Abstract
Background
The “double-faced” effect of nitric oxide (NO) is thought to play an important role in triggering and progression of glaucoma.
Material/Methods
Iris samples were obtained during iridectomy in 35 patients (mean age of 65.4±5.3 years) with diagnosed primary open-angle glaucoma (POAG). The controls were collected postmortem from 10 donors with a mean age of 62.2±1.9 years. Visual field defects were evaluated by perimetry. The Hodapp-Parrish-Anderson classification was used to divide patients into 3 visual field defect groups. The intraocular pressure was measured 3 times before surgery using applanation tonometry. The phenotype activity of nitric oxide synthase (NOS) isoenzymes (endothelial – eNOS and inducible – iNOS) and expression of nitrotyrosine in iris vasculature was assessed.
Results
Significant differences were found between glaucoma patients and the controls in eNOS and iNOS activity (Mann-Whitney test, U=35.5, Z=−2.037, p=0.04 and U=21, Z=2.69, p=0.007, respectively). In addition, the results showed an upregulation of nitrotyrosine in the capillary endothelial cells in the study group, which was associated with the duration of diagnosed glaucoma (R-Spearman of 0.33, p=0.0047) and visual field mean defect MD (R-Spearman of 0.29, p=0.019). Moreover, the activity of nitrotyrosine was significantly correlated with iNOS immunoreactivity (R-Spearman of 0.5, p=0.0001). However, the iNOS activity significantly varied among Hodapp-Parrish-Anderson groups (p=0.03).
Conclusions
Our observations confirmed the association between glaucomatous disturbances and upregulation of iNOS, together with increased nitrotyrosine storage.
MeSH Keywords: Glaucoma, Open-Angle; Iris; Nitric Oxide
Background
Glaucoma is a wide group of disturbances mainly causing progressive ganglion cell damage, visual field loss, and, finally, blindness. Despite well developed diagnostic tools and relatively efficient treatment, glaucoma is still the world’s leading cause of irreversible blindness among older people. Glaucomatous neuropathy may progress with elevated or normal (arbitrarily estimated) intraocular pressure (IOP). Therefore, the main known glaucoma risk factor – elevated IOP – is neither sufficient nor necessary to trigger glaucoma neuropathy.
Several observations have suggested the multifactorial character of glaucoma pathogenesis, but the trigger initiating glaucomatous pathology remains unknown.
Recent reports demonstrated a significant role of nitrative stress in glaucomatous pathology. Several studies highlighted the role of nitric oxide (NO) and increased nitrative stress in glaucoma [1].
The NO is synthesized from L-arginine by a family of nitric oxide synthase (NOS) isozymes: neuronal (n)NOS (NOS1), endothelial (e)NOS (NOS3), and inducible-NOS (i)NOS (NOS2). NOS1 have been cloned from human retina [2] and NOS3 mainly from vascular [3] and trabecular meshwork (TM) endothelial cells [4]. NOS2 isoform has been explored in the cornea, iris, ciliary body, neural retina, retinal glial cells, retinal pigment epithelial cells, and optic nerve head under conditions of increased intraocular pressure and uveitis [1,5–7].
The neuronal and endothelial NOS are constitutive, Ca2+/calmodulin-dependent enzymes and are tightly controlled by mechanisms regulating physiological intracellular Ca2+ levels. The iNOS is Ca2+-independent and is induced in response to immunologic or inflammatory stimuli [8]. The observed “double-faced” effect of NO in the eye is thought to play an important role in triggering and progression of glaucoma. On the one hand, eNOS expressed in TM physiologically regulates aqueous outflow in the eye by maintaining endothelial cell function [9,10]. Therefore, upregulation of eNOS lowers IOP [11], reducing the main risk factor, as postulated in the mechanical hypothesis of glaucoma. Additionally, vascular endothelial cell production of eNOS improves optic nerve head (ONH) blood flow velocity, as postulated in the vascular hypothesis of glaucoma [12,13].
