The incidence of thyroid cancer is higher in pediatric thyroid nodules, requiring surgical intervention to a proportionately greater extent than in adults. The objective of this review is to summarize new concepts in clinical disease management of nodular thyroid disease in the pediatric population, including patient history, medical examination, and diagnosis workup.
Keywords: Pediatrics, Thyroid, Nodules, Oncology, Therapeutics, Cancer
Abstract
Thyroid nodules are commonly diagnosed in adults. Although rare in children, the risk for thyroid cancer is much higher in the pediatric population compared with adults. Presenting as either a solitary nodule or a multinodular goiter, thyroid nodular disease in children requires a thorough workup that includes a detailed clinical examination comprised of prior history of thyroid disease in the patient or in their family, history of radiation exposure, careful palpation of the thyroid and lymph nodes, blood tests, ultrasonography, and cytological assessment. Thyroid surgery is the gold-standard treatment for pediatric thyroid nodules; nonetheless, the extent of surgery remains controversial. Because surgery is not without risk, the decision matrix necessitates focus on the benefits of surgery for the child contingent upon all the preoperative exams. New diagnostic technology such as molecular testing with fine needle aspiration biopsy may help distinguish between benign and malignant lesions while potentially decreasing surgery for benign disease. The objective of this review is to summarize new concepts in clinical disease management of nodular thyroid disease in the pediatric population, including patient history, medical examination, and diagnosis workup.
Implications for Practice:
The incidence of thyroid cancer is higher in pediatric thyroid nodules, requiring surgical intervention to a proportionately greater extent than in adults. This review discusses contemporary diagnostic and management parameters for the pediatric thyroid nodule, which aims at improving diagnosis between the benign and malignant nodule to better direct surgical intervention.
Introduction
Thyroid nodules are commonly diagnosed in adults, although they are rare in pediatrics (<18 years of age), affecting only 1%–2% of this population based on clinical examination [1–4]. Risk factors for developing thyroid nodules in children include head and neck irradiation, female gender, iodine deficiency, age of puberty, and family or personal history of thyroid disease [5, 6]. Several nonthyroid diseases such as abscesses, lymphatic or vascular malformations, ectopic thymus, thyroglossal duct cysts, and tumors can mimic thyroid nodules in children. Hashimoto’s thyroiditis creating palpable changes in the gland can also evoke a nodule [7]. Thyroid nodular disease can include different entities: a solitary nodule, multinodular goiter, nodular goiter observed in autoimmune goiter (chronic lymphocytic thyroiditis presenting as Hashimoto thyroiditis or Graves’ disease), and nonpalpable thyroid nodules [8]. The most challenging dilemma in this population is the differentiation of the malignant from benign lesion [1]. The incidence of thyroid cancer is higher in children presenting with thyroid nodules compared with adults, as determined from a surgical cohort [1, 9–11]. In children, thyroid nodules have been reported to be malignant between 9.2% and 50% [12, 13] of the time, with a mean of 26.4%, whereas in adults, the incidence of thyroid nodule malignancy has been reported to be between 5% and 15%. It has been debated whether isolated and multiple thyroid nodules have the same risk of malignancy. The solitary nodule does not seem significantly more likely to be malignant [10], and although cancer can be present in multinodular thyroid disease, the majority of malignant nodules are found in a uninodular glands [3, 9, 14–16]. In fact, thyroid cancer is the most common pediatric endocrine cancer [6], constituting 0.5%–3% of all childhood malignancies [17]. Follicular adenoma is the most frequent nonmalignant diagnosis in children with thyroid nodules [11, 18]. Thyroid carcinomas in childhood are almost always well differentiated [15], with a distribution of malignancy similar to that seen in adults [19, 20]. Papillary thyroid carcinoma (PTC) is reported to be the most frequent malignancy among children with thyroid nodules (5%–47%) [10, 21–24], and overall, up to 72% of the thyroid cancers in children are of the PTC type [25].
Clinical Assessment
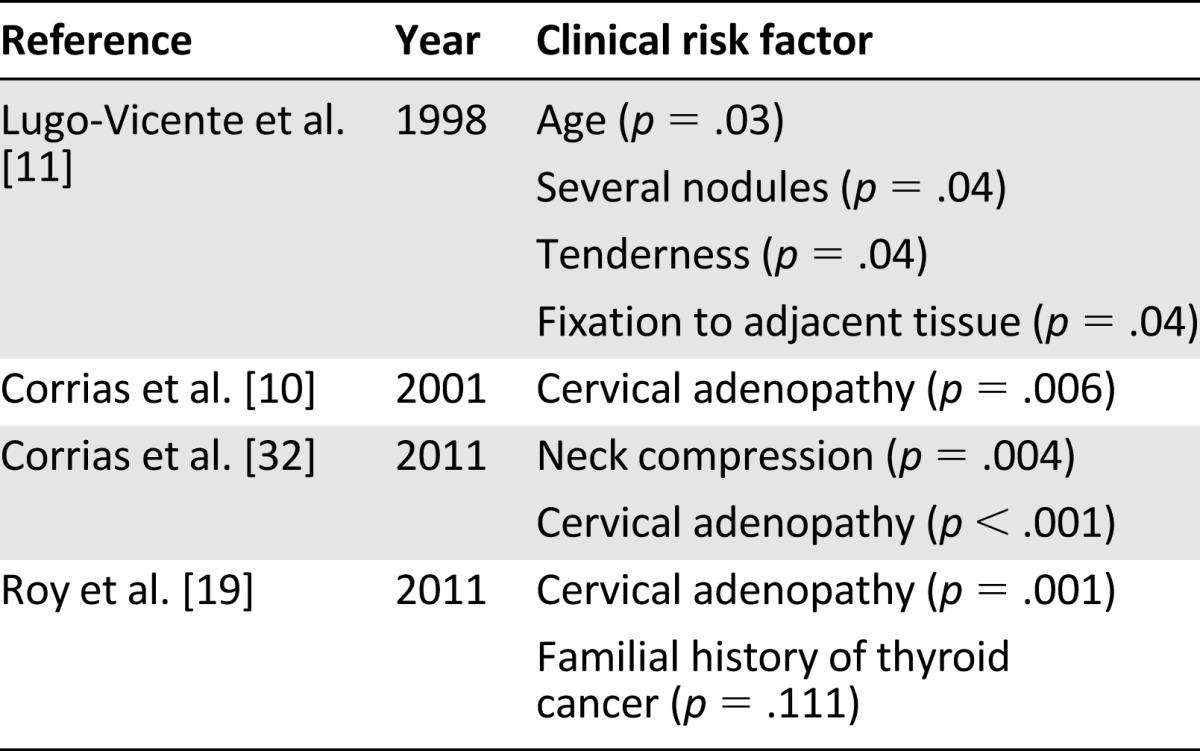
A thyroid nodule can be discovered by a physician during a routine physical examination, discovered by patients themselves, or observed incidentally during imaging of the neck. However, the majority (75%) of pediatric patients with both benign and malignant nodules have asymptomatic neck masses [23]. Clinical symptoms of hyperthyroidism can include tachycardia, increased differential pressure, weight loss with increased appetite, heat intolerance and increased sweating, diarrhea, and exophthalmia [26]. Contrasting clinical symptoms of hypothyroidism include bradycardia, decreased differential pressure, decreased appetite with weight gain, hyperhidrosis, constipation, and tremors [26]. The average duration of clinical symptoms before the diagnosis of thyroid nodule is made is between 5 and 6 months for children presenting with benign and malignant lesions, respectively [27]. Although not corroborated [28], pediatric thyroid nodular disease has been reported to be more frequent in females, with a female/male ratio of 3:1 or more [15, 17, 29]. Scholz et al. [29] reports that this ratio markedly decreases to 1.25/1 in children younger than 10 years with the incidence of nodules and thyroid cancer peaking in adolescence (12–16 years) with a mean and median of 12 and 13 years of age. In the work of Yip et al. [14], male children younger than 12 years (prepubertal) were more likely to have malignancy. Scholz et al. [29] also reports a higher incidence of malignancy observed in younger children with thyroid nodules (48% in children less than 10 years) compared with older children (33% in children older than 10 years). Conversely, it has been reported that older patients are more at risk for cancer with the mean age of patients with malignant lesions being 16.4 versus 14.5 years [14]. It has been suspected that increase sex hormone levels during puberty may contribute to thyroid carcinogenesis [6], because it is also at puberty that the well documented female sex predominance in thyroid disease is first noted [30, 31]. Lack of corroboration in reported gender differences may be related to age of children included, inclusion or exclusion related to pathologic diagnosis, and whether investigations were retrospective versus prospective in nature. Other clinical characteristics that suggest a thyroid malignancy are presented in Table 1.
