Abstract
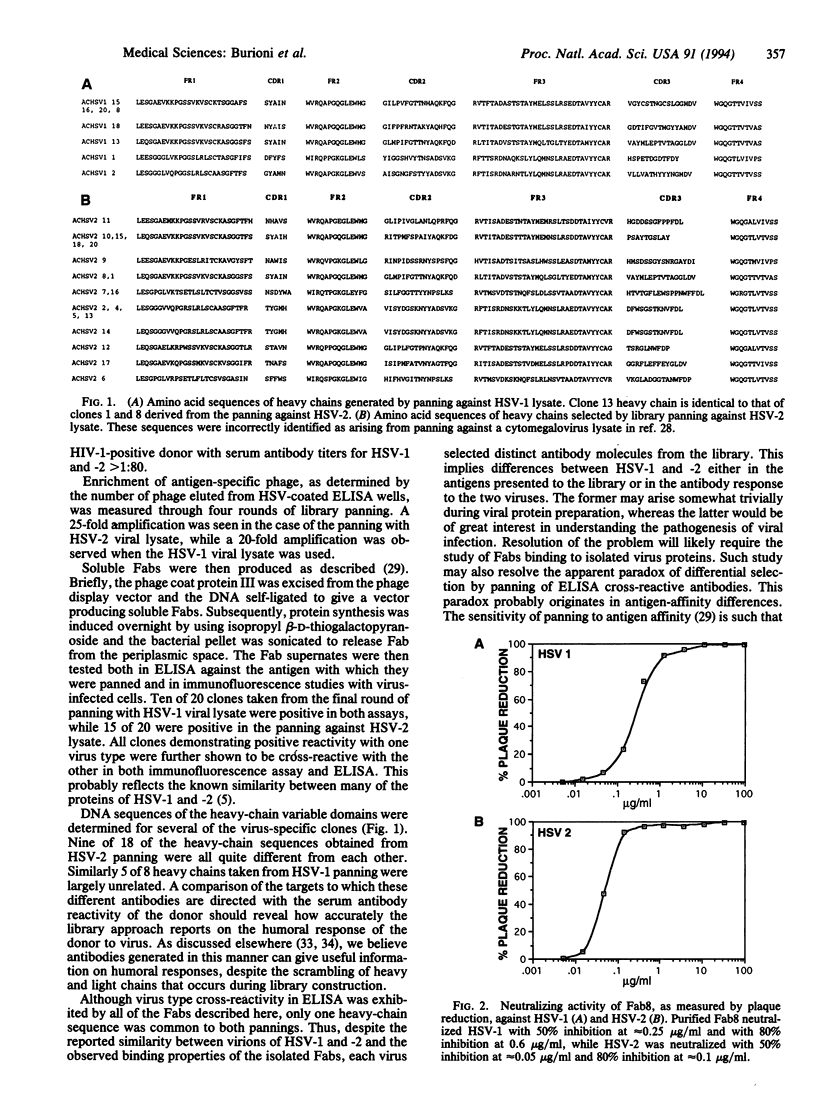
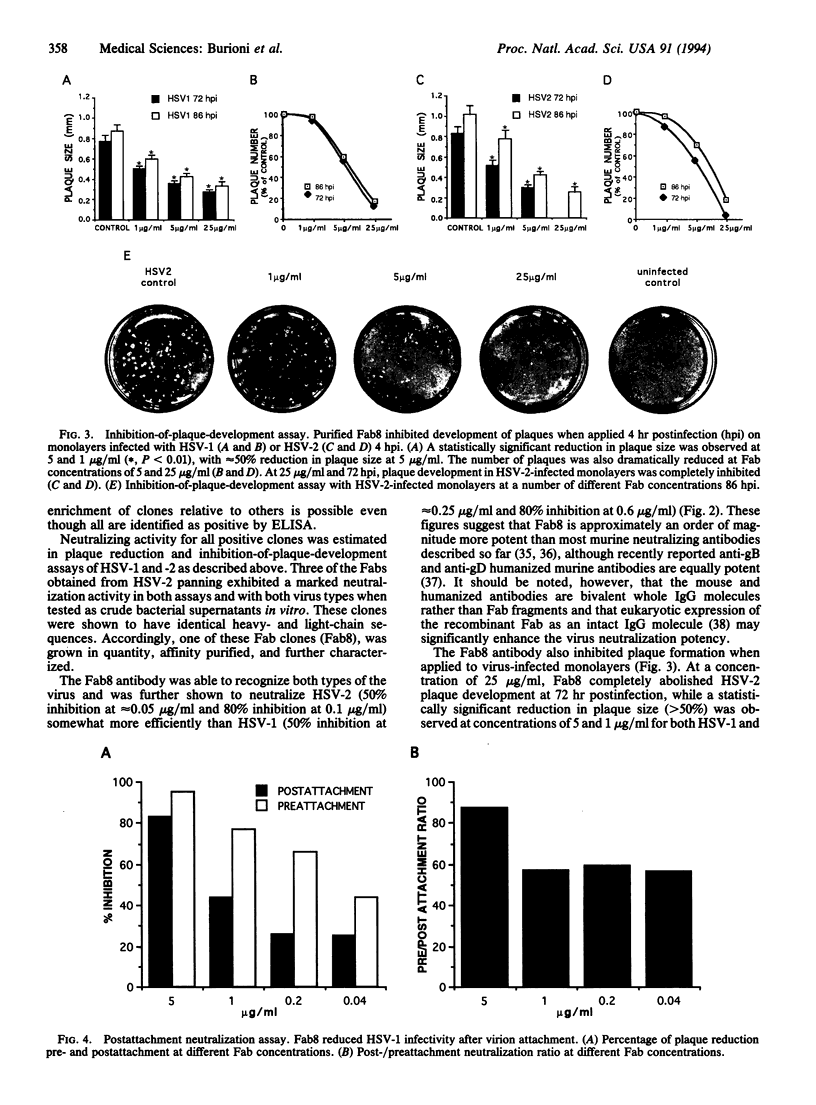
Herpes simplex viruses 1 and 2 (HSV-1 and -2) are associated with a number of conditions of varying severity, which are only partially responsive to current therapies. Human antibodies to the viruses offer a potential alternative. We describe here the generation of panels of human monoclonal Fab fragments to HSV-1 and -2 by panning a phage display combinatorial antibody library against whole lysates from the two viruses. Each lysate selected a largely distinct set of Fabs, although all of the Fabs were cross-reactive with both viruses. In a plaque-reduction assay, one Fab neutralized HSV-1 at 0.25 microgram/ml (50% reduction) and HSV-2 at 0.05 microgram/ml. This Fab also inhibited plaque formation when applied to virus-infected monolayers, completely abolishing HSV-2 plaque development at 25 micrograms/ml 72 hr postinfection, indicating the ability of the Fab to prevent cell-to-cell spread of virus. The Fab was shown to recognize viral glycoprotein D and to neutralize virus primarily by a postattachment mechanism. Recombinant Fabs may be useful for topical administration, although whole antibody will probably be required for systemic use.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Interactions of antibodies, complement components and various cell types in immunity against viruses and pyogenic bacteria. Transplant Rev. 1974;19(0):3–55. doi: 10.1111/j.1600-065x.1974.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Collet T. A., Amberg W., Roben P., Binley J. M., Hoekstra D., Cababa D., Jones T. M., Williamson R. A., Pilkington G. R. Molecular profile of an antibody response to HIV-1 as probed by combinatorial libraries. J Mol Biol. 1993 Apr 5;230(3):812–823. doi: 10.1006/jmbi.1993.1203. [DOI] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Kang A. S., Lerner R. A., Benkovic S. J. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender E., Woof J. M., Atkin J. D., Barker M. D., Bebbington C. R., Burton D. R. Recombinant human antibodies: linkage of an Fab fragment from a combinatorial library to an Fc fragment for expression in mammalian cell culture. Hum Antibodies Hybridomas. 1993 Apr;4(2):74–79. [PubMed] [Google Scholar]
- Burton D. R., Barbas C. F., 3rd Antibodies from libraries. Nature. 1992 Oct 29;359(6398):782–783. doi: 10.1038/359782b0. [DOI] [PubMed] [Google Scholar]
- Burton D. R., Barbas C. F., 3rd, Persson M. A., Koenig S., Chanock R. M., Lerner R. A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss D. H., Scharyj M., White D. R. Visceral herpesvirus infections in leukemic patients receiving cytarabine. JAMA. 1980 May 16;243(19):1903–1905. [PubMed] [Google Scholar]
- Co M. S., Deschamps M., Whitley R. J., Queen C. Humanized antibodies for antiviral therapy. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2869–2873. doi: 10.1073/pnas.88.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M. The implications of resistance to antiviral agents for herpesvirus drug targets and drug therapy. Antiviral Res. 1991 May;15(4):287–300. doi: 10.1016/0166-3542(91)90010-o. [DOI] [PubMed] [Google Scholar]
