Abstract
The host response to intravascular, Gram-negative bacteria includes profound immunologic, hematologic and physiologic changes. Numerous host defense mechanisms are activated by Gram-negative bacteria, including the complement system. Activation of the complement system leads to cleavage of C5 with subsequent generation of the C5a anaphylatoxin peptide. C5a mediates potent, proinflammatory activities by binding to the C5a receptor (C5aR, CD88). In this study, we report the targeted disruption of the murine C5aR gene (C5aR−/− mice) and define the role of the C5aR in a model of Gram-negative bacteremia. Following an intravenous infusion of heat-killed Escherichia coli, the C5aR−/− mice were completely protected from the mortality suffered by their wild-type littermates (P < 0.001). The C5aR−/− mice were also significantly (P = 0.008) more resistant to mortality following an intravenous infusion of purified E. coli endotoxin compared to the wild-type littermates. In addition, the C5aR−/− mice were resistant to the thrombocytopenia and hemoconcentration observed in wild-type animals. Lethality in the wild-type mice was reversed by pre-treatment with either the histamine antagonist diphenhydramine or triprolidine. The wild-type littermates were also rescued following pre-treatment with the basophil and mast cell-stabilizing agent – cromolyn sodium. Collectively, these data demonstrate that not only is the absence of the C5aR protective in E. coli bacteremia, but that C5aR-dependent histamine release plays a major role in shock induced by Gram-negative septicemia. Moreover, they provide additional in vivo evidence that C3a and C5a have divergent biological functions in Gram-negative bacteremia and shock.
Keywords: Anaphylatoxins, Complement, Knock-out Mice, Sepsis, Shock
1. Introduction
Studies dating back to the early 1900s have documented that activation of complement in fresh serum induces a fatal anaphylactoid reaction when infused into the donor animal (Friedberger, 1910). The biological responses observed in these studies were later attributed to the generation of complement secondary reaction products C3a and C5a (reviewed in Hugli and Muller-Eberhard, 1978). Collectively referred to as anaphylatoxins, C3a and C5a have become widely recognized for their broad proinflammatory potential. Both the C3a and C5a peptides are well adapted to hostile, infectious environments; both are stable at extremes of pH and temperature (reviewed in Ember et al., 1998). At 77 and 74 amino acids, respectively, both the C3a and C5a peptides are inactivated by the enzymatic cleavage of the terminal arginine by serum carboxypeptidase N (reviewed in Matthews et al., 2004). Prior to their inactivation, C3a and C5a mediate biological functions after binding to separate, high affinity, septahelical, G protein coupled receptors (reviewed in Wetsel et al., 2000).
The generation of complement anaphylatoxins is thought to contribute to both the physiologic and immunologic dysfunction of septic shock. Numerous clinical reports have documented the generation of complement anaphylatoxins in septic shock patients (Cole et al., 2002; Nakae et al., 1996; Selberg et al., 2000; Stove et al., 1996), and some have reported that circulating levels of complement anaphylatoxins are inversely proportional to patient survival (Groeneveld et al., 2003; Hack et al., 1989; Hecke et al., 1997). The in vivo effects of rapid and acute intravascular C5a production have been studied using intravenous infusion experiments. Infusion of C5a into rabbits (Lundberg et al., 1987) and rats (Smedegard et al., 1989) reproduces many of the changes observed in bacteremic shock, including a rapid drop in mean arterial pressure and decreased peripheral circulation of granulocytes, monocytes and platelets. With the addition of endotoxin, the effects of intravenous C5a are more pronounced, resulting in a synergistic increase in vascular permeability and a severe hypotensive response (Smedegard et al., 1989). Experiments in primates have focused on the therapeutic efficacy of blocking C5a in septic shock. Following intravenous infusion of Escherichia coli, primates pre-treated with anti-C5a antibody recovered promptly and suffered no mortality, while untreated primates experienced 75% mortality (Stevens et al., 1986). Work with the cecal ligation and puncture (CLP) model of sepsis also describes a therapeutic advantage following treatment with a C5a-neutralizing antibody (reviewed in Guo et al., 2004). Mechanistically, results from animal models of CLP-induced shock indicate that C5a contributes to acquired neutrophil dysfunction in sepsis (Guo et al., 2002; Riedemann et al., 2003).
The current study was designed to evaluate the contribution of C5aR activation in mice subjected to Gram-negative shock and to compare the results with those of C3aR−/− mice, which we have previously shown to exhibit increased susceptibility to endotoxemia (Kildsgaard et al., 2000). To facilitate these studies, C5aR−/− mice were generated by targeted gene deletion. Experiments with both endotoxin and heat-killed E. coli revealed that C5aR−/− mice were highly resistant to Gram-negative shock. C5aR−/− mice experienced 100% survival compared to the 21% survival in age and sex-matched wild-type littermates. Compared to C5aR−/− mice, wild-type mice experienced a rapid and significant increase in hematocrit and a decrease in circulating platelets. Following induction of shock, wild-type mice were rescued by pre-treatment with antihistamines or with the basophil and mast cell-stabilizing agent cromolyn sodium. The results strongly suggest that in this model of bacteremic shock, mast cell activation products are potent contributors to host mortality. We have also extended our initial findings that C3aR−/− mice are more sensitive to endotoxic shock by including evidence that C3aR−/− mice are also more susceptible to Gram-negative bacteremia. These results provide evidence that the complement C5aR functions to mediate many of the physiological and cellular responses characteristic of acute shock and that the C3aR functions to temper the immune response in acute shock.
2. Materials and methods
2.1. Reagents and C3aR−/− mice
Unless otherwise specified, chemicals were purchased from Sigma Chemical Co., restriction enzymes and PCR reagents from Roche (Indianapolis, IN), and cell culture reagents from Invitrogen-Gibco (Carlsbad, CA). C3aR−/− mice were generated in our laboratory as previously described (Kildsgaard et al., 2000). The C3aR−/− mice used in these studies were backcrossed 10 times onto a C57BL/6 background, and 8–10-week-old male mice were used. Age- and sex-matched C57BL/6 wild-type controls were purchased from Harlan Laboratories (Houston, TX). All animal care and studies were conducted in accordance with the principles and guidelines approved by the University of Texas Health Science Center Committee for Animal Resources.