Table 1.
Clinical risk factors for malignancy in children
Risk Factors
Prior History of Thyroid Disease
Thyroid nodules are often associated with other thyroid diseases [8] implying a causative relationship. Corrias et al. [33] report thyroid nodules in 31.5% of patients with juvenile autoimmune thyroiditis, and thyroid cancer was present in 9.6% of these nodules with the primary histologic diagnosis being papillary carcinoma. Niedziela et al. [8], reported that a nodule in Hashimoto’s thyroiditis may progress to carcinoma, especially PTC, or it can be associated with a benign neoplasm. Other previous or coexisting thyroid disorders may be a risk factor for thyroid nodule or carcinoma [34]. Congenital hypothyroidism caused by dyshormonogenesis or by an iodine transporter defect increases the risk of nodules in children [35] that can evolve to thyroid cancer of the follicular type [34–37].
Exposure to Radiation
An association between radiation exposure and the development of thyroid cancer was first reported in a 1950 study in which 36% of the children with thyroid malignancy had previous radiation treatment or exposure [30]. In a study of 878 children with thyroid malignancy, 66% had previous irradiation to the head and neck with an average latency time between exposure and diagnosis of thyroid carcinoma of 8.5 years [38]. More recently, investigators have reported decreases in the percentage of malignant thyroid nodules caused by an increase in the awareness of childhood irradiation [3, 6, 39]. These decreases reflect the fact that the dangers of irradiation are now well recognized [40], in addition to decreased doses used in irradiation that are more focused with protective precautions that are commonly used. Because the thyroid gland is one of the most frequent sites of secondary neoplasms in children who receive radiation therapy for other malignancies [25], medical history should include questions about external irradiation to the head, neck, and chest.
Further support for the relationship between radiation exposure in children and thyroid disease comes from studying children exposed to radioactive iodine. In 1986, the Chernobyl accident exposed children in the Ukraine, Belarus, and neighboring regions to high levels of radiation. An increase in thyroid cancer among exposed children from Belarus was detected as early as 4 years after the accident [41]. In the Ukraine, the annual incidence of thyroid cancer in children aged 0–14 has increased since 1989, with the rate in 1993 being five times higher than in 1986 [42]. Interestingly, children below the age of 2 years appear to be the most at risk of radiation-induced PTC [43] because the youngest children at the time of the Chernobyl disaster had the highest incidence of cancer [8, 44]. It should be noted that the extent of the radiation exposure in these historical cases widely surpass doses given to children for medical treatment for Graves’ disease or most thyroid cancers.
Genetic Disease
Approximately 41% of children with thyroid nodules have a family history of thyroid disorders [6]. Roughly 5% of all thyroid cancers with a follicular cell origin in the pediatric population have a familial association, whereas that number increases to nearly 25% for parafollicular cell origin cancers.
Medullary thyroid carcinoma (MTC) comprises 5% of childhood thyroid carcinomas, and children that are symptomatic typically present with thyroid nodules. Approximately 20% of patients with MTC have familial cancer associated with a germline RET (ret proto-oncogene) mutation. Familial MTC may occur in isolation or as a part of multiple endocrine neoplasia (MEN) type II syndrome. MEN IIa (MEN II in the new classification) associates medullary thyroid carcinoma and bilateral pheochromocytoma in up to 50% of cases and hyperparathyroidism (parathyroid hyperplasia or adenoma) in 20%–35% of cases. MEN IIb (MEN III in the new classification) associates MTC with pheochromocytoma in 50% of cases, with marfanoid habitus and mucosal and digestive neurofibromatosis [45]. MTC in MEN IIb presents at younger ages than in MEN IIa and in isolated FMTC and is more aggressive than MTC associated with these two latter designates. In patients with a family history or suspicion of MEN, RET oncogene mutations must be evaluated for a definitive diagnosis.
Pediatric thyroid nodules can reveal a familial thyroid cancer secondary to follicular cells either as an isolated entity or as part of other family cancer syndromes. Isolated familial thyroid cancers from follicular cells accounts for 5% of all thyroid cancers of follicular cell origin and are usually more aggressive than its sporadic form in terms of increased risk of recurrence and has a younger age of onset [46]. The main types are familial papillary and Hurthle cell thyroid cancers. Familial nonmedullary thyroid cancer may include Carney’s complex (PPKAR1A gene mutation), Cowden’s syndrome (PTEN gene mutation) and familial adenomatous polyposis (FAP) (APC gene mutation). Carney’s complex is a multiple neoplasia and lentiginosis syndrome that affects endocrine glands, including the pituitary, adrenals, and testes. Carney’s complex includes a spectrum of thyroid abnormalities ranging from follicular hyperplasia or cystic changes to carcinoma [47]. Thyroid nodules are present in 67% of children presenting with Carney’s complex, and thyroid carcinoma is observed in 3.8% of patients. Cowden’s syndrome, also known as multiple hamartoma syndrome, is a cancer-associated dermatosis. It is characterized by hamartomas of the gastrointestinal tract and cancer of the breast, endometrium, brain, and thyroid [48]. Up to 85% of these patients have multinodular goiters [46], and frequently associated benign tumors harbor a risk of progression to carcinoma [49]. Most of the thyroid cancers are of the papillary or Hurthle cell types. FAP is an inherited familial cancer characterized by an increased predisposition to colorectal cancer and other benign and malignant extracolonic neoplasia. Of patients with FAP, 1%–2% also have thyroid carcinoma.
Physical Examination
Characteristics of a thyroid nodule that need to be assessed include its size, its consistency, whether it induces tenderness or pain, changes over time, growth, and possible fixation to surrounding structures. Cervical lymph nodes should be examined for possible metastasis. PTC mainly metastasizes to regional lymph nodes and lungs, whereas follicular thyroid carcinoma metastasizes hematologically to the lungs and liver. For MTC, the lymphatic route to cervical lymph nodes is the most common [8]. Thyroid cancer is associated with locoregional lymphadenopathy in 27%–83% of cases [50–52]. Compressive symptoms such as dysphagia, hoarseness, discomfort, or shortness of breath should systematically be sought [53] with children presenting with thyroid nodules.
Diagnostic Workup
As in adults, for pediatric thyroid disease, a thorough clinical evaluation is augmented with diagnostic assessments that can include thyroid function tests, scintigraphy, ultrasonography, and fine needle aspiration biopsy (FNAB) with and without genetic testing.
