Abstract
Surfactants are extremely important agents to clean and sanitize various environments. Their biocidal activity is a key factor determined by the interactions between amphiphile structure and the target microbial cells. The object of this study was to analyze the interactions between four structural variants of N-alkyltropinium bromide surfactants with the Gram negative Escherichia coli and the Gram positive Listeria innocua bacteria. Microbiological and conductometric methods with a previously described FTIR bioassay were used to assess the metabolomic damage exerted by these compounds. All surfactants tested showed more biocidal activity in L. innocua than in E. coli. N-tetradecyltropinium bromide was the most effective compound against both species, while all the other variants had a reduced efficacy as biocides, mainly against E. coli cells. In general, the most prominent metabolomic response was observed for the constituents of the cell envelope in the fatty acids (W1) and amides (W2) regions and at the wavenumbers referred to peptidoglycan (W2 and W3 regions). This response was particularly strong and negative in L. innocua, when cells were challenged by N-tetradecyltropinium bromide, and by the variant with a smaller head and a 12C tail (N-dodecylquinuclidinium bromide). Tail length was critical for microbial inhibition especially when acting against E. coli, maybe due the complex nature of Gram negative cell envelope. Statistical analysis allowed us to correlate the induced mortality with the metabolomic cell response, highlighting two different modes of action. In general, gaining insights in the interactions between fine structural properties of surfactants and the microbial diversity can allow tailoring these compounds for the various operative conditions.
Introduction
Surfactants are among the worldly most common chemicals, widely used as detergents [1], paints [2, 3], emulsificants [4], de-mulsificants [5] and metal flotation agents [6]. Their structural characteristics allow the dissolution in both polar and apolar solvents thanks to the presence of hydrophilic head groups (cationic, anionic, zwitterionic or non-ionic) and hydrophobic moiety [7]. Surfactants can be synthesized chemically [8, 9, 10, 11, 12] or can be obtained from bacteria and fungi [13].
The use of surfactants as biocides against bacteria, virus and fungi is one of their most appreciated features [8, 14, 15, 16, 17, 18, 19]; in fact many such molecules are used as antibiotics against Gram negative and Gram positive multi drug-resistant bacteria, e.g. insensitive to colistin and daptomycin [20].
Many studies have been dedicated to elucidate the mechanisms governing this biocidal activity. In spite of these efforts, there is not yet a full consensus on their mode of action. According to a long lasting model, the primary targets of cationic surfactants are supposedly the phospholipids components of the bacterial cell membrane, somehow destabilized by surfactants, leading to membrane distortion followed by decompartimentalization and cell lysis [21, 22, 23]. Additionally, the negative charge on the bacterial cells seems to be involved in this process through an ion exchange mechanism by the cytoplasmic membrane and the cationic surfactants [24]. It was also suggested that the length, rather than the composition of the alkyl chain, plays a primary role in surfactants biocidal activity [25, 26]. The antimicrobial surfactants effect depends from their hydrophilic/lipophilic balance, but the efficacy appears to be modulated by the composition of the cell envelope, and particularly of the cell membrane, in a strain-dependent manner [8]. Additionally, endo-metabolites (e.g. superoxides) can be produced in response to surfactants [27]. Altogether, it seems that the mode of action is the complex result of surfactant structure and cell type used as target.
Fourier Transform InfraRed Spectroscopy (FTIR) has been applied in microbiological studies to whole cell analysis [12, 28, 29, 30, 31]. A FTIR-based bioassay was developed in our laboratory to determine the presence and the extent of cellular stress, with the rationale that stressing conditions can alter the cell metabolome before and after cell death [32]. This assay was tested primarily against yeast cells, to detect the types of molecules more involved in the stress phenomenon [33], but was also extended to mammal cells [34] similarly to other approaches aiming at determining environmental stress [35, 36]. These features make FTIR metabolomics fingerprint a tool of choice to characterize the biological activity exerted by surfactants.
A practical approach to the study of surfactants antimicrobial properties is to consider that there are many contaminants in environments and situations calling for simultaneous cleaning and effective microbial inactivation. These two activities can be exerted by surfactants, provided that their structure combines effectively detergency and biocidal effect against the typical spoilers and contaminants of the environment to sanitize. E. coli and L. innocua represent dangerous bacterial contaminants [37, 38, 39] and a model to study biological effects against Gram negative and Gram positive bacteria, respectively.
A series of structural variants of N-alkyltropinium bromide surfactants has been previously synthesized and tested against not pathogenic or opportunistic yeasts, demonstrating that alkyl tail length and head-group size affected their anti-microbial activity [40]. Four of these structural variants were employed in the present study to test whether FTIR spectroscopy can differentiate their modes of action at the metabolomic level and evaluate their efficacy against a Gram + and Gram—bacterial species. Cell damage was evaluated after 1 hr exposition in order to exclude any problem deriving from the limitations due to compounds diffusion in the agar medium and to exclude the mortality and physiological changes deriving from a long lasting deprivation of nutrients [41]. Statistical analysis allowed to propose a typing of the mode of action based on the biocidal activity and on the metabolomic damage exerted by stressing compounds. The possibility to generalize the typing obtained in this study will require more insight with compounds other than those considered in this paper. This is potentially important for high-throughput first level screening of novel putative biocidal compounds. An analysis of the functional FTIR data significance showed that it is possible to dissect a ante mortem from a post mortem cell metabolomic damage.
Materials and Methods
Bacterial strains and growth conditions
Bacterial strains E. coli BCF 2 and L. innocua BCF 13 were obtained from the internal collection of the Microbial Genetics and Phylogenetics Laboratory of the Department of Pharmaceutical Sciences (University of Perugia). They are deposited in the Netherlands Culture Collection of Bacteria (NCCB) as NCCB 100509 and NCCB 100510 respectively.
Pre-cultures and subsequent cultures were inoculated at OD600 = 0.2 in 100 mL of BHI medium (Brain Heart Infusion—Biolife Italiana S.r.l., Milan, Italy) and grown 24 h at 37°C, with 150 rpm shaking, leading the cultures to cell densities around 4*108 cells/mL. The biomass amount for the FTIR analysis was normalized using the optical densities at 600 nm, taking care to read the cell suspensions in the OD600 = 0.1 to 0.5 to guarantee the linearity between the cells and the optical density. The actual optical density was calculated by multiplying the dilution factor by the recorded OD600.
Synthesis and purification of quaternary ammonium salts
The tropinium-head surfactants were synthesized by quaternization of the tropine with the corresponding n-alkyl bromide heated to reflux in acetonitrile. The 23S-sh surfactant was synthesized from quinuclidine and n-dodecyl bromide in acetonitrile at room temperature (Table 1 and S1 Table). All detailed synthetic procedures and characterizations were reported in previous papers [40, 42].
