum imaged at ×60, the magnification we use when recording. The somatic and neuropil layers and the border between them can be easily distinguished.
Fig. 1.
The horizontal brain slice preparation. A: an overhead view of a stage 49 Xenopus tadpole with the brain in focus. The skin overlying the intact brain has been removed, the 1st step in the dissection. B: boxed area in A with the optic tectum (OT), neuropil, and soma layers labeled. The dorsal-ventral axis is lying in the z-coordinate. C: ×10 image illustrating the 1st step in the horizontal brain slice preparation; at this stage in the dissection, the brain has been isolated, filleted along the midline (such that the part of tectum that was dorsal is now lateral), and secured onto the Sylgard by placing 1 insect pin (labeled here with a white asterisk) in the olfactory bulb (OB) and 1 in the hindbrain (HB; data not shown). Next, a cut is made along the most lateral 1/4th of the OT (indicated by the dashed line and scissors) using a razor blade. D: the final step involves repinning the brain onto the side of the block of Sylgard such that the sliced side is facing up. Image was captured using a ×5 objective. The sets of double white lines represent the retinal ganglion cell (RGC) and HB stimulating electrodes. E: boxed area in D is enlarged here (60×) to show the different somatic layers and the neuropil of the tectum. Notice how the somatic layers and the neuropil are visible and readily distinguishable. The model cell drawn in black depicts the orientation of the somas and the dendrites. Dashed line indicates the border between somatic and neuropil layers. OC, optic chiasm; R, rostral; C, caudal; M, medial; L, lateral; D, dorsal; V, ventral.
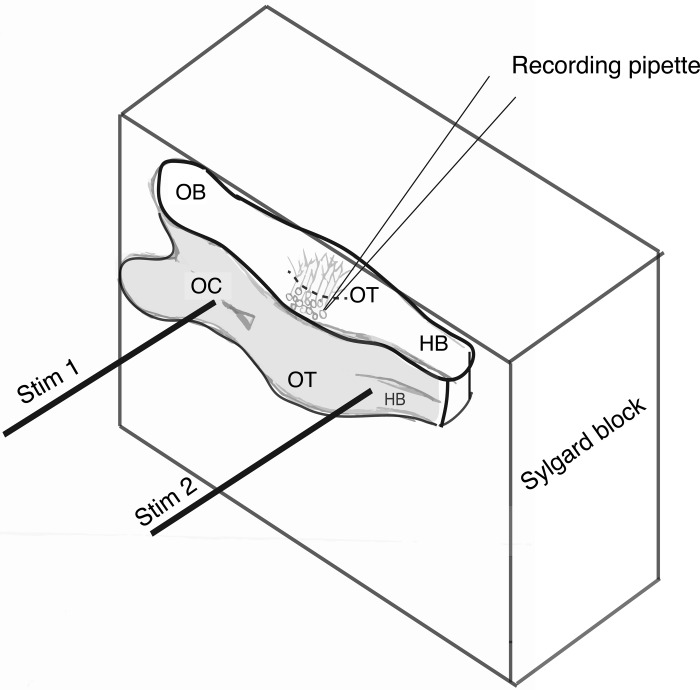
Fig. 2.
A schematic showing the horizontal brain slice preparation attached to a Sylgard block and ready for recording. The sliced side of the preparation (shown in white) is facing up such that tectal neurons and neuropil across the medial-lateral axis of the tectum (i.e., the horizontal plane, or axis, in vivo) can be readily visualized and accessed for whole cell patch-clamp or field-potential (FP) recordings. The ventricle side of the preparation is facing away from the Sylgard block so that a stimulating electrode (“Stim 1”) can be placed on the OC and another (“Stim 2”) on the HB so that the RGC axonal inputs and HB inputs, respectively, can be activated and resulting synaptic responses recorded from tectal neurons or the neuropil. Dashed line indicates the border between soma and neuropil layers. This schematic corresponds to Fig. 1D.
Once mastered, the entire dissection can be completed within 10 min on average. In addition, unlike the whole brain preparation, there is no ventricular membrane to be removed before neurons can be recorded. We find that the horizontal brain slice preparation stays healthy on the rig for ∼2 h (no perfusion required).
