Abstract
Objective
To evaluate the effect of a digital dispenser’s acoustic alarm function on adherence to ethinylestradiol (EE) 20 μg/drospirenone 3 mg in a flexible extended regimen (EE/drospirenoneFlex) among women in five European countries (France, Germany, Italy, Spain, UK) seeking oral contraception.
Study design
Randomized, parallel-group open-label study.
Methods
Women aged 18–35 years received EE/drospirenoneFlex administered in a regimen with cycle lengths of their choice with the aid of a digital pill dispenser over 1 year. In group A (N=250), the dispenser’s acoustic alarm was activated (ie, acoustic alarm + visual reminder). In group B (N=249), the acoustic alarm was deactivated (ie, visual reminder only). In addition, the women recorded pill intake daily in diary cards. The primary efficacy variable was the mean delay of daily pill release after the dispenser reminded the woman to take a pill (reference time). Secondary efficacy variables included number of missed pills, contraceptive efficacy, bleeding pattern, tolerability, and user satisfaction.
Results
Dispenser data showed a mean (standard deviation [SD]) daily delay in pill release of 88 (126) minutes in group A vs 178 (140) minutes in group B (P<0.0001). Median (lower quartile, Q1; upper quartile, Q3) number of missed pills was 0 (0; 1) in group A vs 4 (1; 9) in group B (P<0.0001). Diary card results revealed similar trends; however, underreporting of missed pills was evident in both groups. No pregnancies were reported during 424 women-years of exposure. Across the two groups, the mean (SD) EE/drospirenoneFlex cycle length was 51.0 (31.8) days with strong regional differences, and the mean (SD) number of bleeding/spotting days was 50.4 (33.0) days. EE/drospirenoneFlex was well tolerated, and 80% of women were satisfied with treatment.
Conclusion
The dispenser’s activated acoustic alarm improved adherence with daily tablet intake of EE/drospirenoneFlex, reducing missed pills. EE/drospirenoneFlex provided effective contraception and a good tolerability profile.
Keywords: contraception, efficacy, pill dispenser, tolerability, drospirenone, compliance
Introduction
Most available combined oral contraceptives (COCs) are based on a 28-day cycle usually comprising 21 days or 24 days of hormone intake followed by a hormone-free interval resulting in a withdrawal bleed every 4 weeks. COCs taken in fixed extended regimens are also available and have been shown to be equally effective and with similar safety and tolerability profiles compared to conventional 28-day cycle COCs.1–3 These regimens have been found to decrease the number of bleeding days and improve menstrual symptoms,4 and may also have beneficial effects on sexual behavior and quality of life.5 Extended regimens may be advocated for women who prefer reduced frequency in their bleeding,6 but may be associated with increased frequency of breakthrough bleeding vs conventional 28-day cycle COCs.1 A flexible extended regimen enables women to extend pill intake and reduce the number of menstrual periods per year according to their needs, while providing a convenient strategy to manage breakthrough bleeding.7 The flexible extended regimen comprising ethinylestradiol (EE) 20 μg/drospirenone 3 mg including 4-day pill breaks after 24–120 days of tablet intake as required (EE/drospirenoneFlex; YAZ Flex®) has been approved in the European Union and in countries outside Europe. EE/drospirenoneFlex has been shown to be well tolerated and provide good contraceptive efficacy with fewer bleeding days over time compared with a conventional 28-day and a long-cycle 124-day COC regimen.8,9 The pregnancy rates reported with EE/drospirenoneFlex in these studies8,9 were similar to those for EE/drospirenone in a 24/4 regimen (YAZ),10,11 with 1-year Pearl Indices ranging between 0.49 and 1.65.
Poor compliance is a common issue in COC users. A study of 17,091 COC users in Spain found that 71% reported noncompliant behavior.12 In a retrospective cohort study in France (N=3,316), 42% of missed pills occurred during the first week after the pill-free interval; of these, 18% occurred on the first day after the pill-free interval.13 Unintended pregnancies are common (44% of pregnancies in Europe and 48% in the USA in 2008)14 and a high proportion can be attributed to poor compliance with contraception. In European women aged 16–30 years (N=6,676), those who missed one pill a month were 2.6 times more likely to have an unintended pregnancy than women who missed no pills.15
EE/drospirenoneFlex has now been made available with the Clyk™ (Figure 1) digital dispenser to guide women with the flexible pill regimen to ensure correct and consistent use. This refillable pill dispenser guides users through the intake cycle and the 4-day pill break. It provides a daily reminder when pill intake is due, both visually on the dispenser screen and with an acoustic alarm. The time at which the dispenser reminds the woman to take a pill each day (reference time) is set by the first pill release but can be changed. The dispenser also tracks missed pills and assesses the intake pattern according to missed pill rules,16 displaying a warning (“Use additional contraception” [UAC] symbol) on the screen when back-up contraception should be used. The warning appears when there are more than 7 days with irregular or no tablet intake and it stays on the screen until there are 7 days of uninterrupted tablet intake.
Figure 1.
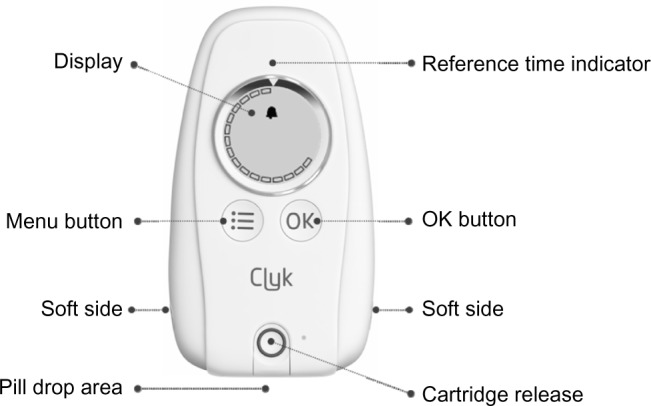
The adherence-aiding dispenser.