On the other hand, increased IOP induced iNOS upregulation at the transcription level [14,15]. The overexpression of iNOS leads to production of large amounts of potentially cytotoxic NO [8]. The cytotoxicity associated with high levels of NO is due to the formation of the powerful oxidant peroxynitrite (ONOO−) by its interaction with superoxide anion (O2−). Peroxynitrite is a highly reactive molecule that can cause extensive damage to proteins, lipids, and, especially, DNA molecules. Nitrative modification of proteins leads to important structural and functional alterations involved in trabecular meshwork degeneration and retinal ganglion cells (RGC) [16]. A major reaction peroxynitrite with proteins is the formation of 3-nitrotyrosine (NO2Tyr). NO2Tyr is commonly used as a diagnostic marker of NO-derived oxidants in both human disease states and animal models [1,17]. Positive NT immunoreactivity indicates long-term exposure to peroxynitrite [18].
The purpose of our study was to estimate the association iNOS, eNOS expression, and 3-NT accumulation in human iris sampled in vivo with clinical feature of patients with POAG.
Material and Methods
The study protocol was approved by the Ethics Committee of the Medical University of Silesia, Katowice (permission number: KNW/0022/KB1/123/10) and adhered to the tenets of the Declaration of Helsinki for experiments involving human tissue and samples.
Participants
Patients who were planned for deep sclerectomy with basal iridectomy were recruited to our study. The inclusion criteria were: (1) Caucasians with diagnosed and treated primary open-angle glaucoma (POAG), (2) age 65–75 years, (3) nonsmokers, (4) normal or normalized blood pressure, and (5) no retinal or neurologic disease that may have affected the visual field. The exclusion criteria were: (1) glaucoma other than POAG type, (2) diabetes mellitus, (3) incidences of inflammation process in ocular tissue, (4) infective disorders, (5) antithrombotic or vasoactive cardiovascular therapy, (6) intrabulbar surgery during the last 12 months, and (7) Raynaud’ disease.
POAG was defined as the presence of a reproducible visual field defect consistent with glaucoma and the appearance of the optic disc, along with a pretreatment IOP of 21 mm Hg or more, and an opened angle with no signs of secondary causes of glaucoma. The type of glaucoma was confirmed by 2 experienced ophthalmologists based on gonioscopy, ophthalmoscopy, tonometry, visual field examination (Octopus 301 HS, Interzeag) and policlinic history analysis including the last 6 months of glaucoma eye drops use. The Hodapp-Parrish-Anderson classification [19] was used to stage glaucoma defects in patients. The average IOP was determined by 3 measurements using Goldmann applanation tonometry (Haag-Streit, Bern, Switzerland) under topical anesthesia with 0.5% Alcaine (Alcon) eye drops. The first one was measured at 14–21±3.1 days before surgery, when the patient presented at our policlinic. The third measurement was made on the day of surgery (before administration of any intravenous osmotic agents) and the second measurement was made during the control test 3–5±1.3 days after the first measurement. All IOP measurements were made during morning hours (between 8 AM and 11 AM).
Following written consent, each patient underwent the surgical procedure, in which a full-thickness piece of iris (approximately 1×1 mm) was removed using iridectomy scissors.
Controls
The control sections were obtained at autopsy and processed within 8 hours after death. The methodology was similar, through corneal incision full-thickness piece of iris was sampled. The age-matched donors were involved when inclusion/exclusion criteria (as above) were fulfilled in their health history.
Sample collection
Each sample was gently irrigated with 10 ml of Ringer’s solution, then was placed into an Eppendorf 2-ml Safe-Lock test tube (Eppendorf Biopur®) containing 99.5% acetone for dehydration for 5 min at 4°C. Afterwards, the acetone was poured off and the test tube with sample inside was filled with tissue-freezing medium (OCT Compound, Miles). The specimens were placed in a −70°C freezer until sectioning.
Immunohistochemistry
For immunohistochemistry, frozen sections were incubated with murine antihuman antibodies: monoclonal anti-eNOS (clone RN5 from ABCAM), polyclonal anti-iNOS (MILLIPORE), and monoclonal anti-Nitrotyrosine (clone HM11 from INVITROGEN).