2.2. C5aR targeting vector and the generation of C5aR null mice
A genomic clone containing exon 2 of the murine C5aR gene was isolated from a mouse 129Svj genomic phage library. The insert of the lambda clone was excised with SalI and sub-cloned into pBluescript. After removing the BamHI site of pBluescript, the insert-containing plasmid was digested with BamHI, thereby removing bases 57–1050 of the murine C5aR. The lox containing plasmid PBS 246 (Gibco) was digested with BamHI and HindIII. A neomcycin-resistance cassette (Lexicon Genetics, The Woodlands, TX) was cloned into the BamHI and HindIII sites of PBS 246. The lox flanked neomycin cassette was then excised with NotI, blunted with Klenow enzyme, and cloned between the Klenow blunted sites of the BamHI cut genomic plasmid. RW-4 embryonic stem cells (Hug et al., 1996) were transfected with linearized targeting vector. Targeted clones were identified using southern blotting. Homologous recombinants were screened for multiple vector insertions and those that contained only a single vector insertion were injected into C57BL/6 blastocysts. The resulting chimeric males were mated with C57BL/6 females, and all progeny were screened for germline transmission via southern blotting.
2.3. Southern blot analysis
DNA was isolated from neomycin (G418)-resistant embryonic stem cells or from mouse tail clippings and digested with XhoI. Standard southern blotting was performed with a 175 base pair (bp), 32P-radiolabeled, 5′ probe which was external to the targeting vector. The probe was generated with the following oligonucleotides: 5′-TCACCCTGTCCTTTTCGTGACTTCA and 5′-GTGCTGGGATCACAGTTATGCAGTGC.
2.4. PCR genotyping
DNA was isolated from mouse tail clippings. The isolated DNA was used as a template for PCR with the primers P1 (5′-GACCCATAGATAACAGCAGCTTTG), P2 (5′-CCCTCGAGCTAGAGGTACCCTAG), and P3 (5′-ACCCACCATCAGCCGCAGGATGGC). The primers P1 and P2 amplify a 260 bp fragment corresponding to targeted mice. Primers P1 and P3 amplify a 580 bp fragment specific for the wild-type allele.
2.5. Flow cytometry
An anti-mouse C5aR monoclonal antibody (D9.3) was raised to a peptide corresponding to the first extracellular domain of the C5aR, as previously described (Drouin et al., 2001; Riedemann et al., 2002). By ELISA it was determined that the mouse antibody specifically recognizes the C5aR peptide. For flow cytometry, thioglycollate-stimulated peritoneal macrophages were obtained from C5aR−/− mice and their wild-type littermates as described (Michell, 1980). Cells were washed briefly in PBS, and stained as follows: non-specific blocking with 5% goat serum (Jackson Immunoresearch), followed by 1 μg/106 cells of either anti-mC5aR antibody (D9.3) or purified murine IgG (Sigma Chemical Corp.), the cells were washed twice with cold PBS, and then a 1:250 dilution of FITC-conjugated goat anti-mouse F(ab)2 antibody (Sigma) was added. Cells were again washed twice with cold PBS, resuspended, and analyzed using CellQuest software with BD FACSCalibur (BD Biosciences) instrumentation. Analysis was performed on 10,000-gated events based on FSS and SSC profiles. Similar FSS and SSC profiles as well as cell numbers were noted in both the C5aR−/− mice and their wild-type littermates (data not shown).
2.6. Gram-negative bacteremia
E. coli serotype 0127: K63 (B8) was purchased from the American Type Culture Collection (ATCC 12740). Prior to each experiment, bacteria were cultured in Luria-Burtani (LB) broth at 37 °C with shaking. Bacteria were grown to mid-logarithmic phase, harvested by centrifugation, washed one time in sterile phosphate-buffered saline (PBS), and resuspended in sterile PBS to a concentration of 3 × 1010 colony forming units (CFU)/ml. Both optical densities at 600 nm and dilution plating with CFU counting were used to quantify bacterial concentration. Bacteria were heat inactivated by incubation in a water bath at 85 °C for 1 h. Eight to 10-week-old C5aR-deficient mice and their wild-type littermates (F2 mice on a C57BL/6 background) were anesthetized with isoflurane and injected intravenously with 6 × 109 CFU of heat-killed E. coli as a 200 μl bolus in PBS. For experiments requiring pharmacological antagonism, intraperitoneal (i.p.) injections of diphenhydramine (10 mg/kg), triprolidine (10 mg/kg), or cromolyn sodium (100 mg/kg) were given 30 min prior to bacterial challenge. Mortality was continuously monitored for the first 2–3 h and then every 4–8 h for 3 days.
2.7. Endotoxic shock
E. coli serotype 0111:B4 endotoxin was purchased from List Biological Laboratories, Inc. (#20139A1, Campbell, CA). Endotoxin was resuspended in PBS to a stock concentration of 2.5 mg/ml. Mice were weighed and the endotoxin stock solution was diluted appropriately in PBS to inject 3 mg/kg in a 200 μl bolus. Eight to 10-week-old C5aR−/− mice and their wild-type littermates (F2 mice on a C57BL/6 background) were anesthetized with isoflurane and injected intravenously at time zero. Mortality was continuously monitored for the first 2–3 h and then every 4–8 h for 3 days.
2.8. Hematologic measurements
At time zero and at both 5 and 10 min after induction of Gram-negative bacteremia, mice were anesthetized, and blood was harvested via cardiac puncture using EDTA anticoagulation. Samples were immediately analyzed on a Hemavet cell counter and blood smears were analyzed in a blinded fashion.