Note: A prototype of the dispenser was used in the clinical study.
This study evaluated the effect of the dispenser’s acoustic alarm on adherence (or sometime referred to as compliance) with EE/drospirenoneFlex. The efficacy and tolerability of EE/drospirenoneFlex and user satisfaction were also assessed.
Materials and methods
Study design
This open-label, randomized, two-arm, parallel-group multicenter study was conducted at 42 centers in France, Germany, Italy, Spain, and the UK (ClinicalTrials.gov identifier: NCT01257984) from December 2010 to September 2012.
Subjects
Women aged 18–35 years (smokers up to age 30 years) in good general health and requesting contraception were eligible to participate in the study. The women were also required to have a normal cervical smear test result at screening or in the 6 months prior to study start. Exclusion criteria included: pregnancy or lactation (<3 months since delivery, abortion, or lactation before start of treatment); continued use of other contraceptive methods or sterilization; body mass index ≥30 kg/m2; undiagnosed abnormal genital bleeding or abnormal menstrual history indicative of infertility; known hypersensitivity to any of the study drug ingredients; any disease or condition that could interfere with the study medication or worsen with hormonal administration, consistent with the absolute and relative contraindications for COC use; any disease or condition that might interfere with the conduct of the study or the interpretation of the results; abuse of alcohol, drugs, or medicines; use of any medication that could have resulted in excessive accumulation, impaired metabolism, or altered excretion of the study drug; simultaneous participation in another clinical trial; major surgery scheduled during the study period; close affiliation with the investigational site; administration of an investigational product within 1 month before or during the study; and inability to cooperate with the study procedures for any reason.
All women provided written informed consent before enrollment. The study was approved by local Independent Ethics Committees and Institutional Review Boards, and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines.
Treatment
Women were randomized 1:1 to one of two groups using an interactive voice response system/interactive web recognition system. The investigator called the interactive voice response system/interactive web recognition system to find out the subject’s allocated treatment group. In group A, the acoustic alarm on the dispenser was functional and activated (by default). In this group, women were reminded to take their pill at the reference time by both an acoustic alarm and a visual symbol on the dispenser screen. Women in this group could turn off the acoustic alarm if they wished. In group B, the acoustic alarm was permanently deactivated; women were reminded to take their pill by a visual symbol only. In both groups, the dispenser automatically recorded the time of pill release. Women also recorded pill intake in a daily diary card.
The women were given written instructions for use of the digital pill dispenser. The women were also given a sufficient number of pre-filled cartridges (enough to last them until the next scheduled study visit) containing 30 EE/drospirenoneFlex pills for use with the digital dispenser. The digital pill dispenser was activated when the first pre-filled cartridge was inserted into the system, and once activated, guided the women through their pill cycle, advised and reminded them when to take their pill, assisted them to get back on track in cases of late or missed pills, and advised when additional back-up barrier methods of contraception should be used (ie, UAC warning).
Study medication was to start on the first day of menstrual bleeding for new COC starters or on the day of the next treatment cycle for those changing from other combined hormonal contraceptives. Women switching from other methods including mini-pills, implant, or intrauterine system could start on the same day (or at the latest, the day after) as discontinuation. A wash-out period of three times the injection interval was required for those switching from depot medroxyprogesterone acetate. The women were instructed to take EE/drospirenoneFlex for at least 24 days (“mandatory phase”). Thereafter, the women could continue pill intake for up to a maximum of 120 days, followed by a 4-day pill break. However, the women could decide to take a 4-day pill-free interval at any time between days 25 and 120 (“flexible phase”) as desired. In cases where the women experienced 3 consecutive days of intracyclic bleeding during the flexible phase, they were advised to take the 4-day break. This strategy has been shown to reduce the number of bleeding days over time.9 After each 4-day pill-free interval, regardless of any ongoing bleeding, a new intake cycle with a mandatory 24-day intake phase would start as already described, and the whole process repeated for up to 1 year.
Study visits were scheduled for weeks 3, 11, 23, 35, and 51 following initiation of study medication, and 18–25 days after completion of study medication or on premature discontinuation. A urine pregnancy test was performed routinely at all study visits, and at home by the women if they felt the need for it, for example, in the event of forgotten pills, diarrhea/vomiting, or antibiotic treatment, and at the start of a new cartridge. At each visit during the study, the investigators documented information on the use of back-up contraceptive measures (eg, condoms), concomitant medication use, adverse event (AE) occurrence, checked documents and digital dispenser, and dispensed home pregnancy test kits. Vital signs and body weight were also assessed at scheduled study visits at weeks 3, 23, and 51, and at follow-up.
Outcomes
The primary efficacy variable was the daily mean (standard deviation [SD]) time delay of pill release compared with the reference time, ie, the set time of the daily call (with/without acoustic alarm) for pill intake for the entire 1 year. The delay was calculated using the dispenser’s log data and could be up to a maximum of 1,440 minutes (24 hours). If there was no delay in pill release, the difference was zero. The dispenser enabled women to dispense the planned pill up to 12 hours early if required; for the purposes of the study, pills that were dispensed early were considered to be dispensed at the reference time (ie, difference of zero vs the reference time). Any additional release within 12 hours after the reference time, however, was considered a replacement, for example, in case of vomiting or if the pill got lost after release.
Secondary efficacy variables included number of missed pills over 1 year. A pill was defined as missed if it was not released within 24 hours after the reference time (excluding the 4-day pill break). Missed pills were assessed using data recorded in the dispenser and daily diary cards in parallel. In case of missed pills during the flexible phase of the intake cycle between days 25 and 120, the subject had the option to initiate a 4-day break, ie, to continue with a pill-free interval to induce withdrawal bleeding. In this scenario, the missed tablets were counted as part of pill-free interval so long as they added up to 4 days in total. If pills were missed without indicating a break on the digital dispenser, a delay of 24 hours was set for each missed pill, but after day 24 of the cycle only for up to two consecutively missed pills. In this scenario, the third and fourth consecutively missed pills were interpreted as part of a pill break and excluded from the analysis.
