Acidic lipids act as coreceptors with Ypt7p to bind the HOPS complex to support membrane tethering and fusion. After phosphorylation by the vacuolar kinase Yck3p, phospho-HOPS needs both Ypt7p:GTP and acidic lipids to support fusion.
Abstract
Fusion of yeast vacuoles requires the Rab GTPase Ypt7p, four SNAREs (soluble N-ethylmaleimide–sensitive factor attachment protein receptors), the SNARE disassembly chaperones Sec17p/Sec18p, vacuolar lipids, and the Rab-effector complex HOPS (homotypic fusion and vacuole protein sorting). Two HOPS subunits have direct affinity for Ypt7p. Although vacuolar fusion has been reconstituted with purified components, the functional relationships between individual lipids and Ypt7p:GTP have remained unclear. We now report that acidic lipids function with Ypt7p as coreceptors for HOPS, supporting membrane tethering and fusion. After phosphorylation by the vacuolar kinase Yck3p, phospho-HOPS needs both Ypt7p:GTP and acidic lipids to support fusion.
INTRODUCTION
Biological membrane fusion, which underlies cell growth, hormone secretion, intracellular vesicular trafficking, and neurotransmission, occurs by mechanisms that have been conserved from yeast to humans. Before fusion, membranes adhere through the interactions of Rab-family GTPases with their binding proteins, which serve as intermembrane tethers (Grosshans et al., 2006). Proteins and lipids required for fusion become enriched in fusion microdomains. Prominent among these are soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) proteins (Jahn and Scheller, 2006). These membrane proteins, defined by their α-helical SNARE domains with characteristic heptad repeats, are found in conserved families termed R-, Qa-, Qb-, or Qc-SNAREs according to whether they have a conserved arginyl or glutaminyl residue at the center of their SNARE domain (Fasshauer et al., 1998; Bock et al., 2001). On membrane tethering, four SNAREs, one from each family, assemble into a coiled-coil four-helical structure anchored to each membrane termed the trans-SNARE complex. After fusion, these complexes remain anchored to the single, fused membrane and are then termed cis-SNARE complexes. These complexes can be disassembled by Sec18p/NSF, an ATP-driven chaperone, and its cochaperone, Sec17p/α-SNAP, to allow subsequent rounds of fusion. Trans-SNARE complexes bring membranes into closer apposition than is achieved by tethers and have been proposed to strain the bilayers to facilitate lipid rearrangements that constitute fusion (McNew et al., 2000), although it is unclear whether strain is always required (Jun et al., 2007; Zhou et al., 2013). Key lipids, such as phosphoinositides, diacylglycerol, and sterols, are also enriched at fusion sites (Fratti et al., 2004). These proteins and lipids are interdependent for their fusion-domain enrichment and are both essential for the fusion process (Fratti et al., 2004). The interrelationships among these lipids and proteins for their enrichment and action at fusion microdomains are unknown.
We study membrane fusion with vacuoles (lysosomes) from Saccharomyces cerevisiae (Wickner, 2010). Vacuoles undergo constant cycles of fission and homotypic fusion, regulating their size and aggregate volume in response to growth medium osmolarity (LaGrassa and Ungermann, 2005). Genetic interruption of fusion, accompanied by unimpeded fission, gives a striking phenotype of multiple small vacuoles instead of one or a few large organelles. Early genetic screens for this altered vacuole morphology were the basis for the definition of nine vam genes, each required for homotypic vacuole fusion (Wada et al., 1992). A quantitative colorimetric assay of the fusion of purified vacuoles (Haas et al., 1994) allowed definition of the proteins and lipids required at each reaction stage. On incubation with ATP, vacuoles undergo “priming”—the synthesis of phosphoinositides and ATP-driven disassembly of cis-SNARE complexes—releasing Sec17p (Mayer et al., 1996) and the soluble Qc-SNARE Vam7p (Boeddinghaus et al., 2002). Primed vacuoles then tether (Mayer and Wickner, 1997). Tethering requires the vacuolar Rab-family GTPase Ypt7p and its effector complex homotypic fusion and vacuole protein sorting complex (HOPS; Stroupe et al., 2006). HOPS is a large hexameric complex comprising Vps11p, Vps16p, Vps18p, Vps33p, Vps39p, and Vps41p. HOPS, or its Vps39p and Vps41p subunits, can bind directly to Ypt7p (Brett et al., 2008), although there have been contradictory reports on whether Ypt7p is truly required for fusion (Mima et al., 2008). One HOPS subunit, Vps33p, is a member of the SNARE-binding Sec1/Munc18 family, but little is known of how HOPS relates to SNAREs and SNARE complex assembly.
In addition to genetic studies of vacuole fusion in vivo and extensive studies of the fusion of the purified organelle, there has been iterative progress in reconstituting this fusion event with purified and defined components. At first, a vacuole detergent extract was reconstituted by detergent dialysis to form proteoliposomes that could fuse with intact vacuoles (Sato and Wickner, 1998). Fractionation of this detergent extract before reconstitution showed that it contained at least two essential components—the Rab Ypt7p and a complex of the vacuolar SNAREs. A second approach (Fukuda et al., 2000) reported apparent lipid mixing between two complementary proteoliposome preparations bearing either the recombinant R-SNARE Nyv1p or the three Q-SNAREs, Vam3p (Qa), Vti1p (Qb), and Vam7p (Qc). When all four SNAREs were present on each fusion partner as cis-SNARE complexes, as is the case for the organelle itself, purified HOPS, Sec17p, and Sec18p were also required (Mima et al., 2008). In some studies, recombinant Ypt7p was also required (Stroupe et al., 2009; Zick and Wickner, 2012), although the factors underlying this requirement have remained unclear. The rigor of assay of reconstituted fusion has also increased in successive studies. Early assays used proteoliposome pairs in which one partner bore two fluorescent lipids, which quenched through fluorescence resonance energy transfer (FRET), whereas the other partner was nonfluorescent (Struck et al., 1981). Proteoliposome interactions led to dequenching, some due to fusion, but some due to lysis/reannealing (Zucchi and Zick, 2011) or other, ill-defined trans interactions (Zick and Wickner, 2014). An assay of protected lumenal compartment mixing allowed more rigorous measures of fusion and lysis along with lipid mixing (Zucchi and Zick, 2011).
Prior work led to apparently contradictory findings that either a very simple lipid composition suffices for fusion (Fukuda et al., 2000) or that there are complex lipid requirements for fusion (Fratti et al., 2004) and that either Ypt7p is dispensable for fusion (Mima et al., 2008) or is required for fusion (Stroupe et al., 2009; Zick and Wickner, 2012). We now exploit the power of the chemically defined reconstituted fusion reaction to resolve the interrelated requirements for lipids and Ypt7p for tethering and fusion. Acidic lipids are an important component of the HOPS membrane receptor and can support tethering and the ensuing fusion. With high levels of Ypt7p in its active, GTP-bound state, acidic lipids are not required for tethering or fusion. Either with lower levels of Ypt7p and at mildly elevated ionic strength or after phosphorylation of HOPS by the vacuolar kinase Yck3p, fusion requires both Ypt7p:GTP and acidic lipids.
RESULTS
Proteoliposomes were formed by dialysis from detergent-mixed micellar solutions of various combinations of the four SNAREs, Ypt7p, and purified lipids. To allow assay of lumenal compartment mixing upon fusion, either of two fluorescent proteins, biotinylated phycoerythrin or Cy5-derivatized streptavidin, was trapped inside the proteoliposomes during their preparation (Zucchi and Zick, 2011). Isolated proteoliposomes of all lipid compositions had consistent protein compositions (Supplemental Figure S1). Reconstituted proteoliposomes bearing Ypt7p were preincubated to exchange the Rab into its GTP-bound form. Proteoliposomes with identical protein and lipid composition but bearing complementary lumenal proteins were assayed for fusion in the presence of Sec17p, Sec18p, ATP, and HOPS. Fusion and the resulting lumenal compartment mixing results in a strong FRET signal due to the high-affinity binding of lumenal Cy5-streptavidin to biotin-phycoerythrin, whereas the presence of a large external excess of nonfluorescent streptavidin blocks any signal due to lysis (Zucchi and Zick, 2011; Zick and Wickner, 2014).