A minor drawback of this preparation is that, even though the afferent sensory inputs are preserved, removing the most outside edge of tectum (which corresponds to the most dorsal portion of the intact tectum) may result in a certain amount of intratectal connectivity to be lost. This could potentially impact the frequency of spontaneous synaptic events appearing to be received by a given tectal neuron during recording. However, compared with deep-layer neurons recorded using the original whole brain preparation, we have observed no noticeable decrease in the frequency of spontaneous synaptic events recorded from these deep-layer neurons using the horizontal brain slice preparation. The frequency of spontaneous synaptic events using either the whole brain preparation or horizontal brain slice preparation was observed to be in the range of one to seven events per second, and no preparation-dependent trends in synaptic frequencies have been observed. A similar concern was that, since the pattern and strength of evoked polysynaptic activity is determined by local intratectal connectivity, it is conceivable that removing a portion of the tectum may also stunt the polysynaptic portion of the evoked response. As observed with the frequency of spontaneous synaptic events, however, polysynaptic responses recorded from deep-layer neurons using the horizontal brain slice preparation, thus far, appear quite similar in shape and size to those recorded using the original whole brain preparation. This is probably because the horizontal brain slice preparation preserves the local tectal connectivity across layers, which constitutes the majority of intratectal projections.
Whole cell electrophysiology.
We visualized tectal cells using a Zeiss light microscope with a ×60 water-immersion objective in series with a Hamamatsu infrared CCD camera. Whole cell recordings were made using borosilicate glass micropipettes filled with K+-gluconate internal recording saline (in mM: 100 K-gluconate, 8 KCl, 5 NaCl, 1.5 MgCl2, 20 HEPES, 10 EGTA, 2 ATP, and 0.3 GTP, pH 7.2, osmolarity 255 mosM). Pipette resistances were in the range of 8–12 MΩ. Electrophysiological recordings were done using Axon Instruments MultiClamp 700B Microelectrode Amplifier (Molecular Devices, Sunnyvale, CA), digitized at 10 kHz using a Digidata 1322A digitizer, and acquired using pCLAMP software. Leak current was subtracted in real-time using the acquisition software. For electrical stimulation of visual and mechanosensory inputs, we delivered 0.2-ms pulses using a bipolar stimulating electrode (FHC) placed on the optic chiasm (to simulate RGC axons) or HB (to stimulate mechanosensory inputs) with an ISO-Flex stimulator (A.M.P.I.). All data were analyzed using AxoGraph X and graphed using IGOR Pro software (WaveMetrics).
Extracellular FP recordings.
For FP recordings, we used glass micropipettes that had resistances in the range of 1–2 MΩ. Pipettes were filled with external recording saline, and evoked FP recordings were obtained at 10-μm intervals from the most outer/distal part of the neuropil (corresponding to the most lateral portion of the intact tectum) to the deepest somatic layer (corresponding to the most medial portion of the intact tectum) while stimulating the visual or mechanosensory inputs. A scale bar was superimposed onto the video monitor to allow accurate placements of the recording pipette. Importantly, with this horizontal brain slice preparation, the medial-lateral axis of the neuropil (corresponding to the proximal-distal axis of the tectal neuron dendritic arbors), and all layers of somata, all lie in the same horizontal plane or x-coordinate. This means that to record FPs at different points along this axis, the recording pipette can simply be raised up off of the tissue, moved 10 μm along the x-coordinate, and lowered back down onto the tectum. Lifting and lowering the pipette to different points along the dendrites avoids inherent caveats associated with typical FP recordings that require the recording pipette to be advanced vertically down (along the z-coordinate) and recording at different depths. Advancing the pipette down to different depths of the tissue creates depth measurement errors due to dimpling or dragging of the tissue as the pipette advances. This becomes especially significant when attempting to record FPs from a thin brain structure such as the tadpole tectum (Chung et al. 1974b). Furthermore, since neither the pipette nor the tissue is visible below the surface, it is not possible to know whether and to what extent this sort of drag is occurring. In addition, using the horizontal brain slice preparation described here, the location of the pipette in relationship to the somatic layers and neuropil is readily identified. The visual and mechanosensory inputs were stimulated using bipolar electrodes as described above in the Whole cell electrophysiology section of materials and methods. At the start of each experiment, exploratory FP responses were obtained from across the rostral-caudal axis in the midneuropil region. Once a reliable response was identified, FPs were collected from the most outer (lateral) to the most inner (medial) layer.
