An electronic survey was used to assess current practice of dose adjustment of chemotherapy in obese and overweight patients undergoing hematopoietic stem cell transplantation. It revealed large diversity among transplant centers regarding dose-adjustment practice. This novel survey is an important step toward defining the right dose adjustment for pretransplantation conditioning to improve efficacy, to reduce toxicity, and thus to improve transplantation outcome.
Keywords: Obesity, Dose adjustment, Hematopoietic stem cell transplantation, Conditioning
Abstract
Background.
Appropriate chemotherapy dosing for obese patients with malignant diseases is a significant challenge because limiting chemotherapy doses in these patients may negatively influence outcome. There is a paucity of information addressing high-dose chemotherapy in obese patients undergoing hematopoietic stem cell transplantation (HSCT).
Methods.
The Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT) designed an electronic survey to assess current practice of dose adjustment of chemotherapy in obese patients undergoing HSCT.
Results.
A total of 56 EBMT centers from 27 countries responded to the online survey. Overall, 45 centers declared that they routinely adjust chemotherapy doses for obese patients (80.5%), and only 11 (19.5%) declared they do not adjust dose. Among the former group, most used body mass index as the parameter for defining obesity (28 centers, 62%). The method for determining the weight for chemotherapy calculation was actual body weight (ABW) in 16 centers, ideal body weight (IBW) in 10 centers, IBW plus 25% of the difference between IBW and ABW in 16 centers, and other methods for the rest. Among centers that used dose adjustment, 44% also capped the dose at 2 m2 for a chemotherapy dose based on body surface area (BSA), whereas 56% did not cap. Interestingly, most of the centers (9 of 11) that did not adjust dose for weight also did not cap the BSA at 2 m2.
Conclusion.
This EBMT survey revealed large diversity among transplant centers regarding dose-adjustment practice for high-dose conditioning chemotherapy. Our next step is to analyze outcomes of transplantation according to dose-adjustment practice and, subsequently, to formulate a methodology for future prospective studies.
Implications for Practice:
There is a paucity of information addressing the pharmacokinetics of high-dose chemotherapy in obese patients undergoing hematopoietic stem cell transplantation (HSCT). For the first time, an electronic survey was used to assess current practice of dose adjustment of chemotherapy in obese and overweight patients undergoing HSCT. This novel survey revealed large diversity among transplant centers regarding dose-adjustment practice. This survey is an important step toward defining the right dose adjustment for pretransplantation conditioning to improve efficacy, to reduce toxicity, and thus to improve transplantation outcome.
Introduction
The incidence of obesity has substantially increased in recent years [1–4]. Among cancer patients, obesity is associated with greater overall and cancer-specific mortality [4, 5]. Obese patients are more likely to suffer from comorbidities, such as cardiovascular disease and diabetes, that might greatly affect complications during chemotherapy and thus increase mortality [6]. Pharmacokinetics of drugs including chemotherapy is different in obese patients because of alteration in renal and hepatic clearance, increased volume of distribution of lipophilic drugs, and increased protein binding [7]. Consequently, appropriate chemotherapy dosing for obese patients with malignant diseases is a significant challenge. In addition, limiting chemotherapy doses in overweight and obese patients may negatively influence the outcomes in these patients, whereas overdosing might increase toxicity.
The current practice for determining chemotherapy dose uses body surface area (BSA) and was proposed based on animal and human studies performed decades ago [8]. Some chemotherapeutic drug dosage is based on body weight. In either case, weight for calculation should be determined in obese patients.
The American Society of Clinical Oncology (ASCO) recently published clinical practice guidelines for conventional chemotherapy dosing for obese patients with cancer indicating that up to 40% of obese patients received reduced chemotherapy doses that are not based on actual body weight (ABW) [9]. Many oncologists continue to use either ideal body weight (IBW), adjusted IBW, or capping the BSA at, for example, 2.0 m2 rather than using ABW to calculate BSA. This is mainly the result of traditional concerns about toxicity or overdosing in obese patients if the ABW were used. Thus, the ASCO panel recommended that full weight-based cytotoxic chemotherapy doses must be used to treat obese patients with cancer because no evidence shows that short- or long-term toxicity is increased among obese patients receiving full weight-based doses. Of note, most data indicated that myelosuppression is similar or less pronounced among obese patients compared with nonobese patients [9].
