This study evaluated age-related changes in pharmacokinetic and pharmacodynamic parameters of nanoparticle albumin-bound paclitaxel in patients with metastatic breast cancer. A borderline significant relationship exists between age and 24-hour area under the curve, and there is an association between toxicity risk score and grade ≥3 chemotherapy toxicity and pharmacokinetic variables. The treatment is well tolerated across all age groups.
Keywords: Older adult, Pharmacokinetics, Pharmacodynamics, Geriatric oncology, Taxane
Abstract
Purpose.
This study evaluated age-related changes in pharmacokinetic and pharmacodynamic parameters of nanoparticle albumin-bound paclitaxel (nab-paclitaxel) in patients with metastatic breast cancer.
Methods.
Forty patients received nab-paclitaxel (100 mg/m2 weekly for 3 weeks followed by a 1-week break) as first- or second-line chemotherapy. Blood samples were collected for analysis, and response was assessed every two cycles. Planned statistical analyses included linear regression to examine the relationship between age and pharmacokinetic variables (ln clearance [CL] and ln area under the curve [AUC]) and two-sided two-sample t tests to evaluate age differences in pharmacodynamic variables. The association between chemotherapy toxicity risk scores and pharmacokinetic and pharmacodynamic variables including grade ≥3 toxicity were examined post hoc.
Results.
Of 40 patients enrolled, 39 (98%) were evaluable (mean age: 60 years; range: 30–81 years). A partial response was achieved in 31%, and 38% had stable disease. There was a borderline positive association between age and 24-hour ln AUC (slope = 0.011; SE = 0.006; p = .055). Grade 3 toxicity was experienced by 26% (8% hematologic, 18% nonhematologic). There were no differences in age based on the presence of grade 3 toxicity (p = .75), dose reductions (p = .38), or dose omissions (p = .15). A significant association was noted between chemotherapy toxicity risk score category and presence of grade 3 toxicity (toxicity rate by risk score category: low, 5 of 30 patients; medium, 3 of 6 patients; high, 2 of 3 patients; p = .041).
Conclusion.
A borderline significant relationship exists between age and 24-hour AUC, but no differences were noted for pharmacodynamic variables (grade 3 toxicity, dose reductions, or dose omissions) based on age. There is an association between toxicity risk score and grade ≥3 chemotherapy toxicity and pharmacokinetic variables. The treatment is well tolerated across all age groups.
Implications for Practice:
Older adults have been under-represented on oncology clinical trials, and few studies have evaluated how older adults tolerate cancer therapy compared with younger adults. The objective of this study was to evaluate the drug levels of nanoparticle albumin-bound paclitaxel (nab-paclitaxel) across the aging spectrum. The study demonstrated a borderline association between patient age and nab-paclitaxel drug levels, suggesting that drug levels increase with age; however, there was no significant difference in toxicity, dose reduction, or dose omissions in older versus younger adults. The treatment was well tolerated across all age groups.
Introduction
Cancer is a disease of aging, with older adults composing approximately 60% of the patients with cancer [1]. Although chemotherapy has been shown to benefit both younger and older adults [2, 3], age is a risk factor for experiencing chemotherapy toxicity [4–10]. Despite the association of cancer and aging and the association between age and treatment toxicity risk, the majority of patients enrolled in clinical studies are younger than the mean age of the population of patients with the disease [11]. This under-representation of older adults [12, 13] leads to a shortage of data on efficacy and toxicity of cancer therapy in this population.
A possible explanation for age-related differences in chemotherapy toxicity is variation in drug pharmacokinetics. The majority of studies to date have reported small or no differences in the pharmacokinetics of cancer therapy with increased age; however, most of these studies focused on patients aged ≥65 years and did not include patients across the age spectrum to truly identify whether age played a role [14, 15]. Furthermore, factors in addition to increasing age are associated with an increased risk of chemotherapy toxicity. In a study of 500 patients with cancer receiving standard-of-care chemotherapy, 10 factors in addition to age were identified that placed a patient at increased risk of chemotherapy toxicity. Of the 500-patient cohort, 179 patients were treated with a taxane-based chemotherapy regimen. A predictive model was developed assigning a risk score to each variable. The sum of this score can be used to place patients in a low-, medium-, or high-risk category for toxicity. This score provides insight into the functional age of an older adult; however, the association of these factors and drug pharmacokinetics is unknown.
The overall goal of this study was to evaluate age-related changes in the pharmacokinetics and pharmacodynamics of nanoparticle albumin-bound paclitaxel (nab-paclitaxel), which is commonly used in the treatment of metastatic breast cancer, non-small cell lung cancer, and pancreatic cancer. Nab-paclitaxel consists of nanoparticles of hydrophobic paclitaxel encased in human serum albumin. This composition obviates the need for steroid premedication and is particularly attractive for older adults who may have diabetes or who are at risk of delirium with steroid use. However, the geriatric usage section of the nab-paclitaxel package insert (Abraxane; Celgene, Summit, NJ, http://www.celgene.com) states that only 13% of the 229 patients in the registration clinical trial were aged ≥65 years, and <2% were aged ≥75 years [16]. This study attempts to fill the gap in knowledge of age-related differences in nab-paclitaxel pharmacokinetics and pharmacodynamics and to explore how factors associated with chemotherapy toxicity risk (functional age) are associated with nab-paclitaxel pharmacokinetics.
