Abstract
Many fungi use membrane vesicles to transport complex molecules across their cell walls. Like mammalian exosomes, fungal vesicles contain lipids, proteins, and polysaccharides, many of which are associated with virulence. Here we identify and characterize extracellular vesicles (EVs) in Alternaria infectoria, a ubiquitous, environmental filamentous fungus that is also an opportunistic human pathogen. Examination of the A. infectoria EVs revealed a morphology similar to that of vesicles described in other fungal species. Of note, proteomic analysis detected a reduced number of vesicle-associated proteins. There were two prevalent categories among the 20 identified proteins, including the polysaccharide metabolism group, probably related to plant host invasion or biosynthesis/degradation of cell wall components, and the nuclear proteins, especially DNA repair enzymes. We also found enzymes related to pigment synthesis, adhesion to the host cell, and trafficking of vesicles/organelles/molecules. This is the first time EV secretions have been identified in a filamentous fungus. We believe that these vesicles might have a role in virulence.
Keywords: Alternaria infectoria, extracellular vesicles, secreted proteins
Introduction
Alternaria infectoria is an environmental fungus of the order Pleosporales that includes the cell wall melanin-containing fungi, the Dematiaceae. The genus Alternaria includes ubiquitous, saprophytic fungi that are part of the endogenous and exogenous microbiota of wheat grains. Many species are considered important phytopathogenic agents that cause spoilage and disease of food crops. The Dematiaceae filamentous fungi are increasingly found in the environment, with the genus Alternaria found most frequently in outdoor air [1,2]. Recently, Alternaria spores have been associated with the development of allergic rhinitis and asthma [3]. In addition, the members of this genus have proven to be rare opportunistic agents that cause phaeohyphomycosis involving cutaneous or subcutaneous infections and characterized by the presence of dark-walled hyphae or yeast-like cells in affected tissues. Of note, the number of infections in immunocompromised patients is increasing, including infections caused by common species such as A. alternata and A. infectoria [4–6].
Despite its increasing medical and agricultural importance, little is known about the physiology, biochemistry, and genetics of A. infectoria. Recently, we identified several A. infectoria genes related to the synthesis of cell wall components. Some of the genes were completely characterized [7], while in others only deposited the partial sequence at the NCBI database (GenBank database, accession number JF742672). Additional proteomic and secretome-related studies are needed to evaluate the presence of secreted proteins and enzymes associated with growth, differentiation, or infection in order to clarify the biology of A. infectoria.
In addition to classic secretion systems, many bacteria use extracellular vesicles (EVs) as platforms for the delivery of virulence factors to host cells. In microbes, EVs were initially described in gram-negative bacteria [8], but recent reports indicate that they are also ubiquitous in gram-positive bacteria [9–12]. EVs are closed spherical structures that are released from the outermost compartments; their diameters range from 10 to 300 nm. Gram-negative bacteria membrane vesicles contain phospholipids, outer membrane molecules, lipopolysaccharide, and some periplasmic components. These membrane vesicles have been detected in bacteria that grow under many different situations, including culture media, biofilms, during in vitro infections, and even in tissues of infected hosts [12]. The vesicular transport system in fungi was first described in Cryptococcus neoformans [13]. Subsequent investigations of the presence of these vesicles in culture supernatants of Saccharomyces cerevisiae, Candida albicans, Candida parapsilosis, Histoplasma capsulatum, and Sporothrix schenckii suggest that this phenomenon was conserved in fungi [14]. Fungal EVs are involved in the transport of lipids, polysaccharides, and protein components associated with cell wall biogenesis and with virulence across cell walls [15–17]. Because of their capacity to carry molecules related to virulence, EVs have been referred to as “virulence factor bags” [15].
In this study, we detected the presence of structures that are highly suggestive of EVs in A. infectoria cultures. Structural studies, including the use of scanning electron microscopy (SEM), revealed A. infectoria hyphae releasing vesicle-like structures into the media. Moreover, images using transmission electron microcopy of isolated vesicles were consistent with a bilayered membrane. A proteomic study of these vesicles revealed an atypical low diversity in the families of enzymes present as compared with other fungi that are more commonly associated with human disease. This is the first report on membrane vesicles in a filamentous fungus.
