Abstract
Background
The role of the multidrug resistance-1 (MDR1 or ABCB1) gene polymorphisms 1236T>C, 2677T>G, and 3435T>C was studied in relation to susceptibility, demographics, and pathological characteristics, as well as their role in the therapeutic response (TR) to prednisone treatment in children with idiopathic nephrotic syndrome (INS).
Material/Methods
The polymorphisms were analyzed using the polymerase chain reaction-restriction fragment length polymorphism method in 46 children with INS and in 100 healthy controls. Different genetic models (codominant, dominant, recessive, and overdominant) were used for testing of associations between polymorphisms and phenotypes.
Results
Statistical analysis showed a significantly increased chance of TR in children carrying 3435TC genotype (OR=5.13, 95% CI=1.18–22.25; overdominant model). Moreover, INS patients under 6 years of age had significantly decreased frequencies of MDR1 1236CC (7.7% vs. 35%, p=0.029) or 2677GG (3.8% vs. 30.0%, p=0.033) genotypes. We also observed that patients with minimal change in disease and patients under 6 years of age at the onset of INS were initial responders more frequently when compared with children with focal segmental glomerulosclerosis and patients ≥6 years old at the onset (p=0.0001, p=0.027, respectively).
Conclusions
These data suggest that prednisone TR may be influenced by histology, age at the onset of INS, and MDR1 3435T>C polymorphism. The MDR1 1236T>C and 2677T>G polymorphisms were significantly associated with age at onset. Larger multicenter studies and studies across other ethnic groups are needed to elucidate the contradictory implications of MDR1 polymorphisms with INS in children.
MeSH Keywords: Genes, MDR; P-Glycoprotein; Polymerase Chain Reaction
Background
Nephrotic syndrome (NS) is the most common glomerular pathological condition encountered in children. In contrast to NS in adulthood, when NS is often secondary to another disorder such as diabetes mellitus, cancer, and chronic inflammatory diseases [1,2], NS in childhood (>12 months age) is largely primary or idiopathic (INS) [3]. The exact reason for this is still not understood. Generally, INS patients present diverse pathological features (e.g., minimal change disease-MCD, focal segmental glomerulosclerosis-FSGS, and mesangial proliferative glomerulonephritis-MesPGN). Variable response to immunosuppressive therapy is observed when glucocorticoids (GC) are recommended as the first-line therapy due to the immune-inflammatory characteristics of the disease. Therapeutic response (TR) to initial steroid therapy has been reported to be 60–95% of children, depending on the geographic area [4,5]. Despite the high initial response rate, relapses occur in 60–90% of the initial responders and some patients remain steroid-dependent. Clinical experience has demonstrated that patients with a poor response to steroids have an unfavorable prognosis and often develop end-stage renal failure [6]. Therefore, it is essential to identify potential factors that contribute to immunosuppressive therapy failure in order to optimize and individualize the treatment of INS.
Changes in the levels and/or function of P-glycoprotein (P-gp) are one of the possible mechanisms of drug resistance that have been published in the literature [7]. P-gp is the product of the MDR1 (alias ABCB1) gene and is an active transmembrane efflux pump for a variety of toxins and many drugs. GC (including prednisone) are also transported by P-gp and may induce P-gp expression [8]. P-gp is found in many organs and tissues (e.g., luminal cells of the lower digestive system, liver, proximal renal tubules, endothelial cells in the blood–brain barrier, placenta, and hematopoietic cells), and the tissue distribution suggests that P-gp plays an important role in excreting potentially toxic or unnecessary xenobiotics and metabolites from the body and the cells, and has significant pharmacokinetic and pharmacodynamic implications for P-gp substrates [9]. P-gp may also actively participate in the chronic inflammation in autoimmune diseases; it is up-regulated by cytokines, probably participates in releasing certain inflammatory mediators, and is probably involved in the cell activation/death pathways [10,11].
Recent experimental and clinical studies have shown that gene expression and the activity of P-gp may be affected by MDR1 gene polymorphisms. Three single-nucleotide polymorphisms (SNPs) seem to have the most important clinical relevance: 1236T>C (rs1128503, MAF/minor allele frequency: T=0.422, silent SNP); 2677T>G/A (rs2032582, MAF: T=0.340, Ser893Ala/Thr); and 3435T>C (rs1045642, MAF: T=0.397, silent SNP). The silent 3435T>C polymorphism may have some effect on DNA structure, RNA stability, and P-gp function, or it is in linkage disequilibrium with other functional MDR1 polymorphisms [12–14]. In most studies, the T allele is associated with decreased P-gp function. The non-synonymous SNP (2677T>G/A) has also been found to be associated with altered expression, activity and the substrate specificity of P-gp [15,16]. It is speculated that patients who carry the T/A are more resistant to drugs [17]. The silent 1236T>C polymorphism may affect translation regulation, RNA stability, or other molecular mechanisms. It may also have an indirect effect, being linked to a causal variant. In a recent study, it was shown that TT genotype minimizes P-gp activity in a substrate-dependent manner [18].
Only a few studies in different ethnic populations have evaluated the distribution of the MDR1 polymorphisms (1236T>C, 2677T>G/A, and 3435T>C) in children with NS and controls to investigate their usefulness as markers of responsiveness of the disease to steroids, and they have shown inconsistent results [19–22].
On the basis of published data, our study focused on determining the relevance of the MDR1 (1236T>C, 2677T>G, and 3435T>C) polymorphisms and haplotypes to childhood INS susceptibility, selected demographic and clinical characteristics of INS, and TR to prednisone-based therapy in Slovak children.
