This study used a nationwide population-based retrospective cohort study to explore the relationship between polycystic ovary syndrome (PCOS) and the subsequent development of gynecological cancers including uterine, breast, or ovarian cancer. The results indicate that PCOS might increase the risk of subsequent newly diagnosed uterine cancer, but it is critical that further studies be conducted to confirm the association between PCOS and gynecological cancer risk.
Keywords: Polycystic ovary syndrome, Uterine cancer, Ovarian cancer, Breast cancer, Retrospective cohort study
Abstract
Background.
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders among women of reproductive age. We used a nationwide population-based retrospective cohort study to explore the relationship between PCOS and the subsequent development of gynecological cancers including uterine, breast, or ovarian cancer.
Methods.
We identified subjects who were diagnosed with PCOS between January 1, 2000, and December 31, 2004, in the Taiwan National Health Insurance (NHI) Research Database. A comparison cohort was constructed for patients without known PCOS who were also matched according to age. All PCOS and control patients were observed until diagnosed with breast cancer, ovarian cancer, or uterine cancer or until death, withdrawal from the NHI system, or December 31, 2009.
Results.
The PCOS cohort consisted of 3,566 patients, and the comparison cohort consisted of 14,264 matched control patients without PCOS. The adjusted hazard ratio (HR) of uterine cancer and breast cancer in subjects with PCOS were higher (HR: 8.42 [95% confidence interval: 1.62–43.89] and HR: 1.99 [95% confidence interval: 1.05–3.77], respectively) than that of the controls during the follow-up. With the Monte Carlo method, only the mean adjusted HR of 1,000 comparisons for developing uterine cancer during the follow-up period was greater for the PCOS group than for the control groups (HR: 4.71, 95% confidence interval: 1.57–14.11).
Conclusion.
PCOS might increase the risk of subsequent newly diagnosed uterine cancer. It is critical that further large-scale, well-designed studies be conducted to confirm the association between PCOS and gynecological cancer risk.
Abstract
摘要
背景. 多囊卵巢综合征(PCOS)为育龄女性中最常见的内分泌系统疾病之一。我们开展了这项基于全台人群的回顾性队列研究,以探索 PCOS 与后续新发妇科癌症之间的相关性,包括子宫癌、乳腺癌或卵巢癌。
方法. 我们从 2000 年 1 月 1 日∼2004年 12 月 31 日台湾地区国民健康保险(NHI)研究数据库中选择确诊 PCOS 的患者作为研究对象。对照队列为年龄匹配且无已知的 PCOS 患者。对全部 PCOS 患者及其对照组进行观察,直至确诊为乳腺癌、卵巢癌或子宫癌或直至死亡、退出 NHI 系统或 2009 年 12 月 31 日。
结果. PCOS 队列包括3 566例患者,对照组包括 14 264例匹配的非 PCOS 患者。随访期发现,PCOS 患者的子宫癌和乳腺癌校正后风险比(HR)[分别为 HR : 8.42(95%可信区间:1.62 ∼ 43.89)、 HR :1.99(95%可信区间:1.05 ∼ 3.77)]高于对照组。使用 Monte Carlo 方法对随访期间1 000例对照进行校正后,PCOS 组平均校正后HR显示子宫癌的发生风险高于对照组(HR:4.71,95%可信区间:1.57 ∼ 14.11)。
结论. PCOS 可能增加后续新发子宫癌的风险。有必要开展更大规模、设计良好的研究以进一步证实 PCOS 与妇科癌症风险之间的相关性。The Oncologist 2015;20:45–49
Implications for Practice:
Polycystic ovary syndrome (PCOS) is a common endocrine system disorder among women of reproductive age. PCOS may raise a woman's risk of developing uterine cancer and breast cancer. Women with polycystic ovary syndrome should be screened regularly to increase the chances of early detection of the two cancers.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age, affecting 6%–10% of the population [1]. In addition to chronic anovulation, hyperandrogenism, and polycystic ovarian morphology that characterize PCOS, obesity, arterial hypertension, diabetes mellitus type 2, dyslipidemia, and cancer also commonly coexist in women with PCOS [2, 3].