Table 1. Structures, c.m.c. and α values of quaternary ammonium salts.
| M.W. | c.m.c. (M) | α | ||
|---|---|---|---|---|
| 23S-12 | N-dodecyltropinium bromide | 390.4 | 1.14 · 10-2 | 0.30 |
| 23S | N-tetradecyltropinium bromide | 418.5 | 2.62 · 10-3 | 0.28 |
| 23S-16 | N-hexadecyltropinium bromide | 446.5 | [n.a. due to low solubility] | [n.a. due to low solubility] |
| 23S-sh | N-dodecylquinuclidinium bromide | 360.42 | 1.08·10-2 | 0.25 |
FTIR-based bioassay
Each cells suspension was centrifuged (3 min at 5300 g), washed twice with distilled sterile water and re-suspended in six polypropylene tubes with an appropriate amount of distilled water (standardized optical density OD600 = 12). Surfactants were added to the test tubes in order to obtain the concentrations of 0.2, 0.4, 0.6, 0.8 and 1.0 mM. A maximum 1.0 mM concentration was chosen on the basis of the outcomes of a previous work [42]. The control (0.0 mM) was obtained by re-suspending the cells directly in distilled sterile water. All tests were carried out in triplicate. Tubes were incubated 1 hr at 25°C in a shaking incubator set at 50 rpm [41]. After the incubation, 1.5 mL suspension was taken from each sample, centrifuged (5 min at 5,300 g) washed twice with distilled sterile water and re-suspended in 1.5 mL HPLC grade water. 105 µL suspension was sampled for three independent FTIR readings (35 µL each, according to the technique suggested by Manfait and co-workers [43]). The FTIR experiments were carried out with a TENSOR 27 FTIR spectrometer, equipped with HTS-XT accessory for rapid automation of the analysis (BRUKER Optics GmbH, Ettlingen, Germany). FTIR measurements were performed in transmission mode. All spectra were recorded in the range between 4000 and 400 cm-1. Spectral resolution was set at 4 cm-1, sampling 256 scans per sample. The software OPUS version 6.5 (BRUKER Optics GmbH, Ettlingen, Germany) was used to carry out the quality test, baseline correction, vector normalization and the calculation of the first and second derivatives of spectral values.
Spectra statistical analyses
The MSA (Metabolomic Spectral Analysis) script employed in this study was developed in “R” language to carry out the following operations on the matrices of spectral data exported as ASCII text from OPUS 6.5. The analytical process consists in calculating the distance between the spectrum of the cells under test and that of the cells without the stressing agent; this procedure is extended to the five spectral regions in order to differentiate the stress response among the different classes of molecules [32].
In more detail, the procedure could be outlined as follows:
Each single spectrum was normalized in the range spanning from 0 to 1, in a way already suggested by Goodacre and coll. [44]. Average spectra from the three repetitions were calculated.
Response spectra (hereinafter reported as RS) were calculated as difference between each average spectrum and the average spectrum of the same cells maintained in water (defined as control RS). Response spectra of each agent were plotted with the exclusion of the control RS, which is by definition a straight line with RS = 0.
Synthetic stress indexes (hereinafter reported as SI) of metabolomic stress response were calculated as Euclidean distances of the RS under stress and the control RS. SI of the whole spectrum and of the five different spectral regions individuated by Kuemmerle et al. [45] were calculated. The five regions were defined as follows: fatty acids (W1) from 3000 to 2800 cm-1, amides (W2) from 1800 to 1500 cm-1, mixed region (W3) from 1500 to 1200 cm-1, carbohydrates (W4) from 1200 to 900 cm-1 and typing region (W5) from 900 to 700 cm-1. Since the five spectral regions differ in length, their SI were scaled to the length of the whole spectrum, in order to make the different SI comparable on the same scale.
Biocidal activity test
The biocidal activity tests were carried out in parallel with the FTIR based stress bioassay to compare the metabolomic damages with the loss of viability. 100 µL of each cells suspension prepared for the FTIR analysis were serial diluted to determine the viable cell counting, in triplicate, on BHI plates. The biocidal effect of the tested compounds was highlighted as cell mortality induced at different concentrations. The cell mortality (M) was calculated as M = (1-Cv/Ct) x 100, where Cv is the number of viable cells in the tested sample and Ct the number of viable cells in the control suspension.
Conductivity measurements of N-tetradecyltropinium bromide solution with bacterial cells
Conductivity was measured on a CRISON GLP-31 conductivity meter at 25.0 ± 0.1°C (Pharmacia Biotech Multitemp III thermostat). A Watson-Marlow 323 peristaltic pump (with Watson-Marlow tubes) was used for the surfactant solutions and cell suspensions additions. A JASCO V-530 spectrophotometer was used to determine cell suspension concentration. Cell suspensions were prepared by washing cell pellets twice with HCl 5·10-3 M and three times with bi-distilled water to standardize the supernatant conductivity to a value below 10 μS/cm. Each cell suspension was prepared at a standardized optical density OD600 = 5. N-tetradecyltropinium bromide stock solution was prepared at 0.02 M. Conductivity measurements were carried out using the automated method proposed by Tiecco and co-workers [46]. Data were imported in R statistical environment (http://cran.r-project.org/) and treated with the CMC-R script, freely available from the following sites:http://www.bio-aware.com/BioloMICSNews.aspx?Rec=87 and http://docs.google.com/file/d/0Bzj3M187nEU2YlRKbVB5cnBtWEk/edit?usp=sharing.