Other secondary variables assessed included contraceptive efficacy (Pearl Index; ie, the number of pregnancies per 100 woman-years of use), cycle length, number of bleeding/spotting days, frequency of intracyclic bleeding during the first 24 days of each intake cycle, and tolerability as described elsewhere.9 In brief, the number of unintended pregnancies were assessed during the study, and the corresponding Pearl Index and 95% confidence intervals (CIs) calculated. The women also assessed their bleeding daily and recorded any vaginal bleeding episodes in the same diary cards as for pill intake. A bleeding episode was defined as consecutive days with spotting and/or bleeding preceded and followed by at least 2 days without any bleeding. Bleeding intensity was rated as none, spotting, light, normal, or heavy. Spotting was defined as bleeding that did not warrant use of sanitary protection. Where sanitary protection was required, bleeding was rated as light, normal, or heavy bleeding relative to their normal experience. Bleeding/spotting days were analyzed using 90-day reference periods, with the first reference period starting on the first day of pill intake.17 Bleeding/spotting episodes that occurred within close proximity of the hormone-free interval (up to the fourth day of the next cycle) were considered to represent withdrawal bleeding. All other bleeding events were considered intracyclic bleeding.
Safety assessments included monitoring for AEs and for abnormal laboratory findings, and are classified by the study investigator as to their likely relationship to study medication. The intensity of an AE was classified as mild, moderate, or severe, taking into account the possible range of the intensity of the event. A serious AE (SAE) was defined as any untoward medical occurrence that met any of the following criteria: resulted in death; life-threatening; required in-patient hospitalization or prolongation of existing hospitalization; resulted in persistent or significant disability/incapacity; congenital anomaly or birth defect; and any other medically important serious event as judged by the investigator.
At the end of the study (18–25 days after last study drug intake), the women completed a questionnaire to assess ease of understanding the instructions, task ratings, dispenser attributes, technical difficulties/malfunctions experienced, satisfaction with the regimen, and overall treatment satisfaction.
Statistical analysis
A sample size of 250 women in each group was selected to have a sufficiently large number of subjects and study centers in five European countries to not only show superiority of the activated acoustic alarm but also explore secondary endpoints including regional differences with regard to handling and user satisfaction. The sample size was sufficient to show a difference of 0.34 SDs in the mean daily time delay in pill release, with 90% power and a 5% alpha level, assuming a 25% drop-out rate.
The primary variable was analyzed using the full analysis set (FAS; all women who took the study drug and for whom post-baseline data were available) and the per-protocol set (PPS; women without major protocol deviations) and presented by treatment group. A one-factorial analysis of variance (ANOVA) with “activated/deactivated dispenser alarm” as the factor and “mean daily delay in pill release” as the dependent variable was performed; 95% CIs were calculated.
The number of missed pills was calculated for the FAS and PPS and presented descriptively by treatment group for the overall population and by country. Data for all other secondary outcomes are presented descriptively for the FAS using pooled treatment group data.
Post hoc Wilcoxon rank sum test was used to assess differences in the number of missed pills between groups. Fisher’s exact test was used to assess differences in the number of subjects with missed pills (no/yes) between groups.
Results
Subjects
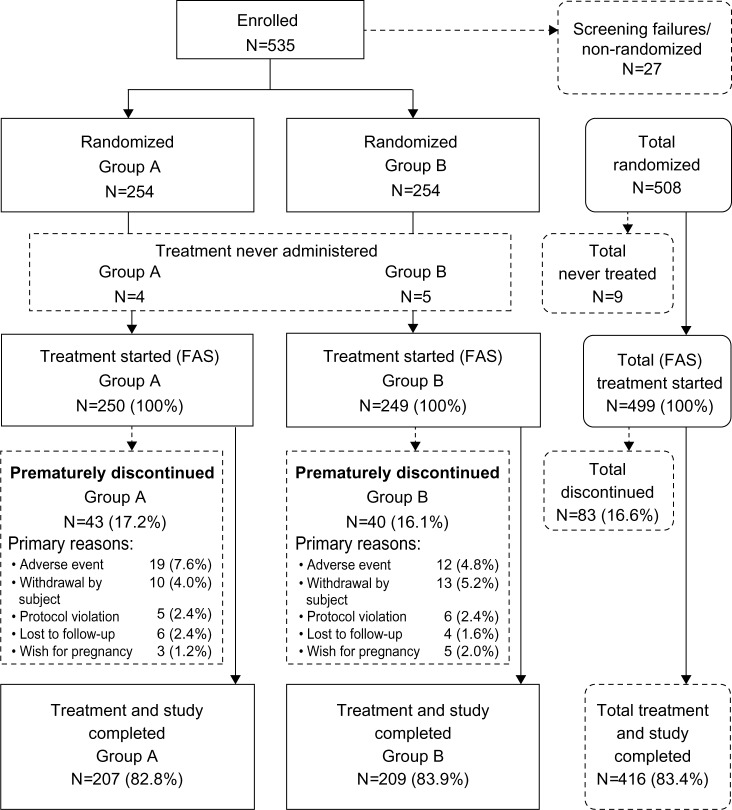
Overall, 535 women were enrolled and screened: 225 at nine centers in Germany, 112 at eleven centers in Italy, 76 at seven centers in Great Britain, 66 at ten centers in France, and 56 at five centers in Spain. Of these, 27 women failed the screening process and were not included in the study. The remaining 508 women were randomized to treatment, but nine never used any study treatment. Thus, the FAS comprised 499 women (group A: N=250; group B: N=249) and the PPS comprised 363 women (group A: N=160; group B: N=203). The main reasons for exclusion from the PPS were that the acoustic alarm was activated for <80% of pills released (only applied to group A: N=59) and that the duration of participation was <80% of that planned (group A: N=33; group B: N=35). Figure 2 shows the flow of subjects through the study.
Figure 2.