Material and Methods
Study population and data collection
The study was performed in 2 groups of participants. The patient group comprised 46 children with a diagnosis of INS treated and/or monitored in the Pediatric Nephrology Centre at the Department of Pediatrics and Adolescent Medicine of the Faculty of Medicine of P. J. Safarik University in Kosice, Slovakia. The control group comprised 100 healthy children without any known chronic disease. Blood samples from both groups were collected during routine control biochemical tests or routine preventive pediatric examination at the same department from January 2010 to January 2014.
Participation was voluntary and could be cancelled by any individual at any time during the study (according to the Helsinki II declaration). The local ethics committee approved the research protocol for this study and all volunteers and/or their parents signed the study informed consent form.
Data evaluation was based on analysis of retrospective charts. All INS patients were followed up for at least 2 years. The inclusion criteria for the patient group were a fulfilled definition of INS (massive proteinuria of ≥40 mg/h/m2 and hypo-albuminemia of ≤2.5 g/dL), absence of secondary cause of nephrotic syndrome, and age 1–18 years. All cases were Slovaks from different regions of Eastern Slovakia.
All patients were treated with the standard initial steroid therapy, consisting of daily dosage of prednisone of 60 mg/m2/day for 4 weeks followed by 40 mg/m2/day on alternate days for an additional 4 weeks. Relapses were treated with daily prednisone dose of 60 mg/m2/day until urine protein test results were negative or trace for 3 consecutive days, followed by 40 mg/m2/day on alternate days for 4 weeks, and finally by various steps of tapering-off on alternate days.
The following definitions were considered for classification of response pattern to steroid therapy [3,4]:
Initial steroid response (RE-responders): attainment of complete remission (CR) within the initial 4 weeks of GC therapy. CR was defined as urinary protein excretion <4 mg/h/m2; nil or trace by dipstick on spot sample for 3 consecutive days.
Initial steroid resistance (NRE-non-responders): failure to achieve complete remission following at least 4 weeks of corticosteroid therapy at a dose 60 mg/m2/day.
Steroid dependence: occurrence of 2 consecutive relapses during steroid therapy or within 14 days of its cessation.
Frequent relapse: 2 or more relapses within the first 6 months of initial response, or 4 or more relapses in any 12-month period.
All steroid-resistant patients were successfully screened for NPHS1 and WT1 mutations, with negative results. Renal biopsy was performed in all patients except 1 (because of a hypoplastic kidney).
DNA extraction and genotyping
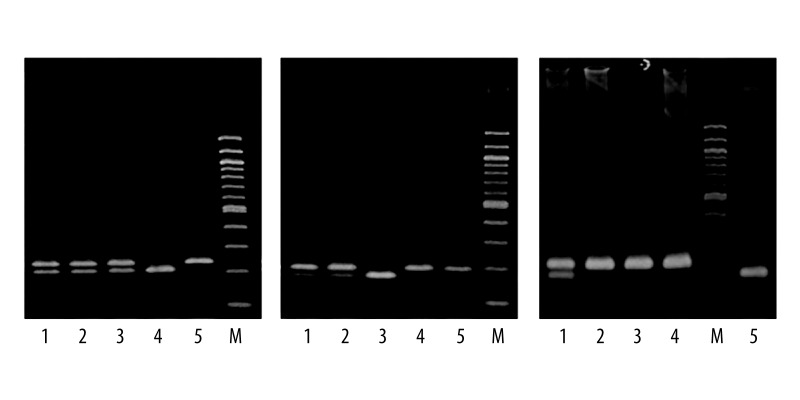
Genomic DNA was extracted from peripheral venous blood by using Wizard® Genomic DNA Purification Kit (Promega Corporation, USA). The SNPs in MDR1 were analyzed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay using the following primer sequences and corresponding restriction enzymes; (1) 1236T>C, 5′-TTACCCATCTCGAAAAGAAGTTAAGGT-3′ – forward, 5′-TGCCCACTCTGCACCTTCATGTTC-3′ – reverse (HaeIII); (2) 2677T>G, 5′-TTACCCAGAATATAGCAAATCTTGG-3′ – forward, 5′-CATATTTAGTTTGACTCACCTTCTCAG-3′ – reverse (Hpy188I); (3) 3435T>C, 5′-TGTTTTCAGCTGCTTGATGG-3′ – forward, R: 5′-AAGGCATGTATGTTGGCCTC-3′ – reverse (BfuCI). Due to the low population frequency of the more recently described A allele of 2677T>G/A polymorphism [23,24], this variant was not genotyped in the present study. The same PCR reaction pattern was used for each SNP. The PCR reaction mixture contained approximately 200 ng of genomic DNA, 1×PCR Buffer with 1.5 mM MgCl2 (Solis BioDyne, Estonia), 200 μM deoxynucleotide triphosphate mix (Jena Bioscience, Germany), 0.4 μM of each primer (Sigma-Aldrich, Germany), and 1.0 U HOT FIREPol® DNA Polymerase (Solis BioDyne, Estonia). PCR-grade water was added, bringing the final volume to 25 μl. The amplification consisted of an initial polymerase activation step for 15 min at 95°C and initial denaturation step for 30 s at 95°C followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 30 s. Terminal elongation was performed at 72°C for 3 min. The PCR products were digested at 37°C overnight using 10 units of appropriate restriction endonuclease (New England BioLabs, UK). The restriction fragments obtained were separated by electrophoresis on a 3% agarose gel for 90 min at 140 V and analyzed after staining with GelRed (Biotium, USA) under ultraviolet light. The electrophoretic pattern for each SNP was: (1) 1236T>C, TT: 234 bp, TC: 234 bp, 207 bp, CC: 207 bp; (2) 2677T>G, TT: 173 bp, TG: 198 bp, 173 bp, GG: 198 bp; and (3) 3435T>C, TT: 197 bp, TC: 197 bp, 158 bp, CC: 158 bp (Figure 1).