Thyroid Function Tests
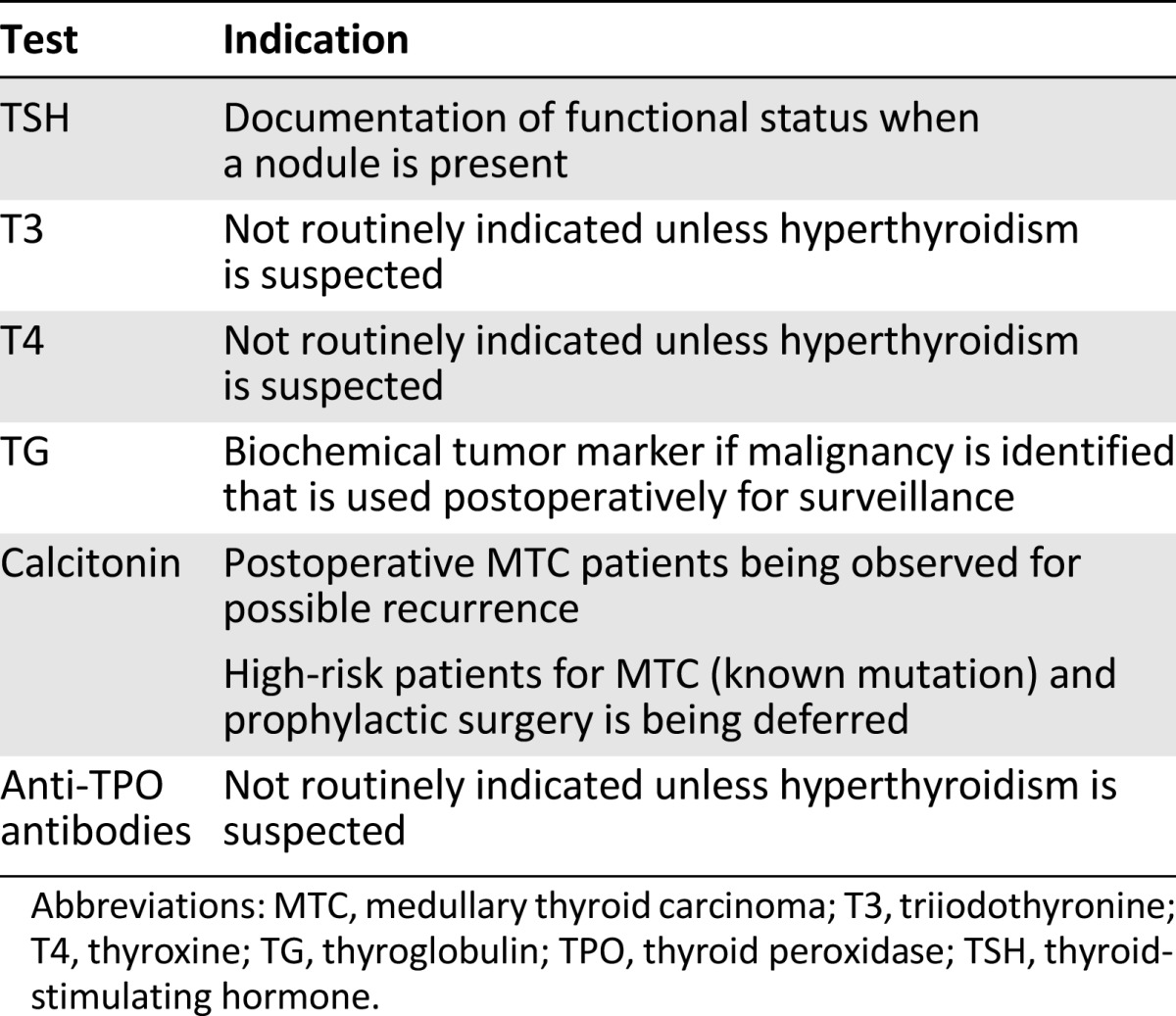
Table 2 describes thyroid function tests and indications for the pediatric population.
Table 2.
Thyroid function tests and indications in pediatrics
Ultrasonography
Radiologic evaluation of thyroid nodules plays an integral part of the diagnosis algorithm. Being noninvasive and inexpensive, ultrasonography (US) is recommended as one of the first diagnostic tests in all children with thyroid nodules. It can easily differentiate between a solid or cystic lesion, a solid nodule being more likely susceptible to malignancy, although most solid lesions are benign, and the presence of a cystic lesion does not exclude malignancy. In adults, a number of other US characteristics are associated with a higher risk of malignancy: solitary solid lesion, multifocal lesions within an otherwise clinically solitary nodule, nodule with hypoechogenic echostructure, subcapsular localization, increased intranodular vascularity (high intranodular flow by Doppler), irregular infiltrative margins, microcalcifications, and suspicious regional lymph nodes accompanying nodule [54–56]. Although not yet corroborated in children, adult criteria are used by practitioners to evaluate pediatric nodules [19, 56, 57].
Scintigraphy
In the 1990s, US was commonly used in Europe to explore thyroid nodules [58], and scintigraphy was more commonly used in North America [59]. Scintigraphy differentiates between functioning and nonfunctioning nodules. For a long period of time, it was considered the first examination to perform on a thyroid nodule with its goal to classify nodules as either “cold” or “hot” depending on their iodine-trapping function. However, this standard is not very effective for differentiating a benign from a malignant nodule in the pediatric population [10, 21, 58]. Historically, cold nodules were often thought to carry a higher malignancy risk. Panneerselvan et al. [60] report that radioactive iodine (RAI) scan findings were concordant with malignancy only 17% of the time. A hot nodule may represent a follicular adenoma, an adenomatous goiter hyperplasia, a chronic lymphocytic thyroiditis, a colloid goiter, or, rarely, a carcinoma. Niedziela et al. [37] differentiates between classic hot nodules (with radionucleide uptake only in the area corresponding to the nodule) and nonclassic hot nodule (with radionucleide uptake in the area corresponding to the nodule and in the extranodular area). Although cold nodules are often benign, the incidence of malignancy ranges from 20% to 60% [61]. Finally, scintigraphy is currently performed in thyrotoxic patients with thyroid nodules [26] and for detecting ectopic thyroid tissue in children [62], and therefore it is not routinely used.
Fine Needle Aspiration Biopsy
The role of FNAB in the evaluation of thyroid nodules is not as well established in children as compared with adults [63]. Many studies have focused on FNAB cytology as a diagnostic test to determine whether a thyroid nodule should be removed or observed in children [24]. Hamberger et al. [64] reported that FNAB decreased the number of patients with thyroid nodules undergoing surgery from 67% to 43%, with a concomitant increase of 14%–29% of patients who had malignancy. Moreover, the use of this exam resulted in a $1,300 cost reduction per adult patient [65], decreasing the overall cost of care by 25% [66]. Although not investigated in children, these values are expected to be similar in the pediatric population [67]. More recently, Bargren et al. [68] report that FNAB should be used in pediatric patients when possible because it can be the most accurate exam to determine the diagnosis of a nodule.
Indications for completion of FNAB in children is similar to adults [9]. Suspicious lesions should undergo FNAB. The examination may be more challenging, and increased cooperation is required because children may have more discomfort and fear during the procedure than adults [69]. It is not uncommon for a child to require sedation compared with adults. Arguments against the routine use of FNAB in children include the anatomical/physical limitation of the patient, the need for an experienced cytopathologist [11], and side effects such as papillary endothelial hyperplasia, hemorrhage, vascular proliferation, vascular thrombosis, fibrosis, cystic change, infarction, and abscess [1, 70–72]. Not all of these aforementioned arguments are a concern in the adult population.