Disposition of subjects (FAS).
Notes: Group A (activated acoustic alarm) and group B (deactivated acoustic alarm).
Abbreviation: FAS, full analysis set.
The characteristics of the women at baseline were similar between treatment groups (Table 1).
Table 1.
Baseline demographics and clinical characteristics (full analysis set)
| Group A (activated acoustic alarm) | Group B (deactivate acoustic alarm) | Total | |
|---|---|---|---|
| Mean age (years) (SD) | 25.3 (4.3) | 25.7 (4.0) | 25.5 (4.1) |
| Mean BMI (SD) | 22.7 (2.9) | 22.4 (3.1) | 22.5 (3.0) |
| Caucasian, N (%) | 215 (86.0) | 216 (86.7) | 431 (86.4) |
| Smoking | |||
| Never, N (%) | 146 (58.4) | 144 (57.8) | 290 (58.1) |
| Former, N (%) | 30 (12.0) | 30 (12.0) | 60 (12.0) |
| Current, N (%) | 74 (29.6) | 75 (30.1) | 149 (29.9) |
| Race, N (%) | |||
| White | 215 (86) | 216 (86.7) | 431 (86.4) |
| Black/African | 2 (0.8) | 0 | 2 (0.4) |
| American Asian | 0 | 1 (0.4) | 1 (0.2) |
| Missing/not reported | 33 (13.2) | 32 (12.4) | 65 (12.8) |
| Educational level, N (%) | |||
| Elementary | 5 (2) | 5 (2) | 10 (2) |
| Secondary | 109 (43.6) | 95 (38.2) | 204 (40.9) |
| College/university | 136 (54.4) | 149 (59.8) | 285 (57.1) |
| Alcohol consumption, N (%) | |||
| Abstinent | 74 (29.6) | 76 (30.5) | 150 (30.1) |
| Light | 173 (69.2) | 165 (66.3) | 338 (67.7) |
| Moderate | 3 (1.2) | 8 (3.2) | 11 (2.2) |
| Number of pregnancies, N (%) | |||
| 0 | 193 (77.2) | 184 (73.9) | 377 (75.6) |
| ≥1 | 57 (22.8) | 65 (26.1) | 122 (24.4) |
| Number of births, N (%) | |||
| 0 | 205 (82.0%) | 198 (79.5%) | 403 (80.8) |
| ≥1 | 45 (18.0) | 51 (20.5) | 96 (19.2) |
| Previous contraception, N (%) | |||
| None | 12 (4.8) | 12 (4.8) | 24 (4.8) |
| Oral | 205 (82.0) | 213 (85.5) | 418 (83.8) |
| Vaginal | 4 (1.6) | 3 (1.2) | 7 (1.4) |
| Transdermal | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| Barrier | 28 (11.2) | 19 (7.6) | 47 (9.4) |
| Implant | 0 | 1 (0.4) | 1 (0.2) |
Note: Totals may not add up to 100% due to rounding.
Abbreviations: BMI, body mass index; SD, standard deviation.
Primary outcome
In the FAS, the mean (SD) daily delay in pill release was 88 (126) minutes in group A vs 178 (140) minutes in group B. This corresponded to a treatment difference of −90 minutes (95% CI: −66 to −113; P<0.0001 in favor of group A, ANOVA). In the PPS, these values were 74 (77) minutes in group A vs 179 (138) minutes in group B (treatment difference of −104 minutes [95% CI: −80 to −128; P<0.0001, ANOVA]).
Secondary outcomes
Missed pills
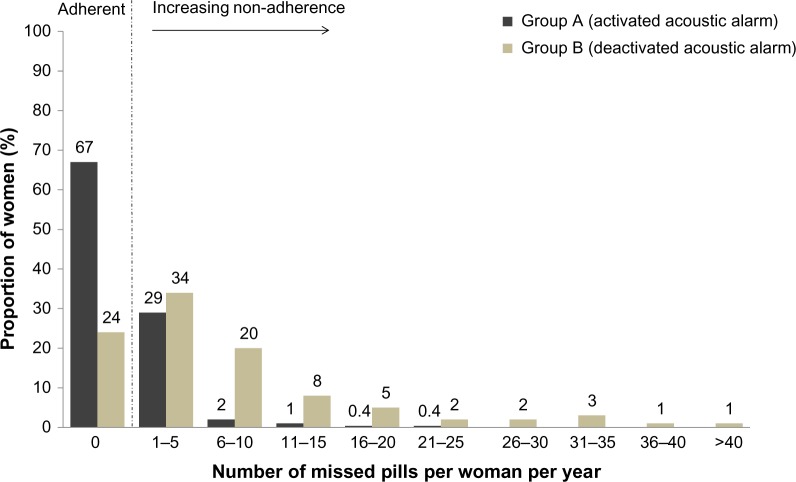
According to dispenser data, in the FAS, the number of missed pills over 1 year was lower in group A than in group B (Figure 3; P<0.0001, Wilcoxon rank sum test). The benefit of the acoustic alarm was particularly evident among subjects with low adherence (Figure 4). Approximately 70% of women in group A did not miss any pills, compared with only 24% in group B (P<0.0001, Fisher’s exact test). While there were no women in group A who missed ≥30 pills, in group B this value was 5%. Diary card results also favored group A; however, underreporting of missed pills was evident. Similar trends were seen in the PPS (data not shown).
Figure 3.