Proteoliposomes of phosphatidylcholine (PC) alone did not fuse (Figure 1, A and B, open circles), reflecting the absence of small–head group lipids, which are needed for progression from the docked state to fusion (Zick et al., 2014). Reconstituted proteoliposomes (RPLs) with a lipid composition of PC/phosphatidylethanolamine (PE)/ergosterol (Erg)/diacylglycerol (DAG), with the full complement of these small–head group lipids but lacking acidic lipids, fused only when bearing Ypt7p:GTP (Figure 1, A and B, open triangles; fusion in the first 5 min for three replicate experiments is presented in Figure 1C, lanes 5 and 6). In contrast, when the acidic lipids phosphatidylinositol (PI), phosphatidylserine (PS), and phosphatidic acid (PA) were also present, that is, with the full vacuolar mimic lipids (VMLs; Zick et al., 2014), fusion was even seen in the absence of Ypt7p (Figure 1A, diamonds). If the proteoliposomes simply bore a higher concentration of PI, the most abundant vacuolar acidic lipid, as well as PC/PE/Erg/DAG, fusion was again seen without Ypt7p (Figure 1, A and B, filled triangles, and C, lane 7). Thus fusion can be seen with acidic lipids and without Ypt7p:GTP (lanes 7 and 9) and at a low but measurable rate with Ypt7p without acidic lipids (lane 6). When proteoliposomes comprised 70% PC and 30% PS (Figure 1, A and B, filled circles, and C, lanes 3 and 4), in which the PS fulfilled the roles of both small–head group lipid and acidic lipid, Ypt7p:GTP gave rapid and complete fusion (lane 4), which was not seen with proteoliposomes of PC alone (lane 2).
FIGURE 1:

Lipid composition and Ypt7p:GTP control fusion. Fusion of RPLs of various lipid compositions, each with the four vacuolar SNAREs and either without Ypt7p (A) or with Ypt7p:GTP (B), was assayed as protected lumenal compartment mixing. RPLs, ATP, streptavidin, and buffer were mixed and preincubated for 10 min at 27ºC with GTP and EDTA in 7 μl to specifically exchange the Ypt7p-bound guanine nucleotide, then mixed with 3 μl of HOPS, Sec17p, Sec18p, and MgCl2 to initiate the fusion reaction. (C) The amount of lumenal compartment mixing after 5 min is shown as average ± SD for three independent experiments. Final concentrations in 10 μl: 1.3 mM ATP, 2.6 mM EDTA, 25.6 μM GTP, 12.6 μM streptavidin, 1.1 mM lipid, 3 mM MgCl2, 177 nM Sec17p, 907 nM Sec18p, and 218 nM HOPS.
Because purified Ypt7p and HOPS cooperate to support proteoliposome tethering (Hickey and Wickner, 2010), the finding that acidic lipids can substitute for Ypt7p suggests that these lipids might also bind HOPS and support tethering. However, there are other possibilities; for example, acidic lipids might affect the assembly of fusogenic microdomains such as those seen on intact vacuoles (Wang et al., 2002, 2003; Fratti et al., 2004) or might alter the asymmetric distribution across the bilayer of proteins or lipids during reconstitution. We therefore compared the fusion of PC/PE/Erg/DAG/4SNARE proteoliposomes, prepared without Ypt7p or with Ypt7p that had been exchanged to the GTP-bound form, when incubated without supplement or with added dioctanoyl PA (diC8-PA). This acidic lipid has the useful properties of substantial water solubility and a strong propensity to partition into membranes. Acidic lipids also contribute to the membrane association of the soluble SNARE Vam7p (Lee et al., 2006; Karunakaran and Wickner, 2013), and additional Vam7p (beyond what was present on the liposomes in cis-4SNARE complex) was therefore also tested as a supplement, alone or in combination with diC8-PA. Proteoliposomes of PC/PE/Erg/DAG/4SNARE without Ypt7p were still able to undergo fusion but only when given diC8-PA (Figure 2A, circles vs. squares). With Ypt7p:GTP, slow fusion was seen without added lipid or Vam7p, but either or both caused strong stimulation (Figure 2B). In all cases, fusion required HOPS, Sec17p, Sec18p, and ATP, added at time 0, and thus reflects the physiological dependence on these SNARE chaperones. We conclude that acidic lipids play a direct role in fusion in a manner that is at least partially redundant with Ypt7p.
FIGURE 2:

Fusion is restored to RPLs that lack acidic lipids by the addition of diC8-PA or excess Vam7p. Fusion of RPLs composed of PC, PE, Erg, and DAG with the four vacuolar SNAREs and either without Ypt7p (A) or with Ypt7p:GTP (B) was assayed as protected lumenal compartment mixing. RPLs were preincubated without addition or with 2 μM Vam7p and/or 110 μM diC8-PA, as indicated, along with ATP, streptavidin, EDTA, and GTP in 7 μl for 10 min at 27ºC before the fusion reaction was started by addition of 3 μl of HOPS, Sec17p, Sec18p, and MgCl2. (C) The amount of lumenal compartment mixing after 5 min is shown as average ± SD for three independent experiments. Final concentrations in 10 μl: 0.9 mM ATP, 1.8 mM EDTA, 18 μM GTP, 9 μM streptavidin, 0.8 mM lipid, 2.5 mM MgCl2, 156 nM Sec17p, 800 nM Sec18p, and 193 nM HOPS.
Because acidic lipids form part of the membrane receptor of the soluble SNARE Vam7p, we tested whether there was a dependence on either acidic lipid or the Rab GTPase when Vam7p was firmly membrane anchored by a hydrophobic transmembrane domain. Proteoliposomes of a PC/PE/Erg/DAG composition were prepared with three wild-type vacuolar SNAREs and a previously characterized membrane-anchored Vam7p (Xu and Wickner, 2012). Coomassie- stained gels showed that the absence of Ypt7p or its presence had no effect on SNARE reconstitution into these proteoliposomes (Supplemental Figure S2). Without Ypt7p, these proteoliposomes showed no fusion at all, but detectable fusion occurred in the presence of added diC8-PA (Figure 3A, open and filled circles). With Ypt7p:GTP, there was rapid and efficient fusion (triangles). The finding that diC8-PA stimulated the fusion of PC/PE/Erg/DAG RPLs bearing Ypt7p:GTP with wild-type Vam7p (Figure 2B, circles and squares) but not with transmembrane-anchored Vam7p, which showed rapid fusion even in the absence of diC8-PA (Figure 3A, open and closed triangles), and the finding that extra Vam7p substantially enhanced the fusion of PC/PE/Erg/DAG RPLs bearing Ypt7p:GTP (Figure 2B, circles vs. triangles) confirm that acidic lipids participate in the recruitment of Vam7p. However, since diC8-PA still had an effect in the absence of Ypt7p:GTP when Vam7p was transmembrane anchored (Figure 3A, compare open vs. closed circles), the function of acidic lipids extends beyond their role as a component of the Vam7p membrane receptor.