Results
Metabolomic characterization of the stress induced by N-alkyltropinium bromide structural variants
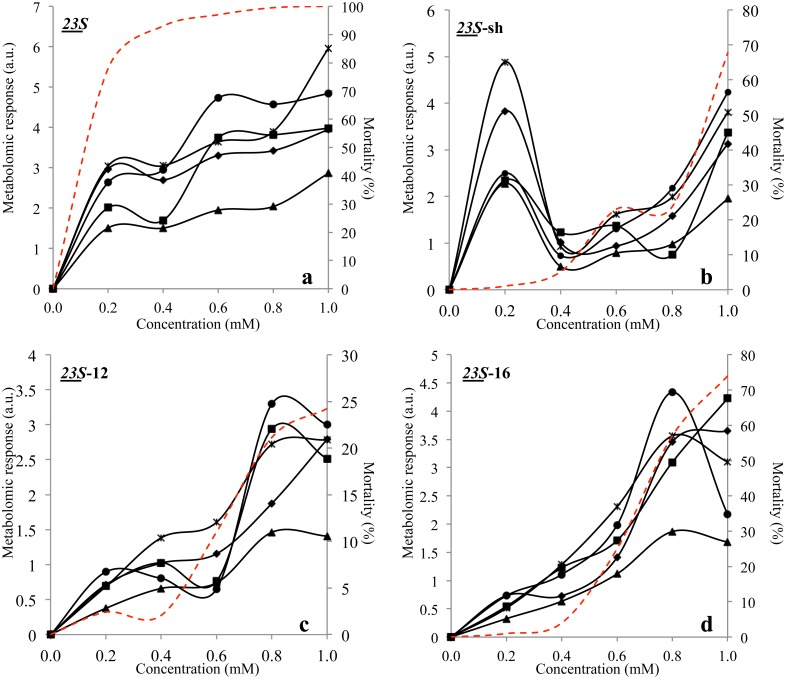
The analysis of the stress induced by the 23S surfactant and the other variants on E. coli and L. innocua cells was carried out by calculating Stress Indexes previously defined as the variation of specific spectral areas caused by the exposition of cells to each surfactant [32]. For this analysis, only four regions were considered out of the five analyzed with MSA. The typing region (900–700 cm-1) was omitted because its response did not appear correlated with these specific stressing conditions. 23S proved the most effective against both bacterial species, with a greater biocidal efficacy in L. innocua in respect to E. coli (Figs. 1a and 2a). In fact, it caused 100% mortality to L. innocua already at 0.2 mM, with SI ranging from 0 to 15, representing approximately the double of the corresponding figures obtained with E. coli. All SIs increased from 0.1 mM with similar trends. Interestingly, the steepest increase was observed from 0.0 and 0.2 mM, although the SIs from W3 and W4 showed a secondary steep increase between 0.4 and 0.6 mM and W1 from 0.8 and 1.0 mM. The steep increase from 0.0 to 0.2 mM corresponds to a similar raise of the mortality from 0 to ca. 80%, suggesting that all regions registered the stress causing the mortality of most of the cells (Fig. 1a).
Figure 1. Stress Indexes of E. coli cells subjected to the action of N-alkyltropinium bromide structural variants at 0.2, 0.4, 0.6, 0.8 and 1.0 mM.
a.u. stands for “arbitrary units”; triangles represent the whole spectrum, asterisks W1 region, diamonds W2 region, squares W3 region, circles W4 region; dashed line represents mortality. The degree of variability between replicas throughout the FTIR spectra ranged around 2.3*10-2.
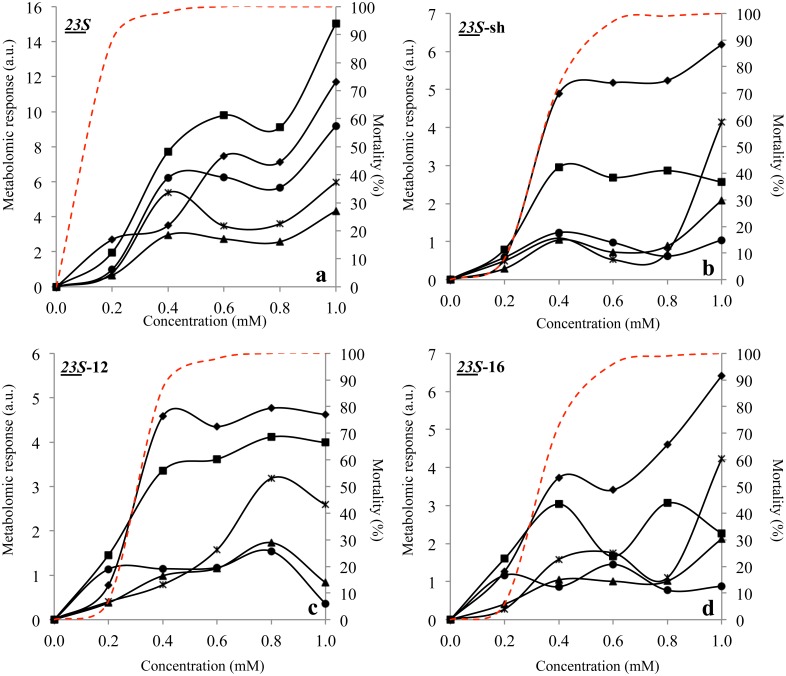
Figure 2. Stress Indexes of L. innocua cells subjected to the action of N-alkyltropinium bromide structural variants at 0.2, 0.4, 0.6, 0.8 and 1.0 mM.
a.u. stands for “arbitrary units”; triangles represent the whole spectrum, asterisks W1 region, diamonds W2 region, squares W3 region, circles W4 region; dashed line represents mortality. The degree of variability between replicas throughout the FTIR spectra ranged around 2.7*10-2.
All the SIs curves showed an increasing trend from 0.0 to 0.4 mM, a concentration causing 100% mortality. As for E. coli, all regions registered the stress leading the cells to die. Interestingly, there is another increase of all SIs from 0.8 to 1.0 mM clearly due to post mortem effects (Fig. 2a). In both species the Global Stress Index referred to the whole spectrum resulted GSI > 1.0 at every concentration of the surfactant, confirming that this index is a reliable marker of effective cell stress when reaching values above 1.0 [30, 32].
23S-sh induced low mortality rates in E coli ranging from 0 at 0.2 mM to less than 70% at 1 mM, presenting a local peak of 25% mortality at 0.6 mM (Fig. 1b). Interestingly, all SIs add a maximum at 0.2 mM, decreased at 0.5 mM and increased slowly at 1.0 mM, reaching values similar or lower than those observed at 0.2 mM. This phenomenon can be explained by the fact that, at low surfactant concentration, cells responded actively to the presence of the surfactant without suffering significant mortality. Interestingly, the most prominent reaction was observed in the fatty acids (W1) and amides (W2) region, suggesting that the reaction occurred mainly at level of the cell envelope, which in Gram negative bacteria is formed by the outer membrane, peptidoglycan cell wall and inner membrane.
This surfactant in L. innocua induced 100% mortality already at 0.6 mM and at higher concentrations. All stress indexes showed a peak at 0.4 mM and remained on the same values up to 1.0 mM, with the exception of the fatty acids which increased fourfold from 0.8 to 1.0 mM (Fig. 2b). The fact that amides and fatty acids SIs showed higher values than other regions, further fortifies the concept that the cell envelope, containing both types of molecules, was the primary target of this surfactant. In general, 23S-sh induced the same level of metabolomic reaction in both species, with a size ranging from 0.0 to 6.0, differently from 23S that was twice more effective in L. innocua.
23S-12 was the least effective compound in E. coli causing a maximum of 25% cell mortality at 1 mM. Carbohydrates (W4), mixed region (W3) and fatty acids (W1) SIs curves showed a peak at 0.8 mM and slightly lower at 1.0 mM. As a confirm that GSI >1 indicates effective cell stress, this index showed values over 1.0 only at 0.8 and 1.0 mM, with 21% mortality (Fig. 1c).