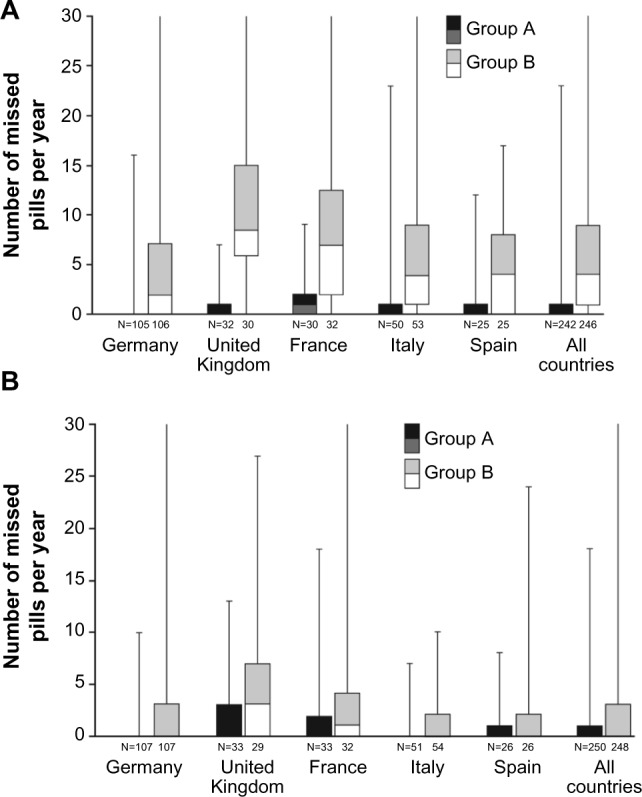
Box and whisker plot for number of missed pills per year in group A (activated acoustic alarm) and group B (deactivated acoustic alarm) according to dispenser (A) and diary card data (full analysis set) (B).
Notes: Boxes range from Q1 to median (light) and from median to Q3 (dark) and whiskers range from minimum to maximum (cutoff at 30). If no box is displayed then Q3 is zero.
Abbreviation: Q, quartile.
Figure 4.
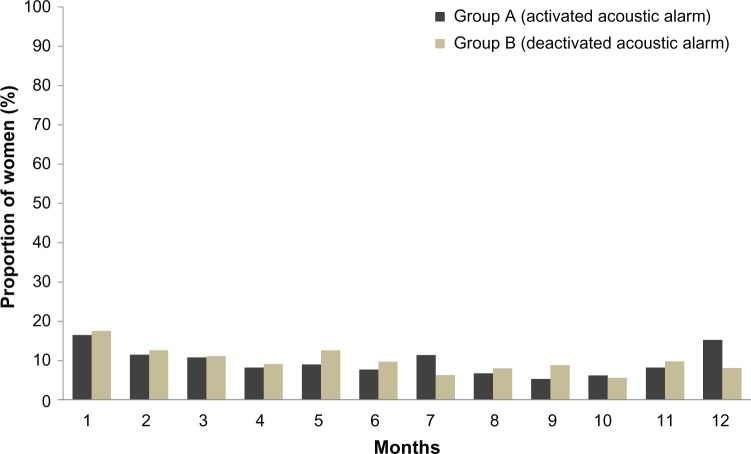
Proportion of women with missed pills over 1 year in group A (acoustic alarm activated) and group B (acoustic alarm deactivated) (full analysis set).
The benefit of the acoustic alarm on missed pills was evident across all countries (Figure 3).
Frequency of UAC warning
Overall, in the PPS, 31% of women in group A and 61% of women in group B received ≥1 UAC warning from the dispenser. A total of 46% of women in group B had multiple UAC warnings vs only 14% in group A.
Contraceptive efficacy
No pregnancies were reported (424 women-years for both treatment groups combined), yielding a Pearl Index of 0.00 (95% CI: 0.00–0.87).
Cycle length
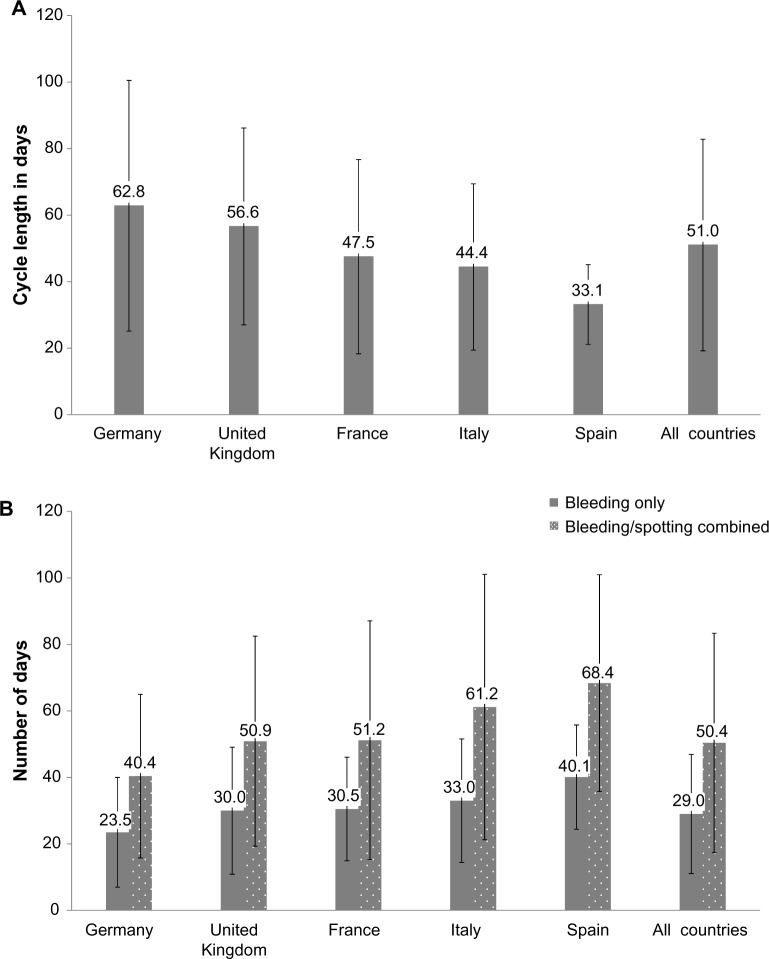
Mean cycle length was 51.0 days (SD: 31.8 days) overall and was longest in Germany. There were huge regional differences with regard to cycle lengths that women chose to have (Figure 5).