FIGURE 3:

Stimulation of fusion of PC/PE/Erg/DAG RPLs with four vacuolar SNAREs, including a transmembrane anchored form of Vam7p (Xu and Wickner, 2012), by addition of diC8-PA. RPLs were prepared as described in Materials and Methods, except that Ypt7p was stripped and reloaded with GTPγS before reconstitution, as described (Zick and Wickner, 2012). (A) RPLs without Ypt7p (circles) or with Ypt7p:GTPγS (triangles) were preincubated without addition (open symbols) or with 110 μM diC8-PA (filled symbols), along with MgCl2, ATP, and streptavidin, in 14 μl for 10 min at 27ºC before the fusion reaction was started by addition of 6 μl of HOPS, Sec17p, and Sec18p. Final concentrations in 20 μl: 1 mM ATP, 9.6 μM streptavidin, 0.5 mM lipid, 1 mM MgCl2, 100 nM Sec17p, 1 μM Sec18p, and 165 nM HOPS. (B) Fusion under these conditions after 5 min.
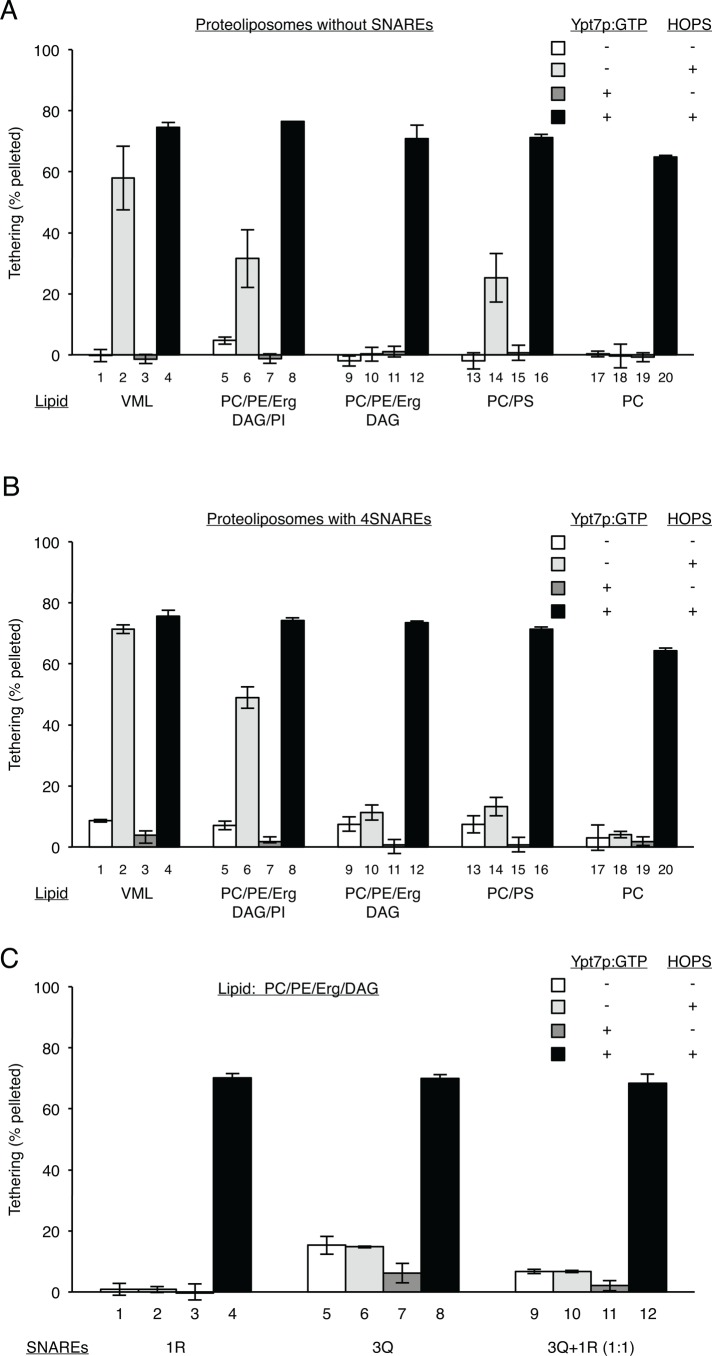
A floatation assay was used to directly compare the roles of acidic lipid and Ypt7p in promoting HOPS membrane association. Without membrane proteins, HOPS only bound stably to liposomes that bore acidic lipids, whether PS, PI, or those of the full vacuolar mimic lipids (Figure 4, columns 1, 5, and 13 with acidic lipids vs. 9 and 17 without). All proteoliposomes with Ypt7p:GTP were able to stably bind HOPS (dark gray and black columns). The presence of the four vacuolar SNAREs on proteoliposomes caused very little increase in HOPS binding in the absence of Ypt7p or acidic lipids (light gray columns).
FIGURE 4:
Acidic lipids and/or Ypt7p facilitate HOPS binding to membranes. Liposomes of various lipid compositions with or without the four vacuolar SNAREs and either without Ypt7p or with Ypt7p:GTP were assayed for their ability to bind HOPS as described in Materials and Methods.
Because Ypt7p, SNAREs, and acidic lipids share the capacity to bind HOPS, is this directly reflected in tethering? To explore the relationship between HOPS binding, tethering, and fusion, we developed a direct, simple, and quantitative assay of tethering. Isolated proteoliposomes require HOPS for assembly into large clusters (Hickey and Wickner, 2010). Such liposome clusters sediment readily upon centrifugation. After a 10-min incubation at 27ºC, proteoliposomes with Ypt7p:GTP supported HOPS-dependent sedimentation without regard to lipid composition (Figure 5A, dark gray vs. black columns). Without acidic lipids or Ypt7p, there was little or no HOPS-dependent tethering (bars 10 and 18), whereas even a single acidic lipid such as PI supported tethering (bar 6). The presence of the four vacuolar SNAREs on these proteoliposomes had little effect on HOPS-dependent tethering (compare Figure 5, A and B). The introduction of fusion-competent 3Q and 1R SNAREs also failed to promote tethering (Figure 5C), even when PC/PE/Erg/DAG proteoliposomes bearing 3Q and 1R SNAREs were mixed in equal proportions (compare Figure 5, C, columns 9 and 10, with B, columns 9 and 10). Tethering is thus established as a truly HOPS-dependent and SNARE-independent first step, required (Zick and Wickner, 2014) for efficient progress from SNARE pairing to later fusion.
FIGURE 5:
HOPS requires acidic lipids and/or Ypt7p to induce membrane clustering. (A) Liposomes of various lipid compositions bearing either no Ypt7p or Ypt7p:GTP were assayed for HOPS-dependent tethering as described in Materials and Methods. (B, C) SNAREs do not make a major contribution to tethering. Liposomes of various lipid compositions with either all four vacuolar SNAREs (B) or only the R-SNARE or the three Q-SNAREs (C), which bore either no Ypt7p or Ypt7p:GTP, were assayed for HOPS-dependent tethering as described in Materials and Methods.
Even in the presence of the full vacuolar lipid mixture, the contributions of lipid and Ypt7p:GTP to tethering and fusion could be distinguished. At 150 mM NaCl, the vacuolar lipids sufficed to support substantial tethering in the absence of Ypt7p (Figure 6A, open circles). At 250 mM NaCl, vacuolar lipids alone could no longer support tethering, which then required Ypt7p (filled squares). Even in the absence of acidic lipids, tethering that was supported by Ypt7p:GTP showed substantial resistance to high salt (Figure 6B).
FIGURE 6:

Salt sensitivity of tethering. (A) VML liposomes with either no Ypt7p or Ypt7p:GTP or (B) PC/PE/Erg/DAG liposomes with Ypt7p:GTP were assayed for HOPS-dependent tethering as described in Materials and Methods in the presence of increasing concentrations of NaCl.