Patients and Methods
Study Design
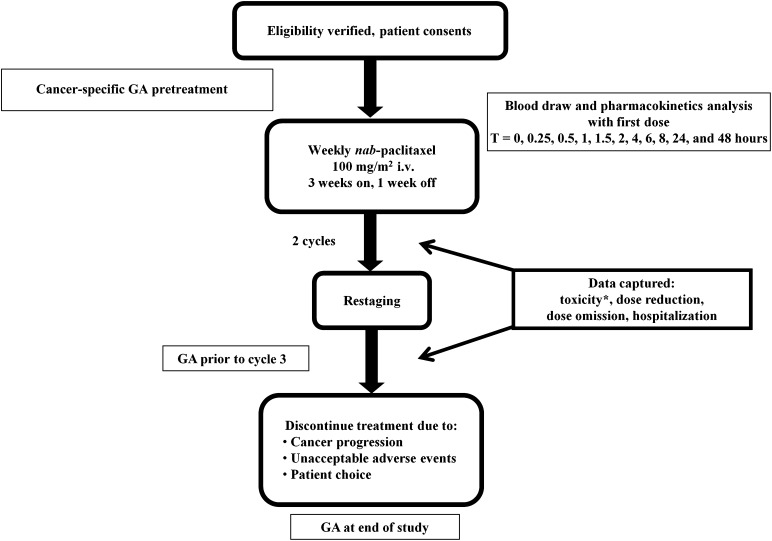
This was an open-label, stratified, single-arm, clinical trial to assess the relationship between age and the pharmacokinetic variables (ln clearance [ln CL] and ln area under the curve [ln AUC]). Forty patients with metastatic breast cancer receiving first- or second-line chemotherapy with weekly nab-paclitaxel were enrolled in this study (Fig. 1). Patients were accrued from four age strata: <50, 50–59, 60–69, and ≥70 years. The primary objective of the study was to determine age-related changes in the pharmacokinetics and pharmacodynamics of weekly nab-paclitaxel. The secondary objectives were to determine response and time to event (progression or toxicity that led to the patient being taken off treatment) and to explore predictors of grade 3 or 4 toxicity or the need for dose reductions and dose omissions. We also explored how the factors associated with chemotherapy toxicity risk [5] are associated with changes in the pharmacokinetics and pharmacodynamics of nab-paclitaxel therapy. This study was approved by the City of Hope National Medical Center institutional review board. All study participants provided written informed consent before enrollment.
Figure 1.
Study schema. ∗, Based on National Cancer Institute Common Terminology Criteria for Adverse Events v3.0: 1, mild; 2, moderate; 3, severe; 4, life-threatening/disabling; 5, death.
Abbreviations: GA, geriatric assessment; nab, nanoparticle albumin-bound; T, time point.
Eligibility Criteria
Patients were eligible if they were aged ≥18 years with metastatic breast cancer, were receiving first- or second-line chemotherapy treatment, and met the following criteria: Karnofsky performance status (KPS) ≥70%; resolution of grade ≥2 toxicity from prior therapy (other than alopecia); grade ≤1 peripheral neuropathy; white blood cell (WBC) count ≥3,000 cells/mm3, absolute neutrophil count (ANC) ≥1,500 cells/mm3, platelets ≥100,000 cells/mm3, and hemoglobin ≥9.0 g/dL; adequate hepatic and renal function (aspartate aminotransferase and alanine aminotransferase ≤2.5 times the institutional upper limit of normal [ULN], alkaline phosphatase ≤2.5 times the ULN unless bone metastases were present in the absence of liver metastases, bilirubin ≤1.5 mg/dL, and creatinine clearance ≥30 mL/min); negative pregnancy test in patients of childbearing potential; and the ability to understand and willingness to sign a written informed consent document. Patients were ineligible if they were receiving any other investigational agents, had untreated brain metastases or symptomatic brain metastases requiring escalating doses of corticosteroids, had a known history of allergic reactions to paclitaxel, had any serious or uncontrolled infection, were lactating, or had received a taxane for adjuvant therapy or metastatic disease in the last 12 months.
Drug Treatment and Sample Collection
Patients were administered i.v. nab-paclitaxel at 100 mg/m2 weekly for 3 weeks followed by a 1-week break, which constituted 1 cycle. Blood samples were collected for pharmacokinetic analysis with the first dose of nab-paclitaxel. Patients were seen prior to treatment in week 1 and week 3 of each cycle. Toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (v3.0) and was adjudicated as attributable to nab-paclitaxel if it was possibly, probably, or definitely related. Treatment was held and the dose was omitted if a patient experienced grade ≥2 sensory neuropathy; subsequently, treatment was resumed at the next lower dose level after the sensory neuropathy improved to grade ≤1. If toxicities grade ≥3 occurred, except for anemia, treatment was withheld until resolution to grade ≤1 or baseline if baseline was higher than grade 1, then reinstituted, if medically appropriate, at the next lower dose level. If the blood counts did not meet the criteria for eligibility (i.e., WBC <3,000 cells/mm3, ANC <1,500 cells/mm3) on the day of treatment, then the treatment was held and granulocyte-colony stimulating factor was given with subsequent treatments. Treatments skipped because of toxicity were omitted, and total cycle length remained the same. Restaging was performed every two cycles. Response was assessed by the Response Evaluation Criteria in Solid Tumors; however, response was not confirmed via repeated computed tomography scans within 4 weeks of response criteria being met. Patients continued on the study until disease progression, unacceptable toxicity, or physician or patient choice to discontinue treatment. Blood for pharmacokinetic sampling was collected during cycle 1, week 1, at the following time points: 0, 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 24, and 48 hours.
Pharmacokinetic Sample and Data Analyses
Total and free paclitaxel in plasma were measured using a modification of the liquid chromatography and tandem mass spectrometry method of Gardner et al. [17]. Briefly, after the addition of d5-paclitaxel (Cambridge Isotope Laboratories, Tewksbury, MA, http://www.isotope.com) as an internal standard, total paclitaxel was extracted from plasma by protein precipitation and free paclitaxel was extracted by ultracentrifugation using a Centrifree micropartition device (EMD Millipore, Billerica, MA, http://www.emdmillipore.com). Following extraction, paclitaxel concentrations were determined by reversed-phase liquid chromatography and tandem mass spectrometry. The lower limit of quantitation for paclitaxel was 4 ng/mL, and the intra- and interday accuracy and precision of the assay were within ±10% of target values.