Figure 5.
Mean (standard deviation [SD]) cycle length (A) and mean (SD) number of days with bleeding (excluding spotting), and bleeding and spotting combined over 1 year by country (B) (diary card data for pooled treatment groups; full analysis set).
Notes: Cycle length includes the 4-day break. The last cycle for each subject was calculated including a 4-day break.
Bleeding pattern indices
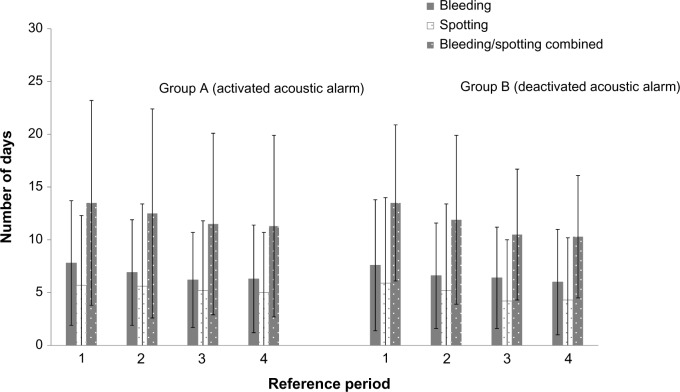
Differences in adherence between treatment groups (shown earlier) did not lead to differences in the number of bleeding/spotting days experienced (Figure 6). When treatment groups were pooled, the mean (SD) number of bleeding/spotting days over 1 year was 50.4 (33.0) days overall.
Figure 6.
Mean number (standard deviation) of bleeding, spotting, and bleeding/spotting days per 90-day reference period over 1 year of ethinylestradiol/drospirenoneFlex use in the two groups (reference periods 1–4) (full analysis set).
The number of bleeding/spotting days was inversely associated with length of the intake cycle (Figure 5). Therefore, the mean number of bleeding/spotting days was lowest in Germany and highest in Spain (Figure 5). In the FAS, four women discontinued due to bleeding-related disorders.
Overall, 6%–12% of women had intracyclic bleeding in the first 24 days (“mandatory phase”) of cycles 2–12 (Figure 7).
Figure 7.
Proportion of women with intracyclic bleeding during the first 24 days of each intake cycle in groups A and B (full analysis set).
Tolerability
EE/drospirenoneFlex was well tolerated. Overall, 61.5% (N=307) of women had ≥1 AE and 17.6% (n=88) of women had drug-related AEs, of which the most common were headache (3.2% of women), breast pain (2.6%), and nausea (2.2%). Twelve women (2.4%) reported SAEs, none of which was deemed to be drug related (investigator classified). No deaths were reported. AEs tended to be mild (19.2%) or moderate (37.1%) in intensity (investigator classified). Severe AEs occurred in 5.2% (n=26) of women; 0.8% of severe AEs were considered drug related. Overall, 6.0% (n=30) of women discontinued due to AEs. Two women discontinued due to SAEs; one of them discontinued due to deep vein thrombosis and pulmonary embolism, and at follow-up was found to have a coagulopathy with lupus anticoagulant antibodies, which may explain the development of thromboembolic disease. The other woman had preexisting Crohn’s disease and discontinued due to ileal stenosis.
During the study, 19 women had cervical dysplasia or “cervical smear abnormal”; these cases were considered unrelated to study drug. In one subject, mild tachycardia was reported and led to study discontinuation.
User satisfaction
Overall (ie, for pooled treatment groups), 80.0% of women reported that they were much satisfied (54.3%) or very much satisfied (25.7%) with EE/drospirenoneFlex and 78.0% would recommend it to a friend. In general, women found the regimen “easy to use and easy to understand”; 93.5% responded that they “Disagree” (49.8%) or “Strongly disagree” (43.7%) with the statement that “The intake regimen is too complicated.” The proportion of women who had a positive or neutral rating for dispenser attributes was as follows: size of the dispenser, 67.9%; visual appeal, 81.4%; and display clarity, 96.3%. Detailed results have been presented elsewhere.18
Discussion
This large study in women seeking contraception showed that an acoustic alarm on the digital pill dispenser reduced the mean delay time for daily pill intake by 1.5 hours compared with visual reminders only. A few hours delay in taking COCs would not be expected to adversely affect contraceptive efficacy. The daily delay in pill intake, however, was chosen as a surrogate endpoint for adherence with daily pill intake as it is reasonable to assume that behavior characterized by frequent delays in pill intake may likely be associated with increased risk of missed pills (delayed intake >24 hours). Our results support this association; the number of missed pills over the duration of the study was consistently lower in the group with the activated acoustic alarm reminder (group A) than in the group (group B) with visual reminders only as determined by data from the pill dispenser and diary card recordings.
A previous randomized controlled trial, which assessed the use of daily text messages on COC adherence, reported no improvement in pill adherence over 3 months with the daily text messages.19 Both the daily text messages group and the control group missed about five pills per cycle. The lack of a significant difference between the groups could be attributed to the frequent use of alternative reminder systems in the control group. At the beginning of the study, all women were given routine contraceptive counseling, including the possibility of using reminder methods to help them to remember to take their pill. The high rate of reminder system use in the control arm, particularly electronic systems such as cell phone alarms that mimicked the study intervention, could contribute to the lack of difference between the groups.
Daily text messages were shown to improve oral contraceptive continuation rates over 6 months in another randomized controlled trial.20 In that study of 962 participants, those who received daily text messages were more likely to continue OC use than control participants at 6 months (75% vs 54%, P=0.003; odds ratio 1.44, 95% CI: 1.03–2.00). In addition, the use of reminder cards was shown to significantly improve compliance to OC use in another study that included 975 women requesting contraception (repeat prescription or first-time use).21 However, that study was not randomized, and the decision of whether or not to provide the women with a reminder card was left to the gynecologist, and therefore, subject to bias.
