Endogenous thyroxine may be safely and effectively reduced or depleted in end-stage solid tumor patients and replaced with exogenous triiodothyronine (T3). This change served to stabilize or improve the clinical status of patients with advanced cancers. Therapeutic induction of euthyroid hypothyroxinemia was safe and appeared to be compatible with conventional end-stage anticancer measures or used as sole therapy. This novel treatment paradigm is immediately available to medical practitioners involved in palliative cancer care.
Keywords: Hypothyroxinemia, Triiodothyronine, Cancer, Survival
Abstract
Background.
Clinical studies have shown that interventional lowering of serum free thyroxine (FT4) may be associated with extended survival in patients with some terminal cancers. The report of success with this approach in glioblastoma multiforme caused involvement of the author (A.H.) in the prospective consultative management of 23 end-stage solid tumor patients in whom hypothyroxinemia was induced to prolong life.
Patients and Methods.
Patients were self-referred or recommended by attending physicians to the author (A.H.) and had advanced cancers of the brain, ovary, lung, pancreas, salivary gland, and breast or had mesothelioma or soft-tissue sarcoma. Hypothyroxinemia was achieved in euthyroid patients by using methimazole, with the addition of 3,3′,5-triiodo-l-thyronine (l-T3) to prevent hypothyroidism and suppress endogenous thyrotropin (TSH). In patients with pre-existent primary hypothyroidism, T3 administration was substituted for T4 replacement. Serum FT4 and TSH concentrations were serially monitored to enable adjustments to drug therapy and prevent clinical hypothyroidism. Survival was measured from the date of hypothyroxinemia induction with T3 or methimazole plus T3. Outcomes were compared with the odds of death based on the Surveillance Epidemiology and End Results and American Joint Committee on Cancer databases and literature reports.
Results.
The survival time of 83% (19 of 23) of patients exceeded the 20% expected 1-year survival for this hypothyroxinemic, end-stage cancer group. The difference between actual and expected survival was significant.
Conclusion.
Although this is an uncontrolled observational experience with frank limitations, compassionate medical induction of hypothyroxinemia should be considered for patients with advanced cancers to whom other avenues of treatment are closed.
Implications for Practice:
There is increasing documentation, but still limited general appreciation, of the proliferative effect of circulating l-thyroxine (l-T4) on solid tumors. The novelty of the current set of case histories is that endogenous T4 may be safely and effectively reduced or depleted in end-stage solid tumor patients and replaced with exogenous triiodothyronine (T3). This change served to stabilize or improve the clinical status of patients with advanced cancers. In the 23 patient cases collected in this report, therapeutic induction of euthyroid hypothyroxinemia was safe and appeared to be compatible with conventional end-stage anticancer measures or used as sole therapy. This novel treatment paradigm, which features generic T3, usually with generic methimazole, is immediately available to medical practitioners involved in palliative cancer care.
Introduction
Thyroid hormone plays a major role in physiologic processes crucial to growth, maturation, and metabolism [1]. It has been hypothesized that thyroid hormone is an endogenous modulator of malignant tumors that may affect disease prognosis [2, 3], and supportive clinical literature has recently been reviewed by Moeller and Fuhrer [4]. In a phase II clinical trial of recurrent glioblastoma multiforme (GBM) conducted by the author (A.H.), medically induced hypothyroidism was associated with significantly longer progression-free survival and overall survival rates [5, 6]. In that study, it was found that prolongation of survival correlated significantly and independently with circulating free thyroxine (FT4) levels that had declined to values below the lower limit of the reference range. The drawbacks in this GBM study were delay in depletion of blood FT4—50% of patients died prior to reaching that FT4 target—and clinical morbidity, principally fatigue, in those who became hypothyroid. A result of the publication of this GBM study was informal consultation of the author (A.H.) by a panel of patients with terminal cancers and by caregivers for such patients. This panel of cancer patients forms the basis for the current report.
The association between thyroid hormone and cancer has become mechanistically better understood with the description of receptors for l-thyroxine (l-T4) and 3,3′,5-triiodo-l-thyronine (l-T3) on plasma membrane integrin αvβ3 [7]. The integrin is amply expressed by cancer cells and rapidly dividing endothelial cells and is not well expressed by or activated in quiescent, nonmalignant cells [3]. Among the nongenomic actions of T4 and T3 initiated at αvβ3 are stimulation of angiogenesis by multiple mechanisms [8], of tumor cell proliferation, and of antiapoptotic tumor defenses [9, 10]. These actions are inhibited by the deaminated analog of T4, tetraiodothyroacetic acid (tetrac), which inhibits binding of iodothyronines to αvβ3 [3, 10].