The dilution of the primary antibody was verified in our laboratory in a series of pilot experiments. Antibodies were diluted in TRIS-buffered saline, pH 7.6, and samples were incubated for 60 min at room temperature in a humidified chamber. Prior to staining, frozen sections were incubated for 10 min at room temperature with a serum-free protein block (DAKO A/S) to block nonspecific staining. The EnVision method (DAKO EnVision Kit®/Alkaline Phosphatase detection system) was used according to the manufacturer’s instructions. The bound primary antibody was detected using the New Fuchsin Substrate System (DAKO A/S). The primary antibody was omitted from negative control slides. As a positive control, heart biopsy specimens from patients with heart failure were used. To suppress nonspecific staining due to endogenous alkaline phosphatase activity, Levamisole was used at a final concentration of 0.2 mM. The sections were counterstained with Mayer’s hematoxylin. The immunohistological examination of biopsy specimens was made by 2 investigators independently, blinded to clinical features and histopathological diagnosis. Each specimen was evaluated qualitatively and semiquantitatively. The semiquantitative scoring system was defined as: 0, lack of or weak focal staining on the endothelial cells; 1+, weak, multifocal staining on the interstitial cells; 2+, increased, multifocal staining; and 3+, strong endothelial staining (Figures 1 and 2).
Figure 1.
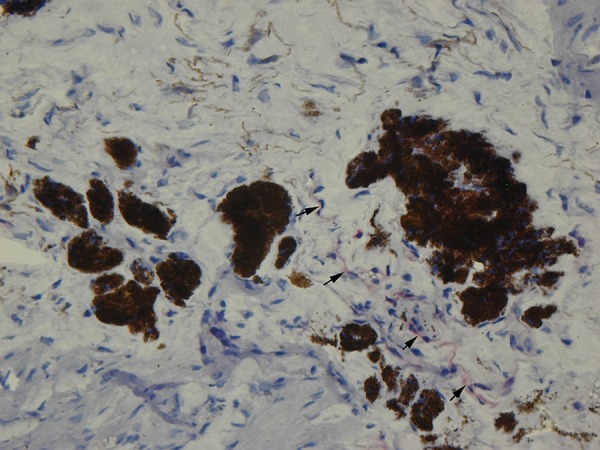
Weak immunostaining for iNOS in sparse iris endothelial cells (arrows) in a control subject (original magnification 400×).
Figure 2.
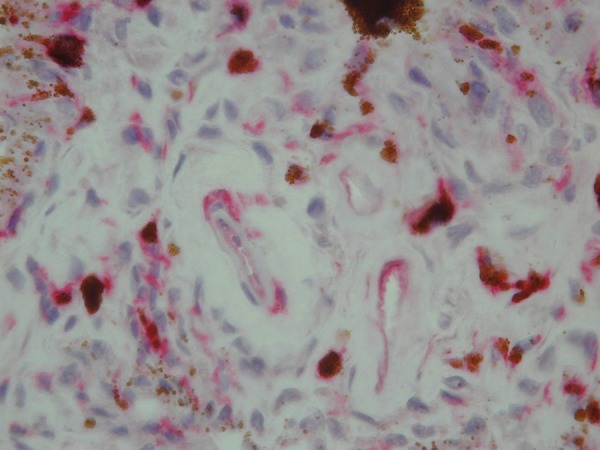
Up-regulation of iNOS by microvascular iris endothelium in a study group subject (red color) (original magnification 400×).
Statistics
The non-parametric Spearman correlation was calculated for rank variables. In case of comparison between multiple groups, the non-parametric Kruskall-Wallis or Mann-Whitney test were used, respectively. The p-value <0.05 was considered statistically significant.
Results
Table 1 summarizes the demographic distribution. Only 1 eye per patient was included in the study.
Table 1.
Demographic characteristics of patients enrolled in the study.
| Glaucoma patients n=35 | Controls n=10 | |||||
|---|---|---|---|---|---|---|
| Sex (men/female) | ♂ n=15 | ♀ n=20 | ♂ n=4 | ♀ n=6 | ||
| Age (years) | 65.8±4.9 | 65.1±5.6 | 62.5±3.5 | 62.0±1.0 | ||
| 65.4±5.3 | 62.2±1.92 | |||||
| Median duration of known glaucoma (years) | 1–15 5.6±3.5 | Ø | ||||
| Glaucoma drugs | β-b* | CAI* | α-m* | PG* | Ø | |
| 27 | 22 | 20 | 2 | |||
β-b – β blockers; CAI – carbonic anhydrase inhibitors; α-m – α2 agonists; PG – prostaglandin analogues.