In L. innocua 23S-12 killed all cells already at 0.4 mM, proving to be less effective than 23S and as active as the other two variants (Fig. 2c). Amides (W2), mixed region (W3) and fatty acid (W1) SIs increased following the mortality curve trend while the carbohydrates (W4) SI followed consistently that of GSI.
23S-16 showed the same biocidal activity of 23S-sh in E. coli, inducing low mortality rates ranging from 0 at 0.2 mM to less than 75% at 1 mM. All indexes followed the mortality curve trend with the exception of the carbohydrates region (W4), with a maximum peak at 0.8 mM followed by a steep decrease at 1 mM. GSI index showed values over 1.0 only at 0.8 and 1.0 mM, corresponding to 60–75% mortality (Fig. 1d). This compound in L. innocua induced 100% mortality at 0.6 mM although, at 0.4 mM, this value was already 80%. GSI showed values equal to 1.0 from 0.4 to 0.8 mM and >1 at 1.0 mM, corresponding to a mortality range from 70 to 100%. The amides (W2) SIs was the most sensitive index for the stress induced by this surfactant, showing the highest values with a size ranging from 0.0 to 6.0 with a general trend similar to that of GSI. The other curves were rather out of phase in comparison with GSI and W2, in fact fatty acids SIs increased from 0.0 to 0.6 mM, decreased at 0.8 mM and then rapidly increased again from 0.8 to 1 mM while carbohydrates were rather low a minimum at 0.4 mM and a maximum at 0.6 mM (Fig. 2d). These data suggest that this surfactant attacks Gram positive and Gram negative cells with rather different mechanism and efficacy, although the cell envelope was one of the targets, as proved by the high response of polysaccharides around 1.0 mM (Figs. 1d and 2d).
Response Spectra analysis
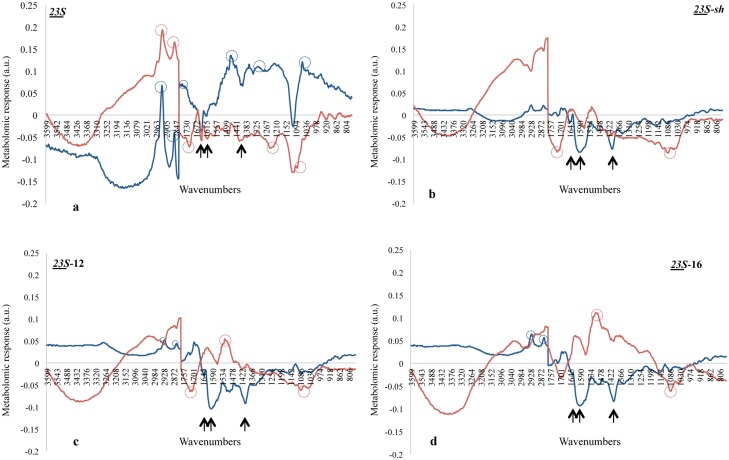
The metabolomic effects caused by these surfactants on bacterial cells was more closely studied by displaying the Response Spectra (RS), obtained as difference between the normalized spectrum of cells subject to the tested compound and that of the control maintained in water in the same experimental conditions. This analysis considered only the RSs at concentration causing in each species 100% mortality (see legend of Fig. 3). The degree of variability between RS replicas throughout the FTIR spectra ranged around 3∙10-3 for E. coli and 3∙10-4 for L. innocua.
Figure 3. Comparison between the Response Spectra of E. coli and L. innocua at the surfactants concentrations that determine, respectively, 100% mortality.
a.u. stands for “arbitrary units”; Panel a) RS of 23S at 0.4 mM for L. innocua and 0.6 mM for E. coli; Panel b) RS of 23S-sh at 0.4 mM for L. innocua and 1 mM for E. coli; Panel c) RS of 23S-12 at 0.4 mM for L. innocua and 1 mM for E. coli; Panel d) RS of 23S-16 at 0.4 mM for L. innocua and 1 mM for E. coli; Arrows correspond to the wavenumbers referred to peptidoglycan while circles to the wavenumbers referred to the major response peaks in each species. Blue, L. innocua; Red, E. coli.
The RSs obtained with the four tested compounds confirmed that 23S was the most efficient in producing a metabolomic response, whereas 23S-sh produced a comparatively high response only in the fatty acids region. The general trend of these RSs was compared by Spearman correlation, as a mean to determine the trend similarity independently of the response intensity.
According to this analysis RSs of E. coli with 23S as a similar trend to that generated by 23S-sh (correlation = 0.875); similarly the correlation of the RSs generated by this species with 23S-12 and 23S-16 was 0.811. L. innocua produced two similar RSs with 23S-sh and 23S-12 (0.882). Interestingly 23S-12 and 23S-16 yielded to almost opposite RSs (-0.819) with E. coli and L. innocua. Weaker correlations were observed in E. coli with 23S-12 against 23S and 23S-sh (0.711 and 0.757 respectively); and in L. innocua challenged by 23S-12 and 23S-16 (0.737). A negative correlation (-0.768) was observed between the RSs obtained by exposing the two species to 23S-16.
According to the SI analysis presented above, the response to these compounds were concentrated in the W1 fatty acids and in the W2 amides; more rarely some peaks were detected in the W3 and W4 representing mixed region and carbohydrates, respectively. The RSs presented significant reactions of the cells in the W1 region when the cells were exposed to 23S at 0.6 mM and 0.4 mM (and higher concentrations), respectively in E. coli and L. innocua (Fig. 3a) and when the latter was challenged by 23S-12 and 23S-16 at concentrations from 0.6 mM to 1.0 mM (Fig. 3c and Fig. 3d).