Endometrial (uterine) cancer is now the most common gynecological malignancy in Europe and North America [4]. In a 1949 case-series study, Speert [5] first suggested an association between PCOS and endometrial carcinoma. Since then, a number of epidemiologic investigations have noted a link between PCOS and endometrial cancer [6–10], although not by all [11]. In a meta-analyses of five comparative studies, Haoula et al. [12] showed that women with PCOS are approximately three times more likely to develop endometrial cancer compared with women without it. Similarly another meta-analyses of five studies (138 women with PCOS and 5,593 non-PCOS controls) demonstrated that women with PCOS were at a significantly increased risk of endometrial cancer (odds ratio, 2.79) [13].
Ovarian cancer is the second most common gynecological cancer and has the highest mortality. Few studies have explored the association between PCOS and ovarian cancer. Meta-analysis of three studies in a recent report demonstrated that the risk of ovarian cancers was not significantly increased in women with PCOS [13]. Similarly, the study also found no increased risk of breast cancer in women with PCOS from meta-analysis of another three studies [13].
To our knowledge, most of the studies that explored the risk of endometrial cancer, ovarian cancer, and breast cancer in women with PCOS had case-control study designs. However, there are problems with these case-control studies, such as recall bias, interviewer bias, and inaccuracy of recorded information about exposure. This is because this information is collected by self-report, interview, or form. Compared with case-control studies, in cohort studies, exposure is usually identified before the outcome. Therefore, cohort study design provides a temporal framework to assess causality and is thus able to provide the strongest scientific evidence. Furthermore, most of the case-control studies were done in Western populations. Thus, we did a large-scale, population-based retrospective cohort study in Taiwan to investigate whether PCOS raises the risk of developing uterine, ovarian, or breast cancer in women.
Materials and Methods
Data Source
Instituted in 1995, the National Health Insurance (NHI) program is a mandatory health insurance program that offers comprehensive medical care coverage, including outpatient, inpatient, emergency, and traditional Chinese medicine to all residents of Taiwan, with a coverage rate of up to 98% [14–17]. The NHI research database (NHIRD) contains comprehensive information regarding clinical visits, including prescription details and diagnostic codes based on the International Classification of Disease, ninth revision, Clinical Modification (ICD-9-CM). The NHIRD is managed by the National Health Research Institute (NHRI), and confidentiality is maintained according to the directives of the Bureau of NHI. For our study, we used as the data source the Longitudinal Health Insurance Database 2005 (LHID 2005), which is a data set released by the NHRI that contains all original claims data for 1 million randomly selected beneficiaries in the 2005 Registry of Beneficiaries. The NHRI of Taiwan reports that there were no significant differences in gender distribution, age distribution, or average insured payroll-related amount between the patients in the LHID 2005 and those in the original NHIRD [18].
Standard Protocol Approvals, Registrations, and Patient Consents
The Institutional Review Board of Taipei Veterans General Hospital approved this study. Written consent from study objects was not obtained, because the NHI data set consists of deidentified secondary data for research purposes, and the Institutional Review Board of Taipei Veterans General Hospital issued a formal written waiver for the need for consent.