Within the cell, T4 serves as a prohormone for T3, and T3 is the metabolically and genomically important form of thyroid hormone [1]. At the integrin, in contrast, the thyroid hormone receptor affinity for T4 is higher than that for T3 [7], and T4 is a more potent inducer of tumor cell proliferation than is T3 [9]. On the basis of these data [3, 9], exogenous l-T3 was administered as compassionate care to a patient with rapidly progressive GBM whose daily propylthiouracil had failed to induce hypothyroidism. Addition of T3 rapidly reduced serum thyrotropin (TSH) and FT4 levels and was associated with rapid clinical and radiologic improvement [11]. The current report is a retrospective analysis of a group of 23 patients with advanced, incurable solid tumors in whom T3 was substituted for T4 for coexistent hypothyroidism or was added to methimazole to securely reduce serum FT4 via suppression of pituitary TSH secretion but to maintain eumetabolism. This approach was taken in response to patient or caregiver requests from widely dispersed geographic sites to treat poor-prognosis patients on a compassionate-need basis. Some patients were on conventional treatments under oncologic care, and others were on nononcologic, palliative support and managed or supervised by a personal physician, internist, or endocrinologist.
Patients and Methods
Patients
Patients with stage IV or recurrent, progressive malignancy deemed incurable by conventional means were identified to the author (A.H.) by their primary care physicians or oncologists. Several patients contacted the author directly. T3-substitution treatment was offered by the author (A.H.) as compassionate care to patients without interrupting any ongoing conventional oncologic treatment. Patients were presented with detailed evidence to support the rationale for the use of thyroid-related medications to modulate blood thyroid hormone levels in an effort to prolong survival and with the risks of the proposed treatment. Adjustments in thyroid medications were made as needed through the professional caregivers. No formal research protocol was submitted to an institutional review board, and no consent form was signed by the investigator and each patient. Patients were at geographically dispersed sites within and outside the U.S. Communication was by e-mail, telephone, and fax. Imaging study (computed tomography, positron emission tomography, and magnetic resonance imaging) reports and, in some cases, the CD-ROM discs were made available for central radiologic review by one of the authors (R.E.J.).
Blood Thyroid Function Tests