Figure 1.
Electrophoretic patterns for MDR1 polymorphisms evaluated by PCR-RFLP-based assay, M (Marker, 100 bp ladder): (A) 1236T>C: TT genotype (lane 5), TC genotype (lane 1, 2, 3), CC genotype (lane 4); (B) 2677T>G: TT genotype (lane 3), TG genotype (lane 1, 2), GG genotype (lane 4, 5); (C) 3435T>C: TT genotype (lane 2, 3, 4), TC genotype (lane 1), CC genotype (lane 5).
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, Inc., USA) was used for most of statistical analyses. A p value of <0.05 was taken as statistically significant. Chi-square or Fisher’s exact tests were performed to compare contingency tables. The Hardy-Weinberg equilibrium (HWE) assumption was assessed for the tested group by comparing the observed numbers of each genotype with those expected under the HWE for the estimated allele frequency. Codominant, dominant, recessive, and overdominant genetic models were used to analyze the association between a polymorphism and phenotype. The most commonly used model, the codominant model, is presented for all SNPs. The best-fitting genetic model was selected on the basis of Akaike’s information criterion. Results from the model chosen were then discussed. Odds ratios (OR) and the corresponding 95% confidence intervals (95% CI) were used for calculating the relative associations. The SNPStats program was used to examine haplotype frequency estimates [25].
Results
Clinical characteristics of the participants
Of the 46 patients enrolled in the study, 32 (69.57%) were males and 14 (30.43%) were females (male: female ratio: 2.29:1). The mean age at onset of INS was 6.42±4.72 years. Twenty-six children were <6 years old at the onset of disease. Among the total of 46 children with INS, 33 were initial RE (71.74%) and 13 were initial NRE (28.26%). From initial RE, 27 participants (81.82%) were frequent relapsers and/or steroid-dependent INS patients.
MCD was found in 32/46 (69.57%) children, FSGS in 11/46 (23.91%), and MesPGN in 2/46 (4.35%, 1 male and 1 female) of the remaining children.
Clinical and pathological characteristics of RE and NRE are shown in Table 1. We observed significant association between histological pattern and age at onset of INS with the initial steroid response. Patients with MCD were initial RE more frequently when compared with children with FSGS (p≤0.0001). Moreover, a significantly higher frequency of RE was observed in patients younger than 6 years of age at onset of INS (p=0.027).
Table 1.
Clinical characteristics of patients with idiopathic nephrotic syndrome.
| Variable | RE N=33 (%) |
NRE N=13 (%) |
OR (95% CI) | p value | |
|---|---|---|---|---|---|
| Age: | <6 years | 22 (66.7) | 4 (30.8) | 1.00 (Ref.) | |
| ≥6 years | 11 (33.3) | 9 (69.2) | 4.5 (1.13–17.94) | 0.027* | |
| Sex: | Males | 24 (72.7) | 8 (61.5) | 1.00 (Ref.) | |
| Females | 9 (27.3) | 5 (38.5) | 1.67 (0.43–6.46) | 0.493 | |
| Histology: | MCD | 29 (87.9) | 3 (23.1) | 1.00 (Ref.) | FSGS vs. MCD |
| FSGS | 2 (6.1) | 9 (69.2) | 43.50 (6.25–302.6) | <0.0001* | |
| MesPGN | 1 (3.0) | 1 (7.7) | |||
| Origin: | Town | 14 (42.4) | 9 (69.2) | 1.00 (Ref.) | |
| Village | 19 (57.6) | 4 (30.8) | 0.33 (0.08–1.28) | 0.102 | |
RE – responders; NRE – non-responders; OR – odds ratio; CI – confidence interval; MCD – minimal change disease; FSGS – focal segmental glomerulosclerosis; MesPGN – mesangial proliferative glomerulonephritis; Ref. – reference;
a significant association.
The control group comprised 100 healthy children (54 males and 46 females) with a mean age of 7.89±6.31. There were no significant differences in distributions of age and sex between controls and INS patients.
Genotype and allelic distribution of MDR1 polymorphisms
All obtained blood samples were successfully genotyped for MDR1 SNPs (3435T>C, 2677T>G, and 1236T>C). Overall genotype and allele frequencies for the tested MDR1 polymorphisms in patients and control subjects are shown in Table 2. The frequency of MDR1 1236T and 3435T alleles in Slovak control participants was higher when compared with 1236C and 3435C alleles. For the 2677T>G polymorphism, the G allele was more frequent than the T allele in the control group.
Table 2.