Study Population
Using data extracted from the LHID 2005, we conducted a retrospective cohort study of patients who were newly diagnosed with PCOS by an obstetrician-gynecologist between January 1, 2000, and December 31, 2004. In Taiwan, most obstetrician-gynecologists use guidelines suggested by the U.S. National Institutes of Health to diagnose PCOS. According to the guidelines, a person has PCOS if she has all of the following: oligo-ovulation, signs of excessive androgen (clinical or biochemical), and exclusion of other disorders that can result in menstrual irregularity and hyperandrogenism. The patients with PCOS were defined as having polycystic ovaries (ICD-9-CM code 256.4). We excluded patients who were diagnosed with PCOS (ICD-9-CM code 256.4) between January 1, 1996, and December 31, 1999. We also excluded patients who were diagnosed with malignancies (ICD-9-CM codes 140–208) before they were diagnosed with PCOS. For each PCOS patient included in the final cohort, four age-matched control patients without malignancies were randomly selected from the LHID 2005. All PCOS and control patients were observed until diagnosed with breast cancer (ICD-9-CM code 174 [malignant neoplasm of female breast]), ovarian cancer (ICD-9-CM code 183 [malignant neoplasm of ovary and other uterine adnexa]), or uterine cancer (ICD-9-CM codes 179 [malignant neoplasm of uterus, part unspecified], 181 [malignant neoplasm of placenta], and 182 [malignant neoplasm of body of uterus]) or until death, withdrawal from the NHI system, or December 31, 2009. The main dependent variable was occurrence of breast cancer, ovarian cancer, or uterine cancer, as reported in the Registry for Catastrophic Illness. For a diagnosis of cancer to be reported in the registry, histological confirmation is required. Common comorbidities, including hypertension, diabetes mellitus, dyslipidemia, congestive heart failure, chronic pulmonary diseases, coronary artery diseases, and cerebrovascular diseases, were also compared between PCOS and control patients.
Statistical Analysis
The incidence of newly diagnosed breast cancer, ovarian cancer, or uterine cancer in the PCOS and control patients was calculated, and independent t tests and χ2 tests were used to examine the differences in the demographic characteristics between the PCOS and control patients. A Cox proportional-hazards regression model was constructed to calculate the hazard ratio (HR) of breast cancer, ovarian cancer, or uterine cancer of the PCOS cohort and control cohort. Control variables, such as age; common comorbidities, including hypertension, diabetes mellitus, dyslipidemia, congestive heart failure, chronic pulmonary diseases, coronary artery diseases, and cerebrovascular diseases; urbanization; and monthly income were included as covariates in the multivariate model to calculate adjusted HR. Insurance premiums, calculated according to the beneficiary’s total income, were used to estimate monthly income. Monthly income was grouped into low income (monthly income < 20,000 New Taiwan Dollar [NTD]), median income (20,000 NTD ≤ monthly income < 40,000 NTD), and high income (monthly income ≥ 40,000 NTD). Urbanization was divided into three groups: urban, suburban, and rural. Because NHIRD does not provide detailed information on patients such as tobacco use, alcohol consumption, body mass index (BMI), fertility data, and so on, we were unable to adjust these potentially confounding factors. Because occurrence of cancer is a rare event, we used the Monte Carlo method to retest the results in our work and to strengthen the power of our study. We repeated the match process 1,000 times randomly to create 1,000 matched groups. The adjusted HR of each group and the mean adjusted HR of all comparisons were determined.
The SAS statistical software for Windows, version 9.3 (SAS Institute, Cary, NC, http://www.sas.com), was used for data extraction, computation, data linkage, processing, and sampling. All other statistical analyses were performed using the SPSS statistical software for Windows, version 20 (IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/). The results of comparisons with a p value of less than .05 were considered to indicate a statistically significant relationship.