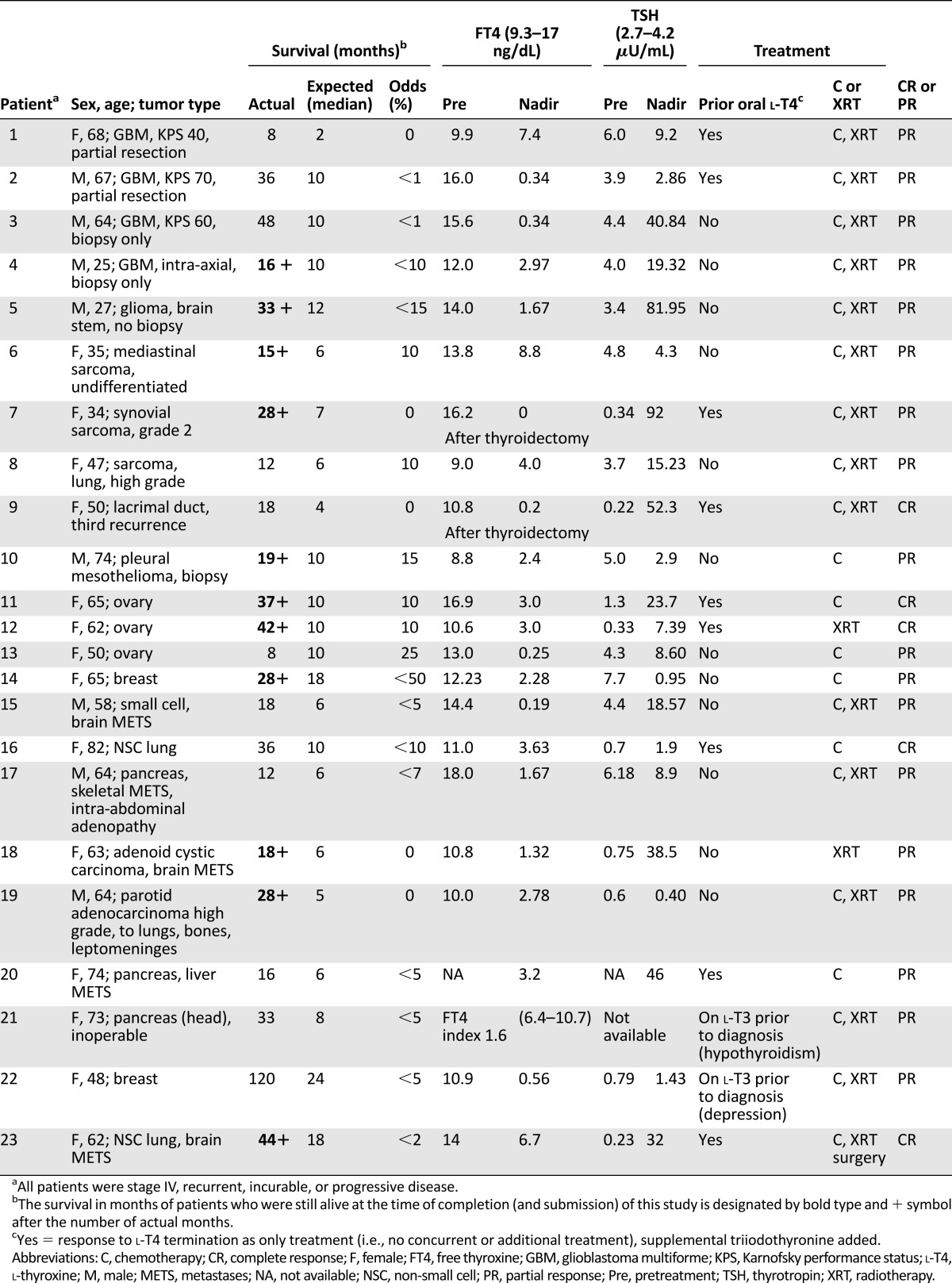
Serum TSH and FT4 levels and, as feasible, FT3 were obtained at regular intervals, usually every 3–6 weeks. The pretreatment and maximally suppressed (nadir) serum FT4 levels are reported in Table 1. TSH levels are also reported at these points but are insufficient in number to describe mean time to nadir.
Table 1.
Patient survival in relation to thyroxine depletion: overall survival, statistical survival comparisons, FT4/TSH response, and tumor response
Adjustment of Patients’ FT4 Levels
Passive T4 Depletion Following Discontinuation of l-T4 Replacement for Pre-Existing Hypothyroidism in Cancer Patients
Patients were converted to T3 abruptly from l-T4 (50–88 μg daily). After a 1-week washout period, exogenous l-T3 15–37.5 μg/day was begun in 2 or 3 daily divided doses. In all patients, serum FT4 levels decreased to below the lower limit of the reference range and to a nadir by 7 weeks after the start of T3 (range: 3–7 weeks; n = 9) (Table 1). Patient comfort level and functional status, not the serum TSH values, determined the dose of l-T3.
Active Depletion of T4 With Methimazole and l-T3
The antithyroid thionamide methimazole was used to interrupt production of T4 by the thyroid gland. T3 suppressed endogenous TSH and maintained the clinical euthyroid state. Methimazole has been in clinical use for >50 years and has a well-documented clinical safety record and lower toxicity compared with propylthiouracil [12]. This l-T3/methimazole regimen was usually initiated within 3 months of patients’ receipt of the clinical state of terminal or incurable disease or following failure of first- or second-line conventional therapy (n = 12) (Table 1). Methimazole 40–50 mg/day (single dose or divided doses) and l-T3 5–6.25 μg 3 times per day were used initially while adjusting doses at 2- to 4-week intervals until serum TSH concentration was reduced to the lower limit of the reference range and FT4 had fallen below reference range and stabilized. The final doses of methimazole ranged from 20 to 50 mg/day in single or divided doses and l-T3 ranged from 5 to 12.5 μg 2 or 3 times per day up to a maximum of 37.5 μg/day.
Patients on l-T3 at presentation
There was no change in l-T3 supplementation in these patients (n = 2).
Patient exclusion
Three patients with whom communication was inadequate voluntarily left the program. Patients who had begun treatment but did not achieve FT4 depletion for a period of at least 3 months—apparently because of noncompliance—or who discontinued treatment were excluded from this analysis.