Materials and methods
Strain and media
A. infectoria strain IMF006 was obtained from Centraal-bureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre, an institute of the Royal Netherlands Academy of Arts and Sciences (KNAW), Utrecht, the Netherlands (CBS 137.90) and maintained on potato dextrose agar (Difco, BD, New Jersey, USA) for at least 7 d at 30°C with a cycle of 8 h of light with an ultraviolet (UV)-enriched lamp (F15W T8BLB; Grainger, NY, USA) and 16 h of dark. For vesicle isolation, A. infectoria was allowed to grow for 7 d in yeast malt extract liquid media at 30°C under constant orbital shaking.
Vesicle isolation
Vesicle isolation was performed according to previously described protocols [18]. The fungal cells were centrifuged at 15,000 rpm for 30 min to remove all cell debris, with the supernatant filtered through a polyvinylidene difluoride filter with a 0.45μm pore size (Millipore, Billerica, MA) and concentrated about 50 fold using an Amicon ultrafiltration system (Millipore) with a 100-kDa exclusion filter. The final concentrated liquid was ultracentrifuged at 60,000 rpm for 1 h at 4°C and washed twice with phosphate-buffered saline (PBS).
Zeta potential and dynamic light scattering measurements
Zeta potential measurements were performed to determine the surface net charge of EVs. Dynamic light scattering (DLS) measurements were performed to study the size and heterogeneity of the EV population. EVs were suspended in distilled water for zeta potential measurements and suspended in PBS for DLS measurements. Measurements were taken using a Zeta Plus analyzer (Brookhaven Instruments Corporation, Holtsville, NY, USA).
Scanning electron microscopy
For SEM studies, the vesicles were fixed with 2.5% glutaraldehyde, 0.1M sodium cacodylate, 0.2M sucrose, and 5 mM magnesium chloride (pH 7.4) and dehydrated through a graded series of ethanol solutions. The critical point drying was accessed using liquid carbon dioxide in a critical point drier (model 795; Tousimis; Rockville, MD, USA). Sputter was coated with gold–palladium in a vacuum desk-2 sputter coater (Denton; Cherry Hill, NJ, USA). Samples were examined in a supra field emission scanning electron microscope (Carl Zeiss Microscopy, LLC; Thornwood, NY, USA) using an accelerating voltage of 5 kV.
Transmission electron microscopy
For transmission electron microscopy (TEM), the sample was fixed with 4% paraformaldehyde and 5% glutaraldehyde in 2× PBS buffer mixed with growth media (1:1), enrobed in 4% gelatin, post fixed with 1% osmium tetroxide, followed by 1% uranyl acetate, and then dehydrated through a graded series of ethanol and embedded in Spurr resin (Electron Microscopy Sciences; Hatfield, PA, USA). Ultrathin sections were cut on a Reichert Ultracut UCT, stained with uranyl acetate followed by lead citrate, and viewed on a JEOL 1200EX transmission electron microscope at 80 kV.
Protein identification by liquid chromatography–tandem mass spectrometry
Proteins from vesicle suspensions were precipitated by adding 6 volumes of ice-cold acetone and incubating overnight at —20°C. Pellets were dissolved in 100 μL of 25 mM ammonium bicarbonate. Disulfide bonds of proteins were reduced by adding 10 μL of 10 mM dithiothreitol and incubating at room temperature for 1 h. The alkylation of cysteine residues was accomplished by adding 10 μL of 10 mM iodoacetamide prepared in 25 mM ammonium bicarbonate and incubating for 1 h at room temperature in the dark. Protein digestion was carried out by incubating 40 μL of 50 ng/μL trypsin (Promega; Madison, WI, USA; 1:20; w/w) overnight at 37°C and then stopping the reaction by freezing the sample at −80°C for 1 h. The peptide mixture was desalted, concentrated on ziptip (Millipore), and then analyzed using Nanoflow liquid chromatography-tandem mass spectrometry (LC-MS/MS). The peptides were loaded onto a 0.3-mm × 5-mm C18 precolumn (New Objective; Woburn, MA, USA). The elution was made with a linear gradient of 5%–90% acetonitrile in 0.1% aqueous solution of formic acid. The gradient was performed over 120 min using a NanoLC 1D Plus (Eksigent-AB SCIEX; Redwood City, CA, USA) at a flow rate of 200 nL/min through a 75-μm × 15-cm fused silica capillary C18 high-performance liquid chromatography PepMap column (LC Packings; Vernon Hills, Illinois, USA) into a stainless steel Nanobore emitter (Proxeon/Thermo Scientifics; Odense, Denmark). The peptides were scanned and fragmented with an LTQ XL Linear Ion Trap Mass Spectrometer (ThermoFinnigan/Thermo Scientific; San Jose, CA, USA) operated in data-dependent and tandem mass spectrometry MS/MS (mass spectrometry/mass spectrometry) switching mode using the most intense precursor ions detected in a survey scan from 400 to 1600 amu (3 μ scans). Normalized collision energy was set to 35%, and dynamic exclusion was applied during 3-min periods to avoid repetitive fragmentation of ions. Raw files were converted to .dta files for a Mascot database (Mascot software; Matrix Science; Boston, MA, USA) search. A database containing Broad Institute Pyrenophora triticirepentis sequences (12,169 sequences, as of 26 October 2012) was searched using Mascot software (version 2.3) for protein identification. Search criteria included trypsin specificity with one missed cleavage allowed, methionine oxidation as variable modification, a minimum precursor and fragment-ion mass accuracy of 1.2 and 0.3 Da, respectively, and a requirement of at least one bold red peptide (ie, highest score peptide matching to protein with highest total score). Cutoff values for Mascot protein scores were set at 29 (P > 0.05) to be considered an accurate identification. Proteins identified with only one peptide were inspected manually. A combined list of proteins identified in all replicates (n = 5) was condensed to remove redundant IDs such as orthologous sequences, redundant database entries, and indistinguishable isoforms based on observed peptide coverage.