Pharmacokinetic data analyses were performed using noncompartmental methods according to the rule of linear trapezoids. Individual pharmacokinetic parameter estimates for total paclitaxel (maximum serum concentration [Cmax], AUC [0–24 hours], AUC [0–48 hours], AUC [0 to infinity], and CL) and free paclitaxel (Cmax) were determined and tabulated using summary statistics (means with standard deviations and medians with ranges).
Patient Questionnaire
A patient questionnaire was filled out by participants before the start of treatment, prior to the third cycle, and at the end of the study. It consisted of measures of functional status (activities of daily living [18], instrumental activities of daily living [19], self-reported KPS [20], and number of falls in the last 6 months), comorbidity (Older Americans Resources and Services Physical Health Section [21]), cognition (Blessed Orientation-Memory-Concentration Test [22]), psychological state (Hospital Anxiety and Depression Scale [23]), social functioning and social support (Medical Outcomes Study [18, 24]), and nutrition (unintentional weight loss and body mass index).
Chemotherapy Toxicity Risk
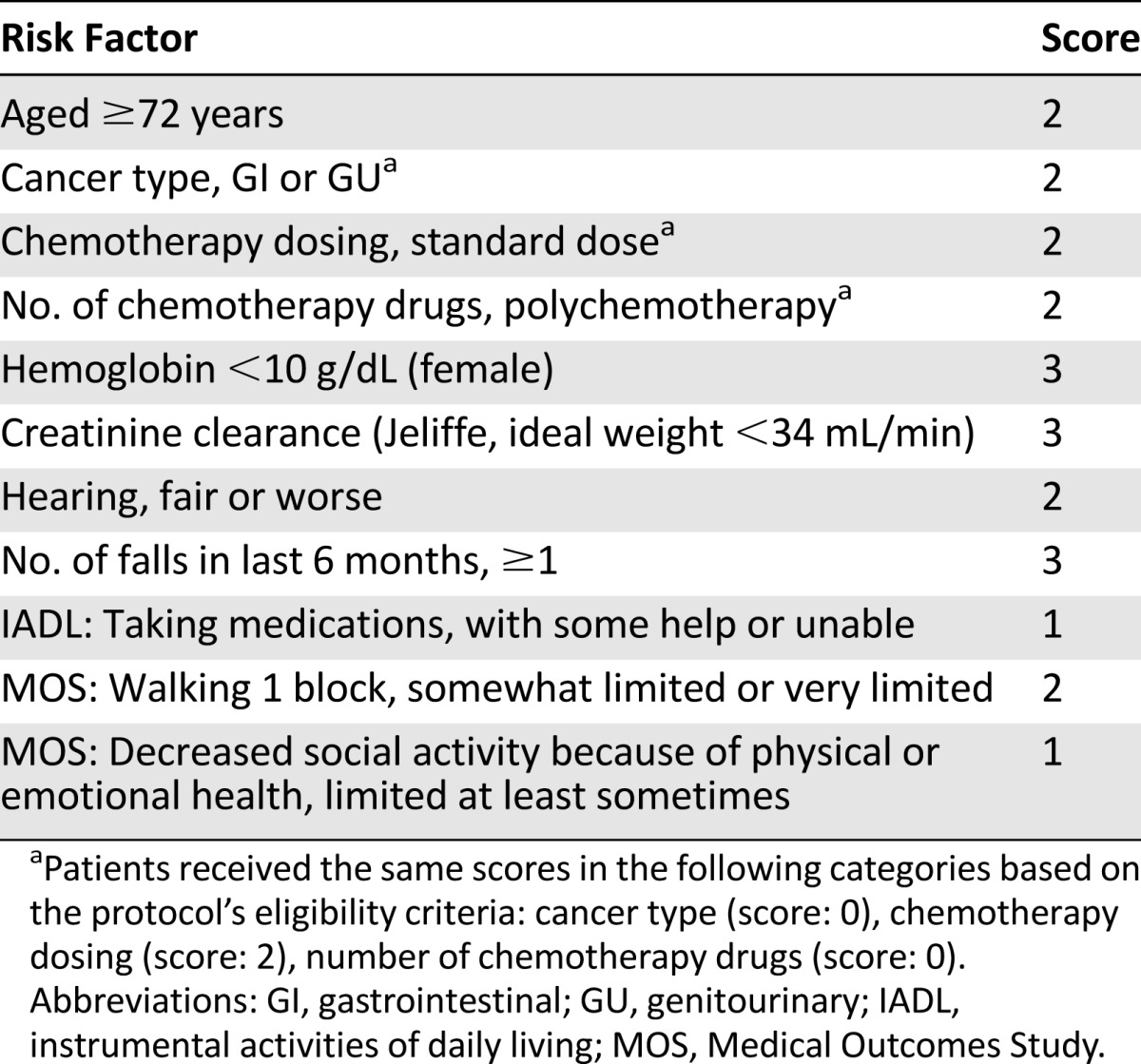
A predictive model for chemotherapy toxicity risk in older adults with cancer has been developed [5]. The variables in the model include patient age (≥72 years); creatinine clearance (<34 mL/min); presence of anemia defined, as <10 g/dL; hearing impairment; falls in the last 6 months; limitations in walking 1 block; the need for assistance with taking medications; and decreased social activities. Additional variables were included in the risk score (number of chemotherapy drugs, chemotherapy dosing [standard vs. dose reduced], tumor type); however, based on the protocol’s eligibility criteria, all participants would have the same score for these specific variables (Table 1).
Table 1.