These responses were detected at 2920 cm-1 and 2852 cm-1 representing the asymmetric and symmetric stretching (ν(CH2)) of the fatty acids chains [47]. Interestingly, these two bands were found to be associated to 1741 cm-1 (ν(C = O) in lipid esters [47]), as an indication of lipids presents increase in human stem cells [48]. This additional band has been detected in our experiments only when L. innocua was exposed to 23S. This data suggested that the presence of these surfactants caused an increase in the lipid content, presumably those of the external and cell membrane. Another constituent of the cell envelope is the cell wall formed by peptidoglycan (PG). Peaks referring to PG in the RSs were detected at 1657 ~ 1600 and ~1400 cm-1 in W2 and W3 regions [49]. Negative peaks at these wavenumbers were detected in both species challenged with 23S and with 23S-sh. When 23S-12 was employed, L. innocua showed all the three negative peaks although the first one was weak; E. coli instead showed only those at ~ 1600 and ~1400 cm-1 (Fig. 3c). Finally, with 23S-16 L. innocua had negative peaks at 1600 and ~1400 cm-1 while E. coli only at ~1400 cm-1. Taken together, these data suggested that surfactants affect negatively the presence of PG in the cell envelope with particular intensity when 23S was used and less effectively with the other three compounds. These data correlated well with the observation presented above indicating that 23S was the most effective of the four compounds, being able to kill 80% of E. coli and 100% of L. innocua at 0.2 mM, whereas at this concentration the other compounds caused much less mortality. Minor variations in the RSs were detected for E. coli in the W2 region. Specifically, as a negative peak at 1708 cm-1 (ν (C = O) H bonded) when cells were exposed to 23S and 23S-sh and as a positive peak at 1515 cm-1, representing the shoulder of proteins [47], when cells were challenged by 23S-12 and 23S-16. Other potential responses were detected for both species in the mixed region (W3), with 23S exposure. Namely, in E. coli there was a peak around 1240 cm-1 (ν (P = O) asymmetric in phospholipids) while in L. innocua at ~ 1460 cm-1 (δ(CH2) of lipids and proteins) and at ~ 1310 cm-1 (Amide III) [47]. Finally, the W4 region of E coli presented a negative peak at 1085 cm-1 (symmetric ν(P = O) in nucleic acids and phospholipids [47]) in all tests. L. innocua showed a positive response around 1050 cm-1 (stretching of phosphate ester [50], only when cells were exposed to 23S.
Physical-chemical behavior of N-tetradecyltropinium bromide in presence of bacterial cells
Conductometric studies were carried out with the highly biocidal 23S to verify whether its physical-chemical properties were changed during the interaction with bacterial cells.
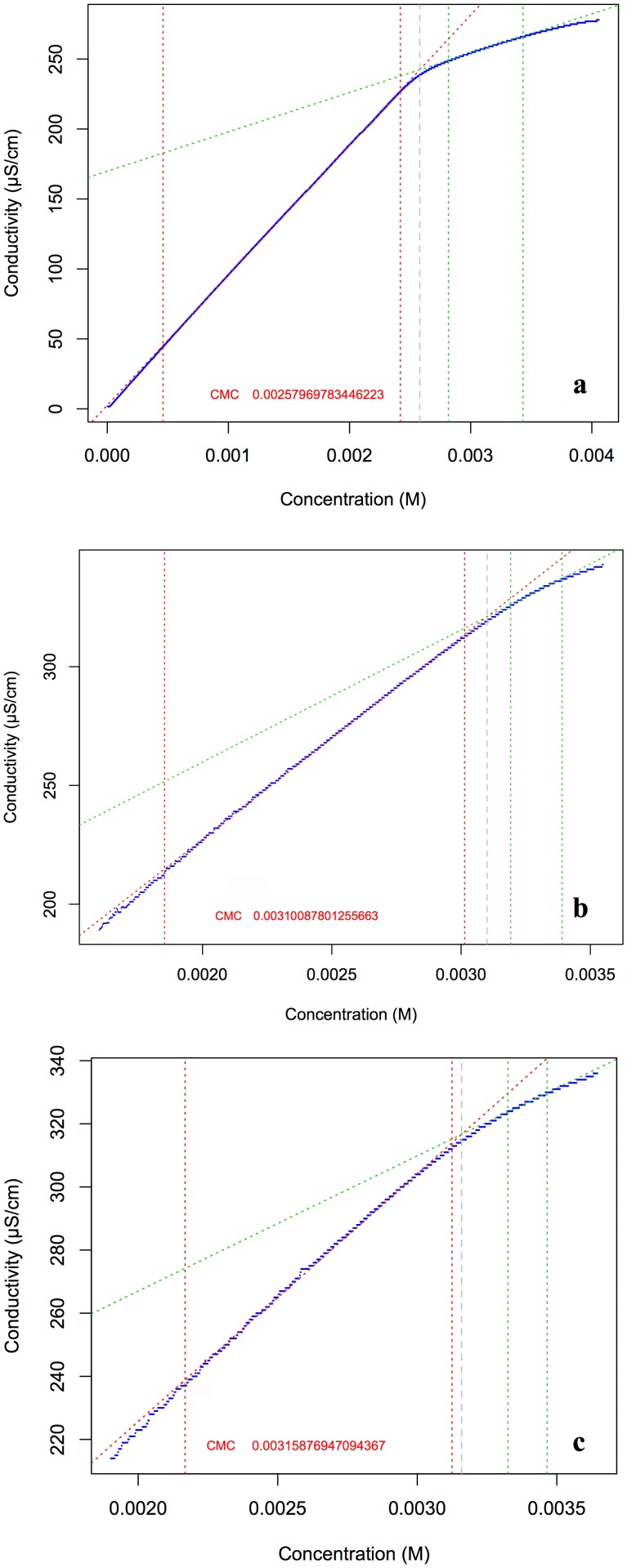
The c.m.c. of the N-tetradecyltropinium bromide solution alone was 2.58·10-3 M, whereas in combination with E. coli and L. innocua suspensions resulted 3.10·10-3 M and 3.16·10-3 M, respectively (Fig. 4). The presence of bacterial cells shifted the c.m.c. by ca 0.5·10-3 M, with both species, indicating that the presence of bacterial cells did not change significantly the micellar formation. The little upshift of c.m.c. could be justified with the sequestration of the surfactant by the cells.
Figure 4. Conductivity profiles of N-tetradecyltropinium bromide solution alone and with bacterial cells.
Conductivity profiles of N-tetradecyltropinium bromide in bidistilled water (a) and in association with E. coli (b) and L. innocua cell suspension at OD600 = 5.0, T = 298.1 K (± 0.1 K). The c.m.c. values calculated with CMC-R script were, respectively, 2.58·10-3 M, 3.10·10-3 M and 3.16·10-3 M. N-tetradecyltropinium bromide variation coefficient resulted 0.00576; E. coli suspension variation coefficient resulted 0.00496; L. innocua suspension variation coefficient resulted 0.00511.
Discussion
The efficacy of the tested surfactants changed according to their tail length and to head size, with a maximum devitalizing effect showed by the surfactant with 14C long tail and the tropinium head (23S). The reduction of head size in the quinuclidinium surfactant (23S-sh) produced a reduction of the antibacterial activity with both species, but particularly with the Gram negative E. coli even in comparison with 23S-12, which has the same tail length. Gram negative bacteria have an external membrane, protecting the peptidoglycan cell wall and the inner membrane, likely to limit the surfactant activity in E. coli more than in the Gram positive L. innocua, which does not present this periplasmatic space and outer membrane. Interestingly, the 23S-sh induced the same reduction (-32%) of both biocidal effect and metabolomics stress response (SI) in E. coli and a generalized decrease of the metabolomics response in both bacteria. Changes in tail length, by increasing or decreasing it with two methylene groups, caused a reduction of the metabolomics response in both species, especially at low concentrations. The biocidal activity exhibited by 23S-12 and 23S-16 was particularly low in E. coli, whereas in L. innocua 100% mortality was obtained over 0.4 mM. These observations indicate that the metabolomic response was specific of the interaction between the compound and the bacterial species, as postulated in the introduction [8]. In fact, even small structural variation caused quite different effects, both as response extent and as spectral perturbation. Results showed that fatty acids and amides were the compounds more intensively reacting to the surfactant. Another interesting cell component was peptidoglycan, the spectral regions of which reacted more actively in L. innocua than in E.coli. Peptidoglycan is the main component of the bacterial cell wall and forms a thick multilayer in Gram positive, whereas is a monolayer in Gram negative bacteria. This means that a stronger signal can be expected from L.innocua peptidoglycan degradation than from E. coli. Moreover, the presence of an external membrane protecting the cell wall in E. coli can further explain the lower FTIR signal attributed to the peptidoglycan degradation.