Data Analysis
Survival was calculated from the day of initiation of the FT4-depletion regimen after diagnosis of recurrence or progression compared with each individual disease type using Surveillance, Epidemiology, and End Results (SEER) and American Joint Committee on Cancer data [12, 13] for tumor-specific overall survival. Although overall survival may be the more significant outcome, it was not feasible to assess in our study. Survival, for example, was 48 months in patient 3 with a GBM versus 10 months in the SEER database. The Kolmogorov-Smirnoff [14] nonparametric test was used to analyze whether the differences between the actual and the (median) database-expected survival shown in Table 1 were statistically significant. Given that there were 14 separate categories of solid tumor types for analysis, another risk factor quantified was odds of survival, defined as the likelihood of death for each patient at 1 year or at last follow-up, expressed as percentage of patients from large databases who were alive at the same time point. Tumor response was evaluated according to Response Evaluation Criteria in Solid Tumors imaging criteria [15].
Results
Results are shown in Table 1. The difference between actual and expected survival was significant at the p < .01 level in comparisons of the total population (men and women, n = 23), men only (n = 8), and women only (n = 15). The odds of surviving at least 12 months for 19 of 23 individual patients were estimated to be lower than 15%–20%, whereas 19 of 23 from the managed group (83%) survived more than 12 months and 12 of 23 (52%) survived more than 24 months versus an estimated 1 of 23 (4.4%). Patients 21 and 22, who had been on exogenous l-T3 at diagnosis for hypothyroidism and depression, respectively, survived significantly beyond expectation. Patient 21 died from a pulmonary embolus and not from disease progression.
The extent of T4 depletion was variable. In the longer surviving patients, the decline became more significant with time. In all patients, serum FT4 levels declined below the reference range, or by at least 50% of baseline by 9 weeks (range: 4–9 weeks), until reaching a nadir. In some, blood thyroid-function tests were carried out at less-than-regular intervals, as long as there was adherence to the medication protocol; these patients maintained low serum FT4 levels long term and reached a nadir point.
All patients reported in this panel had been managed with conventional chemotherapy and radiation.
Radiologically documented tumor regression was observed following exogenous l-T4 withdrawal alone (n = 3). These were unusually rapid and durable responses in association with chemotherapy and/or radiotherapy. Overall response rate (complete and partial [15]) was 100%, that is, there was complete response (CR) in 5 and partial response (PR) in 18. Some patients (10 of 23) had multiple types of chemotherapy and experienced both CR and/or PR after a change in treatment.
Side effects were reported infrequently, and generally the patient responded rapidly to an increase in l-T3 dosage. There were no clear instances of methimazole-related toxicity, such as hepatotoxicity or hematologic (e.g., thrombocytopenia) toxicity, that could be differentiated from concurrent chemotherapy.
Discussion
In this compassionate-need study of terminal patients with a variety of incurable solid tumors, extended survival was observed in a majority of patients using exogenous T3 to induce and maintain hypothyroxinemia. T3 administration prevented symptomatic hypothyroidism. Low odds of survival were surmounted in 19 of 23 patients (83%) who exceeded the expected median survival of literature-reported series used as controls. Conventional modalities of cancer treatment had been exhausted for these patients.
The present observational data are consistent with earlier epidemiologic and clinical evidence suggesting that survival is improved in patients with hypothyroidism harboring various solid tumors, as reviewed by Hercbergs et al. [16] and others [4]. In preclinical studies, T4 enhances cancer cell proliferation, migration, invasion, and angiogenesis [3, 10, 17], apparently acting via the thyroid hormone receptor on cell surface integrin αvβ3 [3, 4]. Supraphysiologic amounts of T3 are required at this receptor to stimulate tumor cell proliferation [3, 9]. The highly unusual instances of rapid tumor regression observed following exogenous l-T4 discontinuation in the case histories provided in this paper in a variety of solid tumors are unusual in non-sex-hormone-dependent tumors (cf., prostate or breast carcinoma). Occlusion of the thyroxine receptors on integrin αvβ3 in a human breast cancer (MDA-MB-231) cell line [18] and medullary thyroid carcinoma cells [19] induces significant modification of cell-survival-pathway gene expression that may be reproduced by acute thyroid hormone depletion. The association of thyroid hormone and cancer has been studied experimentally in additional tumor cell lines and xenografts [20–23].
Other studies have identified and differentiated between the significant pro-oncogenic mitogenicity of physiologic T4 levels and the significantly lower mitogenicity of physiologic T3 levels in human and rodent tumor models, such as glioma [17] and non-small cell lung carcinoma cells [24, 25]. Taken together, these studies show the divergence between the predominantly metabolic actions (with reduced oncogenic potential) of T3 and the pro-oncogenic actions of T4. The divergence constitutes the basis for replacing circulating T4 with exogenous l-T3 in cancer patients.