Chemotherapy toxicity predictive model
Statistical Analysis
This study was designed as an open-label, stratified, single-arm trial to assess the relationship between age and the pharmacokinetic variables ln CL and ln AUC. The sample size of 40 subjects was chosen because it would provide at least 80% power with an α level of 0.05 (R2 can only increase; this study is powered to be comparable to a two-tailed r) to find a relationship between age and ln CL if age predicts at least 17% of the variation in ln CL, assuming a standard deviation of 0.32 [25]. To be assured of getting adequate variability in age, we defined 4 age strata (<50, 50–59, 60–69, and ≥70 years) and required at least 10 patients in the <50 years age group and at least 5 patients in the ≥70 years age group because these two groups were expected to be the most difficult to accrue. Two-sample Student’s t tests were used to compare the mean pharmacokinetic parameters and mean age for differences in the presence of grade ≥3 toxicity, the need for dose reductions, and the need for dose omissions. Mean, median, standard deviation, minimum, and maximum values were provided for the pharmacokinetic parameters. Counts and percentages were provided for maximum-grade toxicities attributable to treatment. Kaplan-Meier methods were used to estimate median event-free survival and overall survival. Post hoc analyses included the use of linear regression analysis to examine the relationship between the chemotherapy toxicity risk score [5], as an explanatory variable, and the pharmacokinetic response variables (ln CL and ln AUC). Fisher’s exact test was used to assess the association between toxicity risk score category and presence of grade ≥3 toxicities attributable to treatment. All analyses were done using the statistical framework R [26].
Results
Patient Characteristics
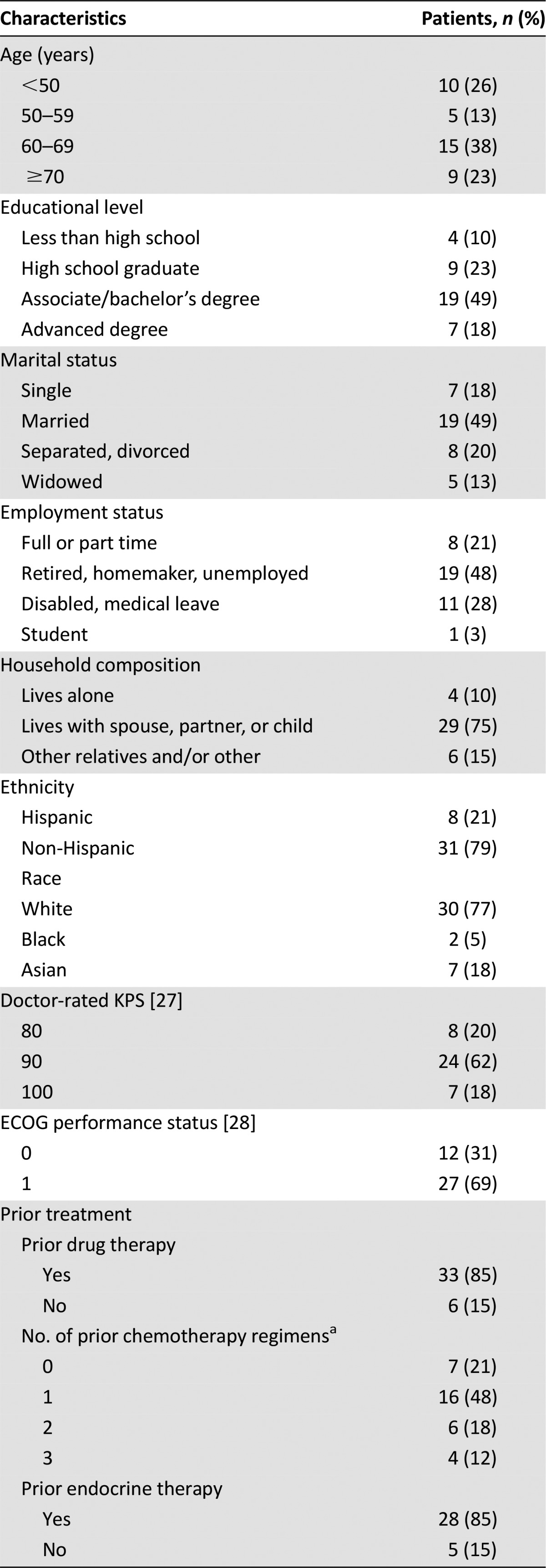
Thirty-nine (mean age: 60 years; range: 30–81 years) of the 40 patients enrolled with metastatic breast cancer who were receiving first- or second-line chemotherapy were evaluable for analysis. One patient was excluded from analysis because she was found to be ineligible after starting study procedures. The sample consisted of 10 patients aged <50 years, 5 patients aged 50–59 years, 15 patients aged 60–69 years, and 9 patients aged ≥70 years. Baseline characteristics are shown in Table 2. Of the 39 evaluable patients, 85% (n = 33) had received prior therapy (of those that received prior therapy 79% [n = 26] had received prior chemotherapy and 85% [n = 28] had received prior endocrine therapy). Seventy-nine percent (n = 31) of the patients had breast cancers that were hormone receptor positive. Our sample was 21% (n = 8) Hispanic and 79% (n = 31) non-Hispanic. The racial breakdown was 18% (n = 7) Asian, 5% (n = 2) black, and 77% (n = 30) white.
Table 2.
