Abstract
Atherosclerosis is a prevalent cardiovascular disease marked by inflammation and the formation of plaque within arterial walls. As the disease progresses, there is an increased risk of major cardiovascular events. Owing to the nature of atherosclerosis, it is imperative to develop methods to further understand the physiological implications and progression of the disease. The combination of positron emission tomography (PET)/computed tomography (CT) has proven to be promising for the evaluation of atherosclerotic plaques and inflammation within the vessel walls. The utilization of the radiopharmaceutical tracer, 18F-fluorodeoxyglucose (18F-FDG), with PET/CT is invaluable in understanding the pathophysiological state involved in atherosclerosis. In this review, we will discuss the use of 18F-FDG-PET/CT imaging for the evaluation of atherosclerosis and inflammation both in preclinical and clinical studies. The potential of more specific novel tracers will be discussed. Finally, we will touch on the potential benefits of using the newly introduced combined PET/magnetic resonance imaging (MRI) for non-invasive imaging of atherosclerosis.
Keywords: atherosclerosis, plaque, inflammation, positron emission tomography, computed tomography, 18F-FDG
Introduction
Atherosclerosis is a cardiovascular disease characterized by inflammation and the buildup of plaques within the arterial walls. These plaques are mainly composed of lipids, calcium, and inflammatory cells.1 The disease involves an ongoing inflammatory response exacerbated by certain cardiovascular risk factors, including elevated basal levels of cytokines, hypertension, diabetes, and obesity. Notably, hypercholesterolemia is a necessary condition for atherogenesis.1–3 The expression of cellular adhesion molecules triggers the recruitment of monocytes and the infiltration of macrophages in the intima of the arterial wall during atherosclerotic plaque development.4–6 Atherosclerotic plaques form over a prolonged period of time, and are usually asymptomatic in the earlier stages of the disease. The initial manifestation of the disease is usually in the form of a severe cardiovascular event such as myocardial infarction, stroke, or acute coronary diseases.1,7–11 Therefore, early, non-invasive diagnosis can have vast clinical significance on the survival of such patient population.
Molecular imaging modalities have allowed for noninvasive diagnosis and study of the progression of this silent disease.12 These include the use of magnetic resonance imaging (MRI) or computed tomography (CT) for visualizing anatomical structures and positron emission tomography (PET) for the observation of molecular and cellular activities.13,14 The co-registration of anatomical and functional images from combined PET/CT is a plausible and reliable method for evaluating the progression of atherosclerosis and inflammation in vivo.15 PET/CT has been used to detect, locate, and quantify the presence of atherosclerotic plaques within the carotids, aortas, and coronary arteries.16–19
There is activation and upregulation of a number of biological markers identifiable to the inflammatory state in atherosclerosis.20,21 These biomarkers associated with atherosclerosis are utilized to detect plaques in PET imaging. Thus far, evidences suggest that this mode of imaging is reliable.22–24 Macrophages inhibit the walls of diseased arteries, and because of their elevated metabolic activities, consume glucose at a high rate. With this knowledge, radioactively labeled glucose analogs have been designed to detect the presence and extent of damage within diseased vessels. Favorable results have been obtained where macrophage density in diseased arterial walls corresponds to the intensity observed with these radioactively labeled tracers in PET imaging.25 CT imaging is useful for anatomical imaging of vessels because of its high spatial resolution. The accessibility of CT and its relatively short acquisition time make it a favorable imaging modality. In comparison to MRI, CT imaging is less prone to motion artifacts.26
18F-FDG-PET/CT for Evaluating Atherosclerosis and Inflammation
One of the main radiopharmaceutical tracers used in PET/CT imaging for atherosclerosis detection is 18F-fluorodeoxyglucose (18F-FDG).27–29 18F-FDG is a radioactively labeled glucose molecule that is readily consumed by the cells of the body, especially in regions of high metabolic activities.27–29 18F-FDG, which is administered intravenously prior to the PET/CT scan, was first used in neurology and oncology for imaging of the brain and tumors, respectively30,31; its use has since been extended to cardiovascular imaging of atherosclerotic plaque and inflammation. In 2001, the first instance of 18F-FDG uptake was noted in the vascular system in a human oncology study.32 From that time,18F-FDG has been extensively explored for understanding the pathological states of atherosclerosis in vivo.32–36 Its effectiveness in locating high levels of metabolic activities in diseased regions of the arterial wall has been observed in preclinical and clinical studies.35
In order for 18F-FDG to be effective with the use of PET/CT for imaging atherosclerotic plaques, cells within the walls of the arteries must have the ability to utilize this glucose analog. This is predominately the case with inflammatory cells found in plaques.21,37 Even though the macrophages involved in the formation of atherosclerotic plaque and inflammation can use fatty acids as an energy source, their high-energy rate restricts them to anaerobic metabolism for the most part. Consequently, glucose becomes the favorable source of energy for these cells through the glycolytic pathway.38–42 The way that 18F-FDG enters the cells is equivalent to the way glucose does through the glucose transporter (GLUT) protein system. 18F-FDG becomes phosphorylated to 18F-FDG-6 phosphate, but it cannot be metabolized further in the glycolytic pathway as illustrated in Figure 1. Thus, it accumulates in the cells proportionally to the metabolic rate.43–45 Higher accumulation of 18F-FDG results in higher release of PET photons, generating the contrast that is observed in the final PET image.
Figure 1.
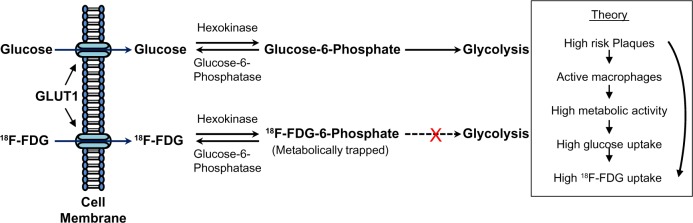
Pathway comparing the uptake and utilization of glucose versus 18F-FDG through the glucose transporter (GLUT1) in a cell.
In order to analyze the signal acquired from 18F-FDG in PET/CT imaging, the data obtained must be quantifiable. The standardized uptake value (SUV), a semi-quantitative metric, has been used extensively in assessing the 18F-FDG signal.14,46–48 The SUV is calculated by dividing the decay-corrected tissue concentration (kilobecquerels per milliliter) by the injected dose of 18F-FDG per body weight (kilobecquerels per gram).49 The target to background ratio (TBR) is also used as a quantitative, analytical measurement for analyzing the extent of atherosclerotic inflammation. The TBR is calculated by dividing the SUV of the artery of interest by that of the venous blood pool. The maximum, minimum, and mean TBR measurements are used to determine inflammatory activities within the arterial walls. The maximum and mean TBR measurements seem to provide the most reliable results for evaluating atherosclerotic inflammation.50
Even though macrophage cells readily take up 18F-FDG for energy, there are certain conditions that can have an effect on the uptake and imaging data. The optimal dosage of 18F-FDG depends on body weight, and administration typically ranges from 307 to 458 MBq. The recommended time for circulation in the body is established to be about two to three hours for cardiovascular imaging.51,52 Additionally, a serum glucose level of <7.0 mmol/L prior to scanning is advisable for obtaining analyzable images.27 These parameters allow for optimal accumulation of 18F-FDG in the arterial walls, while background levels begin to decrease. The time between the injections of 18F-FDG to scanning can notably impact the quality of data obtained.