With such background, challenges for patients receiving high-dose chemotherapy are even bigger. There is a paucity of information addressing the pharmacokinetics of high-dose chemotherapy in obese patients undergoing hematopoietic stem cell transplantation (HSCT). The kinetics are more complicated than those for standard-dose chemotherapy because of metabolizing enzyme saturation, depletion of conjugating substrate, and significant protein binding and changes in volume distribution [10].
A relatively small international survey of drug-dosing schemes among transplant centers revealed that there is no consensus regarding appropriate dose adjustment for obese patients [11]. Stem cell transplantation centers were sent a specific questionnaire for details of dosing for busulfan (Bu), cyclophosphamide (Cy), cyclosporin A, and methotrexate. Data from 33 institutions were evaluable. No single method was used in >30% of transplant centers: 24% and 30% of centers surveyed used actual weight without modification for Bu and Cy, respectively; 15% and 12% used ideal weight only for Bu and Cy, respectively; and ∼20% adjusted dose if actual weight was >20% above ideal weight. The remainder used various dose-adjustment schemes [11].
Moreover, data on outcome in obese versus nonobese patients are limited. Some reports suggested higher treatment-related mortality (TRM), whereas others showed only higher relapse rates. No optimal approach to adjustment is clear from the literature: variable dosing schemes—and consequent under- or overdosing—are used. In addition, different adjustment is probably needed for different drugs [10].
In summary, no standard of practice exists regarding chemotherapy dose adjustment for obese patients undergoing stem cell transplantation. For this reason, the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT) designed an electronic survey for assessing current practice of dose adjustment of chemotherapy in patients undergoing HSCT.
Our survey focusing on chemotherapy dose adjustment in obese patients is the first step in trying to establish standards and evidence-based recommendation for transplant procedure in this population. The need for standardization and harmonization in HSCT is recognized. Lack of standards threatens patients who might be given less efficacious or more toxic conditioning, health care systems that might waste precious resources for inefficacious or toxic therapy, and the professional organizations that might lose their independence if they fail to define optimal conditioning and are forced to use the cheapest approach. Lack of knowledge about how to deal with obese patients is just one aspect and might aggravate other problematic issues in HSCT.
This notion is the basis of recent major efforts by both the EBMT and the Center for International Blood and Marrow Transplant Research (CIBMTR) to enforce the quality-control accreditation programs of transplantation and cellular therapy facilities: the Joint Accreditation Committee-ISCT [International Society for Cellular Therapy] (Europe) and EBMT (JACIE) and the Foundation for the Accreditation of Cellular Therapy (FACT), respectively. In a recent, important paper on behalf of the EBMT and LeukemiaNet, Gratwohl et al. [12] showed that the use of the JACIE quality management system improved outcome after hematopoietic stem cell transplantation. Results became better in all centers over time, but they improved significantly faster and were more pronounced for patients transplanted in the context of accredited programs. Relapse-free survival and overall survival (OS) were significantly higher at 6 years for those patients transplanted in the JACIE-accredited centers.
Materials and Methods
This prospective survey was carried out between February and July 2013. The questionnaire included 27 items regarding the definition of obesity and various aspects of conditioning chemotherapy dose adjustment. EBMT transplant centers were invited to participate, of which 56 centers from 27 countries responded and filled the online survey (supplemental online data). Data were collected by the Acute Leukemia Working Party of the EBMT in Paris, France, and analyzed at Sheba medical center in Israel.