Baseline patient demographics and characteristics and questionnaire results
Plasma Pharmacokinetics
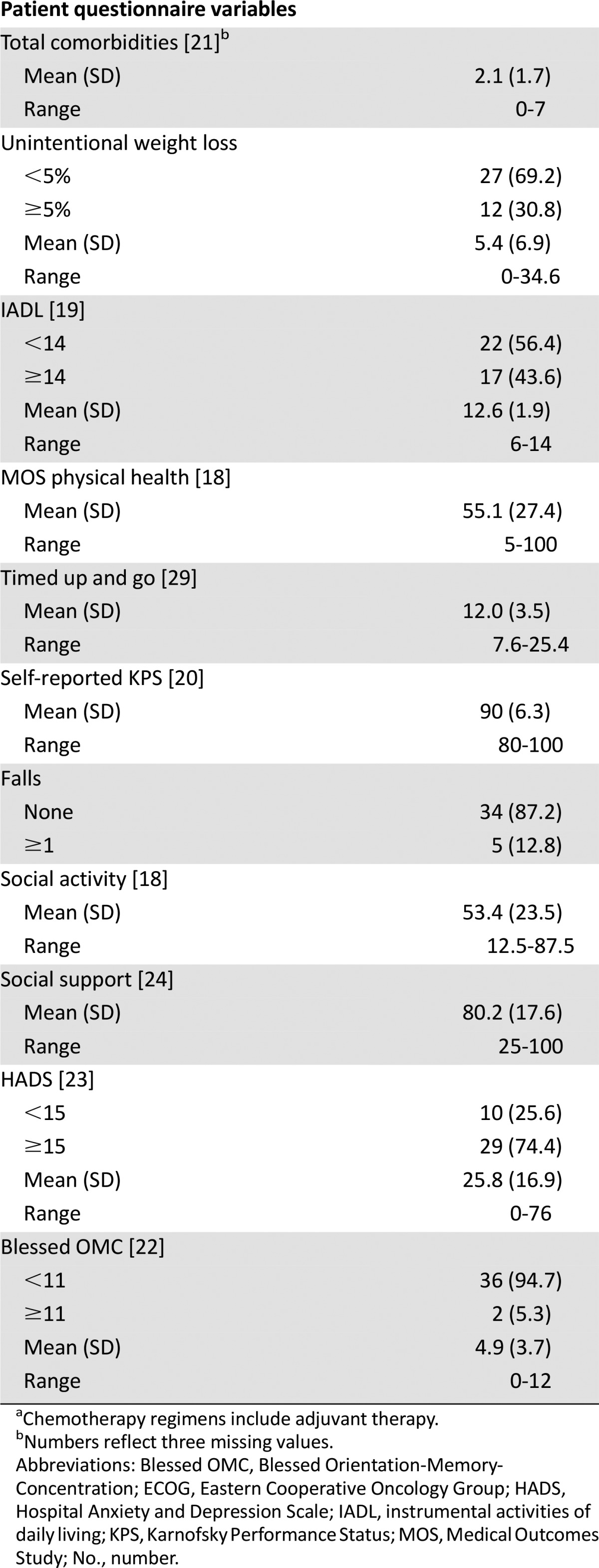
A primary objective of the protocol was to determine whether a relationship existed between the pharmacokinetic parameters and patient age. A borderline significant positive association was noted between patient age and 24-hour ln AUC (ln AUC24; slope = 0.011; standard error [SE] = 0.0057; p = .055; n = 36), whereas the relationship between patient age and ln CL was not significant (slope = −0.0074; SE = 0.0063, p = .25; n = 30). Descriptive statistics for the pharmacokinetic variables for nab-paclitaxel are summarized in Table 3. Of note, data on total plasma paclitaxel AUC (0–24 hours) were available for 36 of 39 subjects enrolled. Total paclitaxel AUC (0–48 hours), AUC (0 to infinity), and CL data were available only in 30 of 39 patients because of incomplete blood sampling. Furthermore, although all collected samples were analyzed for free drug concentrations, the levels of free paclitaxel fell below the limit of quantitation of the assay (4 ng/mL) within 6 hours of the dose in the majority of subjects.
Table 3.
Nanoparticle albumin-bound paclitaxel pharmacokinetic parameters
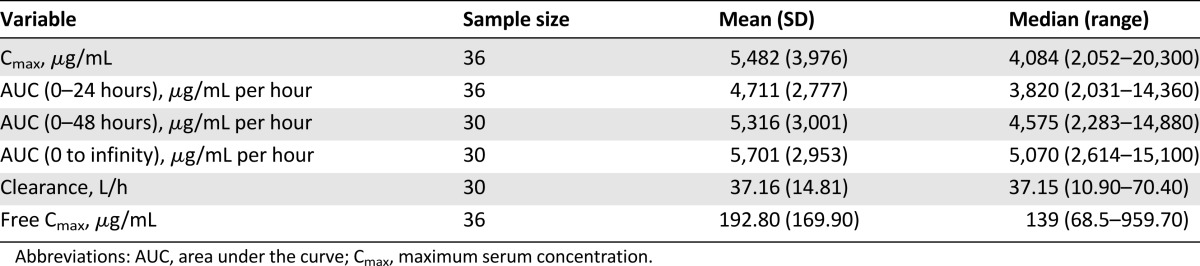
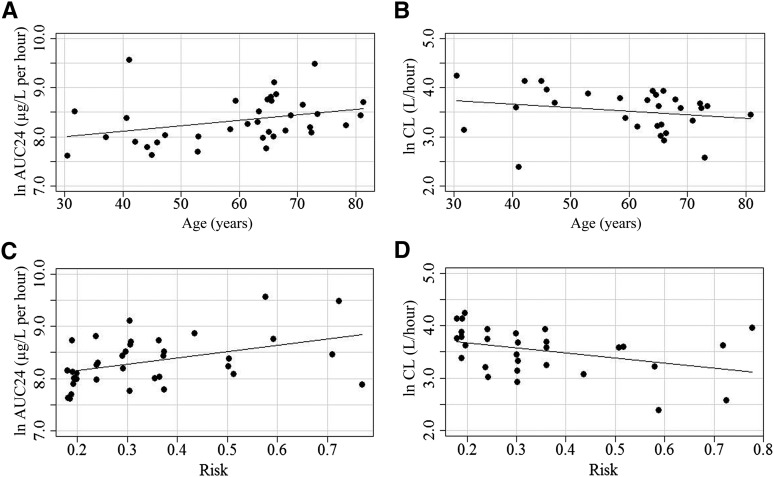
Figure 2 represents the relationship between age and either ln AUC (0–24 hours) and ln CL or between risk score and ln AUC (0–24 hours) and ln CL graphically. In post hoc analyses using univariate linear regression, a significant relationship was found between the chemotherapy toxicity risk score and both ln AUC (0–24 hours; slope = 1.17; SE = 0.45; p = .013; n = 36) (Fig. 2C) and ln CL (slope = −0.96; SE = 0.44; p = .04; n = 30) (Fig. 2D).