Distributions of genotypes and alleles of MDR1 gene polymorphisms among control subjects and INS patients.
| Genotype | Controls N=100 (%) |
INS patients N=46 (%) |
OR (95% CI) | p value |
|---|---|---|---|---|
| 1236T>C | ||||
| TT | 31 (31.0) | 11 (23.9) | 1.00 (Ref.)a | |
| TC | 41 (41.0) | 26 (56.5) | 1.79 (0.77–4.16) | 0.176 |
| CC | 28 (28.0) | 9 (19.6) | 0.91 (0.33–2.51) | 0.849 |
| T allele | 103 (51.5) | 48 (52.2) | 1.00 (Ref.) | |
| C allele | 97 (48.5) | 44 (47.8) | 0.97 (0.59–1.60) | 0.915 |
| 2677T>G | ||||
| TT | 23 (23.0) | 10 (21.7) | 1.00 (Ref.)a | |
| TG | 49 (49.0) | 29 (63.0) | 1.36 (0.57–3.26) | 0.488 |
| GG | 28 (28.0) | 7 (15.2) | 0.58 (0.19–1.75) | 0.327 |
| T allele | 95 (47.5) | 49 (53.3) | 1.00 (Ref.) | |
| G allele | 105 (52.5) | 43 (46.7) | 0.79 (0.48–1.30) | 0.360 |
| 3435T>C | ||||
| TT | 32 (32.0) | 15 (32.6) | 1.00 (Ref.)a | |
| TC | 52 (52.0) | 23 (50.0) | 0.94 (0.43–2.07) | 0.885 |
| CC | 16 (16.0) | 8 (17.4) | 1.07 (0.37–3.04) | 0.904 |
| T allele | 116 (58.0) | 53 (57.6) | 1.00 (Ref.) | |
| C allele | 84 (42.0) | 39 (42.4) | 1.02 (0.62–1.68) | 0.950 |
INS – idiopathic nephrotic syndrome; OR – odds ratio; CI – confidence interval; Ref. – reference;
codominant model.
There was no significant difference in the distribution of genotypes and alleles in all 3 SNPs between the patient group and the control group. The test for HWE showed no significant deviation from the Hardy-Weinberg equilibrium either in the total patient sample (3435T>C: p=0.9872; 2677T>G: p=0.1958; and 1236T>C: p=0.6675) or the control sample (3435T>C: p=0.9335; 2677T>G: p=0.7651; and 1236T>C: p=0.3428).
Patient characteristics according to MDR1 polymorphisms
Distributions of genotypes and alleles of MDR1 gene polymorphisms among INS patients with respect to age at onset are shown in Table 3. INS patients less than 6 years old had decreased frequencies of MDR1 1236CC (7.7% vs. 35%, p=0.029) and MDR1 2677GG (3.8% vs. 30%, p=0.041) genotypes. The risk of early onset of INS was significantly lower among patients carrying 1236CC than patients carrying 1236TT+TC (OR=0.15; 95% CI=0.03–0.86; recessive model). Similarly, patients carrying 2677GG genotypes had lower risk of developing INS at a younger age than patients carrying TG+TT genotype (OR=0.09; 95% CI=0.01–0.86; recessive model). The difference in allele frequencies between INS participants <6 years old and ≥6 years old was not statistically significant.
Table 3.
Distributions of genotypes and alleles of MDR1 gene polymorphisms among INS patients with respect to age at the onset.
| Genotype | Age at onset ≥6 years N=20 (%) |
Age at onset <6 years N=26 (%) |
OR (95% CI) | p value |
|---|---|---|---|---|
| 1236T>C | ||||
| TT | 5 (25.0) | 6 (23.1) | 1.00 (Ref.)a | |
| TC | 8 (40.0) | 18 (69.2) | 1.88 (0.44–8.00) | 0.465 |
| CC | 7 (35.0) | 2 (7.7) | 0.24 (0.03–1.71) | 0.197 |
| TT+TC | 13 (65.0) | 24 (92.3) | 1.00 (Ref.)b | |
| CC | 7 (35.0) | 2 (7.7) | 0.15 (0.03–0.86) | 0.029* |
| TT+CC | 12 (60.0) | 8 (30.8) | 1.00 (Ref.)c | |
| TC | 8 (40.0) | 18 (69.2) | 3.38 (0.99–11.46) | 0.047* |
| T allele | 18 (45.0) | 30 (57.7) | 1.00 (Ref.) | |
| C allele | 22 (55.0) | 22 (42.3) | 0.60 (0.26–1.38) | 0.227 |
| 2677T>G | ||||
| TT | 5 (25.0) | 5 (19.2) | 1.00 (Ref.)a | |
| TG | 9 (45.0) | 20 (76.9) | 2.22 (0.51–9.65) | 0.281 |
| GG | 6 (30.0) | 1 (3.8) | 0.17 (0.01–1.94) | 0.129 |
| TT+TG | 14 (70.0) | 25 (96.2) | 1.00 (Ref.)b | |
| GG | 6 (30.0) | 1 (3.8) | 0.09 (0.01–0.86) | 0.033* |
| TT+GG | 11 (55.0) | 6 (23.1) | 1.00 (Ref.)c | |
| TG | 9 (45.0) | 20 (76.9) | 4.07 (1.15–14.49) | 0.026* |
| T allele | 19 (47.5) | 30 (57.7) | 1.00 (Ref.) | |
| G allele | 21 (52.5) | 22 (42.3) | 0.66 (0.29–1.52) | 0.331 |
| 3435T>C | ||||
| TT | 7 (35.0) | 8 (30.8) | 1.00 (Ref.)a | |
| TC | 7 (35.0) | 16 (61.5) | 2.00 (0.52–7.70) | 0.311 |
| CC | 6 (30.0) | 2 (7.7) | 0.29 (0.04–1.94) | 0.379 |
| T allele | 21 (52.5) | 32 (61.5) | 1.00 (Ref.) | |
| C allele | 19 (47.5) | 20 (38.5) | 0.69 (0.30–1.59) | 0.385 |
INS – idiopathic nephrotic syndrome; OR – odds ratio; CI – confidence interval; Ref. – reference;
codominant model;
recessive model;
overdominant model;
a significant association.