Results
Morphology and physical features of EVs
We allowed A. infectoria mycelia to grow for 7 d in liquid medium to ascertain whether, as in other fungi, it released vesicles. The SEM analysis of cell culture material revealed widely distributed vesicular-like structures on the surface of A. infectoria hyphae (Fig. 1A). Higher magnification examinant revealed structural diversity in both size and granulosity (Fig. 1B–C).
Figure 1.
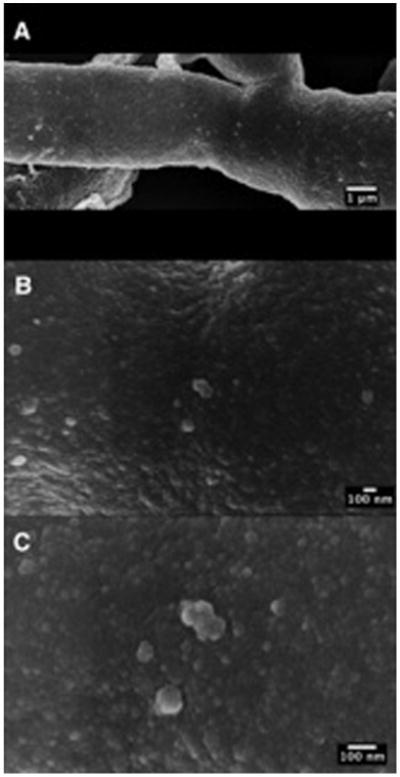
Release of A. infectoria mycelia EVs. Panel of sequential magnifications (A–C) of SEM images revealing the release of vesicles in the surface of A. infectoria hyphae.
Examination of TEM preparations of A. infectoria vesicles from liquid media demonstrated that they were diverse in size and electron density. We were able to visualize membrane-associated electron-dense vesicles (Fig. 2A), heavily pigmented vesicles (Fig. 2B), and electron-dense vesicles (Fig. 2C). Nevertheless, all of the vesicles corresponded to spherical structures enclosed by apparent bilayered membranes.
Figure 2.
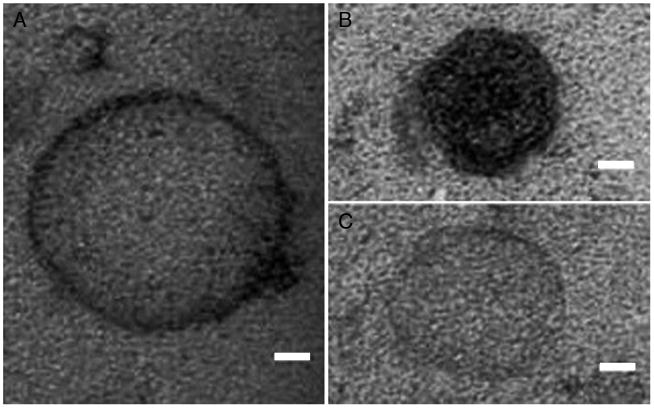
Morphology of A. infectoria EVs. TEM of EVs obtained by ultracentrifugation from A. infectoria grown for 7 d in liquid medium (see Materials and Methods). Three distinct morphologies were found: (A) membrane-associated electron-dense vesicles, (B) heavily pigmented vesicles, and (C) electron-dense vesicles. Scale bar, 20 nm.