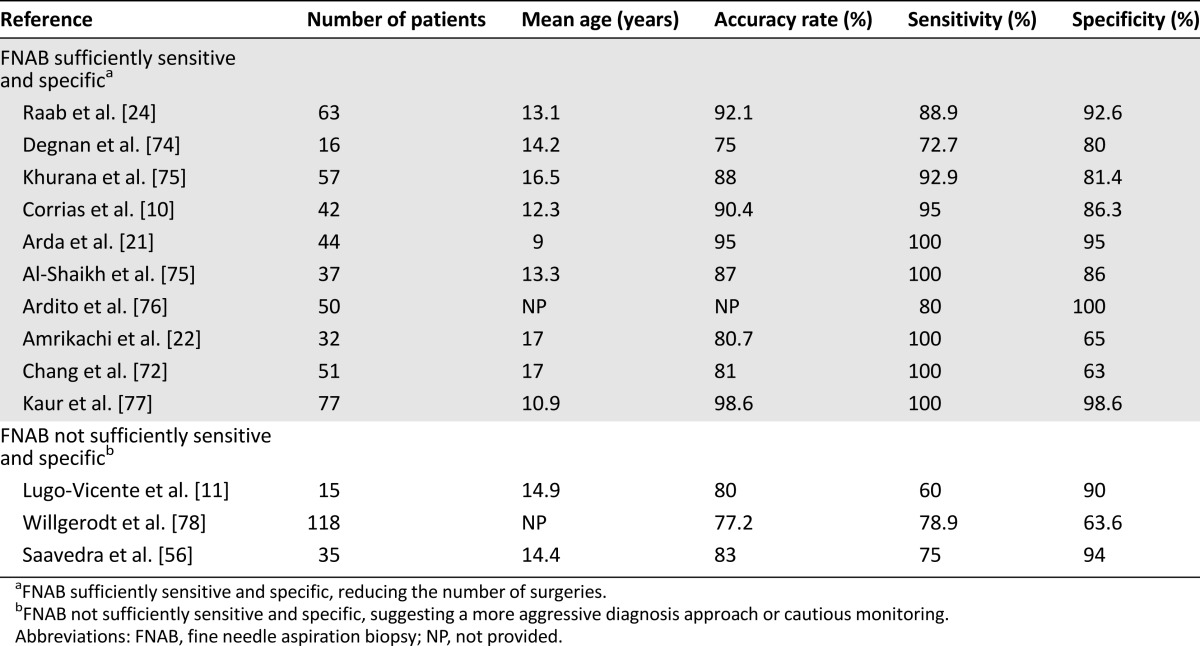
Many studies have found that FNAB is a highly sensitive and accurate test in differentiating benign lesions from malignant nodules [10, 21, 22, 67, 73]. Conversely, others report that this exam is not accurate enough because of its poor sensitivity and underline the need for more radical procedures for definitive diagnosis [11, 24]. The accuracy rate of FNAB has been reported to be between 77.2% and 98.6%. The sensitivity of this exam in different studies has ranged from 60% to 100%; specificity has ranged from 63% to 100%. Results of studies measuring the diagnostic value of FNAB are shown in Table 3 and are divided into two categories depending on the management: studies suggesting that FNAB is sufficiently sensitive and specific to reduce the number of surgeries and studies concluding that FNAB is not sufficiently sensitive and specific, suggesting a more aggressive diagnosis approach or cautious monitoring. FNAB appears to have the same specificity and sensitivity in adults and in children [53]. However, others have suggested that FNAB is not sensitive and specific enough for children and that FNAB assists in determining whether a more aggressive gland removal is needed for the suspicious or malignant cases.
Table 3.
FNAB sensitivity and specificity in the literature
There is a number of categorizing systems for FNAB outcomes. The first typically categorizes outcomes into four designates (a) malignant (carcinoma identified); (b) suspicious for malignancy including follicular neoplasm (hyperplastic nodules, follicular adenoma, follicular carcinoma, and follicular variant of papillary carcinoma) and Hurthle cell neoplasm; (c) benign (adenomatous /colloid nodule, macrophages, and aggregates of normal-appearing uniform thyroid cell, and Hashimoto’s thyroiditis); and (d) unsatisfactory (cyst fluid or few cells identified). A second outcomes scale that is used is the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). This scale was developed for the adult population but recently Smith et al. classified cytological findings in children [80]. This classification includes six categories: (a) nondiagnostic/unsatisfactory, (b) benign, (c) atypia of undetermined significance/follicular lesion of undetermined significance, (d) suspicious for follicular neoplasm, (e) suspicious for malignancy, and (f) malignant.
FNAB is not without limitations, and the use of US as a complement has been suggested to improve diagnosis accuracy. When FNAB suggests benign malignancy, Saavedra et al. [56] recommend the use of thyroid US on follow-up, as a complement to improve diagnosis. The literature may underestimate the potential of FNAB because the majority of studies presenting sensitivity and specificity results do not use US guidance. According to Izquierdo et al. [81], US-guided FNAB in the adult population improved the cytologic diagnostic accuracy, sensitivity, and positive predictive value and reduced the false-negative rate in comparison with palpation-guided FNAB. Gupta et al. [7] recommend that because of the proximity of the esophagus, trachea, and great vessels, US guidance for FNAB should be mandatory in children.
New Diagnostic Methods: Molecular Genetic Studies With FNAB
There are times when the cytological result is indeterminate. Of those patients who undergo a diagnostic lobectomy, only 8%–17% of them have a malignant lesion on histologic analysis. When a malignant diagnosis is confirmed postoperatively, the patient must undergo an additional operation, and the risk of complications such as recurrent laryngeal nerve injury increases. Molecular mutation analysis of several genes involved in the proliferation and progression of cellular cycle on FNAB specimens can serve as an adjunct in this population and are routinely used in clinical practice. Based on the adult population, a molecular mutation assay improves the sensitivity and positive predictive values for malignancy from FNAB specimens [82, 83].
The main genetic alterations associated with PTC are BRAF (B-Raf proto-oncogene) mutation and RET/PTC rearrangements, especially in childhood [84, 85]. BRAF mutations are never found in follicular carcinoma or in benign lesions and appears to be a specific marker for papillary carcinoma [85]. This mutation has a low prevalence in children compared with adults [86]. RET/PTC rearrangements are observed in approximately 20% of sporadic papillary cancer in adults [87], but its frequency is higher in the pediatric population (40%–70%) [88]. A correlation between a gene mutation and tumor aggressiveness has been found by some investigators but is currently debated [89–91]. Monaco et al. [92] found that RET/PTC mutation rearrangements in pediatrics are associated with a favorable prognosis. RAS mutations and PAX8/PPARγ translocation are commonly found in thyroid malignancy but are not specific to a form of cancer. In recent pediatric studies, the presence of a genetic mutation for RAS, BRAF, RET/PTC, PAX8/PPARγ found on indeterminate cytology was correlated with malignancy in all cases [92, 93], elevating the positive predictive value of FNAB exam to nearly 100% and the overall sensitivity and specificity to 80% and 100% [60]. Preoperative molecular studies appear to be an efficient diagnostic tool in children with thyroid nodules helping to guide surgical management [93]. Follow-up may be more or less aggressive depending upon whether mutation information is known [82, 91]. Nevertheless, not all thyroid cancers have a detectable mutation, and a negative mutation should not delay a surgical procedure if other indications are present.
Treatment
Surgery
Different surgical options are used for the management of thyroid diseases including lobectomy, near total thyroidectomy, and total thyroidectomy. Currently in favor is the division of thyroid surgeries into two broad types: lobectomy or total thyroidectomy. In all cases when surgical management is recommended, diagnosis, surgical options, and life-long implications of such management warrant discussion between the clinician and the child’s guardians as a measure of full informed consent.
Management of thyroid nodules initially depends on clinical presentation and the results of diagnostic testing. If a patient has benign cytology without suspicious nodules and has no risk factors for cancer, surgical resection can be avoided [10, 21, 24, 74, 77]. If the nodule increases in size during the follow-up or exceeds 4 cm in size, re-evaluation with FNAB or surgical removal is indicated [26]. A hot nodule usually corresponding to a toxic adenoma can be responsible for symptomatic hyperthyroidism. If it is isolated to the gland, thyroid lobectomy is recommended [23, 27, 94–96]. Although cold nodules may be associated with an increased risk of malignancy, the decision for surgery should be guided by the patient’s overall clinical picture and FNAB.
If a patient has benign cytology without suspicious nodules and has no risk factors for cancer, surgical resection can be avoided. If the nodule increases in size during the follow-up or exceeds 4 cm in size, re-evaluation with FNAB or surgical removal is indicated.
In patients with a suspicious cytological result on FNAB, the goal of surgical intervention is the provision of diagnostic histology [97]. For those children with a unilateral nodule suspicious for follicular neoplasm, a lobectomy is recommended as the initial procedure, followed by total thyroidectomy if the diagnosis of cancer is confirmed by operative histology [7]. In cases of bilateral nodularity, when cancer is clinically suspected and cytologically confirmed, a total thyroidectomy is recommended [98]. Management for well differentiated thyroid cancer (DTC) is primarily total thyroidectomy. However, the extent of surgery for DTC is in some circumstances controversial. Total thyroidectomy allows for a greater ability and increases efficiency for postoperative radioiodine scanning and subsequent ablation by minimizing residual healthy thyroid tissue. Further, total thyroidectomy provides a more accurate determination of serum thyroglobulin level after surgery, which is an important adjunct in identifying recurrent disease [46]. These benefits are especially important in young children who have much higher rates of thyroid cancer recurrence and metastases [47]. Recurrence risks are significantly greater in children treated with lobectomy when compared with children treated with total thyroidectomy [99].