There was no significant association between 3435T>C polymorphism and age of onset under any tested genetic models. No other significant differences were found across clinical and pathological characteristics (sex, histopathology, number of relapses, and origin) differentiated according to genotypes and alleles of MDR1 polymorphisms (data not shown).
MDR1 gene variants and response to glucocorticoids
The association between MDR1 polymorphisms and therapy response to GC was assessed in all patients (n=46). A statistically significant association was found between MDR1 3435T>C polymorphism and successful initial steroid treatment of INS. Statistical analysis showed a significantly increased chance of initial treatment response to GC in children carrying TC genotype (OR=5.13; 95% CI=1.18–22.25; overdominant model) (Table 4). In contrast, we did not observe any significant differences in the distribution of the MDR1 1236T>C and 2677T>G genotypes or alleles between steroid responders and non-responders. No statistically significant associations were found between non-frequent relapsers and frequent relapsers and/or steroid-dependent INS patients and MDR1 genotypes or alleles (data not shown).
Table 4.
Distributions of genotypes and alleles of MDR1 gene polymorphisms among initial steroid responders and steroid non-responders.
| Genotype | NRE N=13 (%) |
RE N=33 (%) |
OR (95% CI) | p value |
|---|---|---|---|---|
| 1236T>C | ||||
| TT | 5 (38.5) | 6 (18.2) | 1.00 (Ref.)a | |
| TC | 6 (46.2) | 20 (60.6) | 2.78 (0.62–12.42) | 0.244 |
| CC | 2 (15.4) | 7 (21.2) | 2.92 (0.41–20.91) | 0.374 |
| T allele | 16 (61.5) | 32 (48.5) | 1.00 (Ref.) | |
| C allele | 10 (38.5) | 34 (51.5) | 1.70 (0.67–4.29) | 0.259 |
| 2677T>G | ||||
| TT | 5 (15.2) | 5 (38.5) | 1.00 (Ref.)a | |
| TG | 23 (69.7) | 6 (46.2) | 0.26 (0.06–1.21) | 0.109 |
| GG | 5 (15.2) | 2 (15.4) | 0.40 (0.05–3.13) | 0.622 |
| T allele | 33 (50.0) | 16 (61.5) | 1.00 (Ref.) | |
| G allele | 33 (50.0) | 10 (38.5) | 0.63 (0.25–1.58) | 0.318 |
| 3435T>C | ||||
| TT | 7 (53.9) | 8 (24.2) | 1.00 (Ref.)a | |
| TC | 3 (23.1) | 20 (60.6) | 5.83 (1.20–28.38) | 0.030* |
| CC | 3 (23.1) | 5 (15.2) | 1.46 (0.25–8.43) | 1.000 |
| TT+CC | 10 (76.9) | 13 (39.4) | 1.00 (Ref.)b | |
| TC | 3 (23.1) | 20 (60.6) | 5.13 (1.18–22.25) | 0.022* |
| T allele | 17 (65.4) | 36 (54.6) | 1.00 (Ref.) | |
| C allele | 9 (34.6) | 30 (45.5) | 1.57 (0.61–4.04) | 0.344 |
INS – idiopathic nephrotic syndrome; RE – responders; NRE – non-responders; OR – odds ratio; CI – confidence interval; Ref. – reference;
codominant model;
overdominant model;
a significant association.
Haplotype analysis
Haplotype analysis of the 3 MDR1 polymorphisms (1236T>C, 2677T>G, and 3435T>C) revealed 7 major haplotypes. TTT haplotype was the most frequent haplotype in the patients and control group. There were no significant differences in the distribution of these haplotypes between INS patients and the controls (Table 5). Moreover, we did not observe any significant difference in the distribution of these haplotypes between initial steroid responders and non-responders (Table 5). Haplotype variations did not influence age at onset of INS or renal pathology (data not shown).
Table 5.
Haplotype frequency distribution of MDR1 gene polymorphisms among control subjects and INS patients and with respect to therapeutic response.
| Haplotype | Controls N=100 |
INS patients N=46 |
p value | RE N=33 |
NRE N=13 |
p value |
|---|---|---|---|---|---|---|
| TTT | 40.3% | 47.5% | 0.240 | 43.5% | 57.5% | 0.235 |
| CGC | 34.1% | 37.7% | 0.502 | 40.5% | 30.6% | 0.367 |
| CGT | 12.8% | 7.9% | 0.177 | 7.9% | 7.9% | 0.951 |
| TTC | 5.6% | 3.6% | 0.433 | 3.4% | 4.1% | 0.827 |
| TGT | 3.3% | 0.0% | 0.310 | 0.0% | 0.0% | – |
| TGC | 2.3% | 1.1% | 0.429 | 1.6% | 0.0% | 0.434 |
| CTT | 1.6% | 2.2% | 0.685 | 3.1% | 0.0% | 0.709 |
INS – idiopathic nephrotic syndrome; RE – responders; NRE – non-responders.
Discussion
Genomic medicine, which is the use of information from genomes and their derivatives to guide medical decision making, is a key component of personalized medicine, which is currently a rapidly advancing field of health care [26].
In the present study, we investigated whether 3 known SNPs in the MDR1 gene (1236T>C, 2677T>G, and 3435T>C) influence INS susceptibility, selected demographics, and pathological characteristics of INS, as well as response to oral prednisone in children from Eastern Slovakia. To our knowledge, this is the first study evaluating MDR1 polymorphisms in childhood INS in a Slovak population.