We used SEM, TEM, and DLS measurement to determine the size distribution of EVs. Five SEM microscopic fields were examined (473 vesicles), and TEM fields were studied corresponding to 1110 vesicles. Using SEM, the majority of the EV population had a diameter between 20 and 40 nm, with a mean size of 28.36 nm (Fig. 3A). The mean size of A. infectoria EVs measured on TEM micrographs was 16.77 nm (Fig. 3B). As noted previously [19], TEM size determinations are underestimated because two-dimensional information is used to generate three-dimensional diameter data. Based on this, a correction factor of 1.27 can be used [20] for such measurements, which results in the mean diameter, measured using TEM, of 21.29 nm, indicating a size distribution closer to that when using SEM. The values of effective diameter provided by DLS suggested two populations of EVs measuring 50 nm and 100 nm (Fig. 3C).
Figure 3.
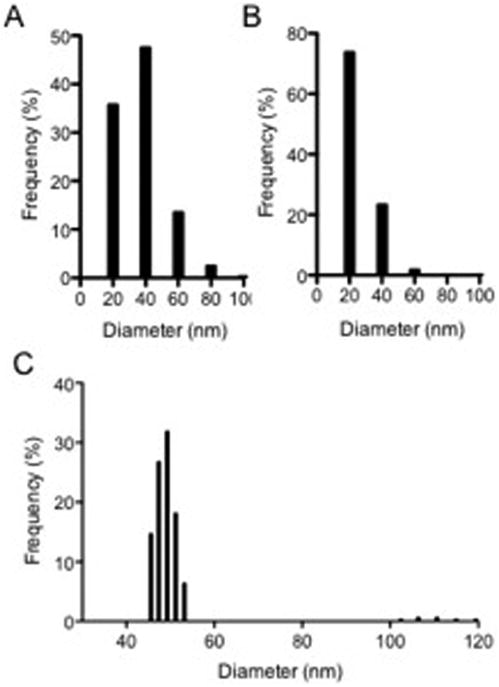
Size distribution of A. infectoria EVs. Vesicles were analyzed in terms of size distribution by SEM (A), TEM (B), and DLS measurement (C). The results are shown as histograms from the analysis of DLS counting and SEM and TEM images.
Proteomic analysis of vesicles
Studies of other fungi have shown that the EVs carry different classes of proteins including those related to metabolic processes and virulence [18,21,22]. The protein content of the EVs of A. infectoria was analyzed using LC-MS/MS. The protocol used allowed us to identify 20 proteins further organized into families according to information from the National Center for Biotechnology Information's Conserved Domain Database CDD (Table 1), including nuclear proteins, proteins related to carbohydrate, lipid and protein metabolism, and pathogenesis. Of note, the number of A. infectoria vesicle–associated proteins was small when compared with other fungal EVs. As many as 76 proteins were detected in C. neoformans EVs [21], 283 in H. capsulatum [23], and 205 in Paracoccidioides brasiliensis [17], while 400 proteins were detected in S. cerevisiae [22]. Seven of the 20 proteins have been described as vesicular proteins, that is, 6-phosphogluconate dehydrogenase 1 [18,22], glyceraldehyde-3-phosphate dehydrogenase [18,21,22], beta-xylosidase [24], heat shock protein 60 [18], Nicotinamide adenine dinucleotide–specific glutamate dehydrogenase [22], which is hypothetical protein that functions as an ATP-binding cassette transporter [25], and plasma membrane ATPase [18].
Table 1.
Protein identification of A. infectoria extracellular vesicles.