2.9. Serum C3 levels
Blood from C5aR−/− mice and their wild-type matched littermates was harvested via cardiac puncture. After centrifugation at 5000 × g for 10 min, serum samples were isolated and stored at −80°C. Serum concentrations of C3 in C5aR−/− mice and their wild-type littermates were evaluated by rocket immunoelectrophoresis. 16 μl of mouse plasma from eight wild-type littermates and 8 C5aR−/− mice were placed into individual wells and subjected to rocket immunoelectrophoresis on 4 × 6-inch glass slides with 1.5% electrophoresis grade agarose (Bio-Rad Laboratory) containing 5.7 mM Tris base, 100 mM glycine, and 8.3 mM tetra-sodium EDTA buffer, pH 8.9 (Tris, glycine, EDTA, pH 8.9 buffer) and 10 μl of polyclonal rabbit anti-mouse C3 antiserum/cm2. Electrophoresis was conducted at 22 °C for 16 h at 6–8 V employing Tris, glycine, EDTA, pH 8.9 in the electrode chambers. After electrophoresis the slides were washed extensively with 0.15 M NaCl, deionized distilled water, dried, and stained with 0.05% amido black in 40% ethanol, 10% acetic acid. Rocket peaks from all 16 samples were identical in shape, size, and in peak distance from well, indicating that there are no significant differences in C3 concentration in the plasma of C5aR−/− mice and their wild-type littermates.
2.10. Measurement of IgM antibody to E. coli
Seventy ml of an overnight culture of E. coli 0127:K63 (B8) was centrifuged, washed one time with PBS, and resuspended in 70 ml of PBS. Each well of seven 96-well plates was coated with 100 μl of E. coli, and the plates were incubated overnight at 4 °C. The next day, the wells were washed three times with 0.05% Tween 20 in PBS and were blocked for 1 h at room temperature with 1% BSA in PBS. Serum samples that had been diluted 1:16, 1:32, 1:64, 1:128, 1:256, 1:512, and 1:1024 in 1% BSA in PBS were added to the wells and were incubated for 2 h at room temperature. Control wells received only 1% BSA in PBS. The wells were washed as above and incubated for 2 h in alkaline phosphatase-conjugated goat anti-mouse IgM diluted 1:5000 (Sigma–Aldrich) in 1% BSA in PBS. The wells were washed as above. Detection was performed using a pNPP liquid substrate system (Sigma–Aldrich) for 30 min, and development was stopped using 2 M NaOH. The absorbance was read at 405 nm on a plate reader.
2.11. Cytokine and nitrate/nitrite measurements
Five minutes after induction of Gram-negative bacteremia, mice were anesthetized and blood was collected via cardiac puncture using EDTA (for cytokines) or heparin (for nitrite/nitrate) anticoagulation. Plasma samples were collected following centrifugation at 5000 × g for 10 min. Serum levels of TNF-α, IL-1β, IL-10 and IL-6 were determined using ELISA kits obtained from R&D Systems (Minneapolis, MN). Plasma nitrite was evaluated from triplicate wells of plasma from individual mice using a kit purchased from Calbiochem.
2.12. Statistical analysis
Analysis of all data was performed using the Graphpad (SanDiego, CA) Prism program package. Survival was compared using the logrank test and hematocrit, platelet, ELISA and nitrate values were compared using a two-tailed, unpaired t-test.
3. Results
3.1. Production and characterization of C5aR−/− mice
The C5aR gene-targeting construct was designed by removing the C5aR coding area and replacing it with a lox flanked neomycin cassette (Fig. 1). During construct design, bases 57–1050 of the murine C5aR gene were excised. Therefore, after homologous recombination 95% of the coding area was removed (Fig. 1). The targeted locus contains only the methionine from exon 1 and 57 base pairs of exon 2. Following transfection and selection of 100 neomycin-resistant embryonic stem cells, 16 clones contained a correctly targeted locus. Three of these clones were prepared and used to microinject blastocyts, one of which integrated into the mouse germline. To confirm that the gene disruption created a null mutation, total RNA was isolated from brain, kidney, liver, lung, and testis of C5aR−/− mice and their wild-type littermates. Quantitative RT-PCR was performed and no C5aR message was identified in any of the C5aR−/− tissues (data not shown). Using antibody to the C5aR, flow cytometry studies confirmed the absence of the receptor on thioglycollate-stimulated peritoneal macrophages (Fig. 2). The C5aR−/− mice developed normally in size, obeyed predicted Mendelian breeding ratios, and no gross anatomical defects were observed. Blood counts and smears from unchallenged C5aR−/− mice were similar to wild-type littermates. In addition, unchallenged C5aR−/− mice and their wild-type littermates had no differences in basal serum levels of C3 and no detectable levels of TNF-α, IL-1β, IL-10, and IL-6.
Fig. 1.
The murine C5aR targeting strategy, Southern blot analysis of targeted embryonic stem cells, and PCR genotyping of targeted mice. (A) Schematic of the C5aR genomic locus, targeting vector, and the targeted locus. Exons are indicated by open boxes while introns are horizontal lines. “L” represents a lox recombination site. The position of the 5′ external screening probe is indicated. (B) Southern blot analysis of XhoI-digested genomic DNA from mouse tail clippings. Bands of 12 and 6 kb correspond to wild-type (W) and targeted (T) alleles, respectively. (C) PCR genotyping of wild-type (+/+), heterozygous (+/−) and C5aR-deficient (−/−) mice. The wild-type and targeted alleles are represented by 580 and 260 base pair (bp) bands, respectively.
Fig. 2.

Flow cytometry histogram of thioglycollate stimulated peritoneal macrophages from C5aR−/− mice and their wild-type littermates. The cells were stained with antimouse C5aR monoclonal antibody (D9.3) and FITC-conjugated goat anti-mouse F(ab)2 antiserum. Minimal non-specific staining was detected in the C5aR−/− mice (dotted line). Compared to C5aR−/− mice stained with anti-C5aR (light gray line), high levels of cell surface C5aR expression was noted in the wild-type littermates (solid black line, n = 2).