Preclinical Evaluation of 18F-FDG-PET/CT in Atherosclerotic Imaging
Numerous animal studies have been performed to evaluate the effectiveness of 18F-FDG-PET/CT. These studies have shown that there is general correspondence in the signal of 18F-FDG uptake when compared to the actual macrophage content within the plaques.47 The validation of PET/CT in the evaluation of atherosclerosis has been extensively evaluated. Assessment of the results is usually done by the comparison of in vivo analysis with histological samples of animal specimens in some cases. These studies are useful in determining that the images acquired with the imaging modalities utilizing 18F-FDG are in fact comparable with actual histological samples.53–56
Preclinical studies that involve animal models exhibiting the diseased state associated with atherosclerosis have been central in understanding the role of 18F-FDG-PET/CT. On the other hand, in vitro explorations have been vital in understanding the uptake of 18F-FDG in mammalian cells. In a study involving cultured mouse peritoneal macrophages, Ogawa et al observed the accumulation of the tracer in the early stage of atherosclerosis marked by macrophage foam cell formation. It was shown that 18F-FDG increases during the formation of foam cells but decreases after differentiation. This change corresponded to hexokinase activities within the cells. It was concluded from the results that 18F-FDG was feasible for detecting the early stages of atherosclerosis with the activity of foam cells.57 Other markers associated with atherosclerosis that can be targeted with the use of 18F-FDG as the disease progresses have been studied as well. For instance, Hag et al investigated whether 18F-FDG can be utilized in vivo for imaging of atherogenesis. Apolipoprotein E (ApoE) mice were recruited for the study and put on a normal or high-fat diet. There were multiple imaging time-points utilizing 18F-FDG-PET/CT. After imaging, mice were euthanized so that the aortas can be analyzed through gamma counting and real-time polymerase chain reaction (qPCR) analysis. The gene expressions of 10 biomarkers involved in atherosclerosis were examined. They included chemo (C-X-C motif) ligand 1 (CXCL-1), monocyte chemoattractant protein (MCP-1), vascular cell adhesion molecule (VCAM-1), cluster of differentiation molecule CD-68, osteopontin (OPN), lectin-like oxidized LDL-receptor (LOX)-1, hypoxia-inducible factor (HIF)-1α, HIF-2α, vascular endothelial growth factor (VEGF) A, and tissue factor (TF). CXCL-1, MCP-1, and VCAM-1 are involved in monocyte and macrophage recruitment. CD-68 is a scavenger receptor, which is expressed by macrophages, and LOX-1 is another scavenger molecule, which is expressed not only by endothelial cells lining the arterial walls and macrophages but also by smooth muscle cells and platelets. OPN is a protein that is expressed during inflammation by several cell types, and has been noted to be highly expressed in human atherosclerosis. HIF-1α and HIF-2α are markers for identifying hypoxia. A hypoxic state is known to exist during advanced human atherosclerosis. VEGF is regulated by the expression of HIF-1α and HIF-2α; it is known to be upregulated by hypoxia and inflammatory mediators. TF is involved in thrombogenicity and expressed by cells in the vessel wall and platelets. Its functions include cell division, angiogenesis, and inflammation. These markers have all been shown to correlate with 18F-FDG uptake. Most importantly, VCAM-1, CD-68, OPN, and TF gene expressions were found to be the most involved in the uptake of 18F-FDG.58 As it has been stated, atherosclerosis is involved with the cascade of inflammatory activity within the arterial walls. To look at inflamed versus stable plaque phenotypes, Wenning et al conducted a study on ApoE mice where carotid artery cuff-model was used to fuel shear-stress-induced atherosclerosis. The implanted device caused inflamed plaques upstream and stable plaques downstream in relation to the location of the cuff. This produced an ideal mode of comparing the uptake of 18F-FDG in plaques of varying compositions. Significant differences were observed in the images of the inflamed and stable plaques. This confirmed that 18F-FDG-PET/CT can be used to differentiate between plaques with inflammation versus phenotypically stable ones.59 Murine models exhibiting atherosclerosis and inflammation have shown that the utilization of 18F-FDG-PET/CT for assessing the disease is feasible. However, there are a few studies showing that the non-specific nature of 18F-FDG can affect the evaluation of atherosclerotic plaques and inflammation in these preclinical studies.60 Other cells found within the arterial walls as well as those in the surrounding anatomical structures can utilize 18F-FDG and, therefore, impede accurate assessment. Cells from periaortic brown adipose tissue, which are inherently distinct from macrophages, may contribute to the observed signal.61
There have been positive studies where rabbit models have proven valuable for developing methods of evaluating atherosclerosis and inflammation.54,62–64 Ishino et al compared data of 18F-FDG uptake using PET/CT imaging versus intravascular ultrasonography in Watanabe heritable hyperlipidemic rabbits with early atherosclerotic lesions. The rabbits were scanned, and histological samples of the aortas were immediately acquired to compare imaging and histopathological findings of the diseased vessel. It was established that 18F-FDG-PET/CT is promising for the evaluation and monitoring of changes in the composition of early atherosclerotic plaques in rabbits as well.54 The efficiency of 18F-FDG for the imaging of atherosclerosis has also been compared with other tracers in animal models. 18F-FDG has been found to be more sensitive as compared to an iron oxide tracer (P904) for the detection of early plaques by Millon et al The results of the study illustrated that 18F-FDG-PET/CT can indeed evaluate changes within the plaques and degree of inflammation by the signal obtained.62 To evaluate the usefulness of 18F-FDG-PET/CT in measuring vascular inflammation, Tawakol et al performed a study on rabbits with atherosclerosis induced through injury of the aortoiliac arterial segment in combination with a high cholesterol diet. In the study, histological analyses were conducted on the diseased arteries of the rabbits and compared with in vivo scanning. From the results obtained, it was verified that 18F-FDG-PET can be used non-invasively to assess vascular inflammation and was shown to be promising for the evaluation of active atherosclerotic plaques.49 Vulnerable and stable plaques can also be evaluated using 18F-FDG as explored by Zhao et al. The rabbits in the study had induced atherosclerosis and pharmacologically, generated thrombosis. PET/CT scans were conducted before and after the triggering of thrombosis. PET/CT scans were performed, and the SUVs were obtained from segments of the aortas before and after the thrombosis was induced in the artery. Plaques were considered to be vulnerable if luminal thrombosis was observed from the images and histological samples. The maximum and mean SUVs were higher in the vulnerable plaques in comparison to the stable plaques. Therefore, it is very plausible that 18F-FDG can be used for quantitatively analyzing vulnerable plaques exhibiting thrombosis. Overall, it has been shown that 18F-FDG with PET/CT can be used to assess atherosclerotic plaques of varying compositions and throughout the different stages of the disease.63 18F-FDG has shown to be functional for understanding the underlying pathology involved in atherosclerosis.