- Corey L., Adams H. G., Brown Z. A., Holmes K. K. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983 Jun;98(6):958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- Desai P. J., Schaffer P. A., Minson A. C. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988 Jun;69(Pt 6):1147–1156. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund J. A., Zimmerman M. E., Swierkosz E. M., Goodman J. L., Scholl D. R., Balfour H. H., Jr Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann Intern Med. 1990 Mar 15;112(6):416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- Erlich K. S., Mills J., Chatis P., Mertz G. J., Busch D. F., Follansbee S. E., Grant R. M., Crumpacker C. S. Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1989 Feb 2;320(5):293–296. doi: 10.1056/NEJM198902023200506. [DOI] [PubMed] [Google Scholar]
- Fujinaga S., Sugano T., Matsumoto Y., Masuho Y., Mori R. Antiviral activities of human monoclonal antibodies to herpes simplex virus. J Infect Dis. 1987 Jan;155(1):45–53. doi: 10.1093/infdis/155.1.45. [DOI] [PubMed] [Google Scholar]
- Fuller A. O., Santos R. E., Spear P. G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989 Aug;63(8):3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Specificities of monoclonal and polyclonal antibodies that inhibit adsorption of herpes simplex virus to cells and lack of inhibition by potent neutralizing antibodies. J Virol. 1985 Aug;55(2):475–482. doi: 10.1128/jvi.55.2.475-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. S., Friedman H., Cohen S. G., Oh S. H., Laster L., Starr S. A comparative study of herpes simplex infections in renal transplant and leukemic patients. J Infect Dis. 1987 Aug;156(2):280–287. doi: 10.1093/infdis/156.2.280. [DOI] [PubMed] [Google Scholar]
- Kapoor A. K., Nash A. A., Wildy P., Phelan J., McLean C. S., Field H. J. Pathogenesis of herpes simplex virus in congenitally athymic mice: the relative roles of cell-mediated and humoral immunity. J Gen Virol. 1982 Jun;60(Pt 2):225–233. doi: 10.1099/0022-1317-60-2-225. [DOI] [PubMed] [Google Scholar]
- Kühn J. E., Dunkler G., Munk K., Braun R. W. Analysis of the IgM and IgG antibody response against herpes simplex virus type 1 (HSV-1) structural and nonstructural proteins. J Med Virol. 1987 Oct;23(2):135–150. doi: 10.1002/jmv.1890230206. [DOI] [PubMed] [Google Scholar]
- Kümel G., Kaerner H. C., Levine M., Schröder C. H., Glorioso J. C. Passive immune protection by herpes simplex virus-specific monoclonal antibodies and monoclonal antibody-resistant mutants altered in pathogenicity. J Virol. 1985 Dec;56(3):930–937. doi: 10.1128/jvi.56.3.930-937.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCafferty J., Griffiths A. D., Winter G., Chiswell D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990 Dec 6;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- Navarro D., Paz P., Pereira L. Domains of herpes simplex virus I glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology. 1992 Jan;186(1):99–112. doi: 10.1016/0042-6822(92)90064-v. [DOI] [PubMed] [Google Scholar]