3.2. C5aR−/− mice are resistant to Gram-negative bacteremia
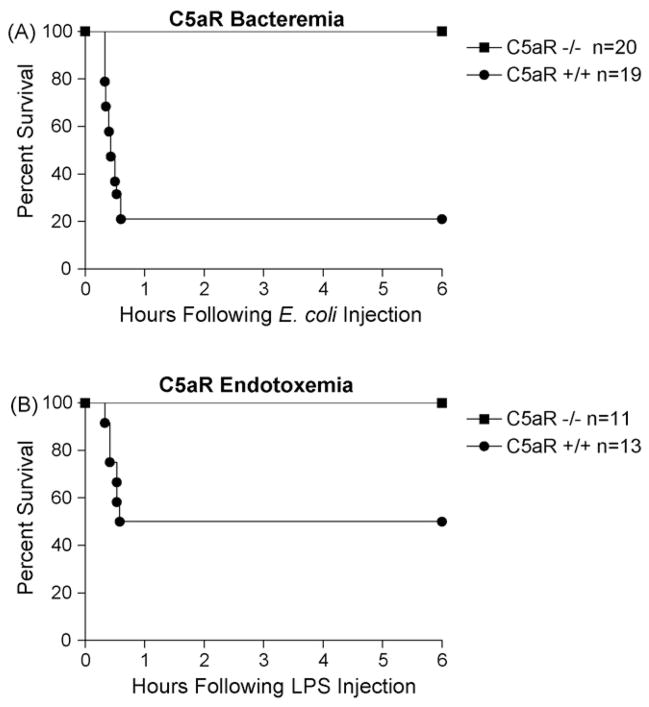
The observation that the complement split products C3a and C5a induce many of the same physiological responses of anaphylactic shock resulted in their categorization as anaphylatoxins. Both the C5a and C3a peptide share similar weights, charge, and peptide properties which imposed early technological limitations on differential purification. Since then, the receptors for C3a and C5a have been identified, cloned, and their respective genes have been disrupted in mice (Hopken et al., 1996; Humbles et al., 2000; Kildsgaard et al., 2000). These advances have allowed us to re-examine the anaphylactoid role of C3a and C5a. To evaluate the contribution of C5a and C3aR activation to the anaphylactic-like response following Gram-negative bacteremia, increasing doses of heat-killed E. coli were injected intravenously into 8–10-week-old male mice. Following injection, all animals recovered promptly from isoflurane anesthetization. At a dose of 6 × 109 CFU per mouse, the C5aR−/− mice were completely protected while their wild-type littermates experienced 79% mortality within 1 h of injection (Fig. 3A). Mice were monitored for 3 days. Both groups experienced no additional mortality beyond that reported.
Fig. 3.
C5aR−/− mice are more resistant to Gram-negative bacteremia and endotoxemia. Panel A, survival of mice receiving 6 × 109 CFU of intravenous, heat-inactivated E. coli. C5aR−/− mice experienced no mortality while their C5aR wild-type littermates suffered 79% mortality (P < 0.001). Panel B, survival of mice receiving 3 mg/kg (50 μg/mouse) of intravenous E. coli endotoxin. C5aR+/+ mice experienced 50% mortality while the C5aR−/− mice were completely protected (P = 0.008). In all experiments, animals were followed for 3 days and no additional mortality was observed.
3.3. C5aR−/− mice are resistant to endotoxemia
Results of the intravenous E. coli experiments suggest that C5a-mediated effects occur rapidly upon injection. While numerous components of the Gram-negative cell wall are capable of activating complement, studies report that endotoxin activates all three pathways of complement (Joiner et al., 1984a,b; Pangburn et al., 1980; Reid et al., 1997; Zhao et al., 2002). In order to compare the effects of endotoxemia in C5aR−/− mice with previous work using C3aR−/− mice, C5aR−/− mice were injected intravenously with increasing doses of endotoxin. Following isoflurane anesthetization and intravenous injection, all animals recovered promptly. At a dose of 50 μg of LPS per mouse, the C5aR−/− mice were completely protected, while their wild-type littermates experienced 50% mortality (Fig. 3B). Mice were monitored for 3 days and no additional mortality was observed.
3.4. C3aR−/− mice are more susceptible to Gram-negative bacteremia
C3aR activation has historically been thought to mediate proinflammatory functions. Reports have since emerged that C3a may have a more diverse role in modulating the proinflammatory cascade (Fischer and Hugli, 1997; Takabayashi et al., 1998; Takabayashi et al., 1996). In fact, we have previously published that C3aR−/− mice are more susceptible to endotoxemia (Kildsgaard et al., 2000). To extend these findings to include responses to whole bacteria, and to compare the acute effects of Gram-negative bacteremia in C3aR−/− and C5aR−/− mice, we subjected C3aR−/− mice to Gram-negative bacteremia. Increasing doses of heat-killed E. coli were injected into C3aR−/− mice and their wild-type controls. Injection of 1.5 × 109 CFU/mouse resulted in 60% survival of C3aR−/−mice compared to 100% survival of wild-type controls (Fig. 4). These results support our earlier studies using a similar strain of E. coli endotoxin in which only 15% of C3aR−/− mice survived compared to 73% of their wild-type controls (Kildsgaard et al., 2000).
Fig. 4.

C3aR−/− mice are more susceptible to Gram-negative bacteremia. Following an intravenous injection of 1.5 × 109 CFU of intravenous, heat-killed E. coli, the C3aR−/− mice suffered 40% mortality while the wild-type controls experienced no mortality (P = 0.022). Mice were followed for 3 days and no additional mortality was observed.
3.5. C5aR−/− mice experience fewer hematologic changes
Gram-negative bacteremia and septic shock are characterized by numerous hematologic changes. It is widely reported that the infusion of Gram-negative bacteria into animals is associated with a rapid fall in circulating platelets and white blood cells and an increase in the hematocrit. The fall in platelets and leukocytes is presumably secondary to sequestration in the lung, liver and spleen (reviewed in Morrison and Ulevitch, 1978). The increase in the hematocrit is an indicator of microvascular dysfunction and subsequent hemoconcentration. Studies have reported that the infusion of C5a into animals induces the same drop in platelets and white blood cells and increase in hematocrit as seen in animals infused with Gram-negative bacteria (Gulbins et al., 1993; Smedegard et al., 1989). In fact, C5a has been reported to act synergistically with LPS, resulting in a more severe change in the hematocrit (Smedegard et al., 1989). To further study these changes, complete blood counts were obtained on C5aR−/− mice and their wild-type littermates prior to and following infusion of heat-killed bacteria. Prior to challenge, no hematologic differences between mice were noted. Following E. coli injection, the C5aR−/− mice were resistant to the hematologic changes experienced by the C5aR wild-type littermates. The wild-type littermates experienced a 38% increase in hematocrit 10 min after an intravenous E. coli infusion, while the deficient mice had a 7% increase (Fig. 5A). The wild-type littermates had a 27% drop in circulating platelets, while the C5aR−/− mice had a 25% increase in circulating platelets 10 min after intravenous E. coli infusion (Fig. 5B). No significant differences were noted in the circulating white blood cells (data not shown).