Dispenser data showed that some women missed a lot of pills; in group B, 5% of women missed ≥30 pills, compared with no women in group A. It is reasonable to assume that women who frequently miss pills are likely to benefit most from the digital pill dispenser. Diary cards showed the same trends but, as expected, there were some discrepancies in missed pills between dispenser and diary cards, especially in group B. Studies assessing adherence with OC using an electronic monitoring device have reported a similar trend.19,22 It is possible that women who are poorly adherent with pill intake are also unlikely to accurately record pill intake in diary cards.22 To err is human but it is also human nature to prefer not to admit every error.
Bleeding pattern indices were similar between treatment groups, but varied depending on how women choose to manage their bleeding (eg, varying cycle lengths, active scheduling of bleeding, management of intracyclic bleeding, long-cycle intake ignoring intracyclic bleeding). The incidence of intracyclic bleeding during days 1–24 was similar to that observed with the standard 24/4 regimen,23 suggesting that management of intracyclic bleeding during cycle extension (ie, by inducing a withdrawal bleed) leads to similar cycle control to a standard COC during the first 24 days of the subsequent cycle.
Between-country differences were evident for length of pill intake and thus number of bleeding/spotting days over 1 year. In Italy and Spain, women tended not to extend cycles and therefore had the highest number of bleeding/spotting days; this may reflect lack of experience with extended regimens and/or a reluctance to postpone bleeding.24
No pregnancies were observed. This could be attributed to the compliance-aiding effect of the dispenser and the advice it gives in case of a missed pill on how to continue intake and if (and for how long) additional contraception is needed. The effective contraception seen in our study is consistent with previous studies with EE/drospirenoneFlex8,9 and EE/drospirenone in a standard 24/4 regimen.10,11,25 Consistent with previous data,9,26 EE/drospirenoneFlex was well tolerated, with a comparable safety profile to other COCs.
Our study was subject to some limitations. For example, women in group A could turn off the acoustic alarm if they wished. To compensate for this, we excluded 59 women from PPS who had the acoustic alarm deactivated for >20% of pill releases. In addition, there was no “non-dispenser” comparative group to allow for an assessment of the overall effect of digital pill dispenser on adherence. However, a “non-dispenser” group would have thrown up additional challenges with regard to primary and secondary endpoints, ie, accurately recording the time delay for pill release and identifying missed pills. A separate digital tool to record pill intake on a daily basis would have influenced adherence by itself and not allowed observation of real-life intake patterns of women taking a product with standard packaging.
A limitation of all clinical trials, however, is that they usually recruit a selected subject population, and as such, the results of this study may not be fully applicable to the general population of women who would use EE/drospirenoneFlex with the digital tablet dispenser in clinical practice. Noteworthy in this respect is that the population recruited were generally well educated with the majority having reached a college/university level. It was interesting to see that good education does not necessarily translate into good pill intake behavior, and improvements with a compliance-aiding device could be shown even in this group of women. A strength of our study, however, was the low loss to follow-up rate (2% across both groups).
A historical comparison of data from paper diaries of a previous study not using the device suggests that the adherence-aiding effect is mainly caused by the acoustic alarm.9 The UAC warning function of the device, however, may also have helped to avoid unplanned pregnancies in women in this study who missed a lot of pills. With the Pearl Index of 0.64 in the previous study, the occurrence of three to four pregnancies could have been a likely outcome.
In conclusion, the activated acoustic alarm of the dispenser improved adherence with EE/drospirenoneFlex, reducing delay of pill intake and number of missed pills. EE/drospirenoneFlex provided effective contraception and demonstrated a similar tolerability profile to other COCs. No pregnancies were reported during 424 women-years of treatment. Between-country usage differences were evident and may reflect familiarity with extended-cycle COCs.
Acknowledgments
This study was funded by Bayer HealthCare Pharmaceuticals. The authors would like to thank Claire Byrne of inScience Communications, Springer Healthcare, who provided medical writing assistance funded by Bayer HealthCare Pharmaceuticals.
Footnotes
Disclosure
Jörg Elliesen, Anja Walzer, and Bodo Kirsch are employees of Bayer HealthCare Pharmaceuticals. During the past 5 years, Inka Wiegratz has had financial relationships (investigator, lecturer, member of advisory boards, and/or consultant) with Bayer Pharma AG, Jenapharm, Merck Sharpe and Dohme, Rottapharm Madaus, and Dr Kade. Anna Maria Paoletti is a full professor of obstetrics and gynecology at the University of Cagliari (Italy) and in the last 5 years she has had financial relationships (member of advisory boards) with Bayer, Teva, and Gedeon Richter. The authors report no other conflicts of interest in this work.
References
- 1.Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68(2):89–96. doi: 10.1016/s0010-7824(03)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.Archer DF, Jensen JT, Johnson JV, Borisute H, Grubb GS, Constantine GD. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74(6):439–445. doi: 10.1016/j.contraception.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Wiegratz I, Stahlberg S, Manthey T, et al. Effects of an oral contraceptive containing 30 mcg ethinyl estradiol and 2 mg dienogest on lipid metabolism during 1 year of conventional or extended-cycle use. Contraception. 2010;81(1):57–61. doi: 10.1016/j.contraception.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Seidman DS, Yeshaya A, Ber A, et al. A prospective follow-up of two 21/7 cycles followed by two extended regimen 84/7 cycles with contraceptive pills containing ethinyl estradiol and drospirenone. Isr Med Assoc J. 2010;12(7):400–405. [PubMed] [Google Scholar]
- 5.Caruso S, Malandrino C, Cicero C, Ciancio F, Cariola M, Cianci A. Quality of sexual life of women on oral contraceptive continued-regimen: pilot study. J Sex Med. 2013;10(2):460–466. doi: 10.1111/j.1743-6109.2012.03004.x. [DOI] [PubMed] [Google Scholar]
- 6.Association of Reproductive Health Professionals What You Need to Know: Menstrual Suppression. 2008. [Accessed June 4, 2014]. Available from: http://www.arhp.org/uploadDocs/menstruationfactsheet.pdf.