The current observations are the outcome of a series of consultations and are not from a prospective controlled study. However, we extended the observations and strategy we made prospectively in end-stage glioma patients to terminally ill patients with other cancers. We recommended to treating physicians the use of a clinical agent, T3, or of T3 and methimazole, which are widely appreciated to be safe at the dosages used. The novel paradigm is off-label drug use in a right-to-try environment in a palliative care patient population. The strategy deserves prospective evaluation.
Conclusion
In this small, compassionate-need, observational study of unselected terminal patients with incurable solid tumors, extended survival was observed in a majority of patients using exogenous l-T3 to simultaneously induce hypothyroxinemia and obviate clinically symptomatic hypothyroidism. Overall, 83% of subjects exceeded the expected (median) survival of reported series. Statistical analysis was limited by the small numbers of patients in each disease category. In a few patients, radiologically documented tumor responses were associated with clinical improvement shortly after discontinuation of exogenous T4 supplementation. These survival outcomes are in line with the in vitro evidence [9, 17] differentiating between the much-reduced pro-oncogenic effects of physiologic T3 from those of T4 on cancer cell growth. An additional shortcoming of the current study is that we do not have estimates of functional status of the treated patients. We suggest that these preliminary results represent a clinically measurable validation of the significant divergence of action of T4 from T3 on tumor cells and may represent a novel, inexpensive, nontoxic approach for improving outcomes in solid tumor patients. The paradigm should be tested in prospective controlled trials in a wider variety of tumor types.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
We thank Harold Sacks (Department of Endocrinology, Veterans Administration, Los Angeles, CA) and Raphael Melmed, F.R.C.P. (Department of Medicine, Hadassah Hospital, Jerusalem, Israel) for their helpful comments. Dr. Kelly Keating (Pharmaceutical Research Institute, Albany College of Pharmacy and Health Sciences) made essential contributions to this article as an advisor to the authors and copyeditor of the text (as part of her regular employment).
Author Contributions
Conception/Design: Aleck Hercbergs, Paul J. Davis
Provision of study material or patients: Aleck Hercbergs, Osnat Ashur-Fabian, David H. Garfield
Collection and/or assembly of data: Aleck Hercbergs, Rebecca E. Johnson, David H. Garfield
Data analysis and interpretation: Aleck Hercbergs, Rebecca E. Johnson, Paul J. Davis
Manuscript writing: Aleck Hercbergs, Paul J. Davis
Final approval of manuscript: Aleck Hercbergs, Rebecca E. Johnson, Osnat Ashur-Fabian, David H. Garfield, Paul J. Davis
Disclosures
Osnat Ashur-Fabian: Patent pending on reducing T4 for cancer treatment (IP). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hercbergs A. The thyroid gland as an intrinsic biologic response-modifier in advanced neoplasia—a novel paradigm. In Vivo. 1996;10:245–247. [PubMed] [Google Scholar]
- 3.Davis PJ, Davis FB, Mousa SA, et al. Membrane receptor for thyroid hormone: Physiologic and pharmacologic implications. Annu Rev Pharmacol Toxicol. 2011;51:99–115. doi: 10.1146/annurev-pharmtox-010510-100512. [DOI] [PubMed] [Google Scholar]
- 4.Moeller LC, Führer D. Thyroid hormone, thyroid hormone receptors, and cancer: A clinical perspective. Endocr Relat Cancer. 2013;20:R19–R29. doi: 10.1530/ERC-12-0219. [DOI] [PubMed] [Google Scholar]
- 5.Hercbergs AA, Goyal LK, Suh JH, et al. Propylthiouracil-induced chemical hypothyroidism with high-dose tamoxifen prolongs survival in recurrent high grade glioma: A phase I/II study. Anticancer Res. 2003;23:617–626. [PubMed] [Google Scholar]
- 6.Hercbergs A, Suh J, Reddy C et al. Early onset propylthiouracil induced hypothyroidism is associated with improved survival in recurrent high grade glioma [abstract 1211]. Presented at: American Association of Cancer Research annual meeting; April 12–16, 2008; San Diego, CA. [Google Scholar]