Current-source density analysis.
We calculated the current-source density (CSD) profile from the set of FPs according to the method described by Mitzdorf and Singer (1977) using a spatial differentiation grid of 20 μm. For this, we calculate the second spatial derivative by subtracting twice the voltage measured at a given position [V(x)] from the sum of the two voltages measured above [V(x+n * Δx)] and below [V(x−n * Δx)] that position and dividing by the square of the distance between the sites of recording (Δx). All calculations were performed using IGOR Pro software.
RESULTS
All of the experiments described here were carried out using stage 48/49 X. laevis tadpoles. Here, using the horizontal brain slice preparation combined with a set of whole cell voltage-clamp and current-clamp electrophysiological recording protocols, we illustrate how synaptic properties, evoked synaptic responses, voltage-gated intrinsic currents, and the ability to fire action potentials (APs) can be quantified and compared between a deep-layer and an outer-layer tectal neuron. Ultimately, data from these experiments will be used to generate or piece together a wiring diagram of the tectum. Next, we demonstrate how this preparation provides a straightforward way to address the spatial pattern of synapses made by the two distinct inputs by recording FPs at different points across the medial-lateral axis of the tectum, from outer, most lateral edge of the neuropil to the inner, most medial cellular layer, in response to RGC axon input and mechanosensory input. The resulting FP traces are converted into CSDs to reveal more precisely the spatial pattern and magnitude of synaptic current generated along the distal-to-proximal axis of the dendrites.
Whole cell recordings from individual tectal neurons residing at different levels of the tectum.
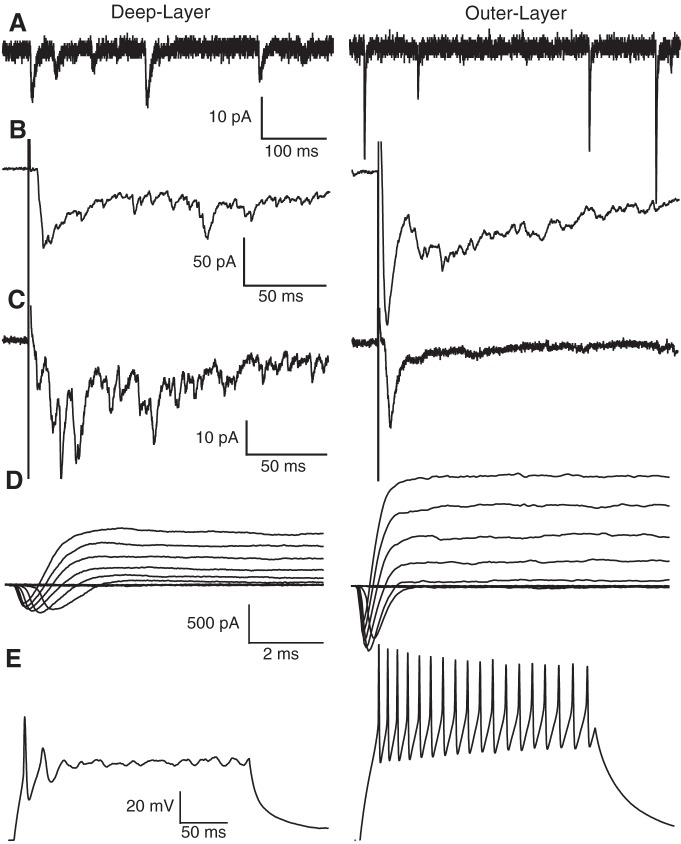
Using the horizontal brain slice preparation, neurons across the entire horizontal plane of the tectum can be readily visualized and recorded in whole cell configuration (Fig. 1E), making it possible, for the first time, to characterize many aspects of synaptic and intrinsic properties displayed by neurons of outer layers of the tectum as well as the pattern and strength of their RGC and mechanosensory inputs. Our initial results show that neurons in the outer layers are, in several ways, electrophysiologically distinct from the well-studied deep-layer tectal neurons. First, to sample excitatory synaptic currents expressed by individual neurons, spontaneous excitatory postsynaptic currents (sEPSCs) were recorded. For this, whole cell voltage-clamp recordings were carried out with the potential of the neuron clamped at −60 mV, and excitatory (AMPA-type glutamate receptor-mediated) currents isolated by blocking spontaneous inhibitory postsynaptic currents (sIPSCs) by including the GABAA antagonist picrotoxin (100 μM) to the external recording solution. Examples of sEPSC recordings from a deep-layer tectal neuron and a neuron residing at the outer-most somatic layer, at the border between somatic and neuropil regions, are shown in Fig. 3A. The outer-layer neuron displays noticeably larger sEPSC amplitudes, a direct measure of synaptic strength. Also, the synaptic events recorded from the outer-layer neuron display noticeably faster rates of decay than those displayed by the deep-layer neuron. This is most likely due to differential receptor subunit composition and/or differences in synapse location in relation to the recording pipette (which is in the soma). Importantly, the synaptic properties displayed by the outer-layer neuron shown here (Fig. 3A) are very similar to a large population of outer-layer neurons that we are currently characterizing and are quite distinct from those expressed by the deep-layer neurons, indicative of different cell types. In fact, with the exception of the morphologically distinct mesencephalic V neuron, a large, unipolar trigeminal sensory neuron that innervates the tentacle (Pratt and Aizenman 2009), the deep-layer tectal neurons appear to be composed of a strikingly homologous population of neurons.