Clinical Evaluation of 18F-FDG-PET/CT in Atherosclerotic Imaging
Clinical trials in human subjects are important and necessary for understanding the extent to which imaging modalities can be used and improved for evaluating atherosclerosis and its inflammatory complex.47,65 One of the first studies mainly focusing on the imaging of human arteries with 18F-FDG-PET/CT was conducted by Rudd et al in 2002. This study is significant in that it demonstrated that the underlying pathological elements involved in the progression of atherosclerosis in humans can be investigated non-invasively alongside the anatomical nature associated with the disease.66
In the clinical studies, it has been observed that 18F-FDG uptake corresponds to macrophage activities within the plaque. There are other factors at play affecting the uptake of 18F-FDG that influence the data obtained from the PET/CT imaging as well. There seems to be a correlation between cardiovascular risk factors and 18F-FDG uptakes. Increased uptake of 18F-FDG has been noted in patients with more cardiovascular risk factors and with preexisting diseases such as metabolic syndrome and diabetes mellitus.67,68 Tahara et al investigated the use of 18F-FDG in a cohort of patients with metabolic syndrome and found a direct relationship between 18F-FDG uptake and increased cardiovascular risk factors.68 In an investigation conducted by Kim et al, the results indicated that high levels of 18F-FDG signal in patients with diabetes mellitus also corresponded to existing vascular inflammation.67 The uptake level of 18F-FDG correlates quantitatively with increased gene expression of specific markers associated with inflammation and atherosclerotic plaque formation.23,44,69,70 These include C-reactive protein (CRP) and pro-inflammatory markers such as matrix metalloproteinase (MMP) 1, 3, and 9.18,25,71–73 Wu et al examined the link between 18F-FDG uptake and the presence of certain circulating biomarkers in patients with atherosclerosis. The study consisted of healthy controls and patients diagnosed with significant carotid stenosis. Leukocyte counts, and CRP and MMP-1 levels were collected and compared. Patients with carotid stenosis had higher 18F-FDG uptake in the vessel walls; they also had higher levels of circulating MMP-1. Accordingly, it is possible to use 18F-FDG-PET/CT for the evaluation of atherosclerotic plaque biology.71 Similar to MMP-1, levels of CRP seem to have a correlation with atherosclerosis development. In a study consisting of a large cohort, Noh et al investigated the relationship of 18F-FDG uptake to levels of CRP and the Framing-ham risk score (a gender-specific algorithm used to assess the risk of cardiovascular disease in 10 years). This study consisted of 1,181 asymptomatic subjects undergoing 18F-FDG-PET/CT scans. The maximum TBR and intima-media thickness were compared to clinical risk factors and levels of CRP. It was found that the 18F-FDG uptake in the carotids corresponds to characteristics of atherosclerotic inflammation, which is separate from CRP levels. Nonetheless, serum levels of CRP can indicate carotid atherosclerosis progression.72
Atherosclerotic plaque and inflammation have been explored extensively in the aortas and carotid arteries using 18F-FDG-PET/CT. The use of 18F-FDG-PET/CT for the imaging of a high-risk plaque in the carotid artery is shown in Figure 2. It is also possible to incorporate 18F-FDG-PET/CT to look at the coronary arteries as well as the iliac, femoral, and popliteal arteries. Multiple vascular beds can be explored simultaneously using this procedure, and it can be used to assess drug therapy over time.50,74–77 For instance, Rudd et al conducted an investigation on the use of 18F-FDG-PET/CT for the imaging of the carotid, iliac, and femoral arteries. The TBR measurement of the 18F-FDG signal was calculated for each of the vessels. From the results, it was concluded that the mean TBR may be efficient for the tracking of systemic arterial drug therapies, while the maximum TBR can be integrated for the detection and examination of local plaques over time.50 Rogers et al investigated the practicability of 18F-FDG for imaging of the coronary arteries to compare acute coronary syndrome and stable angina. The patients were scanned using CT angiography and 18F-FDG-PET. The TBR was calculated for the vessels of interest, and inflammatory activity was observed in the vessels of patients with coronary syndromes.76 The use of 18F-FDG for imaging of coronary arteries has also been investigated.78 One such study was performed by Cheng et al. The study found that the imaging of the coronary arteries with the fusion of PET and CT angiography of the patients with acute myocardial infarction did not detect signal at the diseased area in half of the subjects.79 As can be noted, 18F-FDG is versatile and applicable for imaging of various vessels and for investigating different arterial disease states.
Figure 2.
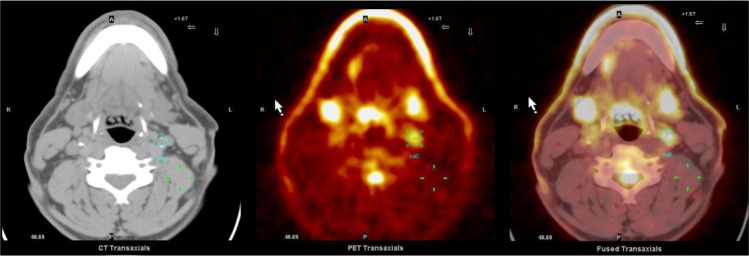
18F-FDG-PET/CT image of a high-risk plaque (circle) in the left carotid of a human.
In order for PET/CT to be reliable for studying atherosclerotic plaques, the data obtained must be consistent and reproducible. This is especially pertinent when evaluating the efficacy of drug treatment for atherosclerosis and for tracking the changes that occur within the plaque over time. By having multiple imaging time-points, one may be able to extrapolate the influences of lifestyle changes and medical intervention on the progression/regression of atherosclerotic plaque lesions and rate of inflammation. There seem to be promising results for inter-observer and intra-observer reproducibility analyses of PET/CT when compared with MR imaging analysis.80 This has been demonstrated in a reproducibility analysis performed by Rudd et al. Statistically favorable results were obtained from both intra- and inter-observer analyses of 18F-FDG-PET/CT images over a 14-day interval.50,81 In one study, 19 patients underwent repeated 18F-FDG-PET/CT scans, and the carotid, iliac, and femoral arteries were analyzed. The reproducibility of the 18F-FDG signal for the carotid and peripheral arteries with the intra-class correlation coefficients (ICC) were all computed to be greater than 0.8, indicating excellent agreement. The mean and maximum TBR measurements were found to be equally reproducible in the quantification of 18F-FDG uptake.50 Statistically reproducible data have been obtained over a three-month time frame from analysis of the carotid arteries also.48 It appears that the signal acquired from 18F-FDG is reliable in quantitatively evaluating atherosclerosis activity within arterial walls as can be seen from the consistent reproducible analyses.
Novel Tracers for Detecting Atherosclerosis and Inflammation
In order to improve on imaging of atherosclerosis and inflammation, it is necessary to explore and design novel radiopharmaceutical tracers to overcome deficits that may be encountered from the preferably used 18F-FDG. The nature of 18F-FDG to be readily taken up by most cells of the body and not just the ones associated with inflammation in atherosclerosis involves limitations. Although, 18F-FDG has been widely used and verified in diagnosing atherosclerotic plaques, there are other radiopharmaceutical tracers that are proven to be useful and more specific as well. Additionally, some may have the potential for allowing the exploration of different physiological aspects of plaque compositions.