The differential salt resistance of tethering also extended to fusion. With proteoliposomes of vacuolar lipid composition, fusion proceeded well at 150 mM NaCl in the absence of Ypt7p but was rapidly lost at higher salt concentrations (Figure 7A, open columns). Fusion persisted at yet higher salt with Ypt7p:GTP (black columns). The vacuolar kinase Yck3p has been reported to confer strict dependence on GTP-bound Ypt7p for fusion (Zick and Wickner, 2012). The soluble form of Yck3p that was used in this study phosphorylated five of the six HOPS subunits and at multiple residues (Supplemental Table S1); substantial further studies will be needed to determine which of these modulate individual HOPS activities (tethering, Vam7p recruitment, proofreading of SNARE complexes, and protection of trans-SNARE complexes from Sec17/Sec18-mediated disassembly). Yck3p blocked, at all ionic strengths tested, the fusion of proteoliposomes of vacuolar lipid composition that lacked Ypt7p (Figure 7B, open columns), which is fully consistent with the observation that Ypt7p:GTP became essential for membrane binding of HOPS after phosphorylation by Yck3p (Supplemental Figure S3). HOPS phosphorylation by Yck3p permitted fusion of proteoliposomes with Ypt7p:GTP at the physiological ionic strength of 150 mM NaCl (black columns), although fusion was inhibited by salt levels such as 300 mM NaCl, which exhibited undiminished fusion with nonphosphorylated HOPS (Figure 7A, black columns). Both phospho-HOPS and nonphosphorylated HOPS are important physiologically, as HOPS phosphorylation by Yck3p and dephosphorylation by as-yet-unidentified phosphatases regulate vacuole fusion in response to growth medium osmolarity (LaGrassa and Ungermann, 2005). Acidic lipids were required for the function of phospho-HOPS with Ypt7p:GTP (Figure 7C). 4-SNARE/Ypt7p:GTP proteoliposomes of PC/PE/Erg/DAG fused at the physiological salt concentration of 150 mM (Figure 7C, light gray columns). This fusion was sensitive to increased NaCl concentrations, whereas addition of the acidic lipid diC8-PA conferred greater salt resistance (dark gray columns). Phosphorylation of HOPS by Yck3p abolished fusion at all salt levels (open columns), but substantial fusion was restored at physiological ionic strength by the addition of acidic lipid (black columns).
FIGURE 7:

Salt sensitivity of fusion. (A, B) Fusion of VML RPLs bearing the four vacuolar SNAREs and either no Ypt7p or Ypt7p:GTP was assayed as protected lumenal compartment mixing in the presence of increasing concentrations of NaCl. RPLs were preincubated with streptavidin and various amounts of NaCl in 15 μl for 10 min at 27ºC before the fusion reaction was started by addition of 5 μl of Sec17p, Sec18p, and HOPS, which had been preincubated for 10 min at 27ºC without (A) or with (B) Yck3p. (C) Fusion of RPLs composed of PC/PE/Erg/DAG, bearing four vacuolar SNAREs, including a transmembrane-anchored form of Vam7p, and Ypt7p:GTP was assayed as protected lumenal compartment mixing in the presence of increasing concentrations of NaCl. RPLs were preincubated without addition or with 100 μM diC8-PA, along with streptavidin and various amounts of NaCl, in 15 μl for 10 min at 27ºC before the fusion reaction was started by addition of 5 μl of Sec17p, Sec18p, and HOPS, preincubated for 10 min at 27ºC with or without Yck3p. Final concentrations in 20 μl: 1 mM ATP, 10 μM streptavidin, 0.5 mM lipid, 1 mM MgCl2, 600 nM Sec17p, 200 nM Sec18p, 100 nM HOPS, and 3 μM Yck3p.
Acidic lipids can cooperate with Ypt7p to support fusion under conditions in which neither alone is sufficient. At 225 mM NaCl, acidic lipids cannot support fusion in the absence of Ypt7p (Figures 7A, open columns, and 8, A and B, black symbols). With the full VML lipid mixture (which includes the acidic lipids PI, PS, and PA), Ypt7p supports similar rates and extents of fusion at molar ratios to lipid ranging from 1:2000 to 1:16,000 (Figure 8, A, open symbols, and C, black columns). In the absence of PI, PS, and PA, 4-SNARE proteoliposomes of PC/PE/Erg/DAG fused well when bearing Ypt7p at a molar ratio to lipids of 1:2000 (Figure 8, B, open circles, and C), but fusion diminished as Ypt7p levels were lowered and was lost at a molar ratio to lipids of 1:16,000 (Figure 8, B, diamonds, and C Thus, as one approaches more physiological concentrations of Ypt7p (see Table 1 in Zick et al., 2014), fusion becomes increasingly dependent on both Ypt7p:GTP and acidic lipids.
FIGURE 8:

Acidic lipids and Ypt7p:GTP can cooperate to support fusion at an elevated ionic strength where neither alone suffices. Proteoliposomes were prepared with (A) VML lipids, Ypt7p at the indicated molar ratios to lipid, and the four vacuolar SNAREs or (B) PC/PE/Erg/DAG lipids, Ypt7p, and the four vacuolar SNAREs, including a transmembrane- anchored form of Vam7p. The RPLs were incubated for 10 min at 27ºC with streptavidin, NaCl, EDTA, and GTP and assayed for fusion upon the addition of HOPS, Sec17p, Sec18p, ATP, and MgCl2. (C) The amount of lumenal compartment mixing after 5 min is shown as average ± SD for three independent experiments. Final concentrations in 20 μl: 0.5 mM lipid, 9.6 μM streptavidin, 225 mM NaCl, 1 mM EDTA, 10 μM GTP, 136 nM HOPS, 600 nM Sec17p, 200 nM Sec18p, 1 mM ATP, and 1 mM MgCl2.
HOPS has four known functions in fusion: tethering membranes (Hickey and Wickner, 2010), catalyzing the entry of Vam7p into SNARE complexes (Zick and Wickner, 2013), proofreading of SNARE complexes (Starai et al., 2008), and protecting trans-SNARE complexes from disassembly by Sec17p/Sec18p (Xu et al., 2010). It associates with membranes to fulfill these functions through several receptors: Ypt7p:GTP, acidic lipids, and SNAREs. These binding modalities and functions can be experimentally distinguished and separately examined. Proteoliposomes were prepared with PC/PE/Erg/DAG, four SNAREs including the transmembrane anchored Vam7p, and without Ypt7p or with Ypt7p in its GTP-bound state. Fusion of such proteoliposomes requires Sec17p/Sec18p to initially disassemble the 4-SNARE cis-complexes and then HOPS to protect trans-SNARE complexes from disassembly by Sec17p/Sec18p. Because acidic lipids are absent, HOPS association depends on Ypt7p, and even when tethering was artificially provided by the addition of polyethylene glycol (PEG), there was almost no fusion without Ypt7p (Figure 9A). With Ypt7p:GTP (Figure 9B), fusion no longer depends on PEG (circles). Even after removing the need for HOPS to tether (by adding PEG) and removing the need for HOPS to recruit Vam7p (through supplying a transmembrane anchor), HOPS is still required to protect trans-SNAREs from Sec17p/Sec18p. With acidic lipids and Ypt7p:GTP (Figure 7B), fusion is resistant to Yck3p. However, without acidic lipids, fusion is sensitive to Yck3p (Figure 9B, triangles). This Yck3p block is reversed by PEG, suggesting that phospho-HOPS is specifically deficient in tethering while fulfilling other Ypt7p-dependent functions.