Fig. 5.
Hematocrit and platelet counts following intravenous E. coli injection. Panel A, C5aR wild-type littermates had a significantly increased hematocrit 10 min after injection when compared to C5aR−/− littermates (P = 0.003). Panel B, C5aR+/+ mice had significantly decreased circulating platelet counts at both 5 and 10 min after challenge compared to C5aR−/− littermates (P = 0.002 at 10 min). Six to seven mice were used in each group at each time point. Results are expressed as mean ± S.E.M. Baseline hematocrit and platelet counts were not significantly different.
3.6. Rescue with histamine antagonism and mast cell stabilization
The change in hematocrit in the wild-type mice following intravenous E. coli infusion suggests considerable hemoconcentration. Mast cell and basophil degranulation is known to liberate numerous mediators, including histamine, which results in rapid extravasation of fluid from vessels. The resulting leakage of fluid from vessels into the interstitial space is reflected by the concentration of intravascular components. C5a is a potent basophil and mast cell degranulating agent (reviewed in Hugli and Muller-Eberhard, 1978). It was therefore hypothesized that the wild-type mouse mortality was dependent on mast cell or basophil mediators. To test this theory, C5aR−/− mice and their wild-type littermates were pre-treated with the mast cell and basophil-stabilizing agent, cromolyn sodium, or with vehicle control. After 30 min the mice were given an intravenous infusion of 6 × 109 CFU heat-killed E. coli. Wild-type littermates treated with vehicle control suffered 60% mortality, while the cromolyn sodium pre-treated wild-type littermates experienced 14% mortality (Fig. 6A). The cromolyn sodium experiment suggested that mast cell and/or basophil degranulation contributed to the mortality observed in the wild-type littermates. While mast cell and basophil degranulation liberates numerous bioactive substances, histamine is known to induce microvascular leakage. To test the hypothesis that histamine was an important mediator in this bacteremia model, mice were pre-treated with the H1 histamine antagonist triprolidine, diphenhydramine or with vehicle control. After 30 min the mice were given an intravenous infusion of heat-killed bacteria. Wild-type littermates treated with vehicle control experienced 60% mortality. C5aR wild-type littermates were completely rescued following pre-treatment with diphenhydramine, i.e. no mortality was observed, and experienced 12.5% mortality with triprolidine pre-treatment (Fig. 6B). The results of these studies suggest that mast cell and/or basophil degranulation with subsequent liberation of histamine is critical for host demise in this bacteremia model.
Fig. 6.
Role of mast cells and histamine in Gram-negative bacteremia. Panel A, survival of mice following pre-treatment with the basophil and mast cell stabilizer, cromolyn sodium (Cro) prior to Gram-negative bacteremia. Following cromolyn sodium pre-treatment, wild-type littermates experienced 14% mortality compared to 60% in the wild-type vehicle control mice (P = 0.022). All data are reported 6 h after intravenous injection of 6 × 109 heat-killed E. coli. Between 8 and 14 mice were used per group. Mice were followed for 72 h with no additional mortality. Panel B, survival of mice receiving pre-treatment with the H1 histamine receptor antagonists triprolidine (tri) or diphenhydramine (dip) prior to Gram-negative bacteremia. C5aR wild-type littermates suffered 60% mortality with vehicle control. Following triprolidine pre-treatment, wild-type mice experienced 12.5% mortality (P = 0.033). Mice were completely rescued with diphenhydramine pre-treatment (P = 0.003). All data are reported 6 h after intravenous injection of heat-killed E. coli.
4. Discussion
To evaluate the function of C5aR activation in acute Gram-negative bacteremia, we generated C5aR−/− mice. The C5aR gene resides on human chromosome 19q13.3-4, which correlates to a syntenic region on mouse chromosome 7 (Bao et al., 1992). Not only has this region been linked to disease pathogenesis in a murine model of allergen-induced asthma (Ewart et al., 2000; Ober et al., 1998) but also adjacent regions have been implicated in spontaneous autoimmunity (Bygrave et al., 2004; Kong et al., 2004; Mohan et al., 1999). Gene ablation constructs, including that used in this study, frequently use strong promoters to drive neomycin expression. In order to limit the effects of inserting a strong promoter on mouse chromosome 7, we flanked the entire neomycin cassette, including the PGK promoter, with lox recognition sites. Mating of these mice with Cre-expressing mice will remove the strong promoter and the neomycin cassette. Our intention was not only to differentiate these mice from an earlier strain of C5aR−/− mice (Hopken et al., 1996) but also to facilitate future disease modeling.
Mice homozygous for the C5aR null allele were completely deficient in both C5aR message and protein (Fig. 2). Extensive baseline characterization of C5aR−/− mice revealed that when compared to their wild-type littermates, the C5aR−/−mice exhibited no difference in fertility, litter size, or growth. Numerous tissues including spleen, liver, lung and bowel were examined in mature mice via hematoxylin and eosin staining, and no detectable abnormalities were noted in the C5aR−/−mice. In addition, C5aR−/− mice and their wild-type littermates had no detectable basal levels of TNF-α, IL-10, IL-6, or IL-1β. Furthermore, both deficient and wild-type littermates had comparable levels of serum C3 and no differences were noted in their baseline complete blood counts or white cell differentials.