| Family | Accession number | Protein name* | Molecular weight (Da) | Function |
|---|---|---|---|---|
| Nuclear proteins | PTRT_01137 | | PTRG_01137 | Pyrenophora tritici-repentis SNF2 family ATP-dependent chromatin-remodeling factor snf21 (1274 aa) | 144369 | ATP-dependent DNA helicase |
| PTRT_01564 | | PTRG_01564 | Pyrenophora tritici-repentis DNA repair protein Rhp41 (1007 aa) | 111820 | DNA repair protein; nucleotide excision repair protein; Rad4 homologue | |
| PTRT_04363 | | PTRG_04363 | Pyrenophora tritici-repentis GINS complex subunit Psf2 (247 aa) | 27242 | DNA replication | |
| PTRT_08220 | | PTRG_08220 | Pyrenophora tritici-repentis conserved hypothetical protein (775 aa) | 85001 | Chromosome segregation protein; SMC | |
| PTRT_09509 | | PTRG_09509 | Pyrenophora tritici-repentis DNA replication licensing factor mcm5 (725 aa) | 80222 | DNA replication initiation | |
| Carbohydrate metabolism | PTRT_00133 | | PTRG_00133 | Pyrenophora tritici-repentis glycogenin-2 (623 aa) | 69322 | Biosynthesis of glycogen; glicosyl transferase family 8 |
| PTRT_02275 | | PTRG_02275 | Pyrenophora tritici-repentis 6-phosphogluconate dehydrogenase 1 (494 aa) | 54197 | Glucose metabolism; descarboxylation of 6-phosphogluconate to ribulose-5-phosphate | |
| PTRT_03700 | | PTRG_03700 | Pyrenophora tritici-repentis glyceraldehyde-3-phosphate dehydrogenase (338 aa) | 36482 | Glucose metabolism; phosphorylation of glyceraldehyde-3-phosphate to D-glycerate 1,3-bisphosphate | |
| PTRT_07360 | | PTRG_07360 | Pyrenophora tritici-repentis beta-xylosidase (1000 aa) | 110996 | Carbohydrate metabolic process; degradation of xylan on hemicellulose | |
| Protein and amino acid metabolism | PTRT_01222 | | PTRG_01222 | Pyrenophora tritici-repentis heat shock protein 60, mitochondrial precursor (576 aa) | 60580 | Chaperonin |
| PTRT_04082 | | PTRG_04082 | Pyrenophora tritici-repentis NADP-specific glutamate dehydrogenase (449 aa) | 48752 | Oxidative deamination | |
| Lipid metabolism | PTRT_04244 | | PTRG_04244 | Pyrenophora tritici-repentis lovastatin nonaketide synthase (3876 aa) | 422260 | Polyketide synthase |
| Pathogenesis | PTRT_11966 | | PTRG_11966 | Pyrenophora tritici-repentis predicted protein (375 aa) | 40808 | S-adenosylmethionine-dependent methyltransferases; O-methyltransferase catalyzing the methylation of vicinal hydroxyl groups of polyphenols |
| Plasma membrane | PTRT_04005 | | PTRG_04005 | Pyrenophora tritici-repentis plasma membrane ATPase (978 aa) | 107657 | ATPase activity |
| Others | PTRT_02703 | | PTRG_02703 | Pyrenophora tritici-repentis C2H2-type zinc finger domain containing protein (469 aa) | 52788 | Fungal specific transcription factor domain; sugar metabolism, amino acid metabolism, gluconeogenesis, respiration, fatty acid catabolism, nitrate assimilation, and similar processes |
| PTRT_02813 | | PTRG_02813 | Pyrenophora tritici-repentis hypothetical protein (147 aa) | 16047 | ABC transporter | |
| PTRT_03263 | | PTRG_03263 | Pyrenophora tritici-repentis conserved hypothetical protein (590 aa) | 65731 | Fungal specific transcription factor domain; sugar and amino acid metabolism, gluconeogenesis, respiration, vitamin synthesis, cell cycle, chromatin remodeling, nitrogen utilization, peroxisome proliferation, drug resistance, stress response | |
| PTRT_04280 | | PTRG_04280 | Pyrenophora tritici-repentis predicted protein (207 aa) | 23715 | Unknown | |
| PTRT_04454 | | PTRG_04454 | Pyrenophora tritici-repentis conserved hypothetical protein (176 aa) | 19309 | Predicted coiled-coil protein | |
| PTRT_11337 | | PTRG_11337 | Pyrenophora tritici-repentis hypothetical protein (527 aa) | 61409 | Unknown |
For protein identification, we searched the database from the Broad Institute at the Massachusetts Institute of Technology, Cambridge, MA, USA, with Pyrenophora tritici-repentis sequences (12,169 sequences, as of 26 October 2012) using Mascot software (version 2.3; Matrix Science).
Among nuclear proteins, we identified Snf21, an ATP-dependent chromatin remodeler [26]; Rhp41, a DNA repair protein homologue to Rad4 [27]; Psf2, a sub-unit of the GINS complex (a component of the eukaryotic replisome) [28]; a structural maintenance of chromosomes (SMC) protein [29]; and Mcm5 protein [30]. Of the class of carbohydrate metabolism proteins, we noted four proteins—two of glucose metabolism, 6-phosphogluconate dehydrogenase 1 and glyceraldehyde-3-phosphate dehydrogenase; one of glycogen biosynthesis, the glycogenin-2 [31]; and one involved In hemicellulose degradation, the beta-xylosidase [32], which was previously classified as a fungi allergen [3].