FIGURE 9:
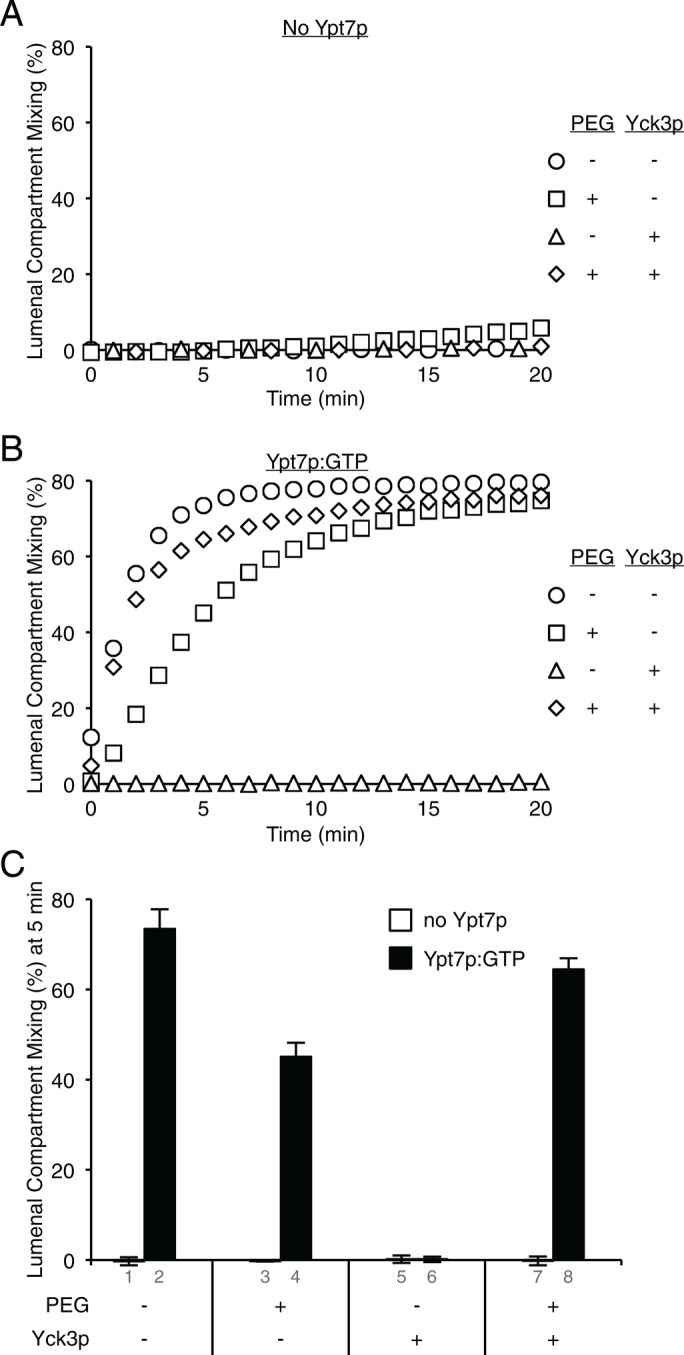
Ypt7p allows phospho-HOPS to fulfill its nontethering functions. Fusion of RPLs composed of PC, PE, Erg, and DAG with the four vacuolar SNAREs, including a transmembrane-anchored form of Vam7p and either without Ypt7p (A) or with Ypt7p:GTP (B), was assayed as protected lumenal compartment mixing. RPLs were preincubated with streptavidin in 10 μl for 10 min at 27ºC before the fusion reaction was started by addition of 10 μl of Sec17p, Sec18p, MgCl2, and ATP, as well as HOPS, preincubated for 10 min at 27ºC with or without Yck3p and PEG, as indicated. (C) The amount of lumenal compartment mixing after 5 min is shown as average ± SD for three independent experiments. Final concentrations in 20 μl: 1 mM ATP, 10 μM streptavidin, 0.5 mM lipid, 1 mM MgCl2, 600 nM Sec17p, 200 nM Sec18p, 100 nM HOPS, 3 μM Yck3p, and 2% (wt/vol) PEG8000.
Is the GTP-bound state of Ypt7p essential for its activity in the absence of acidic lipids? Proteoliposomes were prepared without acidic lipids and bearing Ypt7p and the four SNAREs, and the Ypt7p was loaded with GTP or GTPγS. Incubation of these proteoliposomes with increasing concentrations of the GTPase-activating protein Gyp1-46 (Rak et al., 2000; Wang et al., 2003) fully inactivated fusion when the Ypt7p had been loaded with GTP (Figure 10A) but not when it bore GTPγS (Figure 10B). In the absence of acidic lipids, GTP is strictly required for the activity of Ypt7p.
FIGURE 10:
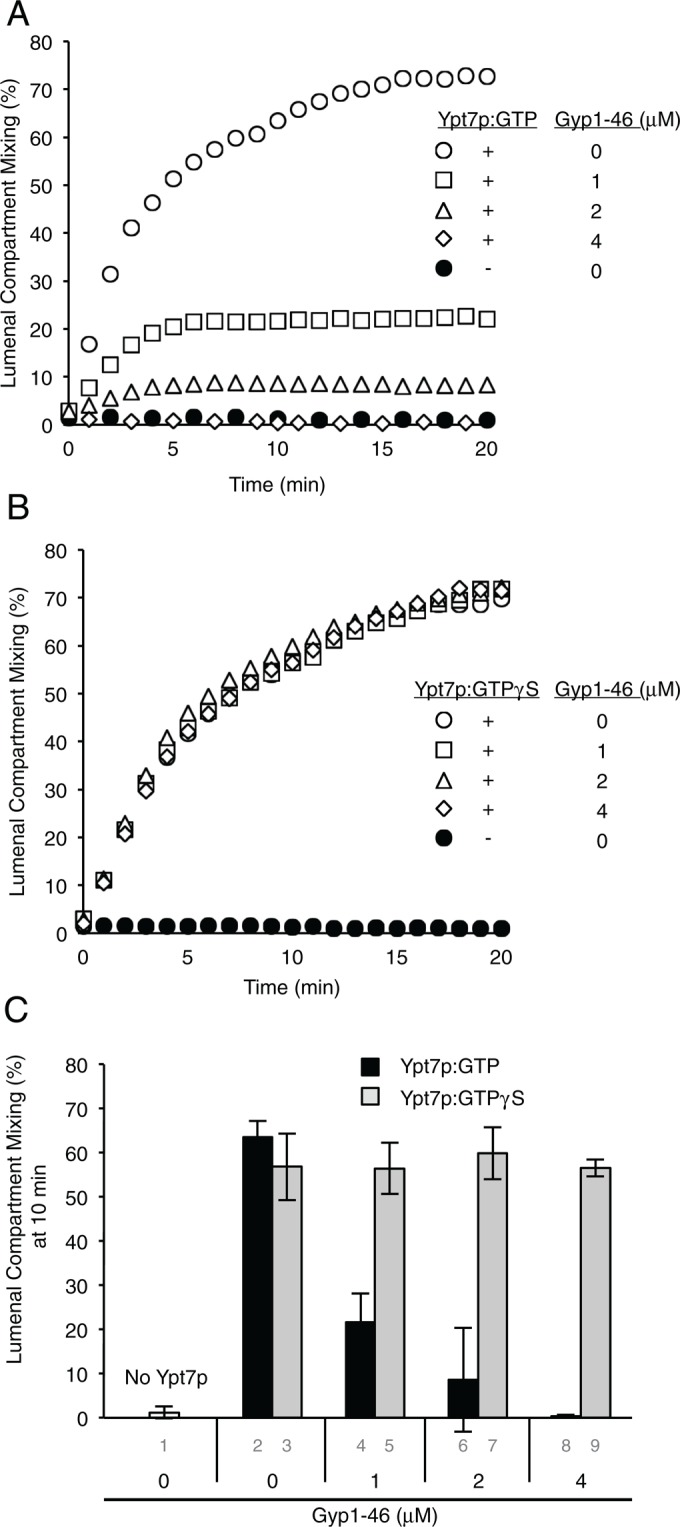
Ypt7p supports fusion only when in its GTP-bound state. Proteoliposomes of PC/PE/DAG/Erg were prepared with or without Ypt7p and with three wild-type vacuolar SNAREs plus the transmembrane-anchored Vam7p. After incubation for 10 min at 27°C with EDTA and either GTP (A) or GTPγS (B) to facilitate nucleotide exchange, they were mixed with MgCl2 and incubated for 10 min at 27°C with the indicated concentration of Gyp1-46. ATP, HOPS, Sec17p, and Sec18p were then added to initiate fusion, as described in Materials and Methods. Final concentrations in 20 μl: 1 mM ATP, 5 μM streptavidin, 0.5 mM lipid, 1 mM EDTA, 10 μM GTP or GTPγS, 100 nM HOPS, 600 nM Sec17p, 200 nM Sec18p, 2.25 mM MgCl2, and 0–4 μM Gyp1-46. (C) Average fusion after 10 min for triplicates of the experiments shown in panels A and B.