The average parametric quantitative index mean defect in the studied group was 9.8±5.6 (1.4–22.7).
Accordingly to Hodapp-Parrish-Anderson classification, the studied group had 11 (31%) patients with early defect, 7 (20%) with moderate defect, and 17 (49%) with severe defect of the visual field.
The average intraocular pressure during the preoperative period was 30.6±7.7 mmHg (range,16–43 mmHg).
The assessment of eNOS, iNOS expression, and NT accumulation in studied specimens was based on immunoreactivity presented by controls (Table 2).
Table 2.
Immunoreactivity of nitric oxide synthase isoenzymes and nitrotyrosine in controls and glaucoma patients.
| eNOS scores 0–3 | iNOS scores 0–3 | NT scores 0–3 | ||||
|---|---|---|---|---|---|---|
| Controls | 1+, n=2; 1.5+, n=6; 2.0+, n=2 | 0+, n=4; 0.5+, n=6 | 0.5+, n=6; 1.0+, n=4 | |||
| Low immunoreactivity | <1.5+ | n=27 (77%) | <0.5+ | n=16 (46%) | <0.5+ | n=13 (37%) |
| Normal immunoreactivity | 1.5+ | n=1 (3%) | 0.5+ | n=9 (26%) | 0.5+ | n=7 (20%) |
| High immunoreactivity | >1.5+ | n=7 (20%) | >1.5+ | n=10 (28%) | >0.5+ | n=15 (43%) |
eNOS – endothelial nitric oxide synthase; iNOS – inducible nitric oxide synthase; NT – nitrotyrosine, n – the number of patients.
The immunoreactivity of eNOS, iNOS, and nitrotyrosine revealed no significant dependence on age (p=0.83, p=0.69, and p=0.66, respectively) or sex (p=0.73, p=0.28, and p=0.89, respectively) of participants.
The iNOS staining significantly varied (p=0.03) among Hodapp-Parrish-Anderson groups. The iNOS expression was significantly different in the severe defect subgroup as compared to the early and moderate defect groups.
The iNOS upregulation was observed in 15 (88%) patients and normal or low activity in 2 (12%) patients with significant defect (HPA III). In the combined first and second HPA groups, 7 (39%) patients had low or normal immunoreactivity and 11 (61%) patients had increased iNOS staining.
The results showed significant dependency (R-Spearman of 0.5, p=0.0001) between iNOS and nitrotyrosine staining.
The nitrotyrosine immunoreactivity was significantly associated with the duration of diagnosed glaucoma (R-Spearman 0.33, p=0.047) and with the visual field mean defect (R-Spearman 0.39, p=0.019).
Similarly, iNOS and eNOS immunoreactivity showed no significant association with diagnosed glaucoma duration (p=0.1 and p=0.44, respectively) or with the visual field mean defect (p=0.1 and p=0.08, respectively).
The eNOS, iNOS, and nitrotyrosine immunoreactivity were not significantly correlated with mean preoperative intraocular pressure (R-Spearman −0.09, p=0.06; R-Spearman −0.3, p=0.085, and R-Spearman −0.12, p=0.48, respectively).
We found significant differences when comparing eNOS (Mann-Whitney test, U=35.5, Z=−2.037, p=0.04) and iNOS (Mann-Whitney test, U=21, Z=2.69, p=0.007) reactivity of examined and control specimens. The differences in nitrotyrosine staining in patients and controls just failed to reach statistical significance (Mann-Whitney test U=44.5, Z= 1.7, p=0.088).
In the examined group we found no significant link between the kind of topical glaucoma medications and the immunoreactivity to iNOS, eNOS, and NT (p=0.04).
Discussion
This study was designed to investigate the role of nitrative stress in glaucoma. We found that nitrative stress appears to be a risk factor for glaucoma.
We examined iNOS, eNOS, and nitrotyrosine immunoreactivity using a somewhat different model compared with earlier experimental protocols, and we used iris tissue, which has not been previously reported. Although this is the first in vivo iris study of nitrative stress in the course of glaucomatous disturbances, we have compared our observation with related articles based on other from iris tissue examination.