Although the mechanisms of pathogenesis of INS are not clear, the justification for selecting the study of the MDR1 gene in INS was supported by evidence of P-gp distribution and expression in many tissues with barrier function, such as intestinal tissue, liver cells, and proximal tubular epithelial cells (impact on efflux of exogenous and endogenous toxins), as well as in the membranes of leukocytes, which play an important role in the pathogenesis of immune-inflammatory diseases. Wasilewska et al. found significantly higher P-gp expression on CD3-positive lymphocytes in patients with INS than in controls [27]. Moreover, association between MDR1 SNPs and inflammation has been reported [28,29].
In the present study, the MDR1 genotype and allele distribution of all 3 tested SNPs were not significantly different between the patient and the control group. There have been only 4 studies conducted to evaluate MDR1 SNPs in children with INS. Similar to our results, other researchers found no association between MDR1 SNPs and INS susceptibility; however, in a Polish study, all INS patients were RE [19,20]. On the other hand, our results are in contrast to studies carried out in different populations, where significant differences in genotype or allele frequencies of MDR1 2677T>G/A and 3435T>C SNPs were observed between INS patients and control subjects. In an Indian population, people with 3435TT genotype and 2677TT+AA genotype appeared to be at increased risk of INS [21]. Similar results were observed in an Egyptian population, where MDR1 2677GT, GA, TT+AA genotypes or T allele, MDR1 3435TT genotype, and T allele genotype frequencies were significantly increased in the INS group [22].
Recently, MDR1 genotyping has attracted research attention to the possibility of personalized treatment through identification of RE and NRE to a certain class of pharmacotherapy. Selection of tested SNPs in INS patients in this analysis was also based on knowledge of the importance of P-gp in pharmacokinetics or pharmacodynamics of the most commonly used GC in the treatment of the INS, including prednisone [8]. Furthermore, the importance of changes in P-gp expression in relation to TR has been confirmed in INS patients. Significantly lower expression of MDR1 and P-gp activity in T lymphocytes was detected in steroid- and cyclosporine-sensitive patients compared with resistant patients [30]. In our study, a significantly increased chance of TR to GC was shown in children carrying the MDR1 3435CT genotype. No significant differences in the distribution of the MDR1 1236T>C and 2677T>G genotypes or alleles between steroid RE and NRE were confirmed. In contrast, Choi et al. found significantly higher frequencies of the MDR1 1236CC genotype and C allele in the initial steroid RE than in NRE [20]. A study from India revealed a significant association between steroid TR in INS children and 2677T>G/A polymorphism [21]; a higher frequency of TT+AA genotypes was detectable in NRE in comparison to RE. Similarly, in an Egyptian study, steroid NRE had significantly higher frequencies of MDR1 2677GT, GA, and TT+AA genotypes than responsive INS patients [22]. In a Polish study evaluating only steroid RE, a strong association was observed between all tested MDR1 polymorphisms and time to initial steroid response [19]. The frequencies of 1236TT, 2677TT, and 3435TT genotypes were higher in late RE (time to remission: ≥7 days) than in early RE (time to remission: <7 days). There is currently no clear understanding of exactly how a heterozygous state in 3435T>C SNP confers GC sensitivity. However, some researchers proposed that heterozygous carriers of certain MDR1 variants are less responsive to infection [31].
The tested MDR1 polymorphisms are in strong linkage disequilibrium, and haplotype analysis was also evaluated. We identified TTT and CGC haplotypes as the most prevalent. Similar observations have been reported in other populations [19,20]. Our results revealed no significant differences in the distribution of MDR1 haplotypes between INS patients and controls. Moreover, we did not observe any significant difference in the distribution of these haplotypes between initial steroid RE and NRE. Haplotype variations did not influence age at onset of INS or renal pathology. Similarly, Jafar et al. did not find a significant correlation between MDR1 haplotypes and TR [21]. However, the haplotype CGC was significantly more common in patients with age at the onset ≥6 years than among patients with an age at the onset <6 years. In contrast, the frequency of the MDR1 TGC haplotype was significantly higher in the initial steroid NRE than in RE in 2 studies [20,22]. In a Polish study, the TTT haplotype was found to be significantly associated with late oral steroid response [19].
We also tested the influence of MDR1 SNPs on age at the onset of INS. Interestingly, MDR1 1236T>C and MDR1 2677T>G SNPs were significantly associated with age at onset. Our preliminary data indicate that genotypes 1236CC or 2677GG could delay early onset of INS but they did not influence the susceptibility to INS. We suppose that these polymorphisms might play a role in pathogenesis of NS in association with some other currently unknown predisposing factor(s). Similarly, Youssef et al. revealed significantly higher frequencies of 2677GG genotype and 3435CC genotype in patients ≥6 years old at the onset of INS [22]. This is in contrast with previous observations showing that age at onset of INS was not influenced by any MDR1 genotype or allele variations in Polish and Korean children [19,20]. In another study, age at onset of INS was correlated only with MDR1 3435T>C SNP [21].
There are a few issues that should be discussed regarding the inconsistent results of studies evaluating P-gp and MDR1 polymorphisms in INS patients. First, studies were done in patients with different ethnicities. It should be noted that P-gp expression and influence of MDR1 SNPs may vary depending on ethnicity and environmental factors [32]. Second, the homogeneity of the tested group is uncertain because of the unknown pathophysiology of the diseases and because of several histological patterns included in the diagnosis of INS. Also, other factors might have influenced the results, such as drug and food interactions [33]. Finally, P-gp has functions other than those directly related to transport activity, and the impact of genetic polymorphisms on these activities may be more pronounced. All steroid-resistant patients were screened for NPHS1 and WT1 mutations, with negative results, but there are also other gene mutations associated with steroid resistance [34].