These data refine our view of tethering. Instead of the Rab GTPase being the only receptor for the tethering effector HOPS, Ypt7p shares that function with acidic lipids. Ypt7p:GTP and acidic lipids could even function in-trans to promote fusion. Proteoliposomes of PC/PE/Erg/DAG with four SNAREs were prepared with each lumenal fluorescent protein and with either PI as acidic lipid, Ypt7p:GTP, or neither and then mixed in all combinations and assayed for fusion in the presence of HOPS, Sec17p, Sec18p, and ATP. In each combination, fusion required either PI or Ypt7p:GTP on each fusion partner (Figure 11).
FIGURE 11:

Ypt7p:GTP and PI can facilitate fusion in both homotypic and heterotypic manners. (A) Fusion of various combinations of RPLs composed of PC, PE, Erg, and DAG with the four vacuolar SNAREs, including a transmembrane-anchored form of Vam7p and Ypt7p:GTP, the acidic lipid PI, or neither, was assayed as protected lumenal compartment mixing. RPLs were preincubated with streptavidin, ATP, EDTA, and GTP in 7 μl for 10 min at 27ºC before the fusion reaction was started by addition of 3 μl of Sec17p, Sec18p, and MgCl2. (B) The amount of lumenal compartment mixing after 5 min is shown as average ±SD for three independent experiments. Final concentrations in 10 μl: 0.9 mM ATP, 1.2 mM EDTA, 17 μM guanine-nucleotide, 8.5 μM streptavidin, 0.8 mM lipid, 2.5 mM MgCl2, 156 nM Sec17p, 800 nM Sec18p, and 193 nM HOPS.
DISCUSSION
Vacuole fusion has been studied intensively in vivo and with the purified organelle (Wickner, 2010), yet some of the very mechanistic questions that led to these studies can be addressed only in fusion reactions of defined chemistry. With reconstituted proteoliposomes of known composition, each component can be independently varied. In early studies with pure components, it appeared that the Rab GTPase Ypt7p was dispensable for fusion (Mima et al., 2008), but the cardiolipin present in these proteoliposomes was later shown to be a major contributor to this bypass (Stroupe, 2012). Cardiolipin is formed in mitochondria, and its presence on vacuolar surfaces is uncertain. We now report that proteoliposomes of vacuolar lipid composition without cardiolipin can still readily fuse in the absence of Ypt7p at physiological ionic strength (Figure 1). Earlier reports of strict Ypt7p-dependence for fusion (Stroupe et al., 2009) may have rested on the elevated ionic strength in those assays due to high MgCl2 levels, as increased ionic strength clearly modulates the requirements for HOPS to be able to facilitate tethering (Figure 6) and fusion (Figure 7). Examination of varied lipid compositions shows that acidic lipids can at least partially substitute for Ypt7p, whereas Ypt7p:GTP can obviate the need for acidic lipids. Separate examination of HOPS binding and proteoliposome tethering shows that either Ypt7p or acidic lipid can contribute to HOPS membrane binding and the consequent tethering reaction. SNAREs alone have little ability to contribute to tethering (Figure 5).
One conclusion from this work is that searches for single receptors can be stymied by the multiplicity of actual binding affinities. For example, a nanomolar binding affinity may be the product of two distinct affinities, one micromolar and one millimolar, and although the loss of either could be seen as an almost complete “off” switch, both contribute to high-affinity binding (e.g., see Kang et al., 1993). Two HOPS subunits, Vps39p and Vps41p, have direct affinity for Ypt7p, but the affinity of each subunit for Ypt7p:GTP, as separate recombinant proteins or in the context of the hexameric HOPS complex, is unknown. The 660-kDa HOPS complex or its subunits have been reported to have direct affinity for the individual SNAREs (Sato et al., 2000; Dulubova et al., 2001; Stroupe et al., 2006; Lobingier and Merz, 2012), the vacuolar SNARE complex (Kramer and Ungermann, 2011; Lobingier and Merz, 2012), Ypt7p:GTP (Seals et al., 2000), and phosphoinositides (Stroupe et al., 2006). In addition, we now show that HOPS binds other acidic lipids, as seen by its binding to protein-free liposomes of PC/PS but not of PC alone (Figure 4) and its binding to protein-free liposomes of PC/PE/Erg/DAG when they also have the acidic lipid PI. Because acidic lipid can support HOPS-dependent tethering and fusion without Ypt7p, it is tempting to speculate that HOPS may have more than one binding site for acidic lipid.
The phosphorylation of HOPS has been shown to regulate the targeting of AP-3 vesicles by regulating its lipid binding affinity (Cabrera et al., 2010), in accord with our present findings that the activity and specificity of HOPS for both Ypt7p and acidic lipids are regulated by Yck3p-dependent phosphorylation (Figure 7). Other Rab effectors, such as Sec2p, show similar regulation (Stalder et al., 2013). As for HOPS, the phosphatase that completes this cycle is unknown, and it remains to be determined whether the phosphorylation by the vacuolar kinase Yck3p only enforces Ypt7p nucleotide specificity (Zick and Wickner, 2012) or also functionally modulates any of the nontethering functions of HOPS. Regulation of membrane traffic by kinases with distinct localizations may prove to be a general aspect of vesicular trafficking.
What is the in vivo relevance of the biochemical findings that lipid head group composition affects fusion through contributing to HOPS membrane association? It is difficult to evaluate the role of each lipid in vacuole fusion in vivo: 1) lipid alterations can alter the several pathways of vesicular delivery of proteins to this organelle and 2) potential shielding of lipid head groups on biological membranes, with their physiological ratio of protein to lipid, is difficult to achieve in reconstitutions, which typically have far lower total protein:lipid ratios. Nonetheless, a survey of strains that each bore the deletion of a known nonessential gene showed that many genes regulating phosphoinositide synthesis, ergosterol biosynthesis, and sphingolipid and glycerophospholipid metabolism are required to avoid vacuole fragmentation, a hallmark of deficient fusion (Table 3 of Seeley et al., 2002). In vitro studies of docked, intact vacuoles showed that sterol, diacylglycerol, and phosphoinositides are highly enriched at the fusion-active vertex ring domain (Fratti et al., 2004), and this enrichment is interdependent with SNARE vertex enrichment. The abundance of the major acidic vacuole lipids in this microdomain and the degree to which acidic lipids are shielded by other proteins on the surface of the intact organelle have yet to be evaluated.
MATERIALS AND METHODS
Protein purification
The purifications of HOPS (Zick and Wickner, 2013), Sec17p (Schwartz and Merz, 2009), Sec18p (Haas and Wickner, 1996), Ypt7p (Zick and Wickner, 2013), and Yck3p (Hickey et al., 2009) were as described. Vacuolar SNAREs were isolated (Mima et al., 2008; Schwartz and Merz, 2009; Zucchi and Zick, 2011) and exchanged into octylglucoside (Zucchi and Zick, 2011). Proteins were frozen in aliquots in liquid nitrogen and stored at −80ºC.