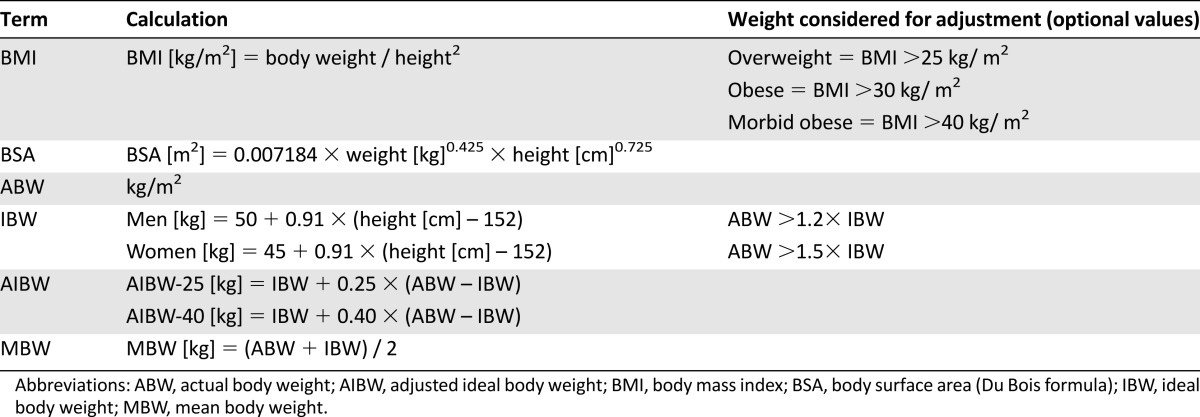
Definitions included in the introduction of the questionnaire to standardize the results are presented in Table 1.
Table 1.
Definitions of obesity
Responders could choose an answer from a proposed list or add free text and remarks if needed.
Results
Among the 56 centers, the percentage of obese patients was <10% at 22 centers (40%), 10%–19% at 23 centers (42%), and >20% in 10 centers (17%) (Table 2). The variation among obesity proportions was not due to variation in the definition of obesity (the same definitions were used among high-prevalence and low-prevalence centers). No center declared excluding obese patients from transplantation; moreover, the proportion of obese patients was not statistically different from the prevalence in the population of the relevant country.
Table 2.
Dose-adjustments parameters and methods for chemotherapy dose calculation
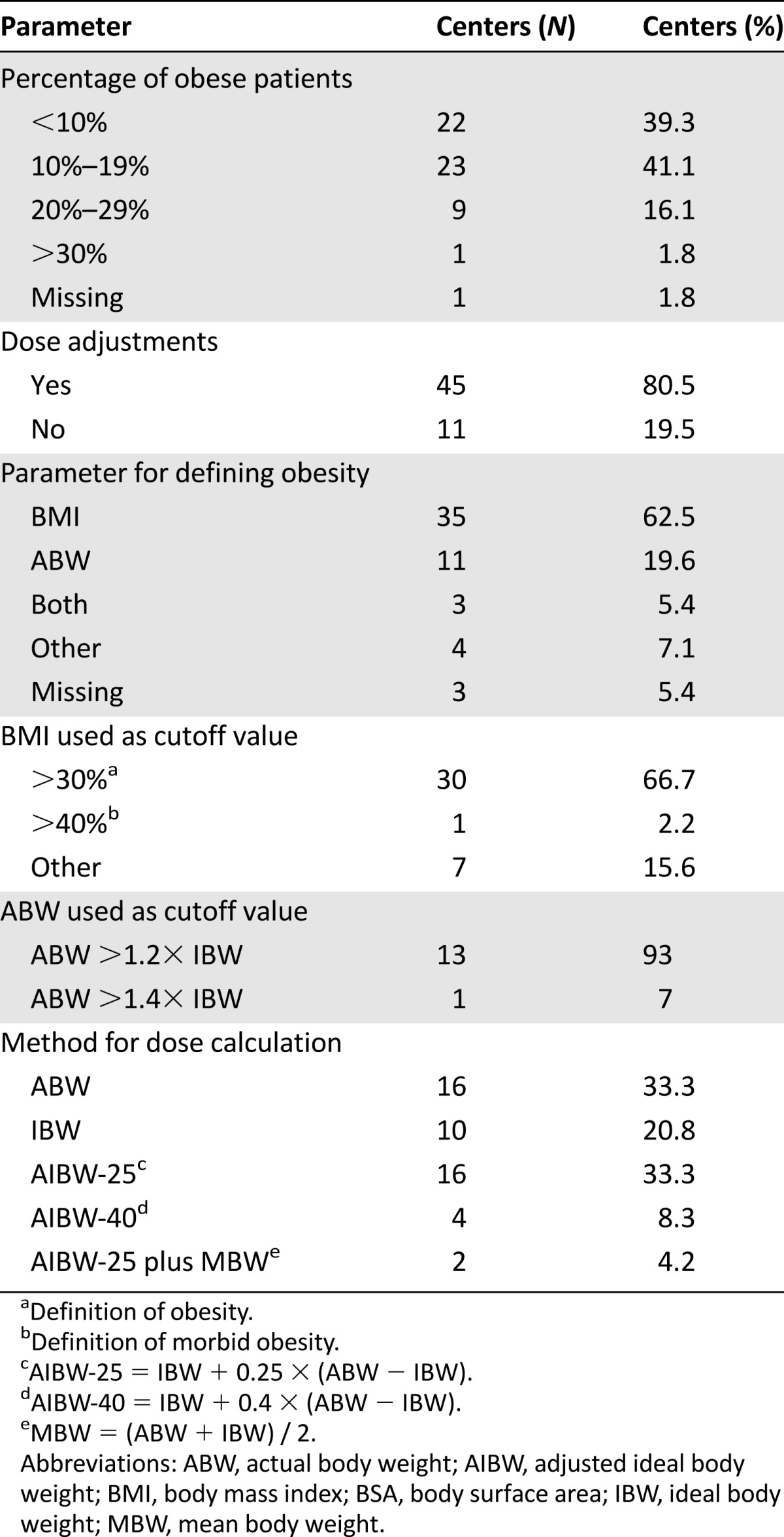