Patients with POAG in our study mainly had acute phase intrabulbar pressure. The present in vivo study of iris tissue increases the understanding of NO responsiveness in glaucomatous/hypertensive conditions. In trabecular meshwork studies [14,15], up-regulation of iNOS was observed, probably in response to elevation of IOP, regardless of experimental protocols. In our examined group, we recorded the mean IOP (approximately 30 mmHg), close to that regulated by perfusion apparatus (30 mmHg) in an in vitro model of Schneemann et al. [14]. Nevertheless, our examined group revealed homogeneity in intraocular pressure and we found no relation among mean IOP and eNOS, iNOS, and NT immunostaining.
Corresponding with previously reported data, we observed an association between iNOS activity and visual field damage. In the study by Fernández-Durango et al. [15] and in the present study, iNOS was significantly correlated with degree of visual field defect. NO overproduction from iNOS in hypoxic-ischemic conditions contributes to cytotoxicity, resulting in cell death and axonal damage to retinal ganglion cells [20], which are associated with visual field changes.
Additionally, in our study nitrotyrosine staining depended on iNOS upregulation, which corresponds with earlier reports [8,18].
Our patients presented significant association between glaucoma duration and increased nitrotyrosine accumulation, which reflects long-term exposure to NO in glaucomatous conditions, probably mainly by upregulation of iNOS. Nevertheless, the iNOS was not directly correlated with the time of diagnosed glaucoma, in our opinion due to its short-term fluctuating activity changing over the course of the disease. However, the duration of treated glaucoma was self-reported by patients and may not be precise. In addition, due to the relatively small number of cases in the study group, such a relationship cannot be excluded (iNOS retained some statistical trend − p=0.1).
Activity of eNOS/iNOS changes in single-sampled glaucomatous conditions was proven by significant differences between examined patients and controls in nitric oxide synthase isoenzymes reactivity, but the nitrotyrosine storage in patients and controls did not reflect these changes. We did not have data on the agonal process of donors, which could have had an impact on postmortem nitrotyrosine accumulation.
Finally, eNOS in iris vasculature revealed poor predictive value. In contrast, patients with POAG present less eNOS activity than that of normal control subjects in the trabecular meshwork [10,11,21]. Findings of eNOS gene polymorphism could be influenced by a small number of cases [22] and/or by the fact that the iris tissue histologically differs from the trabecular meshwork, especially in endothelium percentage.
Conclusions
The results of the present study seem to confirm previous reports describing an association between glaucomatous disturbances and upregulation of iNOS, together with increased nitrotyrosine storage.
Study limitations
– The current study is limited by a relatively small number of patients studied. However, the results are encouraging enough for use in designing a larger study.
– Glaucoma is a disease with various rates of progressions [1,16] and should not be treated as a typical chronic disorder. A prospective follow-up study should be performed.
– The ophthalmic condition of controls was based only on medical history.
– Groups were not genetically investigated.
Footnotes
Disclosure statement
There are no conflicting interests or relationships of the authors of this paper.