Discussion
The transport of substances, including enzymes, across cell walls is essential to biosynthesis of fungal cell surface elements and virulence. In the last decade, this process has been deemed an important mechanism that enables fungal pathogens to be more robustly armed while invading the host tissues and evading the host defenses. Vesicles provide the means by which fungi deliver concentrated “payloads” that contain numerous components to other organisms and to nutritional sources such as decaying vegetation. The advantage of vesicular transport relative to cell surface release is that, in the latter, the components are rapidly diluted as the inverse square of the distance from the cell membrane. Several biologically relevant aspects of extracellular membrane vesicle production, both in mammalian cells and in microbes, have been highlighted in recent years. Rodrigues and colleagues [33] stress that most of the fungi reported to produce vesicles are human pathogens. Although Alternaria spp. do occasionally cause human disease, they are better known as soil inhabitants and, thus, different from the other fungi that have been studied. Nevertheless, we noted many similarities in the extracellular vesicles produced by Alternaria as compared with those of known pathogens such as C. neoformans, H. capsulatum, P. brasiliensis, and S. cerevisiae. For example, our analysis revealed that Alternaria vesicles' proteome had the same protein families as those previously described.
A. infectoria, a filamentous fungus that is ubiquitous in the environment and only rarely causes disease in humans or animals, produces EVs that are similar and comparable to those described in pathogenic fungi. To our knowledge, this is the first time EVs have been identified and characterized in filamentous fungal mycelia. Those found in supernatants of A. infectoria cultures are morphologically similar to vesicles described in other fungi. Using TEM, it was possible to observe vesicle-like structures emerging from the fungal hypha surface. The environmental nature of our organism might explain why the enzymatic profile of A. infectoria EVs found during this work is less rich with regard to the identified proteins. We noted only 20 proteins, significantly fewer than the several dozen to several hundred reported in proteomic studies of EV production in fungi more adapted to mammalian host environments [17,21–33]. On the other hand, the database used for protein identification was from P. tritici-repentis, a species related to A. infectoria, and the degree of homology between their genomes may not be extremely high. In Aspergillus oryzae, a filamentous fungus used in traditional food fermentation of dry seeds such as rice and soybeans, it was demonstrated that the secretion of proteins was much greater in a solid-state culture than in a submerged culture. The authors suggested that these results might be due to the fact that the water activity of solid-state culture is low and differentiation of nutrient components is restricted, causing the fungus to secrete a higher concentration of enzymes in order to obtain essential nutrients [34]. It is worthwhile to note the fact that in our study, as well as others involving filamentous fungi, there was no indication that the extracellular enzymes were enclosed by vesicles. If EV production is a fungal response to stress conditions, we tentatively conclude that the growth conditions that we used correspond to resting conditions, that is, high water content and high nutrient availability. Consequently, there was no need for complex secretion of enzymes. Another factor related to growth conditions is that A. infectoria is adapted to temperatures <30°C, and this might lead to changes in the secretion of extracellular factors. A. alternata, a closely related species, loses a plasmid that is essential for phytopathogenesis when grown at 29°C, becoming less (phyto)pathogenic [35].
A. infectoria EVs (AiEVs) seem to contain a relatively large number of nuclear-related proteins (5/20) when compared with other EV proteomes such as 11/283 in H. capsulatum [18], 2/76 in C. neoformans [21], and 2/205 in P. brasiliensis [17]. The proteins identified are involved in DNA repair and replication, permitting speculation that protein enrichment in the AiEV is related to UV exposure. Among the AiEVs, at least four are clearly involved in polysaccharide metabolism. These, although related to biosynthesis of glycogen, that is, the glucosyl transferase family and a carbohydrate metabolic process, degradation of xylan on hemicellulose can contribute to the following: synthesis of cell wall surface polysaccharides; A. infectoria requirement for the enzymatic machinery to hydrolyze compounds such as xylan or cellulose; the synthesis (elongation and/or ramification) of secreted polysaccharides that can be used as immunomodulators and/or as adhesion factors, as described for other fungi [17,21,36]; and hydrolysis of the cell wall during secretion of the vesicles from the hyphae.