Results
Participant Selection
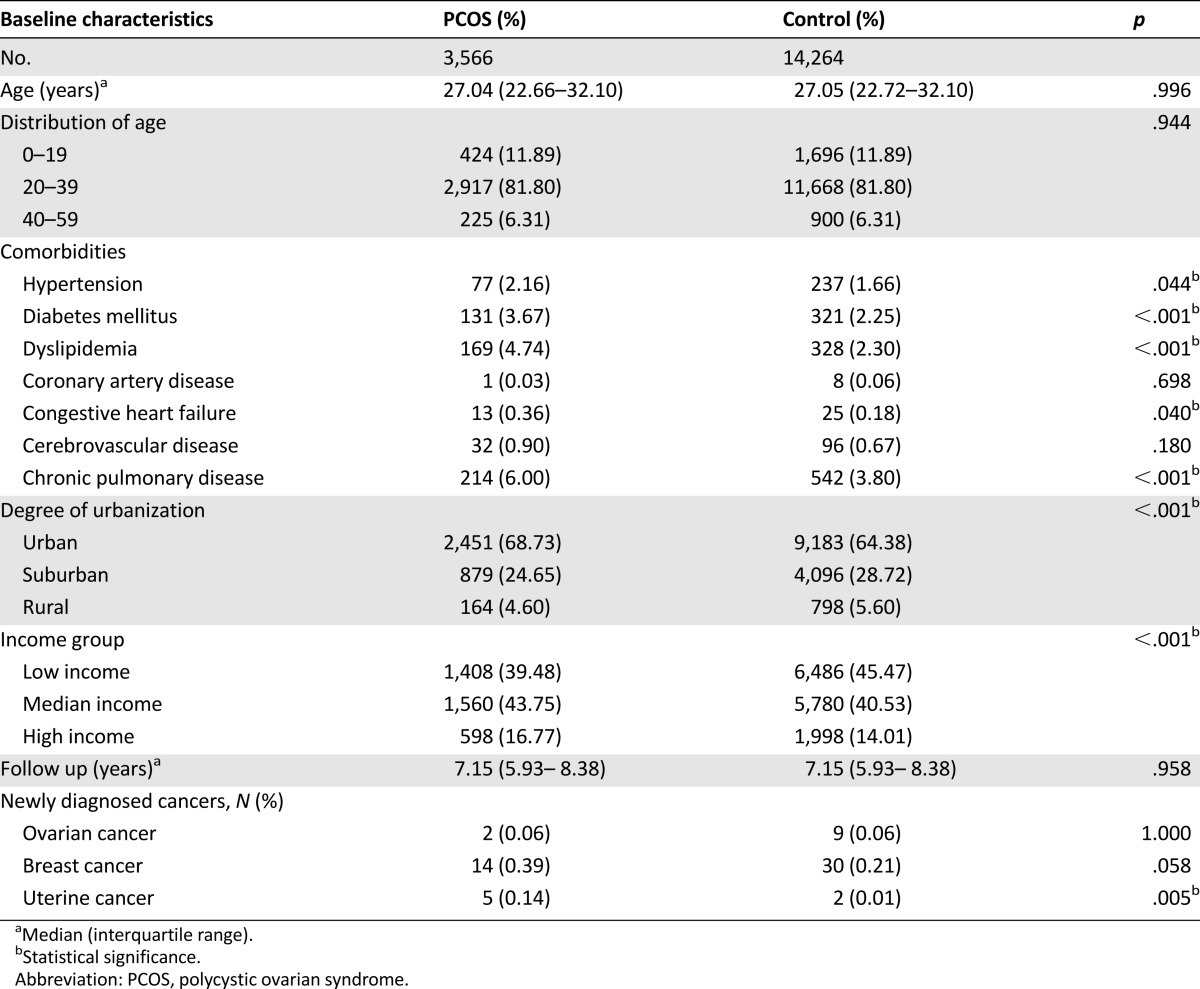
Our study included 3,566 PCOS patients and 14,264 control patients without PCOS. The comparisons of the demographic and clinical variables between the PCOS and control patients are presented in Table 1. The median age at enrollment was 27 years (interquartile range [IQR], 22–32 years), and the median follow-up period was 7.15 years (IQR, 5.93–8.38 years) for both PCOS and control patients. The comorbidities, including hypertension, diabetes mellitus, dyslipidemia, congestive heart failure, and chronic pulmonary disease were more common in PCOS patients than control patients. During the study period, 2 ovarian cancers, 14 breast cancers, and 5 uterine cancers were observed in PCOS group, and 9 ovarian cancers, 30 breast cancers, and 2 uterine cancers were observed in control group. Significantly higher incidence of uterine cancer (p = .005) was observed in the PCOS patients than in the control patients. All the subjects with uterine cancer had ICD-9-CM 182 diagnosis.
Table 1.