Despite the similarities between unchallenged C5aR−/−mice and their wild-type littermates, C5aR−/− mice were significantly protected from Gram-negative bacteremia. At a dose of 6 × 109 CFU/mouse, the survival of C5aR−/− mice was 100% compared to 21% in their wild-type littermates (Fig. 3A). We chose to use a model of acute, severe bacteremia in which mortality occurred rapidly within 1 h of intravenous injection. After the first hour, no additional mortality was observed. Interestingly, after the first hour the C5aR−/− mice had recovered completely from the intravenous bacteremia, resuming normal levels of activity, while the surviving wild-type littermates frequently required 24 h for complete recovery. After the initial mortality differences were recorded, we focused on identifying mechanisms of resistance in the C5aR−/− mice.
Bacteremic shock is frequently accompanied by loss of hematologic homeostasis. The C5aR−/− mice were refractory to the fall in platelets and increase in hematocrit experienced by their wild-type littermates (Fig. 5). In the absence of bacteria or LPS, C5a peptide infusions in dogs (Pavek et al., 1979) and rodents (Gulbins et al., 1993; Smedegard et al., 1989) have reported similar changes in hematocrit and circulating platelets. Our data with C5aR−/− mice correlate well with these earlier reports, and suggest that these effects are due to C5a/C5aR interactions. The infusion experiments also report a rapid drop in circulating leukocytes. Due to a large standard deviation, our data did not reveal significant changes in circulating leukocytes, however (data not shown).
This study has shown that C5aR activation is a critical mediator in the early, fatal response to Gram-negative bacteria. The rapid rise in hematocrit observed in the wild-type animals suggests significant microvascular dysfunction. Numerous mechanisms for microvascular dysfunction exist, including vascular effects of arachidonates, prostaglandins, histamine and endothelial damage via superoxides. While C5a is involved in many of the processes, C5a has long been identified as a potent liberator of histamine through basophil and mast cell degranulation (reviewed in Vogt, 1986 and Hugli and Muller-Eberhard, 1978). Pharmacological treatment with cromolyn sodium is used therapeutically to stabilize mast cell and basophil membranes. We have shown that following pre-treatment with either histamine antagonists or after stabilization of basophils and mast cells with cromolyn sodium, wild-type mice are protected from a lethal dose of intravenous Gram-negative bacteria (Fig. 6A).
It is likely that multiple mechanisms contribute to the mortality observed in this model of bacteremic shock. We have shown that C5a and histamine are critical mediators. Previous work with similar models has reported lethality secondary to pulmonary congestion or edema (Mohr et al., 1998; Stevens et al., 1986). To address these studies, we evaluated lungs from deficient and sufficient mice at 5 and 15 min after intravenous infusion. No evidence existed that wild-type lungs were more congested, edematous or hemorrhagic than C5aR−/− mice (data not shown). We also considered possible cytokine differences between C5aR−/− mice and their wild-type littermates. Since mortality begins at 15 min following injection, we did not favor cytokine production as an explanation for the differential mortality between C5aR−/− mice and their wild-type littermates. Nonetheless, serum levels of IL-1β, IL-6, TNF-α, and IL-10 were measured and as expected no differences were observed between sufficient and deficient mice at 5 and 10 min following intravenous challenge (data not shown). We also evaluated systemic nitrate/nitrite levels in the C5aR−/− and wild-type mice, but no significant differences existed. We considered that circulating levels of pre-formed anti-E. coli IgM may be different between the C5aR−/− mice and their wild-type littermates. Serum anti-E. coli IgM levels were measured in mice at ages 5 weeks, 8 weeks, and 16 weeks, and no substantial differences were noted (data not shown). Lastly, we evaluated baseline levels of serum C3 in C5aR−/− mice and their wild-type littermates, and no differences were observed between groups (data not shown).
In addition to the C5aR data, we have extended our previous findings that C3a is protective in endotoxic shock. Here we show that C3aR activation is protective in Gram-negative bacteremia. The current experiments were designed to focus on acute physiological responses to Gram-negative bacteria, and therefore bacterial loads were used which induce rapid mortality within 15–40 min. The data obtained in these experiments support previous results from both our group and other groups that C3aR activation serves an important immunomodulatory function. The increased mortality in the C3aR−/− mice (Fig. 4) remains to be completely explained. In the endotoxemia experiments, the more susceptible C3aR−/− mice had significantly elevated levels of circulating IL-1β compared to wild-type matched littermates (Kildsgaard et al., 2000). IL-1β converting enzyme deficient mice are incapable of generating IL-1β and they are resistant to the lethal effects of endotoxin (Li et al., 1995). Therefore, we speculated that IL-1β cytokine dysregulation contributed to the increased mortality in the C3aR−/−mice challenged with endotoxin. It is unlikely that this explanation applies to the acute bacteremia results presented here since the bacteremia mortality occurs within 15–45 min, a window unlikely to be affected by cytokine induction. Collectively, these studies show that C5aR activation promotes many of the proinflammatory, physiologic changes associated with bacteremic shock while C3aR activation is important in regulating these changes.
Acknowledgments
This work was supported by U.S. Public Health Service National Institutes of Health Grants RO1 AI025011 (RAW), RO1 HL074333 (to RAW), KO8 DK062197 (MCB), and RO1 DK071057 (MCB). This work was performed by T.J.H in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Immunology, The University of Texas Health Science Center at Houston Graduate School of Biomedical Sciences MD/PhD Program. We thank the members of Dr. Hollmann’s PhD supervisory committee, Drs. Russell Broaddus, Irma Gigli, Henry Strobel, and Norm Weisbrodt.