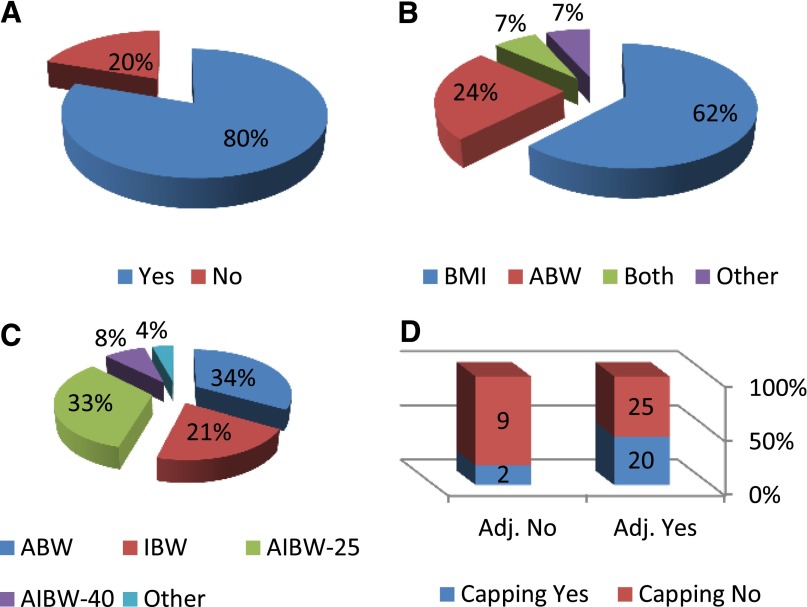
Forty-five centers declared that they adjust chemotherapy dose for obese patients (80.5%), and only 11 centers (19.5%) did not (Table 2; Fig. 1A). Among the former group, most used BMI as the parameter for defining obesity (28 centers, 62%), 24.5% (11 centers) used percentage over ABW, 6.7% (3 centers) used both BMI and ABW, and the remaining 6.7% (3 centers) used other parameters (Table 2; Fig. 1B). In most of the centers that used BMI for dose adjustment, BMI >30 kg/m2 was defined as the cutoff value for the formal definition of obesity. Only one center used morbid obesity (BMI >40 kg/m2), and the remainder used other cutoff values. Among 14 centers that used ABW, 13 used ABW >120% of IBW for adjustment, and only one used ABW >140% of IBW (Table 2). Moreover, 84% of the centers used one level of obesity for adjustment, whereas the rest used two levels and different weight adjustments for each level.