Imaging of the coronary arteries can be a challenge when using 18F-FDG as the surrounding myocardium readily utilizes this glucose analog and interferes with the signal observed. Therefore, the data obtained for the activity in the coronary arteries become non-specific. To suppress the strong signal from the myocardium, patients are subjected to a high-fat, low carbohydrate diet prior to scanning of the coronary arteries with 18F-FDG.82–85 To overcome the challenge encountered, other tracers have been explored. One promising tracer used specifically for imaging of the coronary arteries is 18F-sodium fluoride (18F-NaF). This tracer is used in detection of calcification associated with novel bone formation and remodeling. As calcification is one of the primary features in atherosclerosis, and is substantially present in the diseased coronary arteries, detection of plaques with 18F-NaF may be clinically relevant. In PET/CT study using 18F-NaF, the tracer was shown to be effective in locating plaques of the coronary arteries. The remnants of the vulnerable plaques in the arterial walls after intervention were imaged.16 The comparison of 18F-NaF with 18F-FDG for PET/CT imaging has been investigated by Joshi et al in a prospective clinical study. Notably, 18F-NaF showed to be promising for the identification of both ruptured and high-risk coronary plaques.78
Choline tracers have also been investigated for the use of imaging atherosclerotic plaque and inflammation. These tracers were first used in oncology scanning, and they have proven to be effective in allowing for the detection of macrophages found in active plaques. 18F-fluoromethylcholine (18F-FMCH) and 11C-choline have been tested ex vivo in mouse models exhibiting atherosclerotic plaques and inflammation.86,87 18F-FMCH was first studied in vivo in a sample of patients with prostate cancer where the arteries were assessed. The patients underwent whole-body scans immediately after intravenous administration with the novel tracer. The abdominal aortas and common iliac arteries were segmented and analyzed for the uptake of 18F-FMCH. Several atherosclerotic lesions were identified, which provided promising results to encourage further investigation of this particular tracer for evaluating atherosclerotic plaques.88 11C-Choline was evaluated in a similar cohort by Kato et al, and considerable vascular uptake of the tracer was noted. Whole-body PET/CT scans were conducted with the tracer. The uptake of 11C-choline and calcification in the arterial walls were evaluated; the data were analyzed qualitatively and semi-quantitatively. From the results, it was observed that increased 11C-choline and calcification were deemed to be common in the elderly men in the study as observed throughout the arterial segments analyzed. The aortas and common carotid arteries were mainly focused upon.89 From the current results, it seems that choline tracers have the potential to advance PET/CT imaging of atherosclerotic plaques, but data are still limited.
Another PET tracer that has been developed for oncologic imaging but has the potential of being used for evaluating atherosclerotic plaque and inflammation is 68Ga-DOTATATE. This tracer targets somatostatin receptor 2, which is an inhibitory peptide involved in a large range of biological functions. This protein is known to be overexpressed in active macrophages and also in damaged endothelial cells. These are both primary conditions associated with atherosclerosis.90–92 A number of studies have shown that 68Ga-DOTATATE is readily taken up in the vascular system.93–95 68Ga-DOTATATE has been explored in the imaging of coronary arteries for atherosclerotic plaques as published by Rominger et al. In this study, the uptake of the tracer in the left anterior descending coronary artery was investigated. It was found that 68Ga-DOTATATE was detectable in this particular artery of all the patients analyzed. Prominently, the TBR obtained in the vessel correlated with the presence of calcified plaque. The patients in the study with previous cardiovascular events and calcified plaques had notably increased uptake of the tracer. This shows that 68Ga-DOTATATE has the potential of improving the evaluation of atherosclerosis in the coronary arteries using PET/CT.93 68Ga-DOTATATE has also proven to be of use for the imaging of large arterial vessels. Li et al conducted a study to investigate 68Ga-DOTATATE compared with 18F-FDG for the evaluation of inflammation associated with atherosclerosis, calcium burden, and cardiovascular risk factors. The mean TBR of the tracers was calculated in the large arteries. It was found that the uptake of 68Ga-DOTATATE correlated significantly to the presence of calcification in the arterial plaques when compared to the uptake of 18F-FDG in plaques of similar compositions. There was a stronger association of 68Ga-DOTATATE uptake in the patients with known cardiovascular risk factors in this study. The results suggest that 68Ga-DOTATATE may prove valuable for the imaging of the larger arteries also.95 The fact that it is quite specific in targeting a particular receptor makes it a promising alternative to 18F-FDG. However, further data analysis is needed to decide its efficacy in evaluating atherosclerotic plaques and inflammation.
Targeting biomarkers that are associated with atherosclerosis such as MMP has the potential for advancing PET/CT imaging of atherosclerotic plaque development. MMP are enzymes present at several stages as the disease progresses. They are known to be involved in the earlier stages of the disease where endothelial damage can be seen in the vessel walls, and they also play a role in both stabilizing and destabilizing advanced plaques. Imaging of MMP activities may aid in gaining more in-depth knowledge of the pathophysiological nature of atherosclerotic plaques.96 It also does not require the use of FDG as inflammation within the vessel walls is the main target and gives insight to plaque vulnerability. This can produce more specific imaging of the diseased walls as this approach of imaging MMP focuses on the sites where cells and molecules are located during specified stages of the plaque; this includes vulnerable or rupture-prone plaques.97
Multimodality Imaging of Atherosclerosis and Inflammation: PET/MRI Versus PET/CT
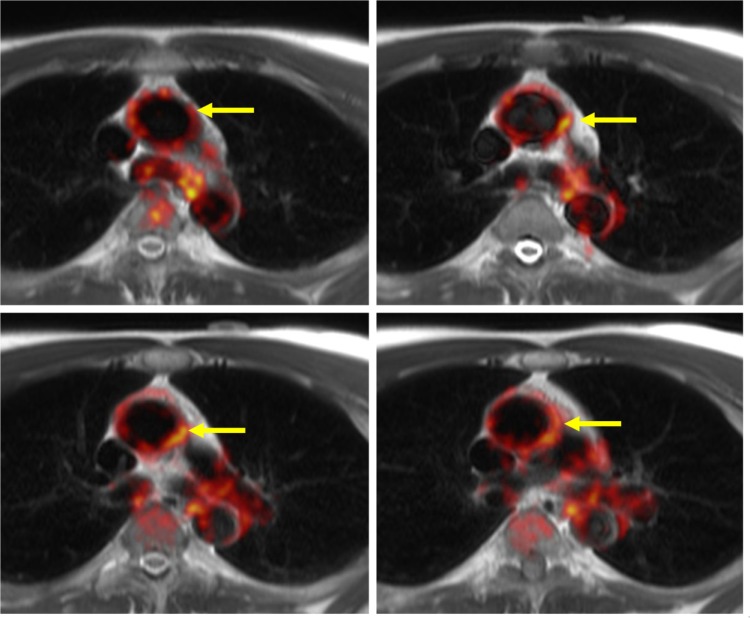
Even though PET/CT is reliable and more accessible, there are some advantages of using MRI co-registered with PET for the imaging of atherosclerosis and inflammation. Importantly, MRI is advantageous in that it provides excellent soft tissue contrast that can allow for a more detailed analysis of the diseased arterial composition. MRI allows for more defined differentiation between normal and abnormal tissues, which can be very beneficial in analyzing the pathology of arterial walls.98 With MRI, it is plausible to study the composition of plaques and to detect the presence of calcium, lipids, or fibrous caps within the diseased arterial walls.24 PET/MRI can be used to study vulnerable plaques and to gain further insight into the composition of the plaques throughout the progression of atherosclerosis.99 Figure 3 shows 18F-FDG-PET/MRI images of the ascending aortas where 18F-FDG uptake can be seen within the vessel wall.
Figure 3.
18F-FDG-PET/MRI images showing the ascending aortas in yellow arrows,18F-FDG uptake in red, and MRI in gray.