Fig. 3.
Sample whole cell recordings from a deep-layer (left) and outer-layer (right) tectal neuron. A: sample spontaneous excitatory postsynaptic currents (sEPSCs) from a deep- and outer-layer neuron. Notice that outer-layer neuron sEPSCs have larger amplitude and faster decay than deep-layer neuron sEPSCs. B and C: sample evoked synaptic responses recorded from a deep- and outer-layer neuron elicited by stimulating the RGC axons (B) and HB axons (C). Both neurons appear to receive monosynaptic input from both sensory modalities followed in time by polysynaptic activity. D: sample mixed-current recordings from the deep- and outer-layer tectal neurons in response to increasingly depolarizing steps. Downward, inactivating deflections represent inward Na+ current, whereas upward inflections represent outward K+ current. Both Na+ and K+ currents in the outer-layer neuron are bigger and faster than the deep-layer neuron. Recordings in A–D were done in voltage-clamp configuration. E: sample current-clamp traces of action potentials (APs) recorded from a deep- and an outer-layer neuron evoked by injection of a square 250-ms current pulse. Notice that the outer-layer neuron can fire many APs and displays less accommodation compared with the deep-layer neuron. Also, the spike height of the AP displayed by the outer-layer neuron is nearly double that of the deep-layer neuron.
In addition to spontaneous synaptic currents, the different sensory inputs (visual and mechanosensory) can be activated, and the resultant postsynaptic currents, referred to as evoked currents, can also be characterized using the horizontal brain slice preparation. Previously, it had been observed that individual deep-layer tectal neurons receive direct synaptic input from both RGCs and the mechanosensory HB inputs (Deeg et al. 2009; Deeg and Aizenman 2011; Pratt and Aizenman 2009). These experiments were carried out using the original/traditional whole brain preparation that allows for the different sensory inputs to be controlled by placing one bipolar stimulating electrode onto the RGC axon tracts at the optic chiasm to activate, at varying intensities, RGC axonal inputs and placing another one of these electrodes on the HB to activate the afferent mechanosensory axons. Evoked synaptic responses are obtained by activating either input while recording in whole cell mode from a postsynaptic tectal neuron (see materials and methods). Since the horizontal brain slice preparation retains these afferent inputs, and since these inputs remain accessible to stimulating electrodes, the same experiments can be done using this horizontal brain slice preparation. Figure 3, B and C, shows examples of synaptic responses of a deep layer and outer-layer neuron to RGC input and HB input stimulation, respectively. Both neurons display a monosynaptic component, indicating that they both receive synaptic drive from both sets of sensory inputs, and polysynaptic input, indicating that they are both integrated into the local tectal-tectal circuitry. Importantly, the RGC evoked responses observed in deep-layer neurons using the horizontal brain slice preparation were similar in amplitude and pattern to those observed using the whole brain preparation. Both preparations yield maximum evoked synaptic amplitudes in the range of 40–60 pA (data not shown). The advantage of carrying out these experiments using the horizontal brain slice preparation is that only with this preparation is it possible to characterize the pattern and strength of the different sensory modalities received as a function of neuron location within the developing layers of the tectum as opposed to using the original whole brain preparation and recording exclusively from the deep-layer neurons. Hence, data using this new preparation will contribute to a more comprehensive understanding about how sensory circuits are built and how they function.