Characteristics of patients with PCOS and comparison subjects
PCOS on Risks of Ovarian Cancer, Breast Cancer, and Uterine Cancer
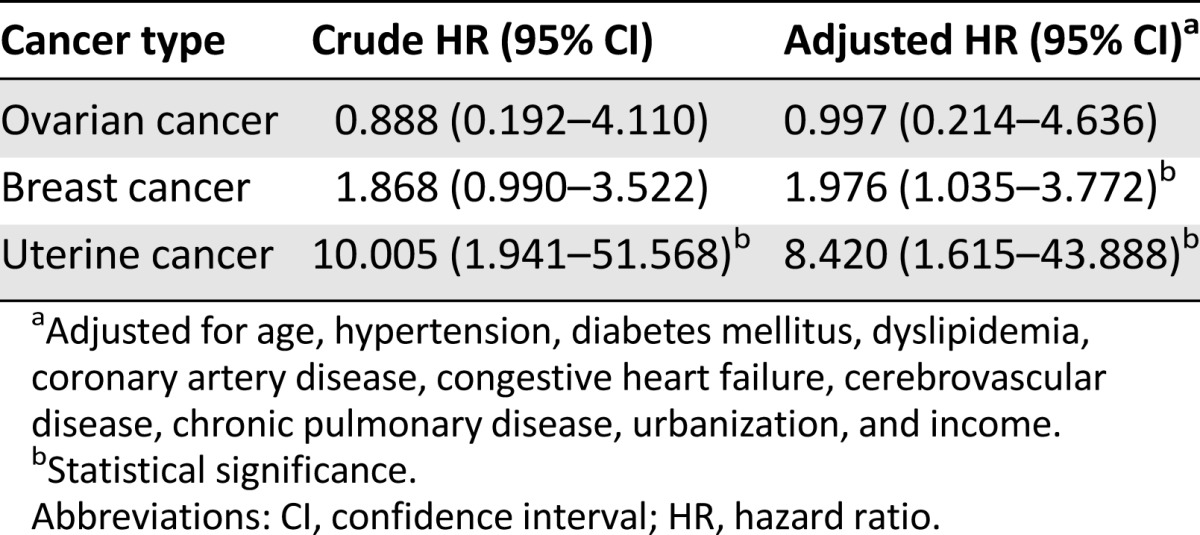
After adjusting for age, comorbidities, urbanization, and monthly income, the HR for developing uterine cancer during the follow-up period was 8.42 times (95% CI: 1.62–43.89) greater for the PCOS patients than for the control patients (Table 2). The adjusted HR for the development of breast cancer was also higher than that of the control patients (adjusted HR: 1.98, 95% CI: 1.04–3.77).
Table 2.
Hazard ratios of time until cancers between patients with polycystic ovarian syndrome and comparison subjects during follow-up period
Results of the Monte Carlo Method
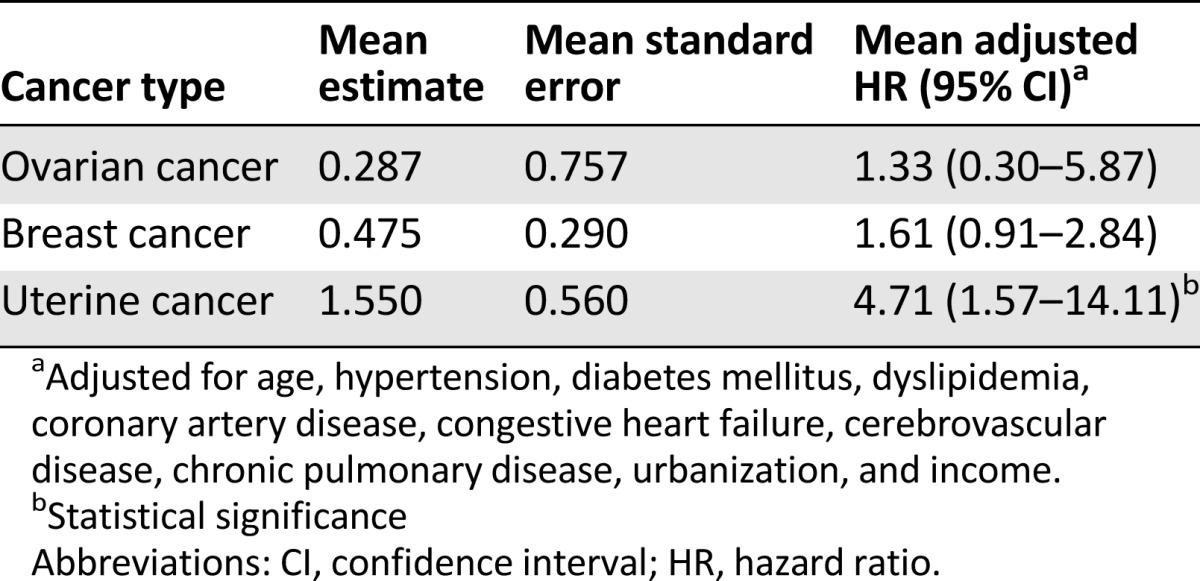
The results of the Monte Carlo method are presented in Table 3. The mean adjusted HR of 1,000 comparisons for developing uterine cancer during the follow-up period was 4.71 times (95% CI: 1.57–14.11) greater for the PCOS group than for the control groups. The mean adjusted HRs for the development of breast cancer and ovary cancer were not higher than that of the control patients (mean adjusted HR: 1.61, 95% CI: 0.91–2.84; and HR: 1.33, 95% CI: 0.30–5.87, respectively).
Table 3.
Mean hazard ratios of time until cancers between patients with polycystic ovarian syndrome and comparison subjects during follow-up period by the Monte Carlo method
Discussion
According to our research, this is the first study to demonstrate the risk of uterine cancer in women with PCOS in a national population-based cohort study. The main finding of our study is that Cox proportional-hazards analysis, after adjusting for age, comorbidities, urbanization, and monthly income, yielded an adjusted HR of uterine cancer that was 8.42 times greater for women with PCOS than for the comparison cohort. This result supports previous reports that PCOS may be a risk factor for developing uterine cancer [6–10]. Women with PCOS have several risk factors for uterine cancer including chronic anovulation, obesity, and hyperinsulinemia and therefore may be at increased risk of developing uterine cancer [19]. Furthermore, our analysis showed that hypertension, dyslipidemia, and diabetes were more prevalent in women with PCOS than in women without PCOS (Table 1), which is consistent with the results of previous studies [20], strengthening the reliability of our findings.