DTC in children is often associated with early cervical lymph node involvement, which is seen in 27%–83% of pediatric cases compared with 20%–50% in adults. The extent of lymph node removal is still debated, and few data are available in children. For patients presenting with a positive central cervical lymph node during a preoperative US evaluation [100], a lymph node resection is more effective at preventing recurrent disease than by cherry picking. Dinauer et al. [101] confirmed this consensus, advocating for children with DTC and positive lymph node disease a total thyroidectomy with uni- and bilateral central compartment lymph node dissection (level VI). This compartment is defined superiorly by the hyoid bone, inferiorly by the substernal notch, laterally by the median portion of the carotid sheath, and dorsally by the prevertebral fascia. Survival rates are similar to those of patients with node negative disease. In a recent report, because of the high prevalence of local lymph node involvement in children with DTC, even if no lymph node metastasis is evident during a preoperative evaluation, prophylactic dissection of the central compartment lymph nodes located on the ipsilateral side of the tumor is recommended secondary to concerns of the potential presence of follicular thyroid cancer [101, 102]. This differs from management in adults in whom follicular thyroid cancer should not be treated with any kind of prophylactic lymph node dissection [9]. Nevertheless, further study is required to confirm this management in pediatrics.
Lymph nodes in the lateral neck can be involved with thyroid cancer in children. Recommendations for management of children are based upon research in adults. Lateral neck dissection must only be performed in patients with evident metastases (clinically or on preoperative US or FNAB) [9, 103], including those with thyroid cancer T1 or more and microcarcinoma (<1 cm). Although this carcinoma is rare in children, the most recent investigation has demonstrated that with the low incidence of recurrence after surgery, there is a high incidence of central or lateral lymph node metastasis [103].
Children with MTC, whatever the size of the nodule, should have a total thyroidectomy and prophylactic bilateral central neck dissection [46, 104]. Indeed, 80% of these carcinomas metastasize to the central ipsilateral cervical lymph nodes, and 40% metastasize to contralateral lymph nodes [105]. If a clinically or radiographically lateral lymphadenopathy is observed with positive FNAB, a lateral compartment lymph node dissection must be performed [106].
Thyroid surgery including lobectomy and total thyroidectomy can have complications. Although prevalence is rare, surgery can be associated with hypoparathyroidism, recurrent laryngeal nerve damage, or bleeding. The risk of complications is higher in younger children, especially in those younger than 4 years old [25], secondary to their smaller anatomy. Risks can be decreased in the hands of surgeons with considerable experience, as defined by those who have performed more than 100 thyroidectomies in 6 years; a surgeon’s experience and operative volumes have been found to be major determinants of morbidity in thyroid surgery [107].
Radioactive Iodine Ablation
In children presenting with DTC, radioiodine ablation is routinely performed 4–6 weeks after total thyroidectomy and lymph node dissection, for remnant ablation or residual disease [101]. Rates of disease recurrence are generally reported to be lower in patients who have received ablation than those who have not [101, 102]. There is a paucity of prospective pediatric radioactive ablation iodine investigations for DTC in pediatrics. In a retrospective investigation, treating children with RAI after surgery compared with treating with only surgery, total thyroidectomy, and radioiodine treatment independently significantly decreased the risk of thyroid bed recurrence. Further the lack of RAI therapy postoperatively increased the risk of recurrence in the thyroid bed 11-fold and increased lymph-node recurrence by a factor of 3.2. Even without supportive prospective data, Rivkees et al. [102] support remnant ablation in children with DTC because children are more prone to recurrent disease. Because these children may have thyroiditis and TgAb, there is little risk associated with remnant ablation in children. There are few low-risk pediatric patients, and knowing whether disease is not present can avoid thyroid-stimulating hormone (TSH)-suppressive regimens. Medical compliance with adolescents and young adults is sporadic, and TSH-suppressive therapy cannot be relied on to prevent DTC recurrence. Jarząb et al. [108] note an exception such that children with no or low (<0.4%) thyroid remnant radioiodine uptake and undetectable or low stimulated thyroglobulin values post-thyroidectomy should not receive radioiodine ablation. There is no agreement in the literature regarding RAI dosing for children; rather, ranges have been provided. Per Rivkees et al. [102], for physically mature children RAI should range from 100 to 200 mCi (3.7–7.4 GBq) and may be corrected for body weight ranging from 1.35 to 2.7 mCi/kg (50–100 MBq/kg) in younger children. Significant research is needed to determine minimal effective dosages applicable to children.
In a retrospective investigation, treating children with RAI after surgery compared with treating with only surgery, total thyroidectomy, and radioiodine treatment independently significantly decreased the risk of thyroid bed recurrence.
Thyroid Hormone Suppression
Levothyroxine (L-T4) suppressive therapy remains a therapeutic approach for benign solid nodules. Only a few studies have been completed in children [109]. Niedziela et al. [8] found that a palpable thyroid nodule should not be treated with L-T4 suppressive therapy because of side effects induced by this treatment such as risk of hyperthyroidism, bone loss, and adverse cardiac effects. Newly created American Thyroid Association (ATA) guidelines provide no recommendation for or against the use suppressive therapy for benign nodules. However, suppression in children with thyroid cancer should be determined by the ATA pediatric risk level and disease status. ATA guidelines suggest that in children with persistent disease, suppression should be maintained when there is no evidence of disease with TSH levels normalized to a low normal range [110].
Conclusion
Although most pediatric thyroid nodules are benign, the initial workup is crucial in determining benign from malignant lesions. Several clinical predictive factors of malignancy have been reported in the literature, and among them palpable cervical lymphadenopathy appears as the strongest predictor of thyroid cancer in children. Though many tools are available and used, the initial workup includes a blood test assessing thyroid function, US, and FNAB. At present, FNAB remains the most accurate exam to make a diagnosis of malignancy and to suggest a surgical approach. Because of an increased risk of cancer in the pediatric population compared with the adult population, surgery is the most effective therapy even if it is associated with more complications in children. Diagnostic and therapeutic procedures for pediatrics are currently debated with controversy regarding management of this disease. Further research including multicenter studies is necessary to attain universal consensus regarding the diagnosis and management of thyroid nodules in the pediatric population.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgment
This work was funded by NIH National Institute on Deafness and Other Communication Disorders Grant R01-4336.
Author Contributions
Conception/Design: Jeremy T. Guille, Susan L. Thibeault, Herbert Chen
Collection and/or assembly of data: Jeremy T. Guille
Data analysis and interpretation: Jeremy T. Guille, Adwoa Opoku-Boateng
Manuscript writing: Jeremy T. Guille, Adwoa Opoku-Boateng, Susan L. Thibeault, Herbert Chen
Final approval of manuscript: Jeremy T. Guille, Adwoa Opoku-Boateng, Susan L. Thibeault, Herbert Chen
Disclosures
The authors indicated no financial relationships.