Using these compounds, we were able to observe three types of metabolomic response. The first and most common type of response was observed in the majority of the experiments when the metabolomics response progressed along with cell mortality, indicating that cells tried to counter the surfactant actively, but were overwhelmed by it. In a second type (23S-sh against E. coli) the response was particularly strong at low surfactant concentration that induced less than 2% mortality, indicating that cells tried somehow to contrast the devitalization, although at higher concentration the trend of cell killing and metabolic responses were almost parallel. A third response type was observed when cell mortality resulted higher than the metabolic response, i.e. with L. innocua challenged by 23S-sh and 23S-16. In the former case the cell mortality was 100% at 0.2 mM with a weak metabolomics response (GSI = 0.650); in the latter the total culture devitalization could be obtained at 0.6 mM with GSI = 1.008. This third type of behavior is likely to indicate the cases in which the surfactant has a strong and immediate action without an effective response of the metabolome.
The above observations on these three different modes of action were further corroborated by correlating the whole spectrum response and the cell mortality at the lowest concentrations inducing 100%, or the highest observed level of mortality. The first type of behavior showed figures ranging from 0.88 and 0.93 in E coli with 23S, 23S-12 and 23S-16. Similarly, 0.78 and 0.82 were obtained with L. innocua challenged by 23S-sh and 23S-12. The second type of response (E. coli with 23S-sh) yielded 0.429 correlation, whereas the third behavior (L. innocua with 23S and 23S-16) was characterized by 0.65 and 0.69 correlation values respectively. Although this aspect requires further systematic investigations, it seems that the first type of response (mortality similar to cell response) can be individuated by correlation values no lower than 0.7, whereas the second (more response than mortality) and the third (more mortality than response) are characterized by correlations respectively below 0.5 and between 0.5 and 0.7. These obviously tentative and indicative thresholds probably split a continuum set of behaviors, as witnessed by the fact that L. innocua with 23S-16 had a borderline correlation (0.69) and in fact the cell mortality grew more rapidly than the metabolomic response, but the GSI reached the 1.008 value. However, a deeper insight in this parameterization can lead to helpful tool to individuate in a quantitative way the relation between mortality and metabolomics response induced by cell-stressing agents. These parameters should be optimize to characterize not only new surfactants, and their variants, but any type of biocidal molecule, in order to maximize the devitalization and reduce as much as possible the “ante mortem” response, which indicates the effect of cell resistance prior to death.
When the surfactants induced the mortality at low concentration (e.g. 23S with both species) it was possible to observe an increase of the metabolomics response at higher concentration, which is not likely due to the metabolic activity, being the cells dead. This suggests that part of the metabolomic change is due to the ante mortem cell response and part to post mortem chemical reactions occurring in the dead cells caused by the direct interaction of the surfactant with the cell materials. Alternatively the mix of various cell components could induce these changes once the surfactant has caused the cell components to mix up. Surfactants in environmental sanitization should couple the high biocidal effect with to low ante mortem and high post mortem metabolomics change, being the latter an evidence of cell disruption, prodromal to the whole cell removal.
The conductometric analyses have shown that the presence of cells did not change the c.m.c. significantly, indicating the physical-chemical properties of the surfactant remained unchanged. The little c.m.c. increase observed could be due to the absorption of surfactant monomers by the cells, thus shifting the formation of micelles. Moreover, the c.m.c. increase can be partly due to the release of salts from the cells to the solution caused by the membrane permeabilization. These findings indicated that the biocidal activity was exerted at concentrations well below the c.m.c. and should therefore be ascribed to the surfactants monomers.
Some of the variants tested in this study gave different results against the two bacterial species. At the same time, the 23S was the most efficient with both bacteria and yeasts [40]. These findings indicate that surfactant structure influences both the efficacy and the spectrum of action, suggesting that compounds tailored for the various situations can be produced once a better understanding will be gained on the relation between structure and biological activity taking in consideration the huge potential of synthetic chemistry combined with microbial biodiversity.
Supporting Information
Chemical structures of the surfactants used in this work, names and acronyms.
(DOCX)
Acknowledgments
MT was supported by 2007–2013 ESF “Competitiveness and Employment objective” Umbrian Regional Operational Programme (ROP), and partially supported by a project of the Bavicchi company (598/94 –Umbria Region). Project partially supported by the FCR grant 2013.0043.021. Work partially supported by the “Green chemistry cluster” project.
Data Availability
All relevant data are within the paper.
Funding Statement
MT was supported by 2007-2013 ESF “Competitiveness and Employment objective” Umbrian Regional Operational Programme (ROP), and partially supported by a project of the Bavicchi company (598/94 – Umbria Region). The project was partially supported by the FCR grant 2013.0043.021. The work was partially supported by the “Green chemistry cluster” project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Breyton C, Pucci B, Popot J-L (2010) Amphipols and fluorinated surfactants: two alternatives to detergents for studying membrane proteins in vitro. Heterologous Expression of Membrane Proteins: Humana Press 601: 219–245. 10.1007/978-1-60761-344-2_14 [DOI] [PubMed] [Google Scholar]
- 2. Woodhall EW, Mesa RL (2012) Masking solutions comprising siloxane-based surfactants for using in painting operations. US Patent App. 13/708,676.