- 7.Bergh JJ, Lin HY, Lansing L, et al. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology. 2005;146:2864–2871. doi: 10.1210/en.2005-0102. [DOI] [PubMed] [Google Scholar]
- 8.Luidens MK, Mousa SA, Davis FB, et al. Thyroid hormone and angiogenesis. Vascul Pharmacol. 2010;52:142–145. doi: 10.1016/j.vph.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Lin HY, Sun M, Tang HY, et al. L-Thyroxine vs. 3,5,3′-triiodo-L-thyronine and cell proliferation: Activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am J Physiol Cell Physiol. 2009;296:C980–C991. doi: 10.1152/ajpcell.00305.2008. [DOI] [PubMed] [Google Scholar]
- 10.Lin HY, Cody V, Davis FB, et al. Identification and functions of the plasma membrane receptor for thyroid hormone analogues. Discov Med. 2011;11:337–347. [PubMed] [Google Scholar]
- 11.Ashur-Fabian O, Blumenthal DT, Bakon M, et al. Long-term response in high-grade optic glioma treated with medically induced hypothyroidism and carboplatin: A case report and review of the literature. Anticancer Drugs. 2013;24:315–323. doi: 10.1097/CAD.0b013e32835c7a47. [DOI] [PubMed] [Google Scholar]
- 12.Ries LAG, Young JL, Keel GE, et al., eds. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001, Patient and Tumor Characteristics 2007Bethesda, MD: National Cancer Institute. [Google Scholar]
- 13.Edge S, Byrd D, Compton C, et al., eds. AJCC Cancer Staging Manual, 20077th ed. New York, NY: Springer. [Google Scholar]
- 14.Tate MW, Clelland RC. Nonparametric and Shortcut Statistics in the Social, Biological, and Medical Sciences. Danville, IL: Interstate Printers and Publishers; 1957. [Google Scholar]
- 15.RECIST: Response Evaluation Criteria in Solid Tumors. Available at http://www.eortc.org/investigators-area/recist. Accessed July 29, 2014.
- 16.Hercbergs AH, Ashur-Fabian O, Garfield D. Thyroid hormones and cancer: Clinical studies of hypothyroidism in oncology. Curr Opin Endocrinol Diabetes Obes. 2010;17:432–436. doi: 10.1097/MED.0b013e32833d9710. [DOI] [PubMed] [Google Scholar]
- 17.Davis FB, Tang HY, Shih A, et al. Acting via a cell surface receptor, thyroid hormone is a growth factor for glioma cells. Cancer Res. 2006;66:7270–7275. doi: 10.1158/0008-5472.CAN-05-4365. [DOI] [PubMed] [Google Scholar]
- 18.Glinskii AB, Glinsky GV, Lin HY, et al. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac) Cell Cycle. 2009;8:3562–3570. doi: 10.4161/cc.8.21.9963. [DOI] [PubMed] [Google Scholar]
- 19.Yalcin M, Dyskin E, Lansing L, et al. Tetraiodothyroacetic acid (tetrac) and nanoparticulate tetrac arrest growth of medullary carcinoma of the thyroid. J Clin Endocrinol Metab. 2010;95:1972–1980. doi: 10.1210/jc.2009-1926. [DOI] [PubMed] [Google Scholar]
- 20.Shoemaker JP, Dagher RK. Remissions of mammary adenocarcinoma in hypothyroid mice given 5-fluorouracil and chloroquine phosphate. J Natl Cancer Inst. 1979;62:1575–1578. [PubMed] [Google Scholar]
- 21.Kumar MS, Chiang T, Deodhar SD. Enhancing effect of thyroxine on tumor growth and metastases in syngeneic mouse tumor systems. Cancer Res. 1979;39:3515–3518. [PubMed] [Google Scholar]
- 22.Mishkin SY, Pollack R, Yalovsky MA, et al. Inhibition of local and metastatic hepatoma growth and prolongation of survival after induction of hypothyroidism. Cancer Res. 1981;41:3040–3045. [PubMed] [Google Scholar]
- 23.Theodossiou C, Skrepnik N, Robert EG, et al. Propylthiouracil-induced hypothyroidism reduces xenograft tumor growth in athymic nude mice. Cancer. 1999;86:1596–1601. [PubMed] [Google Scholar]
- 24.Meng R, Tang HY, Westfall J, et al. Crosstalk between integrin αvβ3 and estrogen receptor-α is involved in thyroid hormone-induced proliferation in human lung carcinoma cells. PLoS One. 2011;6:e27547. doi: 10.1371/journal.pone.0027547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinoshita S, Sone S, Yamashita T, et al. Effects of experimental hyper- and hypothyroidism on natural defense activities against Lewis lung carcinoma and its spontaneous pulmonary metastases in C57BL/6 mice. Tokushima J Exp Med. 1991;38:25–35. [PubMed] [Google Scholar]