In this study, histological pattern and age at onset of INS were significantly associated with the initial steroid response. Patients with MCD were initial RE more frequently when compared with children with FSGS, which is in accordance with the data that has been published earlier [31–33]. Furthermore, a significantly higher frequency of RE was observed in patients younger than 6 years of age at the onset of INS. The higher frequency of MCD in patients younger than 6 years of age could be responsible for better TR.
The main limitation of our study was the relatively small sample size, which was because it was a single-center study and there generally are low numbers of children with INS in small populations. Therefore, we cannot exclude the possibility of bias and our preliminary results must be interpreted with caution. Moreover, because of the retrospective patient data collection from medical records and missing information, the evaluation of other parameters describing the clinical course and TR (such as early or late initial response to steroids) could not be made.
Conclusions
Our findings suggest that prednisone therapeutic response may be influenced by histology, age at onset of INS, and by MDR1 3435T>C polymorphism. The MDR1 1236T>C and 2677T>G polymorphisms were significantly associated with age at onset. Larger multicenter studies and studies across other ethnic groups are needed to elucidate the contradictory implications of MDR1 polymorphisms in children with INS.
Acknowledgements
We thank Marta Vysocka and Kamila Samudovska for their skilful technical assistance.
Footnotes
Conflict of interest
The authors declare no conflict of interest in relation to the article.
Source of support: The project was supported by a grant from Iceland, Liechtenstein, and Norway through the EEA Financial Mechanism and the Norwegian Financial Mechanism, VEGA 1/0715/11 and by the University Scientific Grant Agency (grant No. 5/2011)
References
- 1.Perazella M. A 58-year-old man with diabetes mellitus and nephrotic syndrome. Hosp Physician. 2002;38:39–42. [Google Scholar]
- 2.Orlowska-Kowalik G, Malecka-Massalska T, Ksiazek A. Elevated serum CA-125 levels in a patient with nephrotic syndrome. Am J Case Rep. 2008;9:351–54. [Google Scholar]
- 3.Bagga A, Mantan M. Nephrotic syndrome in children. Indian J Med Res. 2005;122:13–28. [PubMed] [Google Scholar]
- 4.McKinney PA, Feltbower RG, Brocklebank JT, Fitzpatrick MM. Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol. 2001;16:1040–44. doi: 10.1007/s004670100021. [DOI] [PubMed] [Google Scholar]
- 5.Teeninga N, Kist-van Holthe JE, van Rijswijk N, et al. Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome. J Am Soc Nephrol. 2013;24:149–59. doi: 10.1681/ASN.2012070646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortazavi F, Khiavi YS. Steroid response pattern and outcome of pediatric idiopathic nephrotic syndrome: a single-center experience in northwest Iran. Ther Clin Risk Manag. 2011;7:167–71. doi: 10.2147/TCRM.S19751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Zhao Y, Wang C, et al. Inhibition of p38 MAPK diminishes doxorubicin-induced drug resistence associated with P-glycoprotein in human leukemia K562 cells. Med Sci Monit. 2012;18(10):BR383–88. doi: 10.12659/MSM.883477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dilger K, Schwab M, Fromm MF. Identification of budesonide and prednisone as substrates of the intestinal drug efflux pump P-glycoprotein. Inflamm Bowel Dis. 2004;10:578–83. doi: 10.1097/00054725-200409000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Padowski JM, Pollack GM. Pharmacokinetic and pharmacodynamic implications of P-glycoprotein modulation. Methods Mol Biol. 2010;596:359–84. doi: 10.1007/978-1-60761-416-6_16. [DOI] [PubMed] [Google Scholar]
- 10.Richaud-Patin Y, Soto-Vega E, Jakez-Ocampo J, Llorente L. P-glycoprotein in autoimmune diseases. Autoimmun Rev. 2004;3:188–92. doi: 10.1016/j.autrev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Gollapud S, Gupta S. Anti P-glycoprotein antibody-induced apoptosis of activated peripheral blood lymphocytes: a possible role of P-glycoprotein in lymphocyte survival. J Clin Immunol. 2001;21:420–30. doi: 10.1023/a:1013177710941. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473–78. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimchi-Sarfaty C, Oh JM, Kim IW, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–28. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 14.Tang K, Ngoi SM, Gwee PC, et al. Distinct haplotype profiles and strong linkage disequilibrium at the MDR1 multidrug transporter gene locus in three ethnic Asian populations. Pharmacogenetics. 2002;12:437–50. doi: 10.1097/00008571-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kim RB, Leake BF, Choo EF, et al. Identification of functionally variant MDR-1 allele among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–99. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 16.Sakurai A, Onishi I, Hirano H, et al. Quantitative structure-activity relationship analysis and molecular dynamics simulation to functionally validate nonsynonymous polymorphisms of human ABC transporte ABCB1 (P-glycoprotein/MDR1) Biochemistry. 2007;43:7678–93. doi: 10.1021/bi700330b. [DOI] [PubMed] [Google Scholar]