Studies investigating combined PET/MRI have found this multimodality imaging to be feasible both clinically and preclinically. One such experiment was conducted by Pedersen et al. This pilot study investigated the feasibility of using 18F-FDG to perform simultaneous PET/MRI imaging on the Göttingen minipig with diet-induced atherosclerosis. Multiple MRI sequences were acquired and included T1-weighted turbo spin-echo, T2-weighted turbo spin-echo, and proton density imaging. Signal from the 18F-FDG-PET uptake was obtained from a single bed position encompassing the diseased abdominal aorta. Results of the SUV were tabulated, and it was demonstrated that PET/MRI can be a practical approach for imaging of atherosclerosis.100 The first clinical investigation on the feasibility of simultaneous PET/MRI versus PET/CT was conducted by Ripa et al. The study comprised six participants who underwent sequential 18F-FDG-PET/MRI and PET/CT of the carotid arteries. Regions of interest were segmented slice by slice, and SUVs were calculated independently from both data sets. In this study, quantification of 18F-FDG uptake between the PET/MRI and PET/CT had a strong correlation even with differences involved in the two multimodality imaging methods.101 Results from this study also reinforce the feasibility of PET/MRI for evaluating atherosclerosis. Additionally, it has been shown that MRI may be used in the assessment of neovascularization within diseased vessels.102,103
MRI imaging can be deemed to be safer than CT as it does not expose subjects to the ionizing radiation intrinsic to CT scanning. With MRI, overall, it is also more convenient to conduct imaging of larger regions of the body. Multiple vessels throughout the body can be imaged at once. Even though CT can be used to image extended regions of the body also, exposure time to ionizing radiation involves potential risks to patients. However, because of the design of MRI, contraindication with certain metallic implanted materials exists and the scanning time is relatively longer. This increases the likelihood of motion artifacts when compared to CT.98,104 Nonetheless, MRI/PET has the ability to advance the use of multimodality imaging for the assessment of atherosclerotic plaques and inflammation.
A major difference between PET/MRI and PET/CT is that PET/MRI scans can be done simultaneously compared to sequential acquisition in PET/CT. As a result, real-time motion estimates can be measured during the PET acquisition using anatomical MR images and applied to the PET data during the image reconstruction phase to generate motion-corrected PET images. A recent study utilized simulated PET data to show that MR-guided motion correction can improve detectability of atherosclerotic plaques in the coronaries.105 To this date, however, there are no clinical data that support the feasibility of MR-guided motion correction in the heart.
Conclusion
PET/CT is a reliable imaging modality for evaluating atherosclerosis and inflammation. The use of 18F-FDG and other tracers has shown to permit for the assessment of the disease non-invasively. Therefore, further investigation is necessary for advancing the use of these multimodal imaging methods to acquire more precise images and accurate data to reliably evaluate the progression of atherosclerosis as well as other cardiovascular diseases. Multi-imaging methods have valuable perspective for aiding in the development of therapeutic strategies targeting cardiovascular diseases.
Acknowledgments
We wish to thank Dr Philip M. Robson for providing us with the PET/CT and PET/MRI images.
Footnotes
ACADEMIC EDITOR: Thomas E Vanhecke, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript: NA. Contributed to the writing of the manuscript: NA, ME, ZAF, VM. Agreed with manuscript results and conclusions: NA, ME, ZAF, VM. Jointly developed the structure and arguments for the paper: NA, ME, ZAF, VM. Made critical revisions and approved the final version: NA, ME, ZAF, VM. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;32(9):2045–51. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fruchart JC, Nierman MC, Stroes ES, Kastelein JJ, Duriez P. New risk factors for atherosclerosis and patient risk assessment. Circulation. 2004;109(23 suppl 1):III15–9. doi: 10.1161/01.CIR.0000131513.33892.5b. [DOI] [PubMed] [Google Scholar]
- 4.Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19(9):1166–72. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vita JA. Endothelial function. Circulation. 2014;124(25):e906–12. doi: 10.1161/CIRCULATIONAHA.111.078824. [DOI] [PubMed] [Google Scholar]
- 6.Vita JA, Keaney JF, Jr, Larson MG, et al. Brachial artery vasodilator function and systemic inflammation in the Framingham offspring study. Circulation. 2004;110(23):3604–9. doi: 10.1161/01.CIR.0000148821.97162.5E. [DOI] [PubMed] [Google Scholar]
- 7.Baldassarre D, Veglia F, Hamsten A, et al. IMPROVE Study Group Progression of carotid intima-media thickness as predictor of vascular events: results from the IMPROVE study. Arterioscler Thromb Vasc Biol. 2013;33(9):2273–9. doi: 10.1161/ATVBAHA.113.301844. [DOI] [PubMed] [Google Scholar]
- 8.Gepner AD, Korcarz CE, Colangelo LA, et al. Longitudinal effects of a decade of aging on carotid artery stiffness: the multiethnic study of atherosclerosis. Stroke. 2014;45(1):48–53. doi: 10.1161/STROKEAHA.113.002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jickling GC, Chaturvedi S. Carotid plaque inflammation in stroke assessed by PET: a burning issue? Neurology. 2014;82(19):1672–3. doi: 10.1212/WNL.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 10.Davies MJ, Thomas AC. Plaque fissuring – the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985;53(4):363–73. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shalhoub J, Oskrochi Y, Davies AH, Owen DR. Clinical assessment of carotid atherosclerosis inflammation by positron emission tomography. Curr Mol Med. 2013;13(10):1646–52. doi: 10.2174/1566524013666131111130334. [DOI] [PubMed] [Google Scholar]
- 12.Osborn EA, Jaffer FA. The advancing clinical impact of molecular imaging in CVD. JACC Cardiovasc Imaging. 1327;6(12):1327–41. doi: 10.1016/j.jcmg.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res. 2012;111(2):231–44. doi: 10.1161/CIRCRESAHA.112.268144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudd JH, Hyafil F, Fayad ZA. Inflammation imaging in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(7):1009–16. doi: 10.1161/ATVBAHA.108.165563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Townsend DW, Cherry SR. Combining anatomy and function: the path to true image fusion. Eur Radiol. 2001;11(10):1968–74. doi: 10.1007/s003300101007. [DOI] [PubMed] [Google Scholar]