References
- 1.Hung W. Solitary thyroid nodules in 93 children and adolescents: A 35-years experience. Horm Res. 1999;52:15–18. doi: 10.1159/000023426. [DOI] [PubMed] [Google Scholar]
- 2.Telander RL, Zimmerman D, Kaufman BH, et al. Pediatric endocrine surgery. Surg Clin North Am. 1985;65:1551–1587. doi: 10.1016/s0039-6109(16)43786-2. [DOI] [PubMed] [Google Scholar]
- 3.Hung W. Nodular thyroid disease and thyroid carcinoma. Pediatr Ann. 1992;21:50–57. doi: 10.3928/0090-4481-19920101-09. [DOI] [PubMed] [Google Scholar]
- 4.Rallison ML, Dobyns BM, Keating FR, Jr, et al. Thyroid nodularity in children. JAMA. 1975;233:1069–1072. [PubMed] [Google Scholar]
- 5.Josefson J, Zimmerman D. Thyroid nodules and cancers in children. Pediatr Endocrinol Rev. 2008;6:14–23. [PubMed] [Google Scholar]
- 6.Fowler CL, Pokorny WJ, Harberg FJ. Thyroid nodules in children: Current profile of a changing disease. South Med J. 1989;82:1472–1478. doi: 10.1097/00007611-198912000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, Ly S, Castroneves LA, et al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J Clin Endocrinol Metab. 2013;98:3238–3245. doi: 10.1210/jc.2013-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niedziela M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Relat Cancer. 2006;13:427–453. doi: 10.1677/erc.1.00882. [DOI] [PubMed] [Google Scholar]
- 9.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 10.Corrias A, Einaudi S, Chiorboli E, et al. Accuracy of fine needle aspiration biopsy of thyroid nodules in detecting malignancy in childhood: Comparison with conventional clinical, laboratory, and imaging approaches. J Clin Endocrinol Metab. 2001;86:4644–4648. doi: 10.1210/jcem.86.10.7950. [DOI] [PubMed] [Google Scholar]
- 11.Lugo-Vicente H, Ortíz VN, Irizarry H, et al. Pediatric thyroid nodules: Management in the era of fine needle aspiration. J Pediatr Surg. 1998;33:1302–1305. doi: 10.1016/s0022-3468(98)90174-9. [DOI] [PubMed] [Google Scholar]
- 12.Belfiore A, Giuffrida D, La Rosa GL, et al. High frequency of cancer in cold thyroid nodules occurring at young age. Acta Endocrinol (Copenh) 1989;121:197–202. doi: 10.1530/acta.0.1210197. [DOI] [PubMed] [Google Scholar]
- 13.Hayles AB, Kennedy RL, Beahrs OH et al. Management of the child with thyroidal carcinoma. JAMA. 1960;173:21–28. doi: 10.1001/jama.1960.03020190023005. [DOI] [PubMed] [Google Scholar]
- 14.Yip FWK, Reeve TS, Poole AG, et al. Thyroid nodules in childhood and adolescence. Aust N Z J Surg. 1994;64:676–678. doi: 10.1111/j.1445-2197.1994.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 15.Corrias A, Mussa A, Baronio F, et al. Diagnostic features of thyroid nodules in pediatrics. Arch Pediatr Adolesc Med. 2010;164:714–719. doi: 10.1001/archpediatrics.2010.114. [DOI] [PubMed] [Google Scholar]
- 16.Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: Predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 17.Millman B, Pellitteri PK. Thyroid carcinoma in children and adolescents. Arch Otolaryngol Head Neck Surg. 1995;121:1261–1264. doi: 10.1001/archotol.1995.01890110037007. [DOI] [PubMed] [Google Scholar]
- 18.Festen C, Otten BJ, van de Kaa CA. Follicular adenoma of the thyroid gland in children. Eur J Pediatr Surg. 1995;5:262–264. doi: 10.1055/s-2008-1066220. [DOI] [PubMed] [Google Scholar]
- 19.Roy R, Kouniavsky G, Schneider E, et al. Predictive factors of malignancy in pediatric thyroid nodules. Surgery. 2011;150:1228–1233. doi: 10.1016/j.surg.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Tuttle RM, Ball DW, Byrd D, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8:1228–1274. doi: 10.6004/jnccn.2010.0093. [DOI] [PubMed] [Google Scholar]
- 21.Arda IS, Yildirim S, Demirhan B, et al. Fine needle aspiration biopsy of thyroid nodules. Arch Dis Child. 2001;85:313–317. doi: 10.1136/adc.85.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amrikachi M, Ponder TB, Wheeler TM, et al. Thyroid fine-needle aspiration biopsy in children and adolescents: Experience with 218 aspirates. Diagn Cytopathol. 2005;32:189–192. doi: 10.1002/dc.20197. [DOI] [PubMed] [Google Scholar]
- 23.Canadian Pediatric Thyroid Nodule (CaPTN) Study Group The Canadian Pediatric Thyroid Nodule Study: An evaluation of current management practices. J Pediatr Surg. 2008;43:826–830. doi: 10.1016/j.jpedsurg.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Raab SS, Silverman JF, Elsheikh TM, et al. Pediatric thyroid nodules: Disease demographics and clinical management as determined by fine needle aspiration biopsy. Pediatrics. 1995;95:46–49. [PubMed] [Google Scholar]
- 25.Halac I, Zimmerman D. Thyroid nodules and cancers in children. Endocrinol Metab Clin North Am. 2005;34:725–744. doi: 10.1016/j.ecl.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–559. doi: 10.1056/NEJM199302253280807. [DOI] [PubMed] [Google Scholar]
- 27.Millman B, Pellitteri PK. Nodular thyroid disease in children and adolescents. Otolaryngol Head Neck Surg. 1997;116:604–609. doi: 10.1016/S0194-59989770235-3. [DOI] [PubMed] [Google Scholar]
- 28.Harach HR, Williams ED. Childhood thyroid cancer in England and Wales. Br J Cancer. 1995;72:777–783. doi: 10.1038/bjc.1995.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scholz S, Smith JR, Chaignaud B, et al. Thyroid surgery at Children’s Hospital Boston: A 35-year single-institution experience. J Pediatr Surg. 2011;46:437–442. doi: 10.1016/j.jpedsurg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Duffy BJ, Jr, Fitzgerald PJ. Cancer of the thyroid in children: A report of 28 cases. J Clin Endocrinol Metab. 1950;10:1296–1308. doi: 10.1210/jcem-10-10-1296. [DOI] [PubMed] [Google Scholar]
- 31.Kodama T, Fujimoto Y, Obara T, et al. Justification of conservative surgical treatment of childhood thyroid cancer: Report of eleven cases and analysis of Japanese literature. Jpn J Cancer Res. 1986;77:799–807. [PubMed] [Google Scholar]
- 32.Corrias A, Mussa A, Wasniewska M, et al. Levothyroxine treatment in pediatric benign thyroid nodules. Horm Res Paediatr. 2011;75:246–251. doi: 10.1159/000321841. [DOI] [PubMed] [Google Scholar]
- 33.Corrias A, Cassio A, Weber G, et al. Thyroid nodules and cancer in children and adolescents affected by autoimmune thyroiditis. Arch Pediatr Adolesc Med. 2008;162:526–531. doi: 10.1001/archpedi.162.6.526. [DOI] [PubMed] [Google Scholar]
- 34.De Keyser LFM, Van Herle AJ. Differentiated thyroid cancer in children. Head Neck Surg. 1985;8:100–114. doi: 10.1002/hed.2890080207. [DOI] [PubMed] [Google Scholar]
- 35. Neto GAM, Stanbury JB. Inherited Disorders of the Thyroid System. Boca Raton, FL: CRC Press, 1994. [Google Scholar]
- 36.Medeiros-Neto G, Gil-Da-Costa MJ, Santos CL, et al. Metastatic thyroid carcinoma arising from congenital goiter due to mutation in the thyroperoxidase gene. J Clin Endocrinol Metab. 1998;83:4162–4166. doi: 10.1210/jcem.83.11.5264. [DOI] [PubMed] [Google Scholar]
- 37.Niedziela M. Thyroid nodules. J Clin Endocrinol Metab. 2014;28:245–277. doi: 10.1016/j.beem.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Winship T, Rosvall RV.Thyroid carcinoma in childhood: Final report on a 20 year study Clin Proc Child Hosp DC 197026327–349. [Google Scholar]
- 39.Desjardins JG, Khan AH, Montupet P, et al. Management of thyroid nodules in children: A 20-year experience. J Pediatr Surg. 1987;22:736–739. doi: 10.1016/s0022-3468(87)80616-4. [DOI] [PubMed] [Google Scholar]