Source of support: Local government
References
- 1.Aslan M, Cort A, Yucel I. Oxidative and nitrative stress markers in glaucoma. Free Radic Biol Med. 2008;45:367–76. doi: 10.1016/j.freeradbiomed.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Park CS, Pardhasaradhi K, Gianotti C, et al. Human retina expresses both constitutive and inducible isoforms of nitric oxide synthase mRNA. Biochem Biophys Res Commun. 1994;205:85–91. doi: 10.1006/bbrc.1994.2633. [DOI] [PubMed] [Google Scholar]
- 3.Chakravarthy U, Stitt AW, McNally J, et al. Nitric oxide synthase activity and expression in retinal capillary endothelial cells and pericytes. Curr Eye Res. 1995;14:285–94. doi: 10.3109/02713689509033528. [DOI] [PubMed] [Google Scholar]
- 4.Wu RY, Ma N. Expression of nitric oxide synthase and guanylate cyclase in the human ciliary body and trabecular meshwork. Chin Med J (Engl) 2012;125:129–33. [PubMed] [Google Scholar]
- 5.Becquet F, Courtois Y, Goureau O. Nitric oxide in the eye: multifaceted roles and diverse outcomes. Surv Ophthalmol. 1997;42:71–82. doi: 10.1016/s0039-6257(97)84043-x. [DOI] [PubMed] [Google Scholar]
- 6.Aslan M, Yucel I, Ciftcioglu A, et al. Corneal protein nitration in experimental uveitis. Exp Biol Med. 2007;232:1308–13. doi: 10.3181/0702-RM-34. [DOI] [PubMed] [Google Scholar]
- 7.Aslan M, Yucel I, Akar Y, et al. Nitrotyrosine formation and apoptosis in rat models of ocular injury. Free Radic Res. 2006;40:147–53. doi: 10.1080/10715760500456219. [DOI] [PubMed] [Google Scholar]
- 8.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–95. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 9.Gottanka J, Johnson DH, Martus P, Lütjen-Drecoll E. Severity of optic nerve damage in eyes with POAG is correlated with changes in the trabecular meshwork. J Glaucoma. 1997;6:123–33. [PubMed] [Google Scholar]
- 10.Schmetterer L, Polak K. Role of nitric oxide in the control of ocular blood flow. Prog Retin Eye Res. 2001;20:823–47. doi: 10.1016/s1350-9462(01)00014-3. [DOI] [PubMed] [Google Scholar]
- 11.Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995;36:1774–84. [PubMed] [Google Scholar]
- 12.Resch H, Garhofer G, Fuchsjäger-Mayrl G, et al. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009;87:4–12. doi: 10.1111/j.1755-3768.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- 13.Waliszek-Iwanicka A, Waliszek M, Banach M, et al. Assessment of blood flow in posterior ciliary arteries and its correlation with intraocular and arterial blood pressures in patients with open angle glaucoma. Med Sci Monit. 2010;16(10):CR501–9. [PubMed] [Google Scholar]
- 14.Schneemann A, Leusink-Muis A, van den Berg T, et al. Elevation of nitric oxide production in human trabecular meshwork by increased pressure. Graefes Arch Clin Exp Ophthalmol. 2003;241:321–26. doi: 10.1007/s00417-003-0638-4. [DOI] [PubMed] [Google Scholar]
- 15.Fernández-Durango R, Fernández-Martínez A, García-Feijoo J, et al. Expression of nitrotyrosine and oxidative consequences in the trabecular meshwork of patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2008;49:2506–11. doi: 10.1167/iovs.07-1363. [DOI] [PubMed] [Google Scholar]
- 16.Dogan S, Aslan M. The Role of Retinal Oxidative and Nitrative Injury in Glaucomatous Neurodegeneration, Glaucoma – Basic and Clinical Concepts. In: Rumelt Shimon., editor. InTech. 2011. [Google Scholar]
- 17.Aslan M, Ryan TM, Townes TM, et al. Nitric oxide-dependent generation of reactive species in sickle cell disease. Actin tyrosine induces defective cytoskeletal polymerization. J Biol Chem. 2003;278:4194–204. doi: 10.1074/jbc.M208916200. [DOI] [PubMed] [Google Scholar]
- 18.Uttenthal LO, Alonso D, Fernández AP, et al. Neuronal and inducible nitric oxide synthase and nitrotyrosine immunoreactivities in the cerebralcortex of the aging rat. Microsc Res Tech. 1998;43:75–88. doi: 10.1002/(SICI)1097-0029(19981001)43:1<75::AID-JEMT11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Hodapp E, Parrish RK, II, Anderson DR. Clinical decisions in glaucoma. St Louis: The C.V, Mosby Co.; 1993. pp. 52–61. [Google Scholar]
- 20.Charanjit K, Wallace SF, Eng-Ang L. Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol. 2008;2:879–89. doi: 10.2147/opth.s3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarado JA, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–79. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- 22.Logan JF, Chakravarthy U, Hughes AE, et al. Evidence for association of endothelial nitric oxide synthase gene in subjects with glaucoma and a history of migraine. Invest Ophthalmol Vis Sci. 2005;46:3221–26. doi: 10.1167/iovs.05-0368. [DOI] [PubMed] [Google Scholar]