Figure 1.
Distribution of different parameters in transplant center. (A): Dose adjustment for chemotherapy in obese patients. (B): Parameter for defining obesity. (C): Methods used for determining body weight for dose adjustment. (D): Correlation between dose adjustment and capping body surface area at 2 m2.
Abbreviations: Adj., adjusted; ABW, actual body weight; AIBW-25, IBW + 0.25 × (ABW – IBW); AIBW-40, IBW + 0.40 × (ABW – IBW); BMI, body mass index; IBW, ideal body weight.
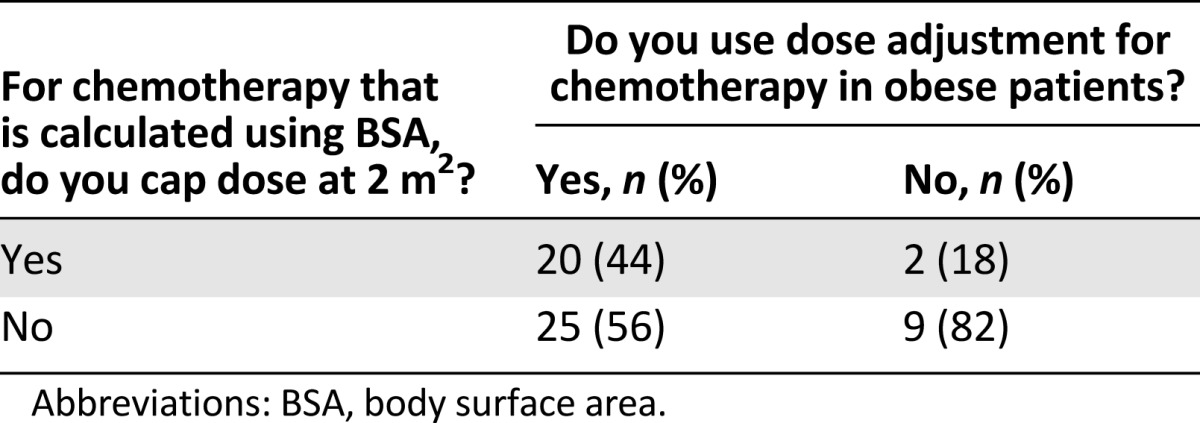
The method of determining the weight for chemotherapy calculation was ABW in 16 centers, IBW in 10 centers, IBW plus 25% of the difference between IBW and ABW (adjusted ideal body weight-25 [AIBW-25]; IBW + 0.25 × [ABW − IBW]) in 16 centers, IBW plus 40% of the difference between IBW and ABW (AIBW-40; IBW + 0.4 × [ABW − IBW]) in 4 centers, and other methods in the rest (n = 2) (Table 2; Fig. 1C). Among centers that used dose adjustment, 44% also capped the dose at 2 m2 for chemotherapy dose based on BSA, whereas 56% did not cap. Interestingly, most of the centers (9 of 11) that did not adjust dose for weight also did not cap BSA at 2 m2. Twelve centers declared that they capped BSA at values >2 m2 (Table 3; Fig. 1D).
Table 3.
Correlation between dose adjustment and capping BSA at 2 m2
Thirty-six centers declared that they used the same approach to dose adjustment in myeloablative (MA) versus reduced-intensity chemotherapy (RIC) or non-MA (NMA) regimens, whereas 8 centers declared they used a smaller dose reduction for RIC or NMA regimens.
For busulfan dosage, only seven centers monitored routinely pharmacokinetics. Eleven centers used IBW for calculation of busulfan dose, 17 used ABW, and 18 corrected weight according to percentage over ABW (15 used AIBW-25 and 3 used AIBW-40). In most transplant centers (86%), formulation of busulfan dose is based on weight, and only the minority used BSA.