- 40.Scott MD, Crawford JD. Solitary thyroid nodules in childhood: Is the incidence of thyroid carcinoma declining? Pediatrics. 1976;58:521–525. [PubMed] [Google Scholar]
- 41.Kazakov VS, Demidchik EP, Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992;359:21. doi: 10.1038/359021a0. [DOI] [PubMed] [Google Scholar]
- 42.Likhtarev IA, Sobolev BG, Kairo IA, et al. Thyroid cancer in the Ukraine. Nature. 1995;375:365. doi: 10.1038/375365a0. [DOI] [PubMed] [Google Scholar]
- 43.Wiersinga WM. Thyroid cancer in children and adolescents: Consequences in later life. J Pediatr Endocrinol Metab. 2001;14(suppl 5):1289–1298. [PubMed] [Google Scholar]
- 44.Nikiforov Y, Gnepp DR, Fagin JA. Thyroid lesions in children and adolescents after the Chernobyl disaster: Implications for the study of radiation tumorigenesis. J Clin Endocrinol Metab. 1996;81:9–14. doi: 10.1210/jcem.81.1.8550800. [DOI] [PubMed] [Google Scholar]
- 45.Bennedbaek FN, Perrild H, Hegedüs L. Diagnosis and treatment of the solitary thyroid nodule. Results of a European survey. Clin Endocrinol (Oxf) 1999;50:357–363. doi: 10.1046/j.1365-2265.1999.00663.x. [DOI] [PubMed] [Google Scholar]
- 46.Alsanea O, Clark OH. Familial thyroid cancer. Curr Opin Oncol. 2001;13:44–51. doi: 10.1097/00001622-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Stratakis CA, Courcoutsakis NA, Abati A, et al. Thyroid gland abnormalities in patients with the syndrome of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas (Carney complex) J Clin Endocrinol Metab. 1997;82:2037–2043. doi: 10.1210/jcem.82.7.4079. [DOI] [PubMed] [Google Scholar]
- 48.Amer M, Mostafa FF, Attwa EM, et al. Cowden’s syndrome: A clinical, immunological, and histopathological study. Int J Dermatol. 2011;50:516–521. doi: 10.1111/j.1365-4632.2010.04669.x. [DOI] [PubMed] [Google Scholar]
- 49.Harach HR, Soubeyran I, Brown A, et al. Thyroid pathologic findings in patients with Cowden disease. Ann Diagn Pathol. 1999;3:331–340. doi: 10.1016/s1092-9134(99)80011-2. [DOI] [PubMed] [Google Scholar]
- 50.Chaukar DA, Rangarajan V, Nair N, et al. Pediatric thyroid cancer. J Surg Oncol. 2005;92:130–133. doi: 10.1002/jso.20339. [DOI] [PubMed] [Google Scholar]
- 51.Haveman JW, van Tol KM, Rouwé CW, et al. Surgical experience in children with differentiated thyroid carcinoma. Ann Surg Oncol. 2003;10:15–20. doi: 10.1245/aso.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Arici C, Erdogan O, Altunbas H, et al. Differentiated thyroid carcinoma in children and adolescents. Clinical characteristics, treatment and outcome of 15 patients. Horm Res. 2002;57:153–156. doi: 10.1159/000058375. [DOI] [PubMed] [Google Scholar]
- 53.Dinauer C, Francis GL. Thyroid cancer in children. Endocrinol Metab Clin North Am. 2007;36:779–806, vii. doi: 10.1016/j.ecl.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Kim E-K, Park CS, Chung WY, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 55.Moon W-J, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation: Multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 56.Saavedra J, Deladoëy J, Saint-Vil D, et al. Is ultrasonography useful in predicting thyroid cancer in children with thyroid nodules and apparently benign cytopathologic features? Horm Res Paediatr. 2011;75:269–275. doi: 10.1159/000322877. [DOI] [PubMed] [Google Scholar]
- 57.Corrias A, Cassio A, Weber G, et al. Thyroid nodules and cancer in children and adolescents affected by autoimmune thyroiditis. Arch Pediatr Adolesc Med. 2008;162:526–531. doi: 10.1001/archpedi.162.6.526. [DOI] [PubMed] [Google Scholar]
- 58.Lawrence W, Jr, Kaplan BJ. Diagnosis and management of patients with thyroid nodules. J Surg Oncol. 2002;80:157–170. doi: 10.1002/jso.10115. [DOI] [PubMed] [Google Scholar]
- 59.Bonnema SJ, Bennedbaek FN, Wiersinga WM, et al. Management of the nontoxic multinodular goitre: A European questionnaire study. Clin Endocrinol (Oxf) 2000;53:5–12. doi: 10.1046/j.1365-2265.2000.01060.x. [DOI] [PubMed] [Google Scholar]
- 60.Panneerselvan R, Schneider DF, Sippel RS, et al. Radioactive iodine scanning is not beneficial but its use persists for euthyroid patients. J Surg Res. 2013;184:269–273. doi: 10.1016/j.jss.2013.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White AK, Smith RJ. Thyroid nodules in children. Otolaryngol Head Neck Surg. 1986;95:70–75. doi: 10.1177/019459988609500114. [DOI] [PubMed] [Google Scholar]
- 62.McHenry CR, Danish R, Murphy T, et al. Atypical thyroglossal duct cyst: A rare cause for a solitary cold thyroid nodule in childhood. Am Surg. 1993;59:223–228. [PubMed] [Google Scholar]
- 63.Gharib H. Changing concepts in the diagnosis and management of thyroid nodules. Endocrinol Metab Clin North Am. 1997;26:777–800. doi: 10.1016/s0889-8529(05)70282-6. [DOI] [PubMed] [Google Scholar]
- 64.Hamberger B, Gharib H, Melton LJ, 3rd, et al. Fine-needle aspiration biopsy of thyroid nodules. Impact on thyroid practice and cost of care. Am J Med. 1982;73:381–384. [PubMed] [Google Scholar]
- 65.Caplan RH, Strutt PJ, Kisken WA, et al. Fine needle aspiration biopsy of thyroid nodules. Wis Med J. 1991;90:285–288. [PubMed] [Google Scholar]
- 66.Belfiore A, La Rosa GL. Fine-needle aspiration biopsy of the thyroid. Endocrinol Metab Clin North Am. 2001;30:361–400. doi: 10.1016/s0889-8529(05)70191-2. [DOI] [PubMed] [Google Scholar]
- 67.Stevens C, Lee JKP, Sadatsafavi M, et al. Pediatric thyroid fine-needle aspiration cytology: A meta-analysis. J Pediatr Surg. 2009;44:2184–2191. doi: 10.1016/j.jpedsurg.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 68.Bargren AE, Meyer-Rochow GY, Sywak MS, et al. Diagnostic utility of fine-needle aspiration cytology in pediatric differentiated thyroid cancer. World J Surg. 2010;34:1254–1260. doi: 10.1007/s00268-010-0391-x. [DOI] [PubMed] [Google Scholar]
- 69.Calkovsky V, Hajtman A. Thyroid diseases in children and adolescents. Bratisl Lek Listy (Tlacene Vyd) 2009;110:31–34. [PubMed] [Google Scholar]
- 70.Gutman PD, Henry M. Fine needle aspiration cytology of the thyroid. Clin Lab Med. 1998;18:461–482, vi. [PubMed] [Google Scholar]
- 71.Geiger JD, Thompson NW. Thyroid tumors in children. Otolaryngol Clin North Am. 1996;29:711–719. [PubMed] [Google Scholar]
- 72.Lafferty AR, Batch JA. Thyroid nodules in childhood and adolescence—thirty years of experience. J Pediatr Endocrinol Metab. 1997;10:479–486. doi: 10.1515/jpem.1997.10.5.479. [DOI] [PubMed] [Google Scholar]
- 73.Chang SH, Joo M, Kim H. Fine needle aspiration biopsy of thyroid nodules in children and adolescents. J Korean Med Sci. 2006;21:469–473. doi: 10.3346/jkms.2006.21.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Degnan BM, McClellan DR, Francis GL. An analysis of fine-needle aspiration biopsy of the thyroid in children and adolescents. J Pediatr Surg. 1996;31:903–907. doi: 10.1016/s0022-3468(96)90407-8. [DOI] [PubMed] [Google Scholar]
- 75.Khurana KK, Labrador E, Izquierdo R, et al. The role of fine-needle aspiration biopsy in the management of thyroid nodules in children, adolescents, and young adults: A multi-institutional study. Thyroid. 1999;9:383–386. doi: 10.1089/thy.1999.9.383. [DOI] [PubMed] [Google Scholar]
- 76.Al-Shaikh A, Ngan B, Daneman A, et al. Fine-needle aspiration biopsy in the management of thyroid nodules in children and adolescents. J Pediatr. 2001;138:140–142. doi: 10.1067/mpd.2001.109609. [DOI] [PubMed] [Google Scholar]