Figure 2.
Graphical representations of the linear regression models predicting plasma pharmacokinetic parameters (ln AUC24, ln CL) as response variables based on the explanatory variables of age and toxicity risk score. The regression line and the data points are presented. (A): ln AUC24 by age: ln AUC = 7.66 + 0.011 × age. Slope = 0.011; SE = 0.0057; p = .055. (B): ln CL by age: ln CL = 3.97 − 0.0074 × age. Slope = −0.0074; SE = 0.0063; p = .25. (C): ln AUC24 by risk: ln AUC = 7.93 + 1.17 × risk. Slope = 1.17; SE = 0.45; p = .013. (D): ln CL by risk: ln CL = 3.87 − 0.96 × risk. Slope = −0.96; SE = 0.44; p = .04.
Abbreviations: AUC24, area under the curve (0–24 hours); CL, clearance; SE, standard error.
Response
The endpoints for this protocol included number of courses completed, reasons for going off treatment, tumor response, event-free survival, and overall survival. The median number of courses completed was 4 (range: 1–28). Sixty-nine percent (n = 27) of patients went off treatment because of progression, 10% (n = 4) went off treatment for toxicity, 8% (n = 3) went off treatment due to physician choice because the patients were symptomatically improved and the doctor and patient wanted a chemotherapy holiday, and 13% (n = 5) went off treatment because of patient desire to discontinue treatment. Best tumor response was 31% (n = 12) with partial response (PR), 38% (n = 15) with stable disease (SD), and 26% (n = 10) with progressive disease, and 5% (n = 2) were too early (i.e., went off treatment without tumor measurement). Consequently, there was a PR rate of 31% (95% confidence interval [CI]: 17%–48%) and a combined PR and SD rate of 69% (95% CI: 52%–83%). Median event-free survival was 5.7 months (95% CI: 3.2–9.2 months), and the median overall survival was 19.4 months (95% CI: 15.4–39.9 months).
Pharmacodynamics and Toxicity
Looking at highest grade toxicity experienced, 59% of study participants (n = 23; 95% CI: 42%–74%) experienced grade 2 toxicities attributed to treatment, whereas 26% (n = 10; 95% CI: 13%–42%) experienced grade 3 toxicities attributed to treatment throughout the treatment courses (8 of the 12 events occurred in cycle 1). We observed 8% (n = 3) grade 3 hematologic toxicities (neutrophils [n = 1; 3%], leukocytes [n = 2; 5%]) and 18% (n = 7) grade 3 nonhematologic toxicities (nausea [n = 1; 3%], hypophosphatemia [n = 1; 3%], diarrhea [n = 1; 3%], infection without neutropenia [n = 3; 8%], fatigue [n = 2; 5%], and hyponatremia [n = 1; 3%]). No grade 3 neurotoxicities occurred, but 8% of patients experienced grade 2 peripheral neuropathy. No patients experienced grade 4 or 5 toxicity. Table 4 summarizes the number and percentage of patients who experienced maximum grade 2 and 3 hematologic and nonhematologic toxicities overall and by toxicity type.
Table 4.
Worst grade of toxicity experienced
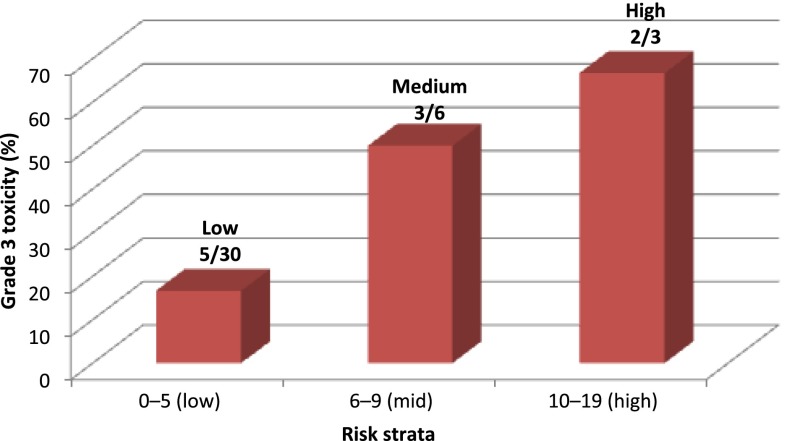
Dose reductions or dose omissions were experienced by 28% (n = 11) and 38% (n = 15), respectively. There were no differences in the mean age based on the presence of grade 3 toxicity (mean difference [MD]: 1.68; 95% CI: −9.3 to 12.7; n = 39; p = .75), need for dose reductions (MD: −4.89; 95% CI: −16.5 to 6.7; n = 39; p = .38), or need for dose omissions (MD: 5.81; 95% CI: −2.3 to 13.9; n = 39; p = .15). There were no differences in the mean CL or mean AUC based on the presence of grade ≥3 toxicity (CL: MD: −0.21; 95% CI: −0.67 to 0.25; n = 30; p = .34; AUC: MD: 0.34; 95% CI: −0.12 to 0.81; n = 36; p = .13), need for dose reductions (CL: MD: −0.063; 95% CI: −0.49 to 0.36; n = 30; p = .75; AUC: MD: 0.058; 95% CI: −0.33 to 0.44; n = 36; p = .76), or need for dose omissions (CL: MD: 0.1; 95% CI: −0.22 to 0.42; n = 30; p = .51; AUC: MD: −0.079; 95% CI: −0.39 to 0.23; n = 36; p = .61). Post hoc analysis using Fisher’s exact test determined that there was a significant association between chemotherapy toxicity risk score category and the presence of grade 3 toxicity attributed to treatment (toxicity rate by risk category: low risk, 5 of 30 patients; medium risk, 3 of 6 patients; high risk, 2 of 3 patients; p = .041) (Fig. 3).