It is worth mentioning that under the A. infectoria growth conditions used in this study, enzymes such as laccase and urease were not detected in the proteomic analysis even though these virulence-related enzymes have been found in other fungi [21]. Since A. infectoria contains melanin in the cell wall and was grown under UV-enriched light, it would be expected that melanin-production machinery would be activated. It has been proposed that melanization in C. neoformans occurs in EVs that are then deposited in the cell wall [19]. Among the proteins associated with AiEVs, we found at least one that was involved in melanin and other microbial pigment synthesis pathways. The enzyme in question is polyketide synthase, which uses acetyl coenzyme A or malonyl coenzyme A as a precursor to produce the melanin intermediate, 1,3,6,8-tetrahydroxynaphthalene, also referred to as T4HN [37]. This enzyme was linked to pathogenesis in several fungi including Penicillium marneffei, C. neoformans, and Fonsecaea pedrosoi [19,37,38]. The O-methyltransferase, also found in AiEVs, is related to both pathogenesis and toxicity, as described for phytopathogenic fungi [39].
Taken together and when compared with results of other studies, our results indicate that most of the enzymes identified in A. infectoria vesicles are likely to be related to phytopathogenesis and to the expected demands of natural environmental habitats rather than to animal pathogenesis. We feel that this is consistent with the fact that this organism is primarily a soil microbe and that, in contrast to other fungi that are soil inhabitants, A. infectoria is much less adapted to the animal/human host and, thus, only very rarely associated with animal/human infections. Nevertheless, we found a chaperonin, the heat shock protein 60, which was previously associated with the immune response to fungi [40] and H. capsulatum vesicles [18]. As such, we identified a glyceraldehyde-3-phosphate dehydrogenase. This is an important glycolytic enzyme that recently was associated with such activities as axonal transport of mitochondria or molecules [41] as well as the adhesion of P. brasiliensis yeast cells to host cells and to extracellular matrix [42]. These two additional functions of this particular enzyme might indicate that in filamentous fungi such as A. infectoria it would be involved not only in adherence to host cells but, most importantly, in the transport of vesicles, organelles, or biomolecules along the hyphae and across the hyphae cell wall and, thus, related to the secretion of the EVs. Several aspects related to the genesis and movement of fungal EVs are still unknown. Recently, an extensive review of these matters highlighted that most likely these enzymes have a cytoplasmic origin and that vesiculation probably occurs through an inverted macropinocytosis mechanism [43].
In summary, we provide the first report of the secretion of EVs in a filamentous fungus as well as one that is an occasional opportunistic human pathogen. Our results suggest that these vesicles carry fewer proteins when compared to other fungal organisms where the proteome of these vesicles was identified. However, this result should be interpreted cautiously since small methodological differences can have large effects on protein detection and since the secretion of proteins to the extracellular milieu is dependent on existing growth conditions. This aspect was highlighted by Rodrigues and colleagues [33] regarding fungi that infect humans and by Oda and colleagues [34] who worked with filamentous fungi grown in liquid and low–water-activity media. The recently described biological function of glyceraldehyde-3-phosphate dehydrogenase [41] suggests that its presence in AiEVs might be related to hyphal trafficking. We believe that our results open new perspectives on the study of vesicle secretions in filamentous fungi and on how these processes can contribute to the adaptation of these organisms to different environments and hosts. Previously, it was described in a lipophilic yeast, Malassezia sympodialis, that EVs have important immunomodulatory functions in atopic eczema patients [44].
Acknowledgments
This study was funded, in part, by an FCT-Fundação para a Ciência e Tecnologia (PTDC/SAU-ESA/108636/2008; co-funded by COMPETE and FEDER and PEst-C/SAU/LA0001/2011) project. This work was also supported by NIH grants HL059842, AI033774, AI033142, and AI052733, and the Center for AIDS Research at Albert Einstein College of Medicine.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Oliveira M, Delgado L, Ribeiro H, Abreu I. Fungal spores from Pleosporales in the atmosphere of urban and rural locations in Portugal. J Environ Mon. 2010;12:1187–1194. doi: 10.1039/b913705j. [DOI] [PubMed] [Google Scholar]
- 2.Sabariego S, Bouso V, Pérez-Badia R. Comparative study of airborne Alternaria conidia levels in two cities in Castilla-La Mancha (central Spain), and correlations with weather-related variables. Ann Agric Environ Med. 2012;19:227–232. [PubMed] [Google Scholar]