Noticeable differences were also observed in voltage-gated intrinsic currents expressed by the deep- and outer-layer neuron. (Fig. 3D). Quantifying voltage gated intrinsic currents is of importance because the combined expression of these currents largely determines the intrinsic excitability of the neuron, the ease in which the neuron generates APs, and thus determines the output function. A classic way to characterize voltage-gated intrinsic currents expressed by a neuron is to construct current-voltage plots for the individual currents. For this, neurons were recorded in whole cell voltage-clamp mode and, starting from a baseline potential of −60 mV, stepped to increasingly more depolarized potentials in increments of 10 mV. This protocol activates both voltage-gated outward K+ and inward Na+ currents (Fig. 3D). The representative outer-layer neuron shown in Fig. 3D displays significantly larger Na+ and K+ currents compared with the deep-layer neuron. From these data, current-voltage plots can be constructed by plotting the maximum (peak) current elicited as a function of voltage step (data not shown). Another common method to characterize intrinsic excitability is to quantify the generation of APs. AP generation can be directly addressed by injecting, in current-clamp mode, different amounts of current and counting the number of APs elicited by this injection. Typically, the data from these experiments are plotted as number of APs fired as a function of the amount of current injected. It is well-established that deep-layer tectal neurons exhibit a great amount of accommodation in response to sustained current injection (Pratt and Aizenman 2007). This is mainly due to the inability of these neurons to repolarize sufficiently to allow for the resetting of Na+ channels. Figure 3E shows AP traces generated by a deep-layer and an outer-layer neuron. Whereas the deep-layer neuron displays robust accommodation, only able to fire a few APs in response to a 250-ms sustained current injection, the representative outer-layer neuron displays essentially no accommodation and fires APs repetitively for the duration of the current injection. These differences between the deep-layer and outer-layer neurons likely reflect the medial-to-lateral developmental gradient (most immature, medial; most mature, lateral) formed by the generation of new neurons in the ventricular proliferative zone that resides at the medial-most end of the axis (Straznicky and Gaze 1972).
Based on data obtained from this set of experiments using the horizontal brain slice preparation, at least two distinct neuron types have been found residing in the outer layers of the tectum thus far. Studies are underway to identify the neurotransmitters they express.
FPs and CSD profiles evoked by RGC and HB stimulation.
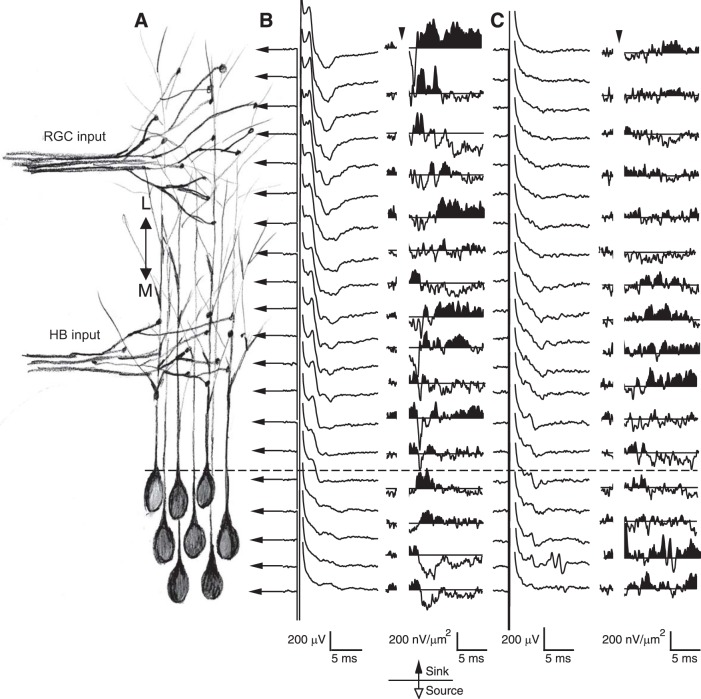
Previously, it has been shown using imaging techniques that axonal projections from the retina and HB onto the tectum display laminar specificity such that RGC axons project to a superficially located, distal lamina in the neuropil, whereas the mechanosensory inputs arriving via the HB project to a deep neuropil lamina located proximally to the tectal cell somata (Fig. 4A; Deeg et al. 2009; Hiramoto and Cline 2009). Although this work demonstrates laminar specificity of sensory axonal inputs onto the tectal neuropil, it is still not clear where the major sites of synaptic activity are located. By stimulating the RGC and HB axonal inputs and recording local FPs from different locations in the neuropil, the sites of major synaptic activities can be identified. From these FP recordings, CSD profiles are derived by calculating the second spatial derivative (see materials and methods). Sample FP recordings obtained from the tectal neuropil in response to RGC and HB stimulation and their corresponding CSD profiles are shown in Fig. 4, B and C, respectively. For CSD profiles, sinks