For other cytotoxic drugs used as part of conditioning regimens, the majority of centers (n = 38) used the same method for weight adjustment for the various drugs. No center used IBW for dose calculation for cytotoxic drugs other than busulfan. The most commonly used adjustment methods were ABW in 15 centers, AIBW-25 in 13 centers, and AIBW-40 in 3 centers.
For drugs used for graft-versus-host disease (GVHD) prophylaxis and treatment and for supportive care, most centers used ABW for dose calculation without adjustment.
Discussion
This EBMT survey revealed large diversity among transplant centers regarding dose-adjustment practice for high-dose-conditioning chemotherapy. Most of the centers used dose adjustment for chemotherapy calculation, whereas BMI >30 kg/m2 is used most commonly as the cutoff value. There was no common method for adjustment and weight calculation, which included IBW, corrected IBW (e.g., IBW plus 25% of the difference between actual and ideal weight), and actual weight. Interestingly, about half of the centers that used dose adjustment also capped BSA at 2 m2, whereas capping was uncommon at the centers that did not adjust doses. Consequently, the range of the final dose might become even wider. Even for busulfan, for which dose is normally calculated according to IBW, the diversity applied to obese patients was striking. For a patient whose height is 170 cm and weight is 125 kg, BMI is >30 kg/m2 (43.3 kg/m2). If dose is not adjusted, BSA is 2.3 m2. If we use ideal body weight for this patient, which is 65 kg, the BSA is 1.8 m2. For standard fludarabine-busulfan conditioning, we get a difference of 27% in fludarabine dose, which is based on BSA, and 92% difference in busulfan dose, which is based on weight. With some method of correction and capping at 2 m2, we can get doses in between these two extreme values.
Clinical practice guidelines for chemotherapy dosing should reduce the large diversity in chemotherapy dosing for obese patients and hopefully improve outcomes. ASCO conducted an exhaustive review of the literature on chemotherapy dosing in obese patients with cancer. Based on this review, clinical practice guidelines have been generated [9]. The ASCO guidelines recommend that obese adult patients with cancer should be treated with chemotherapy doses based on ABW, especially when the goal of treatment is cure. No evidence shows that obese patients receiving chemotherapy would experience increased toxicity if actual weight were used to calculate the dose [13–21]. Clinicians should address treatment-related toxicities in obese patients in the same manner that they would for nonobese patients. The incidence of myelosuppression among obese cancer patients receiving full chemotherapy doses seems to be the same or less compared with healthy-weight patients [13, 17]. It is very important to emphasize that most of the recommendations are based on subgroup analyses of obese patients from random clinical trials and on observational studies comparing results in patients receiving full-dose chemotherapy to those experiencing adjusted weight calculation.
In the field of high-dose chemotherapy, little evidence exists to suggest practice guidelines. Analysis based on scanty data are challenging because of differences in definitions of obesity and adjustments for weight and heterogeneous patient population, diseases, conditioning, and graft sources.
Some studies that compared obese and nonobese patients showed worse outcomes in the former. In a combined adult and pediatric population of 322 patients receiving an allogeneic HSCT at the University of Kentucky, obesity (defined as a weight >120% of IBW) was associated with worse OS, especially among adults and those receiving a human leukocyte antigen-matched graft [22]. The Fred Hutchinson Cancer Research Center in the U.S. reported on 196 patients receiving unrelated donor transplants for chronic-phase chronic myeloid leukemia and found that obesity was associated with higher mortality, with a hazard ratio of 1.6 for each 25% increase in actual weight over ideal weight [23]. In acute myeloid leukemia (AML) patients receiving busulfan-cyclophosphamide and autologous transplant who were dosed based on ABW without adjustments, increased TRM was found [24].
Some other studies showed higher risk of infection in obese patients but no influence on TRM or OS [25, 26]. A registry-based study from Japan including 3,827 patients showed higher acute GVHD and infections but similar rates of relapse and survival [25]. Nikolousis et al. reported on the outcomes of 336 patients with various hematologic malignancies from two transplant centers in the U.K. and found no influence of body weight on outcome, aside from an increased risk of infection in the overweight and obese groups compared with those with normal weight [26].