Proteoliposome preparation
Proteoliposomes of VML composition (Mima et al., 2008) were prepared from octylglucoside mixed micellar solutions as described (Zick et al., 2014), with each SNARE at a 1:1000 M ratio to lipid and Ypt7p at a 1:2000 M ratio to lipid, where indicated. All proteoliposomes throughout this study had 1 mol% diC16–phosphatidylinositol 3-phosphate. PC/PS proteoliposomes had 69 mol% PC and 30 mol% PS. For other lipid compositions, the mole percent of PC was adjusted to compensate for lipids that had been omitted, whereas other lipids were present at their VML molar concentration (PE, 18 mol%; Erg, 8 mol%; DAG, 1 mol%). When PI was incorporated as the only acidic lipid, it was present at 18 mol%. For tethering assays, proteoliposomes were prepared without lumenal fluorescent proteins, and 0.5 mol% rhodamine-PE was incorporated instead of NBD-PE or Marina-Blue-PE.
Tethering assays
Liposomes of the indicated lipid compositions were prepared either without Ypt7p or with Ypt7p as described at a molar ratio of protein:lipid of 1:2000. Ypt7p-bearing liposomes were nucleotide exchanged by incubating a mixture of 3.0 μl of 10 mM EDTA in RB150 (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid–NaOH, pH 7.4, 150 mM NaCl, 10% glycerol), 7.5 μl of 2 mM liposomes in RB150+Mg (RB150 + 1 mM MgCl2), and 3.0 μl of 100 μM disodium GTP in RB150 for 15 min at 27ºC. Liposomes not bearing Ypt7p were treated identically, with RB150 added in place of nucleotide. A mix of 1.8 mM MgCl2 and 0.9 mM HOPS or its buffer was prepared in RB150. Nucleotide-exchanged liposomes (4.5 μl) were transferred into 0.5-ml microcentrifuge tubes, followed by 5.5 μl of the HOPS/MgCl2 mix, for final concentrations of 0.5 mM lipid, 1 mM EDTA, 10 μM nucleotide, 0.5 mM HOPS, and 1.25 mM MgCl2. Reactions were gently vortexed and then incubated at 27ºC for 10 min, diluted with 90 μl of room temperature RB150+Mg, and gently mixed by pipetting. An aliquot (20 μl) of each reaction was transferred to a 384-well plate, and the remaining 80 μl were centrifuged (5 min, 1500 × g). Each tube was carefully removed from the microcentrifuge, and 20 μl were withdrawn from the top portion of the supernatant furthest away from the pellet and transferred into the 384-well plate. Rhodamine fluorescence was assayed in a SpectraMax Gemini XPS (Molecular Devices, Sunnyvale, CA) fluorescence plate reader (emission, 560 nm; excitation, 580 nm; cutoff, 570 nm) for 10 min at 27ºC. The final three readings of each sample were averaged to minimize noise, and data from three identical experiments were averaged and plotted with SDs.
Fusion assay
Fusion was assayed as the FRET signal from the complexes that form between lumenal Cy5-streptavidin and biotin-phycoerythrin upon fusion, all in the presence of an excess of external nonfluorescent streptavidin. Assays were performed as described (Zick et al., 2014). Data were analyzed by subtracting the baseline obtained during the first 10-min incubation from each data point and then expressing the readings as percentage of the maximum possible signal, as determined by solubilization in 0.2% Thesit in the absence of external streptavidin.
HOPS binding assay
Liposomes of the indicated lipid compositions were prepared either with or without Ypt7p as described. Ypt7p-bearing liposomes were nucleotide exchanged by incubating a mixture of 2.8 μl of 10 mM EDTA in RB150, 7 μl of 2 mM liposomes in RB150+ Mg, and 2.8 μl of 100 μM disodium GTP in RB150 for 15 min at 27ºC. Liposomes not bearing Ypt7p were treated identically, with RB150 added in place of nucleotide. A mix of 1.8 mM MgCl2, 0.36% (wt/vol) bovine serum albumin (BSA), and 0.9 mM HOPS was prepared in RB150, and 15.4 μl was added to the nucleotide-exchanged liposomes for final concentrations of 0.5 mM lipid, 1 mM EDTA, 10 μM nucleotide, 0.5 mM HOPS, 0.2% (wt/vol) BSA, and 1.25 mM MgCl2, with the extra 0.25 mM MgCl2 coming from the liposomes. Reactions were gently vortexed, incubated at 27ºC for 1 h, and placed on ice for 5 min. A volume (84 μl) of 54% (wt/vol) Histodenz in iso-osmolar RB150+Mg (containing just 2% [vol/vol] glycerol) was added. Tubes were vortexed gently and manually pipetted to mix. Samples (80 μl) were transferred to 7 × 20 mm polycarbonate centrifuge tubes (Beckman Coulter, Indianapolis, IN) and overlaid with 80 μl of 35% (wt/vol), then 80 μl of 30% (wt/vol) iso-osmolar Histodenz in RB150+Mg, and finally 50 μl of RB150+Mg. The remaining sample was solubilized in 0.1% (vol/vol) Thesit for lipid recovery determination. Tubes were centrifuged in a TLS55 rotor (Beckman Coulter) for 1.5 h at 55,000 rpm and 4ºC. Floated liposomes (80 μl) were harvested from the top of the tube and solubilized in 0.1% (vol/vol) Thesit. Lipid recovery was determined by measuring rhodamine fluorescence (excitation, 560 nm; emission, 580 nm; cutoff, 570 nm) of the starting samples versus the floated samples. Floated samples and a pool of the starting samples were incubated in 2% (wt/vol) SDS for 5 min at 90ºC and then analyzed by SDS–PAGE, with loaded volumes adjusted to account for lipid recovery, followed by immunoblotting for Vps16p. Serial twofold dilutions of the starting sample provided a standard curve for quantifying the amount of bound HOPS using UN-SCAN-IT software (Silk Scientific, Orem, UT).
Supplementary Material
Acknowledgments
We thank Deborah Douville for expert technical assistance. We acknowledge National Institutes of Health Grant S10-0D016212 for the purchase of the Orbitrap Fusion mass spectrometer. This work was supported by National Institutes of Health Grant GM23377-40.
Abbreviations used:
- DAG
diacylglycerol
- Erg
ergosterol
- FRET
fluorescence resonance energy transfer
- HOPS
homotypic fusion and vacuole protein sorting complex
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PI
phosphatidylinositol
- PS
phosphatidylserine
- RPL
reconstituted proteoliposome
- SNARE
soluble N-ethylmaleimide–sensitive factor attachment protein receptor
- VML
vacuolar mimic lipids.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-08-1298) on November 19, 2014.