- 3. Hagan EW, Charalambides MN, Young CR, Learner TJ, Hackney S (2010) Viscoelastic properties of latex paint films in tension: Influence of the inorganic phase and surfactants. Prog Org Coat 69: 73–81. 10.1016/j.porgcoat.2010.05.008 [DOI] [Google Scholar]
- 4. Cheng J, Xia Y, Zhou Y, Guo F, Chen G (2011) Application of an ultrasound-assisted surfactant-enhanced emulsification microextraction method for the analysis of diethofencarb and pyrimethanil fungicides in water and fruit juice samples. Anal Chim Acta 701: 86–91. 10.1016/j.aca.2011.04.058 [DOI] [PubMed] [Google Scholar]
- 5. Jarudilokkul S, Paulsen E, Stuckey DC (2000) The effect of demulsifiers on lysozyme extraction from hen egg white using reverse micelles. Bioseparation 9: 81–91. 10.1023/A:1008183828223 [DOI] [PubMed] [Google Scholar]
- 6. Yuan X, Meng Y, Zeng G, Fang Y, Shi J (2008) Evaluation of tea-derived biosurfactant on removing heavy metal ions from dilute wastewater by ion flotation. Colloids Surf, A 317: 256–261. 10.1016/j.colsurfa.2007.10.024 [DOI] [Google Scholar]
- 7. Zana R (2002) Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review. Adv in Colloid and Interface Sci 97: 205–253. [DOI] [PubMed] [Google Scholar]
- 8. Chaveriat L, Gosselin I, Machut C, Martin P (2013) Synthesis, surface tension properties and antibacterial activities of amphiphilic D-galactopyranose derivatives. Eur J Med Chem 62: 177–186. 10.1016/j.ejmech.2012.12.032 [DOI] [PubMed] [Google Scholar]
- 9. Mandal A, Meda V, Zhang WJ, Farhan KM, Gnanamani A (2012) Synthesis, characterization and comparison of antimicrobial activity of PEG/TritonX-100 capped silver nanoparticles on collagen scaffold. Colloids Surf, B 90: 191–196. 10.1016/j.colsurfb.2011.10.021 [DOI] [PubMed] [Google Scholar]
- 10. Menger FM, Littau CA (1991) Gemini Surfactants- Synthesis and Properties. J Am Chem Soc 113: 1451–1452. 10.1021/ja00004a077 [DOI] [Google Scholar]
- 11. Ng CKL, Singhal V, Widmer F, Wright LC, Sorrell TC, et al. (2007) Synthesis, antifungal and haemolytic activity of a series of bis(pyridinium)alkanes. Bioorg Med Chem 15: 3422–3429. 10.1016/j.bmc.2007.03.018 [DOI] [PubMed] [Google Scholar]
- 12. Zhao H, Kassama Y, Young M, Kell DB, Goodacre R (2004) Differentiation of Micromonospora isolates from a coastal sediment in Wales on the basis of Fourier transform infrared spectroscopy, 16S rRNA sequence analysis, and the amplified fragment length polymorphism technique. Appl Environ Microbiol 70: 6619–6627. 10.1128/AEM.70.11.6619-6627.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh P, Cameotra SS (2004) Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol 22: 142–146. 10.1016/j.tibtech.2004.01.010 [DOI] [PubMed] [Google Scholar]
- 14. Hamouda T, Baker JR, jr. (2000) Antimicrobial mechanism of action of surfactant lipid preparations in enteric Gram-negative bacilli. J Appl Microbiol 89: 397–403. 10.1046/j.1365-2672.2000.01127.x [DOI] [PubMed] [Google Scholar]
- 15. Maillard JY (2002) Bacterial target sites for biocide action. J Appl Microbiol 92 Suppl: 16S–27S. 10.1046/j.1365-2672.92.5s1.3.x [DOI] [PubMed] [Google Scholar]
- 16. Qi L, Kash JC, Dugan VG, Jagger BW, Lau Y-F, et al. (2011) The ability of pandemic influenza virus hemagglutinins to induce lower respiratory pathology is associated with decreased surfactant protein D binding. Virology 412: 426–434. 10.1016/j.virol.2011.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Numata M, Chu HW, Dakhama A, Voelker DR (2010) Pulmonary surfactant phosphatidylglycerol inhibits respiratory syncytial virus–induced inflammation and infection. Proc Natl Acad Sci USA 107: 320–325. 10.1073/pnas.0909361107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Negm NA, El Farargy AF, Mohammad IA, Zaki MF, Khowdiary MM (2013) Synthesis and inhibitory activity of Schiff base surfactants derived from tannic acid and their cobalt (II), manganese (II) and iron (III) complexes against bacteria and fungi. J Surfactants Deterg 16: 767–777. 10.1007/s11743-013-1437-5 [DOI] [Google Scholar]
- 19. Smaoui S, Elleuch L, Bejar W, Karray-Rebai I, Ayadi I, et al. (2010) Inhibition of fungi and gram-negative bacteria by bacteriocin BacTN635 produced by Lactobacillus plantarum sp. TN635. Appl Biochem Biotechnol 162: 1132–1146. 10.1007/s12010-009-8821-7 [DOI] [PubMed] [Google Scholar]
- 20. Falagas ME, Kasiakou SK, Saravolatz LD (2005) Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 40: 1333–1341. 10.1086/429323 [DOI] [PubMed] [Google Scholar]
- 21. Peetla C, Labhasetwar V (2009) Effect of molecular structure of cationic surfactants on biophysical interactions of surfactant-modified nanoparticles with a model membrane and cellular uptake. Langmuir 25: 2369–2377. 10.1021/la803361y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nazari M, Kurdi M, Heerklotz H (2012) Classifying surfactants with respect to their effect on lipid membrane order. Biophys J 102: 498–506. 10.1016/j.bpj.2011.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cabral JP (1992) Mode of antibacterial action of dodine (dodecylguanidine monoacetate) in Pseudomonas syringae . Can J Microbiol 38: 115–123. 10.1139/m92-019 [DOI] [PubMed] [Google Scholar]
- 24. Kügler R, Bouloussa O, Rondelez F (2005) Evidence of a charge-density threshold for optimum efficiency of biocidal cationic surfaces. Microbiology 151: 1341–1348. 10.1099/mic.0.27526-0 [DOI] [PubMed] [Google Scholar]
- 25. Cornellas A, Perez L, Comelles F, Ribosa I, Manresa A, et al. (2011) Self-aggregation and antimicrobial activity of imidazolium and pyridinium based ionic liquids in aqueous solution. J Colloid Interface Sci 355: 164–171. 10.1016/j.jcis.2010.11.063 [DOI] [PubMed] [Google Scholar]
- 26. Kubo I, Xiao P, Nihei K, Fujita K, Yamagiwa Y, et al. (2002) Molecular design of antifungal agents. J Agric Food Chem 50: 3992–3998. 10.1021/jf020088v [DOI] [PubMed] [Google Scholar]
- 27. Nakata K, Tsuchido T, Matsumura Y (2011) Antimicrobial cationic surfactant, cetyltrimethylammonium bromide, induces superoxide stress in Escherichia coli cells. J Appl Microbiol 110: 568–579. 10.1111/j.1365-2672.2010.04912.x [DOI] [PubMed] [Google Scholar]