are upward inflections (black) and represent excitatory synaptic activity, whereas sources are downward deflections (white) representing both the positive current moving back out of the neurons, “postsink,” and/or GABA-mediated influx of negative current. Notice the overall pattern of current sinks and sources are different for RGC and HB inputs and correspond well with the imaging experiments. For RGC inputs, the majority of current sinks occur at the outer lamina of the neuropil, whereas HB sinks occur mostly at the inner lamina and essentially not at all in the outer lamina. Also, notice that the location of the largest sinks generally corresponds to the location of the largest FP. We have observed this input-dependent pattern of sinks to be consistent across preparations. Another recurring pattern we observed is that the HB sinks are consistently smaller than the RGC sinks, which is in agreement with our whole cell recordings that indicate that the maximum postsynaptic responses evoked by RGC axon stimulation are, in general, stronger than responses evoked by HB stimulation. Finally, the CSD sinks appearing later in time most likely reflect polysynaptic activity that is also observed in single-cell recordings.
Fig. 4.
Local FP recordings from the horizontal brain slice preparation. A: simplified schematic of the neuropil showing RGC (distal) and HB (proximal) axonal inputs onto tectal neuron dendrites. FPs were recorded every 10 μm along the distal-proximal axis (indicated by the arrows). B, left: local FPs in response to RGC stimulation. Right: corresponding current-source density (CSD) profile derived using a spatial differentiation of 20 μm. C, left: local FPs in response to HB stimulation. Right: corresponding CSD profile. Upward inflections are current sinks (black), whereas downward deflections are current sources (white). Arrowheads indicate time of stimulation.
Interestingly, both the synaptic FP recordings and whole cell recordings suggest the two sensory inputs evoke different patterns of polysynaptic responses, suggesting that RGC input and mechanosensory input activate distinct intratectal networks.
DISCUSSION
The Xenopus tadpole retinotectal projection is a popular model to study the mechanisms underlying the development of sensory circuits. This is mainly because the entire development of the tadpole, from the moment of fertilization on, all takes place externally (i.e., as opposed to in utero), making it possible to access and observe readily even the earliest stages of neural circuit formation without disrupting the natural environment. In combination with this experimental asset, whole cell recordings of postsynaptic tectal neurons can be readily carried out in vivo to characterize responses to light or, alternatively, using the whole brain preparation that allows for the RGC and HB inputs to be activated in a controlled manner. As a result, the tadpole model has been used to study many aspects of neural circuit development including the maturation of synapses (Wu et al. 1996) and intrinsic currents (Hamodi and Pratt 2014; Pratt and Aizenman 2007), functional refinement (Tao and Poo 2005), direction-selective plasticity (Engert et al. 2002), visual acuity (Aizenman et al. 2003; Dong et al. 2009; Schwartz et al. 2011), and spike-timing-dependent plasticity (Richards et al. 2010; Zhang et al. 1998). This work, however, does not address the neurons that lie beyond the readily accessible periventricular layer. To understand the way in which the tectum functions as a whole, it is necessary to understand the development of the physiological behavior and connectivity of all of its different types of neurons. Thus far, our data from the horizontal brain slice preparation indicate that neurons of the outer layers are electrophysiologically distinct from the homogenous population of neurons of the deep layer and so probably play different roles in sensory processing.