- 17.Anglicheau D, Verstuyft C, Laurent-Puig P, et al. Association of the multidrug resistance-1 gene single nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol. 2003;14:1889–96. doi: 10.1097/01.asn.0000073901.94759.36. [DOI] [PubMed] [Google Scholar]
- 18.Salama NN, Yang Z, Bui T, Ho RJ. MDR-1 haplotypes significantly minimize intracellular uptake and transcellular P-gp substrate transport in recombinant [LC-PK] cells. J Pharm Sci. 2006;95:2293–308. doi: 10.1002/jps.20717. [DOI] [PubMed] [Google Scholar]
- 19.Wasilewska A, Zalewski G, Chyczewski L, Zoch-Zwierz W. MDR-1 gene polymorphisms and clinical course of steroid-responsive nephrotic syndrome in children. Pediatr Nephrol. 2007;22:44–51. doi: 10.1007/s00467-006-0275-3. [DOI] [PubMed] [Google Scholar]
- 20.Choi HJ, Cho HY, Ro H, et al. Polymorphisms of the MDR1 and MIF genes in children with nephrotic syndrome. Pediatr Nephrol. 2011;26:1981–88. doi: 10.1007/s00467-011-1903-0. [DOI] [PubMed] [Google Scholar]
- 21.Jafar T, Prasad N, Agarwal V, et al. MDR-1 gene polymorphisms in steroid-responsive versus steroid-resistant nephrotic syndrome in children. Nephrol Dial Transplant. 2011;26(12):3968–74. doi: 10.1093/ndt/gfr150. [DOI] [PubMed] [Google Scholar]
- 22.Youssef DM, Attia TA, El-Shal AS, Abduelometty FA. Multi-drug resistance-1 gene polymorphisms in nephrotic syndrome: Impact on susceptibility and response to steroids. Gene. 2013;530:201–7. doi: 10.1016/j.gene.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 23.Cascorbi I, Gerloff T, Johne A, et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther. 2001;69:169–74. doi: 10.1067/mcp.2001.114164. [DOI] [PubMed] [Google Scholar]
- 24.Pechandová K, Buzková H, Slanar O, Perlík F. Polymorphisms of the MDR1 gene in the Czech population. Folia Biol. 2006;52:184–89. doi: 10.14712/fb2006052060184. [DOI] [PubMed] [Google Scholar]
- 25.Sole X, Guino E, Valls J, et al. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22:1928–29. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- 26.Ginsburg GS, Willard HF. Genomic and personalized medicine: foundations and applications. Transl Res. 2009;154:277–87. doi: 10.1016/j.trsl.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Wasilewska AM, Zoch-Zwierz WM, Pietruczuk M. Expression of P-glycoprotein in lymphocytes of children with nephrotic syndrome treated with glucocorticoids. Eur J Pediatr. 2006;165:839–44. doi: 10.1007/s00431-006-0177-1. [DOI] [PubMed] [Google Scholar]
- 28.Markova S, Nakamura T, Sakaeda T, et al. Genotype-dependent down-regulation of gene expression and function of MDR1 in human peripheral blood mononuclear cells under acute inflammation. Drug Metab Pharmacokinet. 2006;21:194–200. doi: 10.2133/dmpk.21.194. [DOI] [PubMed] [Google Scholar]
- 29.Pawlik A, Baskiewicz-Masiuk M, Machalinski B, et al. Involvement of C3435T and G2677T multidrug resistance gene polymorphisms in release of cytokines from peripheral blood mononuclear cells treated with methotrexate and dexamethasone. Eur J Pharmacol. 2005;528:27–36. doi: 10.1016/j.ejphar.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 30.Stachowski J, Zanker CB, Runowski D, et al. Resistance to therapy in primary nephrotic syndrome: effect of MDR1 gene activity. Pol Merkuriusz Lek. 2000;8:218–21. [PubMed] [Google Scholar]
- 31.Huebner C, Browning BL, Petermann I, et al. Genetic analysis of MDR1 and inflammatory bowel disease reveals protective effect of heterozygous variants for ulcerative colitis. Inflamm Bowel Dis. 2009;15:1784–93. doi: 10.1002/ibd.21019. [DOI] [PubMed] [Google Scholar]
- 32.Sakaeda T. MDR1 genotype-related pharmacokinetics: fact or fiction? Drug Metabol Pharmacokinet. 2005;20:391–414. doi: 10.2133/dmpk.20.391. [DOI] [PubMed] [Google Scholar]
- 33.Marchetti S, Mazzanti R, Beijnen JH, Schellens JHM. Concise review: clinical relevance of drug-drug and herb-drug interactions mediated by the ABC transporter ABCB1 (MDR1, p-glycoprotein) Oncologist. 2007;12:927–41. doi: 10.1634/theoncologist.12-8-927. [DOI] [PubMed] [Google Scholar]
- 34.Joshi S, Andersen R, Jespersen B, Rittig S. Genetics of steroid-resistant nephrotic syndrome: a review of mutation spectrum and suggested approach for genetic testing. Acta Paediatr. 2013;102:844–56. doi: 10.1111/apa.12317. [DOI] [PubMed] [Google Scholar]
- 35.Mubarak M, Kazi JI, Shakeel S, et al. The spectrum of histopathological lesions in children presenting with steroid-resistant nephrotic syndrome at a single center in Pakistan. Scientific World Journal. 2012;2012:681802. doi: 10.1100/2012/681802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kari JA, Halawani M, Mokhtar G, et al. Pattern of steroid resistant nephrotic syndrome in children living in the kingdomof Saudi Arabia: a single center study. Saudi J Kidney Dis Transpl. 2009;20:854–57. [PubMed] [Google Scholar]
- 37.Seif EI, Ibrahim EAS, Elhefnawy NG, Salman MI. Histological patterns of idiopathic steroid resistant nephrotic syndrome in Egyptian children: A single centre study. J Nephropathology. 2013;2:53–56. doi: 10.5812/nephropathol.8997. [DOI] [PMC free article] [PubMed] [Google Scholar]