- Oakes J. E., Lausch R. N. Monoclonal antibodies suppress replication of herpes simplex virus type 1 in trigeminal ganglia. J Virol. 1984 Sep;51(3):656–661. doi: 10.1128/jvi.51.3.656-661.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass R. F., Whitley R. J., Whelchel J. D., Diethelm A. G., Reynolds D. W., Alford C. A. Identification of patients with increased risk of infection with herpes simplex virus after renal transplantation. J Infect Dis. 1979 Oct;140(4):487–492. doi: 10.1093/infdis/140.4.487. [DOI] [PubMed] [Google Scholar]
- Rand K. H., Rasmussen L. E., Pollard R. B., Arvin A., Merigan T. C. Cellular immunity and herpesvirus infections in cardiac-transplant patients. N Engl J Med. 1977 Jun 16;296(24):1372–1377. doi: 10.1056/NEJM197706162962402. [DOI] [PubMed] [Google Scholar]
- Rector J. T., Lausch R. N., Oakes J. E. Identification of infected cell-specific monoclonal antibodies and their role in host resistance to ocular herpes simplex virus type 1 infection. J Gen Virol. 1984 Mar;65(Pt 3):657–661. doi: 10.1099/0022-1317-65-3-657. [DOI] [PubMed] [Google Scholar]
- Reeves W. C., Corey L., Adams H. G., Vontver L. A., Holmes K. K. Risk of recurrence after first episodes of genital herpes. Relation to HSV type and antibody response. N Engl J Med. 1981 Aug 6;305(6):315–319. doi: 10.1056/NEJM198108063050604. [DOI] [PubMed] [Google Scholar]
- Sacks S. L., Wanklin R. J., Reece D. E., Hicks K. A., Tyler K. L., Coen D. M. Progressive esophagitis from acyclovir-resistant herpes simplex. Clinical roles for DNA polymerase mutants and viral heterogeneity? Ann Intern Med. 1989 Dec 1;111(11):893–899. doi: 10.7326/0003-4819-111-11-893. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Lopez C., Hammer G. S., Brown A. E., Kornfeld S. J., Gold J., Hassett J., Hirschman S. Z., Cunningham-Rundles C., Adelsberg B. R. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981 Dec 10;305(24):1439–1444. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- Simmons A., Nash A. A. Role of antibody in primary and recurrent herpes simplex virus infection. J Virol. 1985 Mar;53(3):944–948. doi: 10.1128/jvi.53.3.944-948.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P. C., Levine M., Glorioso J. C. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science. 1987 May 1;236(4801):576–579. doi: 10.1126/science.3033824. [DOI] [PubMed] [Google Scholar]
- Williamson R. A., Burioni R., Sanna P. P., Partridge L. J., Barbas C. F., 3rd, Burton D. R. Human monoclonal antibodies against a plethora of viral pathogens from single combinatorial libraries. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4141–4145. doi: 10.1073/pnas.90.9.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Milenic D. E., Whitlow M., Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992 Jun 15;52(12):3402–3408. [PubMed] [Google Scholar]