- 7.Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol. 2006;195(4):935–941. doi: 10.1016/j.ajog.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 8.Jensen JT, Garie SG, Trummer D, Elliesen J. Bleeding profile of a flexible extended regimen of ethinylestradiol/drospirenone in US women: an open-label, three-arm, active-controlled, multicenter study. Contraception. 2012;86(2):110–118. doi: 10.1016/j.contraception.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Klipping C, Duijkers I, Fortier MP, et al. Contraceptive efficacy and tolerability of ethinylestradiol 20 mug/drospirenone 3 mg in a flexible extended regimen: an open-label, multicentre, randomised, controlled study. J Fam Plann Reprod Health Care. 2012;38(2):73–83. doi: 10.1136/jfprhc-2011-100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachmann G, Sulak PJ, Sampson-Landers C, Benda N, Marr J. Efficacy and safety of a low-dose 24-day combined oral contraceptive containing 20 micrograms ethinylestradiol and 3 mg drospirenone. Contraception. 2004;70(3):191–198. doi: 10.1016/j.contraception.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Hernadi L, Marr J, Trummer D, De Leo V, Petraglia F. Efficacy and safety of a low-dose combined oral contraceptive containing drospirenone 3 mg and ethinylestradiol 20 mcg in a 24/4-day regimen. Contraception. 2009;80(1):18–24. doi: 10.1016/j.contraception.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Lete I, Doval JL, Pérez-Campos E, et al. Self-described impact of noncompliance among users of a combined hormonal contraceptive method. Contraception. 2008;77(4):276–282. doi: 10.1016/j.contraception.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Aubeny E, Buhler M, Colau JC, Vicaut E, Zadikian M, Childs M. Oral contraception: patterns of non-compliance. The Coraliance Study. Eur J Contracept Reprod Health Care. 2002;7(3):155–161. [PubMed] [Google Scholar]
- 14.Singh S, Sedgh G, Hussain R. Unintended pregnancy: worldwide levels, trends, and outcomes. Stud Fam Plann. 2010;41(4):241–250. doi: 10.1111/j.1728-4465.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg MJ, Waugh MS, Meehan TE. Use and misuse of oral contraceptives: risk indicators for poor pill taking and discontinuation. Contraception. 1995;51(5):283–288. doi: 10.1016/0010-7824(95)00074-k. [DOI] [PubMed] [Google Scholar]
- 16.Bayer Australia Ltd Consumer Medicines Information: YAZ® Flex. 2013. [Accessed June 4, 2014]. Available from: http://www.mydr.com.au/medicines/cmis/yaz-flex-tablets.
- 17.Belsey EM, Farley TM. The analysis of menstrual bleeding patterns: a review. Contraception. 1988;38(2):129–156. doi: 10.1016/0010-7824(88)90035-2. [DOI] [PubMed] [Google Scholar]
- 18.Kirsch B, Elliesen J, Paoletti A, Walzer A, Wiegratz I. User assessments of an adherence-aiding dispenser for daily tablet intake in women receiving the flexible regimen of ethinylestradiol (EE) 20 μg/drospirenone 3 mg; Proceedings of the 10th European Society of Gynecology Congress; September 18–21, 2013; Brussels, Belgium. [Google Scholar]
- 19.Hou MY, Hurwitz S, Kavanagh E, Fortin J, Goldberg AB. Using daily text-message reminders to improve adherence with oral contraceptives: a randomized controlled trial. Obstet Gynecol. 2010;116(3):633–640. doi: 10.1097/AOG.0b013e3181eb6b0f. [DOI] [PubMed] [Google Scholar]
- 20.Castano PM, Bynum JY, Andres R, Lara M, Westhoff C. Effect of daily text messages on oral contraceptive continuation: a randomized controlled trial. Obstet Gynecol. 2012;119(1):14–20. doi: 10.1097/AOG.0b013e31823d4167. [DOI] [PubMed] [Google Scholar]
- 21.Lachowsky M, Levy-Toledano R. Improving compliance in oral contraception: ‘the reminder card’. Eur J Contracept Reprod Health Care. 2002;7(4):210–215. [PubMed] [Google Scholar]
- 22.Potter L, Oakley D, de Leon-Wong E, Canamar R. Measuring compliance among oral contraceptive users. Fam Plann Perspect. 1996;28(4):154–158. [PubMed] [Google Scholar]
- 23.Marr J, Gerlinger C, Kunz M. A historical cycle control comparison of two drospirenone-containing combined oral contraceptives: ethinylestradiol 30 mug/drospirenone 3 mg administered in a 21/7 regimen versus ethinylestradiol 20 mug/drospirenone 3 mg administered in a 24/4 regimen. Eur J Obstet Gynecol Reprod Biol. 2012;162(1):91–95. doi: 10.1016/j.ejogrb.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Szarewski A, von Stenglin A, Rybowski S. Women’s attitudes towards monthly bleeding: results of a global population-based survey. Eur J Contracept Reprod Health Care. 2012;17(4):270–283. doi: 10.3109/13625187.2012.684811. [DOI] [PubMed] [Google Scholar]
- 25.Anttila L, Bachmann G, Hernadi L, et al. Contraceptive efficacy of a combined oral contraceptive containing ethinyloestradiol 20 μg/drospirenone 3mg administered in a 24/4 regimen: a pooled analysis of four open-label studies. Eur J Obstet Gynecol Reprod Biol. 2011;155(2):180–182. doi: 10.1016/j.ejogrb.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Klipping C, Duijkers I, Fortier MP, Marr J, Trummer D, Elliesen J. Long-term tolerability of ethinylestradiol 20 mug/drospirenone 3 mg in a flexible extended regimen: results from a randomised, controlled, multicentre study. J Fam Plann Reprod Health Care. 2012;38(2):84–93. doi: 10.1136/jfprhc-2011-100214. [DOI] [PMC free article] [PubMed] [Google Scholar]