- 28. Adt I, Toubas D, Pinon JM, Manfait M, Sockalingum GD (2006) FTIR spectroscopy as a potential tool to analyse structural modifications during morphogenesis of Candida albicans . Arch Microbiol 185: 277–285. 10.1007/s00203-006-0094-8 [DOI] [PubMed] [Google Scholar]
- 29. Del Bove M, Lattanzi M, Rellini P, Pelliccia C, Fatichenti F, et al. (2009) Comparison of molecular and metabolomic methods as characterization tools of Debaryomyces hansenii cheese isolates. Food Microbiol 26: 453–459. 10.1016/j.fm.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 30. Roscini L, Corte L, Antonielli L, Rellini P, Fatichenti F, et al. (2010) Influence of cell geometry and number of replicas in the reproducibility of whole cell FTIR analysis. Analyst 135: 2099–2105. 10.1039/c0an00127a [DOI] [PubMed] [Google Scholar]
- 31. Szeghalmi A, Kaminskyj S, Gough KM (2007) A synchrotron FTIR microspectroscopy investigation of fungal hyphae grown under optimal and stressed conditions. Anal Bioanal Chem 387: 1779–1789. 10.1007/s00216-006-0850-2 [DOI] [PubMed] [Google Scholar]
- 32. Corte L, Rellini P, Roscini L, Fatichenti F, Cardinali G (2010) Development of a novel, FTIR (Fourier transform infrared spectroscopy) based, yeast bioassay for toxicity testing and stress response study. Anal Chim Acta 659: 258–265. 10.1016/j.aca.2009.11.035 [DOI] [PubMed] [Google Scholar]
- 33. Corte L, Dell’Abate MT, Magini A, Migliore M, Felici B, et al. (2013) Assessment of safety and efficiency of nitrogen organic fertilizers from animal‐based Protein Hydrolysates–a laboratory multidisciplinary approach. J Sci Food Agric 94.2: 235–245. [DOI] [PubMed] [Google Scholar]
- 34. Corte L, Roscini L, Zadra C, Antonielli L, Tancini B, et al. (2012) Effect of pH on potassium metabisulphite biocidic activity against yeast and human cell cultures. Food Chem 134: 1327–1336. 10.1016/j.foodchem.2012.03.025 [DOI] [PubMed] [Google Scholar]
- 35. Kamnev AA (2008) FTIR spectroscopic studies of bacterial cellular responses to environmental factors, plant-bacterial interactions and signalling. Spectroscopy (N. Y., NY, U.S.) 22: 83–95. [Google Scholar]
- 36. Kamnev AA, Sadovnikova JN, Tarantilis PA, Polissiou MG, Antonyuk LP (2008) Responses of Azospirillum brasilense to nitrogen deficiency and to wheat lectin: a diffuse reflectance infrared fourier transform (DRIFT) spectroscopic study. Microb Ecol 56: 615–624. 10.1007/s00248-008-9381-z [DOI] [PubMed] [Google Scholar]
- 37. Fernández MV, Jagus RJ, Mugliaroli SL (2014) Effect of Combined Natural Antimicrobials on Spoilage Microorganisms and Listeria innocua in a Whey Cheese “Ricotta”. Food Bioprocess Technol 7: 1–10. [Google Scholar]
- 38. Noriega E, Shama G, Laca A, Diaz M, Kong MG (2011) Cold atmospheric gas plasma disinfection of chicken meat and chicken skin contaminated with Listeria innocua . Food Microbiol 28: 1293–1300. 10.1016/j.fm.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 39. Winfield MD, Groisman EA (2003) Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli . Appl Environ Microbiol 69: 3687–3694. 10.1128/AEM.69.7.3687-3694.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Corte L, Tiecco M, Roscini L, Germani R, Cardinali G (2014) FTIR analysis of the metabolomic stress response induced by N-alkyltropinium bromide surfactants in the yeasts Saccharomyces cerevisiae and Candida albicans . Colloids Surf, B 116: 761–771. 10.1016/j.colsurfb.2014.01.054 [DOI] [PubMed] [Google Scholar]
- 41. Corte L, Antonielli L, Roscini L, Fatichenti F, Cardinali G (2011) Influence of cell parameters in Fourier transform infrared spectroscopy analysis of whole yeast cells. Analyst 136: 2339–2349. 10.1039/c0an00515k [DOI] [PubMed] [Google Scholar]
- 42. Tiecco M, Cardinali G, Roscini L, Corte L, Germani R (2013) Biocidic and inhibitory activity screening of de novo synthesized surfactants against two eukaryotic and two prokaryotic microbial species Colloids Surf, B 111: 407–417. [DOI] [PubMed] [Google Scholar]
- 43. Essendoubi M, Toubas D, Bouzaggou M, Pinon JM, Manfait M, et al. (2005) Rapid identification of Candida species by FT-IR microspectroscopy. Biochim Biophys Acta 1724: 239–247. 10.1016/j.bbagen.2005.04.019 [DOI] [PubMed] [Google Scholar]
- 44. Huang WE, Hopper D, Goodacre R, Beckmann M, Singer A, et al. (2006) Rapid characterization of microbial biodegradation pathways by FT-IR spectroscopy. J Microbiol Methods. 67.2: 273–280. 10.1016/j.mimet.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 45. Kummerle M, Scherer S, Seiler H (1998) Rapid and reliable identification of food-borne yeasts by Fourier-transform infrared spectroscopy. Appl Environ Microbiol 64: 2207–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tiecco M, Corte L, Roscini L, Colabella C, Germani R and Cardinali G (2014) A novel, semiautomatic method for the conductometric CMC (Critical Micellar Concentration) evaluation of surfactants alone or in combination with other molecules or cells. Chem-Biol Interact. 218: 20–27. 10.1016/j.cbi.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 47. Yu C, Irudayaraj J (2005) Spectroscopic characterization of microorganisms by Fourier transform infrared microspectroscopy. Biopolymers 77: 368–377. 10.1002/bip.20247 [DOI] [PubMed] [Google Scholar]
- 48. Cao J, Ng ES, McNaughton D, Stanley EG, Elefanty AG, et al. (2013) The Characterisation of Pluripotent and Multipotent Stem Cells Using Fourier Transform Infrared Microspectroscopy. Int J Mol Sci 14: 17453–17476. 10.3390/ijms140917453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Naumann D, Barnickel G, Bradaczek H, Labischinski H, Giesbrecht P (1982) Infrared spectroscopy, a tool for probing bacterial peptidoglycan. Eur J Biochem 125: 505–515. 10.1111/j.1432-1033.1982.tb06711.x [DOI] [PubMed] [Google Scholar]
- 50. Bellisola G, Sorio C (2012) Infrared spectroscopy and microscopy in cancer research and diagnosis. Am J Cancer Res 2: 1–21. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chemical structures of the surfactants used in this work, names and acronyms.
(DOCX)
Data Availability Statement
All relevant data are within the paper.