The pattern of connections formed by a neural circuit is a major factor in shaping how the circuit functions. In addition to the well-studied and understood topographic map formed by the RGC inputs and the mechanosensory inputs onto tectum, there exists a less-studied subcellular pattern of connectivity in which RGC inputs target the distal (most lateral) portion of tectal dendrites, whereas mechanosensory axonal inputs target the more proximal region (Deeg et al. 2009; Hiramoto and Cline 2009). Using the horizontal brain slice preparation we are able to quantify this spatial pattern of synaptic activity. The FP recordings and CSD analysis shown in Fig. 4 indicate a spatial pattern of visual and mechanosensory driven synaptic activity that is consistent with the spatial pattern of the axonal inputs.
In addition to the segregation of visual RGC and mechanosensory (HB) inputs in the tadpole tectum, individual axons that constitute the RGC input are further organized within the distal region of dendrite according to the position of the RGC in the eye: RGCs of the dorsal region of the eye project to the more medial portion of the distal dendrites, whereas RGCs of the ventral region of the eye project to the lateral portion (Bollmann and Engert 2009). Similarly, in zebrafish, it has been shown that the spatial pattern of visual inputs is arranged in tectal space according to the direction selectivity of the individual axons (Gabriel et al. 2012). The mechanism(s) underlying how different sets of inputs are consistently guided to specific regions of the dendrite, however, is (are) not completely clear. It has been suggested that synchronous activity of all of the axons associated with specific sensory input could account for “like” axons converging onto a specific focused region of the dendrite (Bollmann and Engert 2009). Consistent with this, in the tadpole tectum, the RGC and mechanosensory inputs arrive simultaneously (Hiramoto and Cline 2009), suggesting the possibility that activity-dependent competition between the different sensory inputs underlies their segregation. Also not clear is the functional consequence of this subcellular arrangement of inputs in the context of sensory integration. In other words, is it important for proper multimodal sensory integration that, for example, the visual inputs to be focused on the distal dendrite and mechanosensory inputs focused closer to the tectal neuron somata? With the capability to activate the different sensory inputs and quantify, at high resolution, the spatial pattern of synaptic activity generated by these inputs in combination with recording responses from individual tectal neurons, the horizontal brain slice preparation provides an improved method to address these questions.
Overall, the experiments that become possible using the newly developed and tested tadpole horizontal brain slice preparation will extend what is known about the functional development of the amphibian tectum in its entirety.
GRANTS
This work was funded by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant P30-GM-32128.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.S.H. and K.G.P. conception and design of research; A.S.H. and K.G.P. performed experiments; A.S.H. and K.G.P. analyzed data; A.S.H. and K.G.P. interpreted results of experiments; A.S.H. and K.G.P. prepared figures; A.S.H. and K.G.P. drafted manuscript; A.S.H. and K.G.P. edited and revised manuscript; A.S.H. and K.G.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Carlos D. Aizenman and Mayank Mehta for their help with the CSD analysis and Dr. Qian-Quan Sun for helpful comments on the manuscript.
REFERENCES
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron 39: 831–842, 2003. [DOI] [PubMed] [Google Scholar]
- Behrend O, Branoner F, Zhivkov Z, Ziehm U. Neural responses to water surface waves in the midbrain of the aquatic predator Xenopus laevis. Eur J Neurosci 23: 729–744, 2006. [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Engert F. Subcellular topography of visually driven dendritic activity in the vertebrate visual system. Neuron 61: 895–905, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Bliss TV, Keating MJ. The synaptic organization of optic afferents in the amphibian tectum. Proc R Soc Lond B Biol Sci 187: 421–447, 1974a. [DOI] [PubMed] [Google Scholar]
- Chung SH, Keating MJ, Bliss TV. Functional synaptic relations during the development of the retino-tectal projection in amphibians. Proc R Soc Lond B Biol Sci 187: 449–459, 1974b. [DOI] [PubMed] [Google Scholar]