The particularly high risks for uterine cancer has often been noted among younger women with PCOS [10, 19]. In our study, the age of onset of uterine cancer for the 5 cases in the PCOS group ranged from 27 to 45 years (mean, 35.5 years; SD, 6.6 years). Endometrial cancer is the most common type of uterine cancer, and studies have shown that most cases of endometrial cancer occur between the ages of 60 and 70 years [21]. High BMI may be a possible factor for early-onset of uterine cancer in patients with PCOS. Such patients are often obese [22], and higher BMI is a huge risk factor for uterine cancer because fat tissues tend to produce higher levels of estrogen [23]. Previous studies have demonstrated that obesity is associated with earlier age at diagnosis of endometrial cancers [24, 25]. The young age of onset of these patients’ uterine cancer highlights the need for additional study to better understand PCOS and to determine what uterine cancer screening and preventive strategies are needed.
Because the case numbers of cancers are relatively small in our work, we used the Monte Carlo method to retest the results in our work. One thousand matched groups were selected, and the mean adjusted HRs of all comparisons were determined. The results confirmed that the mean adjusted HR for developing uterine cancer during the follow-up period was greater for the PCOS group than for the control groups. However, we found the mean adjusted HR of breast cancer did not increase with statistical significance in the Monte Carlo method, which means that the elevated adjusted HR of breast cancer in the original comparison may be due to the random effect in selection of control patients.
In a long-term follow-up of 786 women diagnosed with PCOS, breast cancer was found to be the most common cause of death [25]. In our preliminary analysis, there is a trend of increased breast cancer incidence in PCOS cases, and when we adjusted for age, urbanization, income, and some other physical diseases, the risk of developing breast cancer become statistically significant (adjusted HR: 1.98, 95% CI: 1.04–3.77; Table 2). However, the mean adjusted HR of breast cancer did not increase with statistical significance with the Monte Carlo method. This finding was consistent with previous suggestions that the risk for breast cancer is not significantly greater in women with PCOS [13]. Future population-based prospective studies with large sample sizes are needed to investigate the association between PCOS and the risk of breast cancer.