Figure 3.
Toxicity rate by risk score distribution.
Discussion
This study was designed to elucidate the relationship between patient age and the pharmacokinetics and pharmacodynamics of weekly nab-paclitaxel among patients with metastatic breast cancer. We found a borderline significant relationship between patient age and pharmacokinetics, as measured by the 24-hour AUC. The treatment was well tolerated, and there was no significant relationship between patient age and the risk of toxicity. Post hoc analyses found that the patient’s chemotherapy toxicity risk score [5] was associated with both the risk of toxicity and the pharmacokinetic parameters (24-hour ln AUC, ln CL).
The pharmacokinetics of paclitaxel have been evaluated in other studies. Lichtman et al. evaluated the pharmacokinetics of paclitaxel 175 mg/m2 i.v. over 3 hours, delivered every 3 weeks. The pharmacokinetic data from 122 patients demonstrated that increasing age was associated with increased AUC and decreased CL of paclitaxel. In addition, increasing age was associated with a higher risk of grade ≥3 neutropenia and lower ANC, although there was no increase in hospitalization, receipt of i.v. antibiotics, or fever (body temperature >38°C) [30]. Fidias and colleagues evaluated the pharmacokinetics of weekly paclitaxel in 35 patients aged ≥70 years. Pharmacokinetic sampling was performed in the first and sixth cycles in eight patients. The authors compared these results with values reported in a younger cohort and found no significant difference by age. In addition, no significant difference in paclitaxel pharmacokinetics was seen with repeated dosing [31]. Smorenburg and colleagues evaluated the pharmacokinetics of weekly paclitaxel in 15 patients aged <70 years (paclitaxel 100 mg/m2 i.v. administered over 1 hour) and eight patients aged ≥70 years (paclitaxel 80 mg/m2 i.v. administered over 1 hour) and found that older adults had lower CL of bound and unbound paclitaxel. The older and younger patients experienced similar decreases in ANC and WBC count despite older adults receiving a lower amount of paclitaxel [32]. Our study adds to this literature by evaluating nab-paclitaxel, specifically, recruiting patients across the age spectrum to understand the association of age and pharmacokinetics of nab-paclitaxel. Furthermore, the same dose and schedule of nab-paclitaxel were administered in younger and older patients, allowing for direct comparison of toxicity, dose reductions, and dose omissions.
Although our study did not find an age-related difference in toxicity patterns, numerous studies in the literature demonstrate that older age is a risk factor for toxicity. The study by Lichtman et al. [30] evaluating paclitaxel suggests an age-related increase in myelosuppression. The mechanisms underlying the association between increased chronological age and poorer treatment tolerance is likely multifactorial in nature. First, with aging comes a progressive decrease in physiologic reserve, with a decline in organ function across the age spectrum that occurs at a unique pace for each person [33, 34]. Because either cancer or cancer treatment can be a form of physiologic stress, the age-related decrease in physiologic reserve may affect one’s ability to tolerate cancer treatment. Second, there are age-related changes in drug absorption, distribution, metabolism, and excretion that could affect the pharmacokinetics of cancer treatment and potentially lead to higher drug levels and heightened toxicity. Third, factors in addition to age can identify older patients who are at increased risk of chemotherapy toxicity. These factors give a sense of the functional age of an older individual. However, the predictive value of these factors has not been applied across the age spectrum, and the association of these factors with drug pharmacokinetics is unknown.
We systematically evaluated each of these factors in this study. First, we included patients across the age spectrum (with specific inclusion and exclusion criteria with regard to organ function) to truly understand the association between age and drug pharmacokinetics. In addition, we sought to understand the functional age of the patients by calculating a chemotherapy toxicity risk score. This predictive model for chemotherapy toxicity [5], applied to this cohort, was able to identify patients at higher toxicity risk and those patients with changes in drug pharmacokinetics. These results provide support for the hypothesis that chronological age alone is not an adequate marker of toxicity risk or drug pharmacokinetics, and a more detailed assessment is needed to understand functional age. In fact, metastatic breast cancer or its treatment may geriatricize some younger patients, placing them at even greater risk for toxicity and pharmacokinetic changes than an older, healthy, and fit patient.
Our study has limitations. First, the data about the toxicity risk score was published after our study was already under way. Consequently, these analyses were post hoc and hypothesis generating. Furthermore, the toxicity risk score was developed in patients aged ≥65 years across all tumor types, and in this study, we applied it to patients with breast cancer across all age groups.
Despite these limitations, this study has important strengths. It adds to the literature regarding changes in drug pharmacokinetics and pharmacodynamics across the age spectrum. Furthermore, it provides insight into the potential association between functional age and drug pharmacokinetics. Further studies are needed to establish whether this association is valid.
Acknowledgments
This study was supported by funding from Celgene Corporation (previously Abraxis Bio Science) and was presented at the 35th Annual San Antonio Breast Cancer Symposium, San Antonio, Texas, December 4–8, 2012 (ClinicalTrials.gov identifier NCT00609791). This research was also supported by the Analytical Pharmacology Core Facility of the City of Hope and a Cancer Center Support Grant (CA033572).