- 16.Dweck MR, Chow MW, Joshi NV, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59(17):1539–48. doi: 10.1016/j.jacc.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Menezes LJ, Kayani I, Ben-Haim S, Hutton B, Ell PJ, Groves AM. What is the natural history of 18F-FDG uptake in arterial atheroma on PET/CT? Implications for imaging the vulnerable plaque. Atherosclerosis. 2010;211(1):136–40. doi: 10.1016/j.atherosclerosis.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Myers KS, Rudd JH, Hailman EP, et al. Correlation between arterial FDG uptake and biomarkers in peripheral artery disease. JACC Cardiovasc Imaging. 2012;5(1):38–45. doi: 10.1016/j.jcmg.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang S, Kyung C, Park JS, et al. Subclinical vascular inflammation in subjects with normal weight obesity and its association with body fat: an 18 F-FDG-PET/CT study. Cardiovasc Diabetol. 2014;13:70. doi: 10.1186/1475-2840-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golia E, Limongelli G, Natale F, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. 1007;16(9):435. doi: 10.1007/s11883-014-0435-z. [DOI] [PubMed] [Google Scholar]
- 21.Wong BW, Meredith A, Lin D, McManus BM. The biological role of inflammation in atherosclerosis. Can J Cardiol. 2012;28(6):631–41. doi: 10.1016/j.cjca.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 22.Elkhawad M, Rudd JH. Radiotracer imaging of atherosclerotic plaque biology. Cardiol Clin. 2009;27(2):345–54. doi: 10.1016/j.ccl.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Graebe M, Pedersen SF, Borgwardt L, Hojgaard L, Sillesen H, Kjaer A. Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET) Eur J Vasc Endovasc Surg. 2009;37(6):714–21. doi: 10.1016/j.ejvs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Lairez O, Fayad ZA. Imaging of atherosclerosis: can molecular imaging do more? Arch Cardiovasc Dis. 2013;106(11):551–3. doi: 10.1016/j.acvd.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Rudd JH, Myers KS, Bansilal S, et al. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging. 2009;2(2):107–15. doi: 10.1161/CIRCIMAGING.108.811752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaff MR, Goldmakher GV, Lev MH, Romero JM. Imaging of the carotid arteries: the role of duplex ultrasonography, magnetic resonance arteriography, and computerized tomographic arteriography. Vasc Med. 2008;13(4):281–92. doi: 10.1177/1358863X08091971. [DOI] [PubMed] [Google Scholar]
- 27.Tarkin JM, Joshi FR, Rudd JH. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol. 2014;1038(10):80. doi: 10.1038/nrcardio.2014.80. [DOI] [PubMed] [Google Scholar]
- 28.El-Haddad G, Zhuang H, Gupta N, Alavi A. Evolving role of positron emission tomography in the management of patients with inflammatory and other benign disorders. Semin Nucl Med. 2004;34(4):313–29. doi: 10.1053/j.semnuclmed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Rominger A, Saam T, Wolpers S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50(10):1611–20. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- 30.Fowler JS, Ido T. Initial and subsequent approach for the synthesis of 18FDG. Semin Nucl Med. 2002;32(1):6–12. doi: 10.1053/snuc.2002.29270. [DOI] [PubMed] [Google Scholar]
- 31.Som P, Atkins HL, Bandoypadhyay D, et al. A fluorinated glucose analog, 2-fluoro-2-deoxy-d-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med. 1980;21(7):670–5. [PubMed] [Google Scholar]
- 32.Yun M, Yeh D, Araujo LI, Jang S, Newberg A, Alavi A. F-18 FDG uptake in the large arteries: a new observation. Clin Nucl Med. 2001;26(4):314–9. doi: 10.1097/00003072-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Yun M, Jang S, Cucchiara A, Newberg AB, Alavi A. 18F FDG uptake in the large arteries: a correlation study with the atherogenic risk factors. Semin Nucl Med. 2002;32(1):70–6. doi: 10.1053/snuc.2002.29279. [DOI] [PubMed] [Google Scholar]
- 34.Lederman RJ, Raylman RR, Fisher SJ, et al. Detection of atherosclerosis using a novel positron-sensitive probe and 18-fluorodeoxyglucose (FDG) Nucl Med Commun. 2001;22(7):747–53. doi: 10.1097/00006231-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Haim S, Kupzov E, Tamir A, Israel O. Evaluation of 18F-FDG uptake and arterial wall calcifications using 18F-FDG PET/CT. J Nucl Med. 2004;45(11):1816–21. [PubMed] [Google Scholar]
- 36.Ben-Haim S, Kupzov E, Tamir A, Frenkel A, Israel O. Changing patterns of abnormal vascular wall F-18 fluorodeoxyglucose uptake on follow-up PET/CT studies. J Nucl Cardiol. 2006;13(6):791–800. doi: 10.1016/j.nuclcard.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Weisdorf DJ, Craddock PR, Jacob HS. Granulocytes utilize different energy sources for movement and phagocytosis. Inflammation. 1982;6(3):245–56. doi: 10.1007/BF00916406. [DOI] [PubMed] [Google Scholar]
- 38.Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol. 2009;218(1):7–29. doi: 10.1002/path.2518. [DOI] [PubMed] [Google Scholar]
- 39.Chiong M, Morales P, Torres G, et al. Influence of glucose metabolism on vascular smooth muscle cell proliferation. Vasa. 1024;42(1):8–16. doi: 10.1024/0301-1526/a000243. [DOI] [PubMed] [Google Scholar]
- 40.Newsholme P, Curi R, Gordon S, Newsholme EA. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem J. 1986;239(1):121–5. doi: 10.1042/bj2390121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garedew A, Henderson SO, Moncada S. Activated macrophages utilize glycolytic ATP to maintain mitochondrial membrane potential and prevent apoptotic cell death. Cell Death Differ. 2010;17(10):1540–50. doi: 10.1038/cdd.2010.27. [DOI] [PubMed] [Google Scholar]
- 42.Newsholme P, Gordon S, Newsholme EA. Rates of utilization and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages. Biochem J. 1987;242(3):631–6. doi: 10.1042/bj2420631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu Y, Maianu L, Melbert BR, Garvey WT. Facilitative glucose transporter gene expression in human lymphocytes, monocytes, and macrophages: a role for GLUT isoforms 1, 3, and 5 in the immune response and foam cell formation. Blood Cells Mol Dis. 2004;32(1):182–90. doi: 10.1016/j.bcmd.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Pedersen SF, Graebe M, Fisker Hag AM, Hojgaard L, Sillesen H, Kjaer A. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun. 2010;31(5):423–9. doi: 10.1097/MNM.0b013e32833767e0. [DOI] [PubMed] [Google Scholar]
- 45.Tavakoli S, Zamora D, Ullevig S, Asmis R. Bioenergetic profiles diverge during macrophage polarization: implications for the interpretation of 18F-FDG PET imaging of atherosclerosis. J Nucl Med. 1661;54(9):1661–7. doi: 10.2967/jnumed.112.119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48(9):1818–24. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 47.Hiari N, Rudd JH. FDG PET imaging and cardiovascular inflammation. Curr Cardiol Rep. 2011;13(1):43–8. doi: 10.1007/s11886-010-0150-5. [DOI] [PubMed] [Google Scholar]
- 48.Font MA, Fernandez A, Carvajal A, et al. Imaging of early inflammation in low-to-moderate carotid stenosis by 18-FDG-PET. Front Biosci (Landmark Ed) 2009;14:3352–60. doi: 10.2741/3457. [DOI] [PubMed] [Google Scholar]