In a population-based, case-control study of 476 subjects with histologically confirmed epithelial ovarian cancer, Schildkraut et al. [26] found that ovarian cancer risk was found to increase 2.5-fold (95% CI: 1.1–5.9) among women with PCOS. In this study, we did not find an increased risk of ovarian cancer in PCOS group, which is in line with the recent meta-analysis of three case-control studies that the risk of ovarian cancers was not significantly increased in women with PCOS [13].
The strengths of our study are the large sample size and the diagnosis of PCOS by specialists. In addition, our study design included an unbiased participant selection process. Because participation in the NHI is mandatory and all residents of Taiwan can access health care with low copayments, referral bias is low, and follow-up compliance is high.
Certain limitations to our findings should, however, be considered: (a) The NHIRD does not provide detailed information on patients such as tobacco use, alcohol consumption, and family history of malignancy diseases. They are all major risk factors for cancer development. Specifically, risk factors of uterine cancer such as BMI, metabolic syndrome, and fertility data were also not included in the NHIRD. Thus, we were unable to control for these potentially confounding factors. (b) The follow-up period could be short, considering that most cases of uterine cancer occur after the age of 60 years. (c) The diagnoses of PCOS are identified using the ICD-9 codes from the database, and its prevalence may be underestimated because only subjects seeking medical evaluation can be identified, but this would most likely result in an underestimate of the association between PCOS and gynecological cancers. (d) The stage, histology, and outcome of the uterine cancers found in the PCOS group could not be obtained in the NHIRD, which made the clinical utility of our results in the future somewhat obscure. Further study may be necessary to explore these clinical issues. (e) Although the data we obtained on PCOS and cancer diagnoses were highly reliable, the diagnoses in NHI claims are primarily for administrative billing and do not undergo verification for scientific purposes.
Conclusion
Our nationwide population-based retrospective cohort study provides further evidence of an excessive risk of uterine cancer in PCOS subjects. However, the specific mechanisms that underlie this association remain unknown, and further study is necessary to confirm our findings and explore the underlying pathophysiological mechanisms. Furthermore, the clinical utility of this information is to be determined because the absolute incidences of the cancers were low. Whether intensive screening of cancers and/or prophylactic surgery should be done in patients with PCOS needs further survey for confirmation.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This work was supported by Grant NSC 101-2314-B-075-040 from the National Science Council of Taiwan and Grant V103C-048 from the Taipei Veterans General Hospital. We thank Emily Ting for English editing. The study was based on data from the National Health Insurance Research Database (provided by the Bureau of National Health Insurance of the Department of Health of Taiwan and managed by the National Health Research Institute of Taiwan). The interpretation and conclusions contained in this article do not represent those of the Bureau of National Health Insurance, the Department of Health, or the National Health Research Institute. The authors alone are responsible for the content and writing of the paper.
Author Contributions
Conception/Design: Cheng-Che Shen, Albert C. Yang, Jeng-Hsiu Hung, Li-Yu Hu, Shih-Jen Tsai
Provision of study material or patients: Albert C. Yang, Shih-Jen Tsai
Collection and/or assembly of data: Cheng-Che Shen
Data analysis and interpretation: Cheng-Che Shen, Albert C. Yang, Shih-Jen Tsai
Manuscript writing: Cheng-Che Shen, Shih-Jen Tsai
Final approval of manuscript: Cheng-Che Shen, Albert C. Yang, Jeng-Hsiu Hung, Li-Yu Hu, Shih-Jen Tsai
Disclosures
The authors indicated no financial relationships.