REFERENCES
- Bock JB, Matern HT, Peden AA, Scheller RH. A genomic perspective on membrane compartment organization. Nature. 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- Boeddinghaus C, Merz AJ, Laage R, Ungermann C. A cycle of Vam7p release from and PtdIns 3-P-dependent rebinding to the yeast vacuole is required for homotypic vacuole fusion. J Cell Biol. 2002;157:79–89. doi: 10.1083/jcb.200112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett CL, Plemel RL, Lobingier BT, Vignali M, Fields S, Merz AJ. Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase. J Cell Biol. 2008;182:1141–1151. doi: 10.1083/jcb.200801001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera M, Langemeyer L, Mari M, Rethmeier R, Orban I, Perz A, Brocker C, Griffith J, Klose D, Steinhoff HJ, et al. Phosphorylation of a membrane curvature-sensing motif switches function of the HOPS subunit Vps41 in membrane tethering. J Cell Biol. 2010;191:845–859. doi: 10.1083/jcb.201004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Yamaguchi T, Wang Y, Sudhof TC, Rizo J. Vam3p structure reveals conserved and divergent properties of syntaxins. Nat Struct Biol. 2001;8:258–264. doi: 10.1038/85012. [DOI] [PubMed] [Google Scholar]
- Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti RA, Jun Y, Merz AJ, Margolis N, Wickner W. Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J Cell Biol. 2004;167:1087–1098. doi: 10.1083/jcb.200409068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R, McNew JA, Weber T, Parlati F, Engel T, Nickel W, Rothman JE, Sollner TH. Functional architecture of an intracellular membrane t-SNARE. Nature. 2000;407:198–202. doi: 10.1038/35025084. [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J Cell Biol. 1994;126:87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Wickner W. Homotypic vacuole fusion requires Sec17p (yeast alpha-SNAP) and Sec18p (yeast NSF) EMBO J. 1996;15:3296–3305. [PMC free article] [PubMed] [Google Scholar]
- Hickey CM, Stroupe C, Wickner W. The major role of the Rab Ypt7p in vacuole fusion is supporting HOPS membrane association. J Biol Chem. 2009;284:16118–16125. doi: 10.1074/jbc.M109.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey CM, Wickner W. HOPS initiates vacuole docking by tethering membranes before trans-SNARE complex assembly. Mol Biol Cell. 2010;21:2297–2305. doi: 10.1091/mbc.E10-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jun Y, Xu H, Thorngren N, Wickner W. Sec18p and Vam7p remodel trans-SNARE complexes to permit a lipid-anchored R-SNARE to support yeast vacuole fusion. EMBO J. 2007;26:4935–4945. doi: 10.1038/sj.emboj.7601915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T, Martins T, Sadowski I. Wild type GAL4 binds cooperatively to the GAL1-10 UASG in vitro. J Biol Chem. 1993;268:9629–9635. [PubMed] [Google Scholar]
- Karunakaran V, Wickner W. Fusion proteins and select lipids cooperate as membrane receptors for the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) Vam7p. J Biol Chem. 2013;288:28557–28566. doi: 10.1074/jbc.M113.484410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer L, Ungermann C. HOPS drives vacuole fusion by binding the vacuolar SNARE complex and the Vam7 PX domain via two distinct sites. Mol Biol Cell. 2011;22:2601–2611. doi: 10.1091/mbc.E11-02-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGrassa TJ, Ungermann C. The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex. J Cell Biol. 2005;168:401–414. doi: 10.1083/jcb.200407141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Kovacs J, Stahelin RV, Cheever ML, Overduin M, Setty TG, Burd CG, Cho W, Kutateladze TG. Molecular mechanism of membrane docking by the Vam7p PX domain. J Biol Chem. 2006;281:37091–37101. doi: 10.1074/jbc.M608610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobingier BT, Merz AJ. Sec1/Munc18 protein Vps33 binds to SNARE domains and the quaternary SNARE complex. Mol Biol Cell. 2012;23:4611–4622. doi: 10.1091/mbc.E12-05-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- McNew JA, Weber T, Parlati F, Johnston RJ, Melia TJ, Sollner TH, Rothman JE. Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J Cell Biol. 2000;150:105–117. doi: 10.1083/jcb.150.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima J, Hickey CM, Xu H, Jun Y, Wickner W. Reconstituted membrane fusion requires regulatory lipids, SNAREs and synergistic SNARE chaperones. EMBO J. 2008;27:2031–2042. doi: 10.1038/emboj.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak A, Fedorov R, Alexandrov K, Albert S, Goody RS, Gallwitz D, Scheidig AJ. Crystal structure of the GAP domain of Gyp1p: first insights into interaction with Ypt/Rab proteins. EMBO J. 2000;19:5105–5113. doi: 10.1093/emboj/19.19.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Wickner W. Functional reconstitution of ypt7p GTPase and a purified vacuole SNARE complex. Science. 1998;281:700–702. doi: 10.1126/science.281.5377.700. [DOI] [PubMed] [Google Scholar]
- Sato TK, Rehling P, Peterson MR, Emr SD. Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol Cell. 2000;6:661–671. doi: 10.1016/s1097-2765(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Schwartz ML, Merz AJ. Capture and release of partially zipped trans-SNARE complexes on intact organelles. J Cell Biol. 2009;185:535–549. doi: 10.1083/jcb.200811082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97:9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley ES, Kato M, Margolis N, Wickner W, Eitzen G. Genomic analysis of homotypic vacuole fusion. Mol Biol Cell. 2002;13:782–794. doi: 10.1091/mbc.01-10-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder D, Mizuno-Yamasaki E, Ghassemian M, Novick PJ. Phosphorylation of the Rab exchange factor Sec2p directs a switch in regulatory binding partners. Proc Natl Acad Sci USA. 2013;110:19995–20002. doi: 10.1073/pnas.1320029110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starai VJ, Hickey CM, Wickner W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell. 2008;19:2500–2508. doi: 10.1091/mbc.E08-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe C. The yeast vacuolar Rab GTPase Ypt7p has an activity beyond membrane recruitment of the homotypic fusion and protein sorting-Class C Vps complex. Biochem J. 2012;443:205–211. doi: 10.1042/BJ20110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25:1579–1589. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe C, Hickey CM, Mima J, Burfeind AS, Wickner W. Minimal membrane docking requirements revealed by reconstitution of Rab GTPase-dependent membrane fusion from purified components. Proc Natl Acad Sci USA. 2009;106:17626–17633. doi: 10.1073/pnas.0903801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck DK, Hoekstra D, Pagano RE. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981;20:4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- Wada Y, Ohsumi Y, Anraku Y. Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. I. Isolation and characterization of two classes of vam mutants. J Biol Chem. 1992;267:18665–18670. [PubMed] [Google Scholar]
- Wang L, Merz AJ, Collins KM, Wickner W. Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion. J Cell Biol. 2003;160:365–374. doi: 10.1083/jcb.200209095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Seeley ES, Wickner W, Merz AJ. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell. 2002;108:357–369. doi: 10.1016/s0092-8674(02)00632-3. [DOI] [PubMed] [Google Scholar]
- Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol. 2010;26:115–136. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- Xu H, Jun Y, Thompson J, Yates J, Wickner W. HOPS prevents the disassembly of trans-SNARE complexes by Sec17p/Sec18p during membrane fusion. EMBO J. 2010;29:1948–1960. doi: 10.1038/emboj.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wickner WT. N-terminal domain of vacuolar SNARE Vam7p promotes trans-SNARE complex assembly. Proc Natl Acad Sci USA. 2012;109:17936–17941. doi: 10.1073/pnas.1216201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Bacaj T, Yang X, Pang ZP, Sudhof TC. Lipid-anchored SNAREs lacking transmembrane regions fully support membrane fusion during neurotransmitter release. Neuron. 2013;80:470–483. doi: 10.1016/j.neuron.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick M, Stroupe C, Orr A, Douville D, Wickner WT. Membranes linked by trans-SNARE complexes require lipids prone to non-bilayer structure for progression to fusion. Elife. 2014;3:e01879. doi: 10.7554/eLife.01879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick M, Wickner W. Phosphorylation of the effector complex HOPS by the vacuolar kinase Yck3p confers Rab nucleotide specificity for vacuole docking and fusion. Mol Biol Cell. 2012;23:3429–3437. doi: 10.1091/mbc.E12-04-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick M, Wickner W. The tethering complex HOPS catalyzes assembly of the soluble SNARE Vam7 into fusogenic trans-SNARE complexes. Mol Biol Cell. 2013;24:3746–3753. doi: 10.1091/mbc.E13-07-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick M, Wickner W. A distinct tethering step is vital for vacuole membrane fusion. Elife. 2014;3:e03251. doi: 10.7554/eLife.03251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi PC, Zick M. Membrane fusion catalyzed by a Rab, SNAREs, and SNARE chaperones is accompanied by enhanced permeability to small molecules and by lysis. Mol Biol Cell. 2011;22:4635–4646. doi: 10.1091/mbc.E11-08-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.