- 49.Tawakol A, Migrino RQ, Hoffmann U, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12(3):294–301. doi: 10.1016/j.nuclcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Rudd JH, Myers KS, Bansilal S, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nuc Med. 2008;49(6):871–8. doi: 10.2967/jnumed.107.050294. [DOI] [PubMed] [Google Scholar]
- 51.Bucerius J, Mani V, Moncrieff C, et al. Optimizing 18F-FDG PET/CT imaging of vessel wall inflammation: the impact of 18F-FDG circulation time, injected dose, uptake parameters, and fasting blood glucose levels. Eur J Nucl Med Mol Imaging. 2014;41(2):369–83. doi: 10.1007/s00259-013-2569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oh M, Kim JY, Shin KH, et al. Imaging atherosclerosis in the carotid arteries with F-18-Fluoro-2-deoxy-d-glucose positron emission tomography: effect of imaging time after injection on quantitative measurement. Nucl Med Mol Imaging. 2010;44(4):261–6. doi: 10.1007/s13139-010-0043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyafil F, Cornily JC, Rudd JH, Machac J, Feldman LJ, Fayad ZA. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: a comparison with 18F-FDG PET/CT and histology. J Nucl Med. 2009;50(6):959–65. doi: 10.2967/jnumed.108.060749. [DOI] [PubMed] [Google Scholar]
- 54.Ishino S, Ogawa M, Mori I, et al. 18F-FDG PET and intravascular ultrasonography (IVUS) images compared with histology of atherosclerotic plaques: 18F-FDG accumulates in foamy macrophages. Eur J Nucl Med Mol Imaging. 2014;41(4):624–33. doi: 10.1007/s00259-013-2635-0. [DOI] [PubMed] [Google Scholar]
- 55.Zhao QM, Feng TT, Zhao X, et al. Imaging of atherosclerotic aorta of rabbit model by detection of plaque inflammation with fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. Chin Med J (Engl) 2011;124(6):911–7. [PubMed] [Google Scholar]
- 56.Riou LM, Broisat A, Dimastromatteo J, Pons G, Fagret D, Ghezzi C. Preclinical and clinical evaluation of nuclear tracers for the molecular imaging of vulnerable atherosclerosis: an overview. Curr Med Chem. 2009;16(12):1499–511. doi: 10.2174/092986709787909596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogawa M, Nakamura S, Saito Y, Kosugi M, Magata Y. What can be seen by 18F-FDG PET in atherosclerosis imaging? The effect of foam cell formation on 18F-FDG uptake to macrophages in vitro. J Nucl Med. 2012;53(1):55–8. doi: 10.2967/jnumed.111.092866. [DOI] [PubMed] [Google Scholar]
- 58.Hag AM, Pedersen SF, Christoffersen C, et al. (18)F-FDG PET imaging of murine atherosclerosis: association with gene expression of key molecular markers. PLoS One. 2012;7(11):e50908. doi: 10.1371/journal.pone.0050908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wenning C, Kloth C, Kuhlmann MT, et al. Serial F-18-FDG PET/CT distinguishes inflamed from stable plaque phenotypes in shear-stress induced murine atherosclerosis. Atherosclerosis. 2014;234(2):276–82. doi: 10.1016/j.atherosclerosis.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 60.Laurberg JM, Olsen AK, Hansen SB, et al. Imaging of vulnerable atherosclerotic plaques with FDG-microPET: no FDG accumulation. Atherosclerosis. 2007;192(2):275–82. doi: 10.1016/j.atherosclerosis.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 61.Toczek J, Broisat A, Perret P, et al. Periaortic brown adipose tissue as a major determinant of [(1)(8)F]-fluorodeoxyglucose vascular uptake in atherosclerosis-prone, apoE−/− mice. PLoS One. 2014;9(7):e99441. doi: 10.1371/journal.pone.0099441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Millon A, Dickson SD, Klink A, et al. Monitoring plaque inflammation in atherosclerotic rabbits with an iron oxide (P904) and (18)F-FDG using a combined PET/MR scanner. Atherosclerosis. 2013;228(2):339–45. doi: 10.1016/j.atherosclerosis.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao QM, Zhao X, Feng TT, et al. Detection of vulnerable atherosclerotic plaque and prediction of thrombosis events in a rabbit model using 18F-FDG-PET/CT. PLoS One. 2013;8(4):e61140. doi: 10.1371/journal.pone.0061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calcagno C, Cornily JC, Hyafil F, et al. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol. 2008;28(7):1311–7. doi: 10.1161/ATVBAHA.108.166173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orbay H, Hong H, Zhang Y, Cai W. Positron emission tomography imaging of atherosclerosis. Theranostics. 2013;3(11):894–902. doi: 10.7150/thno.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–11. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 67.Kim TN, Kim S, Yang SJ, et al. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging. 2010;3(2):142–8. doi: 10.1161/CIRCIMAGING.109.888909. [DOI] [PubMed] [Google Scholar]
- 68.Tahara N, Kai H, Yamagishi S, et al. Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am Coll Cardiol. 2007;49(14):1533–9. doi: 10.1016/j.jacc.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 69.Graebe M, Pedersen SF, Hojgaard L, Kjaer A, Sillesen H. 18FDG PET and ultrasound echolucency in carotid artery plaques. JACC Cardiovasc Imaging. 2010;3(3):289–95. doi: 10.1016/j.jcmg.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Khalil A, Orellana MR, Fulop T, Turcotte EE, Bentourkia M. Positron emission tomography imaging for vascular inflammation evaluation in elderly subjects with different risk factors for cardiovascular diseases. Am J Nucl Med Mol Imaging. 2014;4(3):283–92. [PMC free article] [PubMed] [Google Scholar]
- 71.Wu YW, Kao HL, Chen MF, et al. Characterization of plaques using 18F-FDG PET/CT in patients with carotid atherosclerosis and correlation with matrix metalloproteinase−1. J Nucl Med. 2007;48(2):227–33. [PubMed] [Google Scholar]
- 72.Noh TS, Moon SH, Cho YS, et al. Relation of carotid artery 18F-FDG uptake to C-reactive protein and Framingham risk score in a large cohort of asymptomatic adults. J Nucl Med. 2013;54(12):2070–6. doi: 10.2967/jnumed.113.119602. [DOI] [PubMed] [Google Scholar]
- 73.Montagnana M, Danese E, Lippi G. Genetic risk factors of atherothrombosis. Pol Arch Med Wewn. 2014;124(9):474–82. doi: 10.20452/pamw.2409. [DOI] [PubMed] [Google Scholar]
- 74.Basu S, Zhuang H, Alavi A. Imaging of lower extremity artery atherosclerosis in diabetic foot: FDG-PET imaging and histopathological correlates. Clin Nucl Med. 2007;32(7):567–8. doi: 10.1097/RLU.0b013e3180646ac0. [DOI] [PubMed] [Google Scholar]
- 75.Mehta NN, Torigian DA, Gelfand JM, Saboury B, Alavi A. Quantification of atherosclerotic plaque activity and vascular inflammation using [18-F] fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) J Vis Exp. 2012;2(63):e3777. doi: 10.3791/3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rogers IS, Nasir K, Figueroa AL, et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging. 2010;3(4):388–97. doi: 10.1016/j.jcmg.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 77.Strobl FF, Rominger A, Wolpers S, et al. Impact of cardiovascular risk factors on vessel wall inflammation and calcified plaque burden differs across vascular beds: a PET-CT study. Int J Cardiovasc Imaging. 2013;29(8):1899–908. doi: 10.1007/s10554-013-0277-8. [DOI] [PubMed] [Google Scholar]