References
- Bao L, Gerard NP, Eddy RL, Jr, Shows TB, Gerard C. Mapping of genes for the human C5a receptor (C5AR), human FMLP receptor (FPR), and two FMLP receptor homologue orphan receptors (FPRH1, FPRH2) to chromosome 19. Genomics. 1992;13:437–440. doi: 10.1016/0888-7543(92)90265-t. [DOI] [PubMed] [Google Scholar]
- Bygrave AE, Rose KL, Cortes-Hernandez J, Warren J, Rigby RJ, Cook HT, Walport MJ, Vyse TJ, Botto M. Spontaneous autoimmunity in 129 and C57BL/6 mice-implications for autoimmunity described in gene-targeted mice. PLoS Biol. 2004;2:E243. doi: 10.1371/journal.pbio.0020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole L, Bellomo R, Hart G, Journois D, Davenport P, Tipping P, Ronco C. A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Crit Care Med. 2002;30:100–106. doi: 10.1097/00003246-200201000-00016. [DOI] [PubMed] [Google Scholar]
- Drouin SM, Kildsgaard J, Haviland J, Zabner J, Jia HP, McCray PB, Jr, Tack BF, Wetsel RA. Expression of the complement anaphylatoxin C3a and C5a receptors on bronchial epithelial and smooth muscle cells in models of sepsis and asthma. J Immunol. 2001;166:2025–2032. doi: 10.4049/jimmunol.166.3.2025. [DOI] [PubMed] [Google Scholar]
- Ember JA, Jagels MA, Hugli TE. In: Human Complement System in Health and Disease. Frank M, Volanakis JE, editors. Marcel Dekker, Inc; New York: 1998. pp. 241–284. [Google Scholar]
- Ewart SL, Kuperman D, Schadt E, Tankersley C, Grupe A, Shubitowski DM, Peltz G, Wills-Karp M. Quantitative trait loci controlling allergen-induced airway hyperresponsiveness in inbred mice. Am J Respir Cell Mol Biol. 2000;23:537–545. doi: 10.1165/ajrcmb.23.4.4199. [DOI] [PubMed] [Google Scholar]
- Fischer WH, Hugli TE. Regulation of B cell functions by C3a and C3a(desArg): suppression of TNF-alpha, IL-6, and the polyclonal immune response. J Immunol. 1997;159:4279–4286. [PubMed] [Google Scholar]
- Friedberger E. Weitere untersuchungen uber eisissanaphylaxie: IV. Mitteilung Immunitaetaforsch Exp Ther. 1910;4:636–690. [Google Scholar]
- Groeneveld AB, Tacx AN, Bossink AW, van Mierlo GJ, Hack CE. Circulating inflammatory mediators predict shock and mortality in febrile patients with microbial infection. Clin Immunol. 2003;106:106–115. doi: 10.1016/s1521-6616(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Siow Y, Vitale GC. Complement 5a induces in vivo synthesis of cysteinyl leukotrienes in rats. Prostaglandins Leukot Essent Fatty Acids. 1993;48:331–334. doi: 10.1016/0952-3278(93)90226-m. [DOI] [PubMed] [Google Scholar]
- Guo RF, Riedemann NC, Laudes IJ, Sarma VJ, Kunkel RG, Dilley KA, Paulauskis JD, Ward PA. Altered neutrophil trafficking during sepsis. J Immunol. 2002;169:307–314. doi: 10.4049/jimmunol.169.1.307. [DOI] [PubMed] [Google Scholar]
- Guo RF, Riedemann NC, Ward PA. Role of C5a-C5aR interaction in sepsis. Shock. 2004;21:1–7. doi: 10.1097/01.shk.0000105502.75189.5e. [DOI] [PubMed] [Google Scholar]
- Hack CE, Nuijens JH, Felt-Bersma RJ, Schreuder WO, Eerenberg-Belmer AJ, Paardekooper J, Bronsveld W, Thijs LG. Elevated plasma levels of the anaphylatoxins C3a and C4a are associated with a fatal outcome in sepsis. Am J Med. 1989;86:20–26. doi: 10.1016/0002-9343(89)90224-6. [DOI] [PubMed] [Google Scholar]
- Hecke F, Schmidt U, Kola A, Bautsch W, Klos A, Kohl J. Circulating complement proteins in multiple trauma patients—correlation with injury severity, development of sepsis, and outcome. Crit Care Med. 1997;25:2015–2024. doi: 10.1097/00003246-199712000-00019. [DOI] [PubMed] [Google Scholar]
- Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- Hug BA, Wesselschmidt RL, Fiering S, Bender MA, Epner E, Groudine M, Ley TJ. Analysis of mice containing a targeted deletion of beta-globin locus control region 5′ hypersensitive site 3. Mol Cell Biol. 1996;16:2906–2912. doi: 10.1128/mcb.16.6.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugli TE, Muller-Eberhard HJ. Anaphylatoxins: C3a and C5a. Adv Immunol. 1978;26:1–53. doi: 10.1016/s0065-2776(08)60228-x. [DOI] [PubMed] [Google Scholar]
- Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, Gerard NP, Gerard C. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406:998–1001. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- Joiner KA, Brown EJ, Frank MM. Complement and bacteria: chemistry and biology in host defense. Annu Rev Immunol. 1984a;2:461–491. doi: 10.1146/annurev.iy.02.040184.002333. [DOI] [PubMed] [Google Scholar]
- Joiner KA, Goldman R, Schmetz M, Berger M, Hammer CH, Frank MM, Leive L. A quantitative analysis of C3 binding to O-antigen capsule, lipopolysaccharide, and outer membrane protein of E. coli 0111B4. J Immunol. 1984b;132:369–375. [PubMed] [Google Scholar]
- Kildsgaard J, Hollmann TJ, Matthews KW, Bian K, Murad F, Wetsel RA. Cutting edge: targeted disruption of the C3a receptor gene demonstrates a novel protective anti-inflammatory role for C3a in endotoxin-shock. J Immunol. 2000;165:5406–5409. doi: 10.4049/jimmunol.165.10.5406. [DOI] [PubMed] [Google Scholar]
- Kong PL, Morel L, Croker BP, Craft J. The centromeric region of chromosome 7 from MRL mice (Lmb3) is an epistatic modifier of Fas for autoimmune disease expression. J Immunol. 2004;172:2785–2794. doi: 10.4049/jimmunol.172.5.2785. [DOI] [PubMed] [Google Scholar]
- Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Lundberg C, Marceau F, Hugli TE. C5a-induced hemodynamic and hematologic changes in the rabbit. Role of cyclooxygenase products and polymorphonuclear leukocytes. Am J Pathol. 1987;128:471–483. [PMC free article] [PubMed] [Google Scholar]