In contrast, among 262 patients with hematologic malignancies treated with myeloablative conditioning at the Princess Margaret Hospital in Canada, there were no consistent differences in frequency of mucositis, time to relapse, or TRM for extreme quintiles of body weight [27]. Navarro et al. [28] also reported no influence of obesity on nonrelapse mortality, GVHD, or survival when reviewing >4,000 AML patients from the CIBMTR observational database registry.
In a recent review, Weiss et al. suggest using AIBW (defined as IBW plus 25% of the difference between ABW and IBW) as a starting dosing point and conducting dose-escalation studies with pharmacodynamics and pharmacokinetics of agents used in HSCT. These variables can be correlated with fat tissue mass quantitatively, as measured by magnetic resonance imaging scan, and evaluated relative to clinical parameters of graft rejection, acute and chronic toxicity, time to recurrence, disease-free survival, and OS [29]. This suggestion is based on the speculation that ABW is too much and IBW is too little but, unfortunately, is not based on true available data.
Standardization of chemotherapy dose is very important to the individual patient and to the general population of patients. For the individual patient, we must have standard schemes to define the dose that will be enough for efficacy but not too much to avoid severe toxicity. For the general population of patients, we must have standards for comparisons among different conditioning regimens in different transplant centers. As long as obesity is defined and treated so differently, we cannot compare studies and improve conditioning regimens accordingly. Standard of care is a hot topic in many fields of medicine and influences health care policy, so transplant organizations should promote this topic.
Conclusion
This survey raises the important clinical issue and future challenge of lack of evidence-based guidelines for chemotherapy dosing in the setting of HSCT. Unfortunately, we cannot know from current data whether obese patients are under- or overdosed compared with nonobese patients, and we cannot draw any conclusion regarding the best weight adjustment scheme. Our next step will be to analyze outcomes of transplantation according to dose-adjustment practice in the various EBMT transplanting centers. This might help us to draw recommendations on a dose-calculation scheme and, subsequently, to formulate a methodology for future prospective studies. In any event, it is conceivable that our survey is an important step toward delivering pretransplantation conditioning according to dose adjustment, thus improving antileukemic efficacy, reducing toxicity, and improving transplantation outcome for the benefit of the transplanted hematological malignancy patients.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Acknowledgments
The authors acknowledge the 56 participating centers, which are listed in the supplemental online data.
Author Contributions
Conception/Design: Noga Shem-Tov, Arnon Nagler, Mohamad Mohty
Collection and/or assembly of data: Noga Shem-Tov, Myriam Labopin, Leila Moukhtari
Data analysis and interpretation: Noga Shem-Tov, Myriam Labopin, Leila Moukhtari
Manuscript writing: Noga Shem-Tov
Final approval of manuscript: Noga Shem-Tov, Myriam Labopin, Leila Moukhtari, Fabio Ciceri, Jordi Esteve, Sebastian Giebel, Norbert-Claude Gorin, Christopher Schmid, Avichai Shimoni, Arnon Nagler, Mohamad Mohty
Disclosures
The authors indicated no financial relationships.