- 78.Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2013;383(9918):705–13. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- 79.Cheng VY, Slomka PJ, Le Meunier L, et al. Coronary arterial 18F-FDG uptake by fusion of PET and coronary CT angiography at sites of percutaneous stenting for acute myocardial infarction and stable coronary artery disease. J Nucl Med. 2012;53(4):575–83. doi: 10.2967/jnumed.111.097550. [DOI] [PubMed] [Google Scholar]
- 80.Varghese A, Crowe LA, Mohiaddin RH, et al. Inter-study reproducibility of 3D volume selective fast spin echo sequence for quantifying carotid artery wall volume in asymptomatic subjects. Atherosclerosis. 2005;183(2):361–6. doi: 10.1016/j.atherosclerosis.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 81.Rudd JH, Myers KS, Bansilal S, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50(9):892–6. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 82.Fox JJ, Strauss HW. One step closer to imaging vulnerable plaque in the coronary arteries. J Nucl Med. 2009;50(4):497–500. doi: 10.2967/jnumed.108.056325. [DOI] [PubMed] [Google Scholar]
- 83.Saam T, Rominger A, Wolpers S, et al. Association of inflammation of the left anterior descending coronary artery with cardiovascular risk factors, plaque burden and pericardial fat volume: a PET/CT study. Eur J Nucl Med Mol Imaging. 2010;37(6):1203–12. doi: 10.1007/s00259-010-1432-2. [DOI] [PubMed] [Google Scholar]
- 84.Williams G, Kolodny GM. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. AJR Am J Roentgenol. 2008;190(2):W151–6. doi: 10.2214/AJR.07.2409. [DOI] [PubMed] [Google Scholar]
- 85.Williams G, Kolodny GM. Retrospective study of coronary uptake of 18F-fluorodeoxyglucose in association with calcification and coronary artery disease: a preliminary study. Nucl Med Commun. 2009;30(4):287–91. doi: 10.1097/MNM.0b013e328328bfc3. [DOI] [PubMed] [Google Scholar]
- 86.Laitinen IE, Luoto P, Någren K, et al. Uptake of 11C-choline in mouse atherosclerotic plaques. J Nucl Med. 2010;51(5):798–802. doi: 10.2967/jnumed.109.071704. [DOI] [PubMed] [Google Scholar]
- 87.Matter CM, Wyss MT, Meier P, et al. 18F-choline images murine atherosclerotic plaques ex vivo. Arterioscler Thromb Vasc Biol. 2006;26(3):584–9. doi: 10.1161/01.ATV.0000200106.34016.18. [DOI] [PubMed] [Google Scholar]
- 88.Bucerius J, Schmaljohann J, Böhm I, et al. Feasibility of 18F-fluoromethylcholine PET/CT for imaging of vessel wall alterations in humans – first results. Eur J Nucl Med Mol Imaging. 2008;35(4):815–20. doi: 10.1007/s00259-007-0685-x. [DOI] [PubMed] [Google Scholar]
- 89.Kato K, Schober O, Ikeda M, et al. Evaluation and comparison of 11C-choline uptake and calcification in aortic and common carotid arterial walls with combined PET/CT. Eur J Nucl Med Mol Imaging. 2009;36(10):1622–8. doi: 10.1007/s00259-009-1152-7. [DOI] [PubMed] [Google Scholar]
- 90.Armani C, Catalani E, Balbarini A, Bagnoli P, Cervia D. Expression, pharmacology, and functional role of somatostatin receptor subtypes 1 and 2 in human macrophages. J Leukoc Biol. 2007;81(3):845–55. doi: 10.1189/jlb.0606417. [DOI] [PubMed] [Google Scholar]
- 91.Dalm VA, van Hagen PM, van Koetsveld PM, et al. Expression of somatostatin, cortistatin, and somatostatin receptors in human monocytes, macrophages, and dendritic cells. Am J Physiol Endocrinol Metab. 2003;285(2):E344–53. doi: 10.1152/ajpendo.00048.2003. [DOI] [PubMed] [Google Scholar]
- 92.Adams RL, Adams IP, Lindow SW, Zhong W, Atkin SL. Somatostatin receptors 2 and 5 are preferentially expressed in proliferating endothelium. Br J Cancer. 2005;92(8):1493–8. doi: 10.1038/sj.bjc.6602503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rominger A, Saam T, Vogl E, et al. In vivo imaging of macrophage activity in the coronary arteries using 68Ga-DOTATATE PET/CT: correlation with coronary calcium burden and risk factors. J Nucl Med. 2010;51(2):193–7. doi: 10.2967/jnumed.109.070672. [DOI] [PubMed] [Google Scholar]
- 94.Li X, Bauer W, Kreissl MC, et al. Specific somatostatin receptor II expression in arterial plaque: (68)Ga-DOTATATE autoradiographic, immunohistochemical and flow cytometric studies in apoE-deficient mice. Atherosclerosis. 2013;230(1):33–9. doi: 10.1016/j.atherosclerosis.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 95.Li X, Samnick S, Lapa C, et al. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: correlation with18F-FDG, calcium burden and risk factors. EJNMMI Res. 2012;2(1):52. doi: 10.1186/2191-219X-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schafers M, Schober O, Hermann S. Matrix-metalloproteinases as imaging targets for inflammatory activity in atherosclerotic plaques. J Nucl Med. 2010;51(5):663–6. doi: 10.2967/jnumed.109.065698. [DOI] [PubMed] [Google Scholar]
- 97.Hermann S, Starsichova A, Waschkau B, et al. Non-FDG imaging of atherosclerosis: will imaging of MMPs assess plaque vulnerability? J Nucl Cardiol. 2012;19(3):609–17. doi: 10.1007/s12350-012-9553-6. [DOI] [PubMed] [Google Scholar]
- 98.Rischpler C, Nekolla SG, Beer AJ. PET/MR imaging of atherosclerosis: initial experience and outlook. Am J Nucl Med Mol Imaging. 2013;3(5):393–6. [PMC free article] [PubMed] [Google Scholar]
- 99.Rogers WJ, Prichard JW, Hu YL, et al. Characterization of signal properties in atherosclerotic plaque components by intravascular MRI. Arterioscler Thromb Vasc Biol. 2000;20(7):1824–30. doi: 10.1161/01.atv.20.7.1824. [DOI] [PubMed] [Google Scholar]
- 100.Pedersen SF, Ludvigsen TP, Johannesen HH, et al. Feasibility of simultaneous PET/MR in diet-induced atherosclerotic minipig: a pilot study for translational imaging. Am J Nucl Med Mol Imaging. 2014;4(5):448–58. [PMC free article] [PubMed] [Google Scholar]
- 101.Ripa RS, Knudsen A, Hag AM, et al. Feasibility of simultaneous PET/MR of the carotid artery: first clinical experience and comparison to PET/CT. Am J Nucl Med Mol Imaging. 2013;3(4):361–71. [PMC free article] [PubMed] [Google Scholar]
- 102.Calcagno C, Ramachandran S, Izquierdo-Garcia D, et al. The complementary roles of dynamic contrast-enhanced MRI and 18F-fluorodeoxyglucose PET/CT for imaging of carotid atherosclerosis. Eur J Nucl Med Mol Imaging. 2013;40(12):1884–93. doi: 10.1007/s00259-013-2518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Truijman MT, Kwee RM, van Hoof RH, et al. Combined 18F-FDG PET-CT and DCE-MRI to assess inflammation and microvascularization in atherosclerotic plaques. Stroke. 2013;44(12):3568–70. doi: 10.1161/STROKEAHA.113.003140. [DOI] [PubMed] [Google Scholar]
- 104.Adenaw N, Salerno M. PET/MRI: current state of the art and future potential for cardiovascular applications. J Nucl Cardiol. 2013;20(6):976–89. doi: 10.1007/s12350-013-9780-5. [DOI] [PubMed] [Google Scholar]
- 105.Petibon Y, El Fakhri G, Nezafat R, Johnson N, Brady T, Ouyang J. Towards coronary plaque imaging using simultaneous PET-MR: a simulation study. Phys Med Biol. 2014;59(5):1203–22. doi: 10.1088/0031-9155/59/5/1203. [DOI] [PMC free article] [PubMed] [Google Scholar]