- 77.Ardito G, Pintus C, Revelli L, et al. Thyroid tumors in children and adolescents: Preoperative study. Eur J Pediatr Surg. 2001;11:154–157. doi: 10.1055/s-2001-15479. [DOI] [PubMed] [Google Scholar]
- 78.Kaur J, Srinivasan R, Arora SK, et al. Fine-needle aspiration in the evaluation of thyroid lesions in children. Diagn Cytopathol. 2012;40(suppl 1):E33–E37. doi: 10.1002/dc.21568. [DOI] [PubMed] [Google Scholar]
- 79.Willgerodt H, Keller E, Bennek J, et al. Diagnostic value of fine-needle aspiration biopsy of thyroid nodules in children and adolescents. J Pediatr Endocrinol Metab. 2006;19:507–515. [PubMed] [Google Scholar]
- 80.Smith M, Pantanowitz L, Khalbuss WE, et al. Indeterminate pediatric thyroid fine needle aspirations: A study of 68 cases. Acta Cytol. 2013;57:341–348. doi: 10.1159/000351029. [DOI] [PubMed] [Google Scholar]
- 81.Izquierdo R, Arekat MR, Knudson PE, et al. Comparison of palpation-guided versus ultrasound-guided fine-needle aspiration biopsies of thyroid nodules in an outpatient endocrinology practice. Endocr Pract. 2006;12:609–614. doi: 10.4158/EP.12.6.609. [DOI] [PubMed] [Google Scholar]
- 82.Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: A prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96:3390–3397. doi: 10.1210/jc.2011-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 84.Yamashita S, Saenko V. Mechanisms of disease: Molecular genetics of childhood thyroid cancers. Nat Clin Pract Endocrinol Metab. 2007;3:422–429. doi: 10.1038/ncpendmet0499. [DOI] [PubMed] [Google Scholar]
- 85.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 86.Bongarzone I, Fugazzola L, Vigneri P, et al. Age-related activation of the tyrosine kinase receptor protooncogenes RET and NTRK1 in papillary thyroid carcinoma. J Clin Endocrinol Metab. 1996;81:2006–2009. doi: 10.1210/jcem.81.5.8626874. [DOI] [PubMed] [Google Scholar]
- 87.de Groot JWB, Links TP, Plukker JTM, et al. RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocr Rev. 2006;27:535–560. doi: 10.1210/er.2006-0017. [DOI] [PubMed] [Google Scholar]
- 88.Nikiforov YE, Rowland JM, Bove KE, et al. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57:1690–1694. [PubMed] [Google Scholar]
- 89.Collins BJ, Chiappetta G, Schneider AB, et al. RET expression in papillary thyroid cancer from patients irradiated in childhood for benign conditions. J Clin Endocrinol Metab. 2002;87:3941–3946. doi: 10.1210/jcem.87.8.8748. [DOI] [PubMed] [Google Scholar]
- 90.Basolo F, Torregrossa L, Giannini R, et al. Correlation between the BRAF V600E mutation and tumor invasiveness in papillary thyroid carcinomas smaller than 20 millimeters: Analysis of 1060 cases. J Clin Endocrinol Metab. 2010;95:4197–4205. doi: 10.1210/jc.2010-0337. [DOI] [PubMed] [Google Scholar]
- 91.Lassalle S, Hofman V, Marius I, et al. Assessment of morphology, antigenicity, and nucleic acid integrity for diagnostic thyroid pathology using formalin substitute fixatives. Thyroid. 2009;19:1239–1248. doi: 10.1089/thy.2009.0095. [DOI] [PubMed] [Google Scholar]
- 92.Monaco SE, Pantanowitz L, Khalbuss WE, et al. Cytomorphological and molecular genetic findings in pediatric thyroid fine-needle aspiration. Cancer Cytopathol. 2012;120:342–350. doi: 10.1002/cncy.21199. [DOI] [PubMed] [Google Scholar]
- 93.Buryk MA, Monaco SE, Witchel SF, et al. Preoperative cytology with molecular analysis to help guide surgery for pediatric thyroid nodules. Int J Pediatr Otorhinolaryngol. 2013;77:1697–1700. doi: 10.1016/j.ijporl.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 94.Suárez HG. Genetic alterations in human epithelial thyroid tumours. Clin Endocrinol (Oxf) 1998;48:531–546. doi: 10.1046/j.1365-2265.1998.00443.x. [DOI] [PubMed] [Google Scholar]
- 95.Kroll TG, Sarraf P, Pecciarini L, et al. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma. Science. 2000;289:1357–1360. doi: 10.1126/science.289.5483.1357. [published correction appears in Science 2000;289:1474] [DOI] [PubMed] [Google Scholar]
- 96.Vecchio G, Santoro M. Oncogenes and thyroid cancer. Clin Chem Lab Med. 2000;38:113–116. doi: 10.1515/CCLM.2000.017. [DOI] [PubMed] [Google Scholar]
- 97.Schlinkert RT, van Heerden JA, Goellner JR, et al. Factors that predict malignant thyroid lesions when fine-needle aspiration is “suspicious for follicular neoplasm”. Mayo Clin Proc. 1997;72:913–916. doi: 10.1016/S0025-6196(11)63360-0. [DOI] [PubMed] [Google Scholar]
- 98.Breuer C, Tuggle C, Solomon D, et al. Pediatric thyroid disease: When is surgery necessary, and who should be operating on our children? J Clin Res Pediatr Endocrinol. 2013;5(suppl 1):79–85. doi: 10.4274/Jcrpe.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Welch Dinauer CA, Tuttle RM, Robie DK, et al. Extensive surgery improves recurrence-free survival for children and young patients with class I papillary thyroid carcinoma. J Pediatr Surg. 1999;34:1799–1804. doi: 10.1016/s0022-3468(99)90316-0. [DOI] [PubMed] [Google Scholar]
- 100.Kim K-E, Kim E-K, Yoon JH, et al. Preoperative prediction of central lymph node metastasis in thyroid papillary microcarcinoma using clinicopathologic and sonographic features. World J Surg. 2013;37:385–391. doi: 10.1007/s00268-012-1826-3. [DOI] [PubMed] [Google Scholar]
- 101.Dinauer CA, Breuer C, Rivkees SA. Differentiated thyroid cancer in children: Diagnosis and management. Curr Opin Oncol. 2008;20:59–65. doi: 10.1097/CCO.0b013e3282f30220. [DOI] [PubMed] [Google Scholar]
- 102.Rivkees SA, Mazzaferri EL, Verburg FA, et al. The treatment of differentiated thyroid cancer in children: Emphasis on surgical approach and radioactive iodine therapy. Endocr Rev. 2011;32:798–826. doi: 10.1210/er.2011-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park S, Jeong JS, Ryu HR, et al. Differentiated thyroid carcinoma of children and adolescents: 27-year experience in the yonsei university health system. J Korean Med Sci. 2013;28:693–699. doi: 10.3346/jkms.2013.28.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roy M, Chen H, Sippel RS. Current understanding and management of medullary thyroid cancer. The Oncologist. 2013;18:1093–1100. doi: 10.1634/theoncologist.2013-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiménez C, Hu MI-N, Gagel RF. Management of medullary thyroid carcinoma. Endocrinol Metab Clin North Am. 2008;37:481–496. doi: 10.1016/j.ecl.2008.03.001. x–xi. [DOI] [PubMed] [Google Scholar]
- 106.Kloos RT, Eng C, Evans DB, et al. Medullary thyroid cancer: Management guidelines of the American Thyroid Association. Thyroid. 2009;19:565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 107.Sosa JA, Bowman HM, Tielsch JM, et al. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228:320–330. doi: 10.1097/00000658-199809000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jarząb B, Handkiewicz-Junak D, Włoch J. Juvenile differentiated thyroid carcinoma and the role of radioiodine in its treatment: A qualitative review. Endocr Relat Cancer. 2005;12:773–803. doi: 10.1677/erc.1.00880. [DOI] [PubMed] [Google Scholar]
- 109.Papini E, Petrucci L, Guglielmi R, et al. Long-term changes in nodular goiter: A 5-year prospective randomized trial of levothyroxine suppressive therapy for benign cold thyroid nodules. J Clin Endocrinol Metab. 1998;83:780–783. doi: 10.1210/jcem.83.3.4615. [DOI] [PubMed] [Google Scholar]
- 110.American Thyroid Association Available at http://www.thyroid.org. Accessed November 26, 2015.