References
- 1.Asunción M, Calvo RM, San Millán JL, et al. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 2.Cussons AJ, Stuckey BG, Watts GF. Cardiovascular disease in the polycystic ovary syndrome: New insights and perspectives. Atherosclerosis. 2006;185:227–239. doi: 10.1016/j.atherosclerosis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Orio F, Vuolo L, Palomba S, et al. Metabolic and cardiovascular consequences of polycystic ovary syndrome. Minerva Ginecol. 2008;60:39–51. [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.Speert H. Carcinoma of the endometrium in young women. Surg Gynecol Obstet. 1949;88:332–336. [PubMed] [Google Scholar]
- 6.Colafranceschi M, Taddei GL, Scarselli G, et al. Clinico-pathological profile of endometrial carcinoma in young women (under 40 years of age) Eur J Gynaecol Oncol. 1989;10:353–356. [PubMed] [Google Scholar]
- 7.Gadducci A, Gargini A, Palla E, et al. Polycystic ovary syndrome and gynecological cancers: Is there a link? Gynecol Endocrinol. 2005;20:200–208. doi: 10.1080/09513590400021201. [DOI] [PubMed] [Google Scholar]
- 8.Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417–420. [PubMed] [Google Scholar]
- 9.Honoré LH, Davey SJ. Endometrial carcinoma in young women: A report of four cases. J Reprod Med. 1989;34:845–849. [PubMed] [Google Scholar]
- 10.Konishi I, Koshiyama M, Mandai M, et al. Increased expression of LH/hCG receptors in endometrial hyperplasia and carcinoma in anovulatory women. Gynecol Oncol. 1997;65:273–280. doi: 10.1006/gyno.1997.4656. [DOI] [PubMed] [Google Scholar]
- 11.Brinton LA, Moghissi KS, Westhoff CL, et al. Cancer risk among infertile women with androgen excess or menstrual disorders (including polycystic ovary syndrome) Fertil Steril. 2010;94:1787–1792. doi: 10.1016/j.fertnstert.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum Reprod. 2012;27:1327–1331. doi: 10.1093/humrep/des042. [DOI] [PubMed] [Google Scholar]
- 13.Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2014;20:748–758. doi: 10.1093/humupd/dmu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ou SM, Lee YJ, Hu YW, et al. Does Alzheimer’s disease protect against cancers? A nationwide population-based study. Neuroepidemiology. 2013;40:42–49. doi: 10.1159/000341411. [DOI] [PubMed] [Google Scholar]
- 15.Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 16.Chen HL, Hsiao FY. Risk of hospitalization and healthcare cost associated with Diabetes Complication Severity Index in Taiwan’s National Health Insurance Research Database. J Diabetes Complications. 2014;28:612–616. doi: 10.1016/j.jdiacomp.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Chen YC, Yeh HY, Wu JC et al. Taiwan’s National Health Insurance Research Database: Administrative health care database as study object in bibliometrics. Scientometrics 2011;86:365–380.
- 18.Introduction to the National Health Insurance Research Database (NHIRD). Taiwan. Available at http://nhird.nhri.org.tw/date_01.html. Accessed May 1, 2007.
- 19.Hardiman P, Pillay OC, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361:1810–1812. doi: 10.1016/s0140-6736(03)13409-5. [DOI] [PubMed] [Google Scholar]
- 20.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jick H, Walker AM, Rothman KJ. The epidemic of endometrial cancer: A commentary. Am J Public Health. 1980;70:264–267. doi: 10.2105/ajph.70.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nevadunsky NS, Van Arsdale A, Strickler HD, et al. Obesity and age at diagnosis of endometrial cancer. Obstet Gynecol. 2014;124:300–306. doi: 10.1097/AOG.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 24.Semaan A, Ali-Fehmi R, Munkarah AR, et al. Clinical/pathologic features and patient outcome in early onset endometrial carcinoma: A population based analysis and an institutional perspective from the Detroit metropolitan area, Michigan. Gynecol Oncol. 2012;124:265–269. doi: 10.1016/j.ygyno.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Pierpoint T, McKeigue PM, Isaacs AJ, et al. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol. 1998;51:581–586. doi: 10.1016/s0895-4356(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 26.Schildkraut JM, Schwingl PJ, Bastos E, et al. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol. 1996;88:554–559. doi: 10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]