Author Contributions
Conception/Design: Arti Hurria, M. Suzette Blanchard, Timothy W. Synold, George Somlo
Provision of study material or patients: Arti Hurria, Joanne Mortimer, Cathie T. Chung, Thehang Luu, George Somlo
Collection and/or assembly of data: Arti Hurria, M. Suzette Blanchard, Cathie T. Chung, Thehang Luu, Vani Katheria, Arnold J. Rotter, Carol Wong, Rupal Ramani, Jaycen Brown
Data analysis and interpretation: Arti Hurria, M. Suzette Blanchard, Timothy W. Synold, Joanne Mortimer, Vani Katheria, Anthony Choi, Tao Feng, Caroline M. Doan, George Somlo
Manuscript writing: Arti Hurria, M. Suzette Blanchard, Timothy W. Synold, Joanne Mortimer, Cathie T. Chung, Thehang Luu, Vani Katheria, Arnold J. Rotter, Carol Wong, Anthony Choi, Tao Feng, Rupal Ramani, Caroline M. Doan, Jaycen Brown, George Somlo
Final approval of manuscript: Arti Hurria, M. Suzette Blanchard, Timothy W. Synold, Joanne Mortimer, Cathie T. Chung, Thehang Luu, Vani Katheria, Arnold J. Rotter, Carol Wong, Anthony Choi, Tao Feng, Rupal Ramani, Caroline M. Doan, Jaycen Brown, George Somlo
Disclosures
Arti Hurria: Seattle Genetics, GTx, Inc. (C/A); Celgene, GlaxoSmithKline (RF); George Somlo: Celgene, Millennium, Pfizer, Genoptix (C/A); Celgene, Genentech (RF); Cathie T. Chung: Celgene (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Howlander N, Noone NA, Krapcho M et al eds. SEER cancer statistics review, 1975-2010. Available at http://seer.cancer.gov/csr/1975_2010/. Updated June 14, 2013.
- 2.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293:1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 3.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 4.Muss HB, Berry DA, Cirrincione C, et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: The Cancer and Leukemia Group B experience. J Clin Oncol. 2007;25:3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 5.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 7.Aparicio T, Jouve JL, Teillet L, et al. Geriatric factors predict chemotherapy feasibility: Ancillary results of FFCD 2001-02 phase III study in first-line chemotherapy for metastatic colorectal cancer in elderly patients. J Clin Oncol. 2013;31:1464–1470. doi: 10.1200/JCO.2012.42.9894. [DOI] [PubMed] [Google Scholar]
- 8.Wildes TM, Ruwe AP, Fournier C, et al. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J Geriatr Oncol. 2013;4:227–234. doi: 10.1016/j.jgo.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramjaun A, Nassif MO, Krotneva S, et al. Improved targeting of cancer care for older patients: A systematic review of the utility of comprehensive geriatric assessment. J Geriatr Oncol. 2013;4:271–281. doi: 10.1016/j.jgo.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Extermann M, Wedding U. Comorbidity and geriatric assessment for older patients with hematologic malignancies: A review of the evidence. J Geriatr Oncol. 2012;3:49–57. [Google Scholar]
- 11.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 12.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 13.Trimble EL, Carter CL, Cain D, et al. Representation of older patients in cancer treatment trials. Cancer. 1994;74(suppl):2208–2214. doi: 10.1002/1097-0142(19941001)74:7+<2208::aid-cncr2820741737>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Hurria A, Lichtman SM. Pharmacokinetics of chemotherapy in the older patient. Cancer Contr. 2007;14:32–43. doi: 10.1177/107327480701400105. [DOI] [PubMed] [Google Scholar]
- 15.Hurria A, Lichtman SM. Clinical pharmacology of cancer therapies in older adults. Br J Cancer. 2008;98:517–522. doi: 10.1038/sj.bjc.6604201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraxane [package insert]. Summit, NJ: Celgene Corporation, 2013. Available at http://www.abraxane.com/downloads/Abraxane_PrescribingInformation.pdf. Accessed July 8, 2013.
- 17.Gardner ER, Dahut W, Figg WD. Quantitative determination of total and unbound paclitaxel in human plasma following Abraxane treatment. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;862:213–218. doi: 10.1016/j.jchromb.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart AL, Ware JE. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992. [Google Scholar]
- 19.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 20.Loprinzi CL, Laurie JA, Wieand HS, et al. Prospective evaluation of prognostic variables from patient-completed questionnaires. J Clin Oncol. 1994;12:601–607. doi: 10.1200/JCO.1994.12.3.601. [DOI] [PubMed] [Google Scholar]
- 21.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 22.Katzman R, Brown T, Fuld P, et al. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 25.Nyman DW, Campbell KJ, Hersh E, et al. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23:7785–7793. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 26.The R project for statistical computing. Available at http://www.R-project.org.
- 27.Karnofsky D, Burchenal J. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1948. pp. 191–205. [Google Scholar]
- 28.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 29.Podsiadlo D, Richardson S. The timed “up & go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 30.Lichtman SM, Hollis D, Miller AA, et al. Prospective evaluation of the relationship of patient age and paclitaxel clinical pharmacology: Cancer and Leukemia Group B (CALGB 9762) J Clin Oncol. 2006;24:1846–1851. doi: 10.1200/JCO.2005.03.9289. [DOI] [PubMed] [Google Scholar]
- 31.Fidias P, Supko JG, Martins R, et al. A phase II study of weekly paclitaxel in elderly patients with advanced non-small cell lung cancer. Clin Cancer Res. 2001;7:3942–3949. [PubMed] [Google Scholar]
- 32.Smorenburg CH, ten Tije AJ, Verweij J, et al. Altered clearance of unbound paclitaxel in elderly patients with metastatic breast cancer. Eur J Cancer. 2003;39:196–202. doi: 10.1016/s0959-8049(02)00611-1. [DOI] [PubMed] [Google Scholar]
- 33.Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11:449–460. doi: 10.1097/00130404-200511000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Sehl M, Sawhney R, Naeim A. Physiologic aspects of aging: Impact on cancer management and decision making, part II. Cancer J. 2005;11:461–473. doi: 10.1097/00130404-200511000-00005. [DOI] [PubMed] [Google Scholar]