- Deeg KE, Aizenman CD. Sensory modality-specific homeostatic plasticity in the developing optic tectum. Nat Neurosci 14: 548–550, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg KE, Sears IB, Aizenman CD. Development of multisensory convergence in the Xenopus optic tectum. J Neurophysiol 102: 3392–3404, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Lee RH, Xu H, Yang S, Pratt KG, Cao V, Song YK, Nurmikko A, Aizenman CD. Visual avoidance in Xenopus tadpoles is correlated with the maturation of visual responses in the optic tectum. J Neurophysiol 101: 803–815, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert F, Tao HW, Poo MM. Moving visual stimuli rapidly induce direction sensitivity of developing tectal neurons. Nature 419: 470–475, 2002. [DOI] [PubMed] [Google Scholar]
- Gabriel JP, Trivedi CA, Maurer CM, Ryu S, Bollmann JH. Layer-specific targeting of direction-selective neurons in the zebrafish optic tectum. Neuron 76: 1147–1160, 2012. [DOI] [PubMed] [Google Scholar]
- Grusser OJ, Grusser-Cornehls U. Neurophysiology of the anuran visual system. In: Frog Neurobiology, edited by Llinas R and Precht W. New York: Springer, 1976. [Google Scholar]
- Hamodi AS, Pratt KG. Region-specific regulation of voltage-gated intrinsic currents in the developing optic tectum of the Xenopus tadpole. J Neurophysiol 112: 1644–1655, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto M, Cline HT. Convergence of multisensory inputs in Xenopus tadpole tectum. Dev Neurobiol 69: 959–971, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar GY. The development of the optic tectum in Xenopus laevis: a Golgi study. J Anat 116: 347–355, 1973. [PMC free article] [PubMed] [Google Scholar]
- Lowe DA. Organisation of lateral line and auditory areas in the midbrain of Xenopus laevis. J Comp Neurol 245: 498–513, 1986. [DOI] [PubMed] [Google Scholar]
- Miraucourt LS, Santos da Silva J, Burgos K, Li J, Ruthazer ES, Cline HT. GABA expression and regulation by sensory experience in the developing visual system. PLoS One 7: e29086, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U, Singer W. Laminar segregation of afferents to lateral geniculate nucleus of the cat: an analysis of current source density. J Neurophysiol 40: 1227–1244, 1977. [DOI] [PubMed] [Google Scholar]
- Munoz A, Munoz M, Gonzalez A, Ten Donkelaar HJ. Anuran dorsal column nucleus: organization, immunohistochemical characterization, the fiber connections in Rana perezi and Xenopus laevis. J Comp Neurol 363: 197–220, 1995. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Matsumoto N. Current source density analysis of ON/OFF channels in the frog optic tectum. Prog Neurobiol 61: 1–44, 2000. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Matsumoto N. ON and OFF channels of the frog optic tectum revealed by current source density analysis. J Neurophysiol 80: 1886–1899, 1998. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Miyazaki H, Matsumoto N. Principle neuronal organization in the frog optic tectum revealed by a current source density analysis. Vis Neurosci 14: 263–275, 1997. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin). New York: Garland, 1994. [Google Scholar]
- Pratt KG, Aizenman CD. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci 27: 8268–8277, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Aizenman CD. Multisensory integration in the mesencephalic trigeminal neurons in Xenopus tadpoles. J Neurophysiol 102: 399–412, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Dong W, Aizenman CD. Development and spike timing-dependent plasticity of recurrent excitation in the Xenopus optic tectum. Nat Neurosci 11: 467–475, 2008. [DOI] [PubMed] [Google Scholar]
- Richards BA, Aizenman CD, Akerman CJ. In vivo spike-timing-dependent plasticity in the optic tectum of Xenopus laevis. Front Synaptic Neurosci 2: 1–11, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N, Schohl A, Ruthazer ES. Activity-dependent transcription of BDNF enhances visual acuity during development. Neuron 70: 455–467, 2011. [DOI] [PubMed] [Google Scholar]
- Straznicky K, Gaze RM. The development of the tectum in Xenopus laevis: an autoradiographic study. J Embryol Exp Morphol 28: 87–115, 1972. [PubMed] [Google Scholar]
- Tao HW, Poo MM. Activity-dependent matching of excitatory and inhibitory inputs during refinement of visual receptive fields. Neuron 45: 829–836, 2005. [DOI] [PubMed] [Google Scholar]
- Tsurudome K, Li X, Matsumoto N. Intracellular and current source density analysis of somatosensory input to the optic tectum of the frog. Brain Res 1064: 32–41, 2005. [DOI] [PubMed] [Google Scholar]
- Wu G, Malinow R, Cline HT. The maturation of a central glutamatergic synapse. Science 274: 972–976, 1996. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Holt CE, Harris WA, Poo M. A critical window for cooperation and competition among developing retinotectal synapses. Nature 395: 37–44, 1998. [DOI] [PubMed] [Google Scholar]