References
- 1.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42:563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.McTiernan A. Obesity and cancer: The risks, science, and potential management strategies. Oncology (Williston Park) 2005;19:871–881; discussion 881–882, 885–886. [PubMed] [Google Scholar]
- 7.Blouin RA, Kolpek JH, Mann HJ. Influence of obesity on drug disposition. Clin Pharm. 1987;6:706–714. [PubMed] [Google Scholar]
- 8.Pinkel D. The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res. 1958;18:853–856. [PubMed] [Google Scholar]
- 9.Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30:1553–1561. doi: 10.1200/JCO.2011.39.9436. [DOI] [PubMed] [Google Scholar]
- 10.Navarro WH. Impact of obesity in the setting of high-dose chemotherapy. Bone Marrow Transplant. 2003;31:961–966. doi: 10.1038/sj.bmt.1704052. [DOI] [PubMed] [Google Scholar]
- 11.Grigg A, Harun MH, Szer J. Variability in determination of body weight used for dosing busulphan and cyclophosphamide in adult patients: Results of an international survey. Leuk Lymphoma. 1997;25:487–491. doi: 10.3109/10428199709039036. [DOI] [PubMed] [Google Scholar]
- 12.Gratwohl A, Brand R, McGrath E, et al. Use of the quality management system “JACIE” and outcome after hematopoietic stem cell transplantation. Haematologica. 2014;99:908–915. doi: 10.3324/haematol.2013.096461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165:1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 14.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: Findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26:4109–4115. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgiadis MS, Steinberg SM, Hankins LA, et al. Obesity and therapy-related toxicity in patients treated for small-cell lung cancer. J Natl Cancer Inst. 1995;87:361–366. doi: 10.1093/jnci/87.5.361. [DOI] [PubMed] [Google Scholar]
- 16.Poikonen P, Blomqvist C, Joensuu H. Effect of obesity on the leukocyte nadir in women treated with adjuvant cyclophosphamide, methotrexate, and fluorouracil dosed according to body surface area. Acta Oncol. 2001;40:67–71. doi: 10.1080/028418601750071082. [DOI] [PubMed] [Google Scholar]
- 17.Rosner GL, Hargis JB, Hollis DR, et al. Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: Results from Cancer and Leukemia Group B study 8541. J Clin Oncol. 1996;14:3000–3008. doi: 10.1200/JCO.1996.14.11.3000. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz J, Toste B, Dizon DS. Chemotherapy toxicity in gynecologic cancer patients with a body surface area (BSA)>2 m2. Gynecol Oncol. 2009;114:53–56. doi: 10.1016/j.ygyno.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 20.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: Findings from Intergroup Trial 0114. J Clin Oncol. 2004;22:648–657. doi: 10.1200/JCO.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 21.Barrett SV, Paul J, Hay A, et al. Does body mass index affect progression-free or overall survival in patients with ovarian cancer? Results from SCOTROC I trial. Ann Oncol. 2008;19:898–902. doi: 10.1093/annonc/mdm606. [DOI] [PubMed] [Google Scholar]
- 22.Fleming DR, Rayens MK, Garrison J. Impact of obesity on allogeneic hematopoietic cell transplant cell transplant patients: A matched case-controlled study. Am J Med. 1997;102:265–268. doi: 10.1016/S0002-9343(96)00450-0. [DOI] [PubMed] [Google Scholar]
- 23.Hansen JA, Gooley TA, Martin PJ, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338:962–968. doi: 10.1056/NEJM199804023381405. [DOI] [PubMed] [Google Scholar]
- 24.Meloni G, Proia A, Capria S, et al. Obesity and autologous stem cell transplantation in acute myeloid leukemia. Bone Marrow Transplant. 2001;28:365–367. doi: 10.1038/sj.bmt.1703145. [DOI] [PubMed] [Google Scholar]
- 25.Fuji S, Kim SW, Yoshimura K, et al. Possible association between obesity and posttransplantation complications including infectious diseases and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:73–82. doi: 10.1016/j.bbmt.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Nikolousis E, Nagra S, Paneesha S, et al. Allogeneic transplant outcomes are not affected by body mass index (BMI) in patients with haematological malignancies. Ann Hematol. 2010;89:1141–1145. doi: 10.1007/s00277-010-1001-6. [DOI] [PubMed] [Google Scholar]
- 27.Sriharsha L, Lipton JH, Pond G, et al. Examining the safety and efficacy of a chemotherapy dosing method in allogeneic stem cell transplant patients of extreme body size. J Oncol Pharm Pract. 2009;15:201–210. doi: 10.1177/1078155208101960. [DOI] [PubMed] [Google Scholar]
- 28.Navarro WH, Agovi MA, Logan BR, et al. Obesity does not preclude safe and effective myeloablative hematopoietic cell transplantation (HCT) for acute myelogenous leukemia (AML) in adults. Biol Blood Marrow Transplant. 2010;16:1442–1450. doi: 10.1016/j.bbmt.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss BM, Vogl DT, Berger NA, et al. Trimming the fat: Obesity and hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48:1152–1160. doi: 10.1038/bmt.2012.201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.