- Matthews KW, Mueller-Ortiz SL, Wetsel RA. Carboxypeptidase N: a pleiotropic regulator of inflammation. Mol Immunol. 2004;40:785–793. doi: 10.1016/j.molimm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Michell BB. Preparation of mouse cell suspensions. In: Shiigi BBMaSM., editor. Selected Methods in Cellular Immunology. W.H. Freeman and Company; San Francisco: 1980. pp. 3–13. [Google Scholar]
- Mohan C, Yu Y, Morel L, Yang P, Wakeland EK. Genetic dissection of Sle pathogenesis: Sle3 on murine chromosome 7 impacts T cell activation, differentiation, and cell death. J Immunol. 1999;162:6492–6502. [PubMed] [Google Scholar]
- Mohr M, Hopken U, Oppermann M, Mathes C, Goldmann K, Siever S, Gotze O, Burchardi H. Effects of anti-C5a monoclonal antibodies on oxygen use in a porcine model of severe sepsis. Eur J Clin Invest. 1998;28:227–234. doi: 10.1046/j.1365-2362.1998.00260.x. [DOI] [PubMed] [Google Scholar]
- Morrison DC, Ulevitch RJ. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978;93:526–617. [PMC free article] [PubMed] [Google Scholar]
- Nakae H, Endo S, Inada K, Yoshida M. Chronological changes in the complement system in sepsis. Surg Today. 1996;26:225–229. doi: 10.1007/BF00311579. [DOI] [PubMed] [Google Scholar]
- Ober C, Cox NJ, Abney M, Di Rienzo A, Lander ES, Changyaleket B, Gidley H, Kurtz B, Lee J, Nance M, Pettersson A, Prescott J, Richardson A, Schlenker E, Summerhill E, Willadsen S, Parry R. Genome-wide search for asthma susceptibility loci in a founder population. The Collaborative Study on the Genetics of Asthma. Hum Mol Genet. 1998;7:1393–1398. doi: 10.1093/hmg/7.9.1393. [DOI] [PubMed] [Google Scholar]
- Pangburn MK, Morrison DC, Schreiber RD, Muller-Eberhard HJ. Activation of the alternative complement pathway: recognition of surface structures on activators by bound C3b. J Immunol. 1980;124:977–982. [PubMed] [Google Scholar]
- Pavek K, Piper PJ, Smedegard G. Anaphylatoxin-induced shock and two patterns of anaphylactic shock: hemodynamics and mediators. Acta Physiol Scand. 1979;105:393–403. doi: 10.1111/j.1748-1716.1979.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Reid RR, Prodeus AP, Khan W, Hsu T, Rosen FS, Carroll MC. Endotoxin shock in antibody-deficient mice: unraveling the role of natural antibody and complement in the clearance of lipopolysaccharide. J Immunol. 1997;159:970–975. [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Bernacki KD, Reuben JS, Laudes IJ, Neff TA, Gao H, Speyer C, Sarma VJ, Zetoune FS, Ward PA. Regulation by C5a of neutrophil activation during sepsis. Immunity. 2003;19:193–202. doi: 10.1016/s1074-7613(03)00206-1. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Neff TA, Laudes IJ, Keller KA, Sarma VJ, Markiewski MM, Mastellos D, Strey CW, Pierson CL, Lambris JD, Zetoune FS, Ward PA. Increased C5a receptor expression in sepsis. J Clin Invest. 2002;110:101–108. doi: 10.1172/JCI15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selberg O, Hecker H, Martin M, Klos A, Bautsch W, Kohl J. Discrimination of sepsis and systemic inflammatory response syndrome by determination of circulating plasma concentrations of procalcitonin, protein complement 3a, and interleukin-6. Crit Care Med. 2000;28:2793–2798. doi: 10.1097/00003246-200008000-00019. [DOI] [PubMed] [Google Scholar]
- Smedegard G, Cui LX, Hugli TE. Endotoxin-induced shock in the rat. A role for C5a. Am J Pathol. 1989;135:489–497. [PMC free article] [PubMed] [Google Scholar]
- Stevens JH, O’Hanley P, Shapiro JM, Mihm FG, Satoh PS, Collins JA, Raffin TA. Effects of anti-C5a antibodies on the adult respiratory distress syndrome in septic primates. J Clin Invest. 1986;77:1812–1816. doi: 10.1172/JCI112506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stove S, Welte T, Wagner TO, Kola A, Klos A, Bautsch W, Kohl J. Circulating complement proteins in patients with sepsis or systemic inflammatory response syndrome. Clin Diagn Lab Immunol. 1996;3:175–183. doi: 10.1128/cdli.3.2.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabayashi T, Vannier E, Burke JF, Tompkins RG, Gelfand JA, Clark BD. Both C3a and C3a(desArg) regulate interleukin-6 synthesis in human peripheral blood mononuclear cells. J Infect Dis. 1998;177:1622–1628. doi: 10.1086/515316. [DOI] [PubMed] [Google Scholar]
- Takabayashi T, Vannier E, Clark BD, Margolis NH, Dinarello CA, Burke JF, Gelfand JA. A new biologic role for C3a and C3a desArg: regulation of TNF-alpha and IL-1 beta synthesis. J Immunol. 1996;156:3455–3460. [PubMed] [Google Scholar]
- Vogt W. Anaphylatoxins: possible roles in disease. Complement. 1986;3:177–188. doi: 10.1159/000467894. [DOI] [PubMed] [Google Scholar]
- Wetsel RA, Kildsgaard J, Haviland DL. Complement anaphylatoxins (C3a,C4a, and C5a) and their receptors (C3aR, C5aR/CD88) as therapeutic targets in inflammation. In: Lambris JD, Holers VM, editors. Therapeutic Interventions in the Complement System. Humana Press; Totowa, New Jersey: 2000. pp. 113–153. [Google Scholar]
- Zhao L, Ohtaki Y, Yamaguchi K, Matsushita M, Fujita T, Yokochi T, Takada H, Endo Y. LPS-induced platelet response and rapid shock in mice: contribution of O-antigen region of LPS and involvement of the lectin pathway of the complement system. Blood. 2002;100:3233–3239. doi: 10.1182/blood-2002-01-0252. [DOI] [PubMed] [Google Scholar]