- 3.Knutsen AP, Bush RK, Demain JG, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immun. 2012;129:280–291. doi: 10.1016/j.jaci.2011.12.970. [DOI] [PubMed] [Google Scholar]
- 4.Cunha D, Amaro C, Vieira MR, et al. Phaeohyphomycosis caused by Alternaria infectoria presenting as multiple vegetating lesions in a renal transplant patient. Rev Iberoam Micol. 2012;29:44–46. doi: 10.1016/j.riam.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Hipolito E, Faria E, Alves AF, et al. Alternaria infectoria brain abscess in a child with chronic granulomatous disease. Eur J Clin Microbiol Infect Dis. 2008;28:377–380. doi: 10.1007/s10096-008-0623-2. [DOI] [PubMed] [Google Scholar]
- 6.Rammaert B, Aguilar C, Bougnoux ME, et al. Success of posaconazole therapy in a heart transplanted patient with Alternaria infectoria cutaneous infection. Med Mycol. 2012;50:518–521. doi: 10.3109/13693786.2011.641165. [DOI] [PubMed] [Google Scholar]
- 7.Anjos J, Fernandes C, Silva BMA, et al. β(1,3)-glucan synthase complex from Alternaria infectoria, a rare dematiaceous human pathogen. Med Mycol. 2012;50:716–725. doi: 10.3109/13693786.2012.675525. [DOI] [PubMed] [Google Scholar]
- 8.Kuehn MJM, Kesty NCN. Bacterial outer membrane vesicles and the host-pathogen interaction. Gen Dev. 2005;19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- 9.Rivera J, Cordero RJ, Nakouzi AS, et al. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc Nat Acad Sci U S A. 2010;107:19002–19007. doi: 10.1073/pnas.1008843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prados-Rosales RR, Baena AA, Martinez LRL, et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J Clin Invest. 2011;121:1471–1483. doi: 10.1172/JCI44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee EY, Choi DY, Kim DK, et al. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 12.Marsollier LL, Brodin PP, Jackson MM, et al. Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS Pathog. 2007;3:e62. doi: 10.1371/journal.ppat.0030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues ML, Nimrichter L, Oliveira DL, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007;6:48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casadevall A, Nosanchuk JD, Williamson P, Rodrigues ML. Vesicular transport across the fungal cell wall. Trends Microbiol. 2009;17:158–162. doi: 10.1016/j.tim.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues ML, Nimrichter L, Oliveira DL, Nosanchuk JD, Casadevall A. Vesicular trans-cell wall transport in fungi: A mechanism for the delivery of virulence-associated macro-molecules? Lipid Insights. 2008;2:27–40. doi: 10.4137/lpi.s1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nosanchuk JD, Nimrichter L, Casadevall A, Rodrigues ML. A role for vesicular transport of macromolecules across cell walls in fungal pathogenesis. Commun Integr Biol. 2008;1:37–39. doi: 10.4161/cib.1.1.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallejo MCM, Nakayasu ESE, Matsuo ALA, et al. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. J Proteome Res. 2012;11:1676–1685. doi: 10.1021/pr200872s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albuquerque PC, Nakayasu ES, Rodrigues ML, et al. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008;10:1695–1710. doi: 10.1111/j.1462-5822.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenman HCH, Frases SS, Nicola AMA, Rodrigues MLM, Casadevall AA. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology. 2009;155:3860–3867. doi: 10.1099/mic.0.032854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong M, Bhattacharya RN, James C, Basu A. A statistical approach to estimate the 3D size distribution of spheres from 2D size distributions. Geol Soc America Bull. 2005;117:244–249. [Google Scholar]
- 21.Rodrigues ML, Nakayasu ES, Oliveira DL, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira DL, Nakayasu ES, Joffe LS, et al. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS ONE. 2010;5:e11113. doi: 10.1371/journal.pone.0011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holbrook EDE, Edwards JAJ, Youseff BHB, Rappleye CAC. Definition of the extracellular proteome of pathogenic-phase Histoplasma capsulatum. J Proteome Res. 2011;10:1929–1943. doi: 10.1021/pr1011697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Oliveira JMPF, van Passel MWJ, Schaap PJ, de Graaff LH. Proteomic analysis of the secretory response of Aspergillus niger to D-maltose and D-xylose. PLoS ONE. 2011;6:e20865. doi: 10.1371/journal.pone.0020865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.da Silva BA, Sodré CL, Souza-Gonçalves AL, et al. Proteomic analysis of the secretions of Pseudallescheria boydii, a human fungal pathogen with unknown genome. J Proteome Res. 2012;11:172–188. doi: 10.1021/pr200875x. [DOI] [PubMed] [Google Scholar]
- 26.Yamada KK, Hirota KK, Mizuno KIK, Shibata TT, Ohta KK. Essential roles of Snf21, a Swi2/Snf2 family chromatin remodeler, in fission yeast mitosis. Genes Genet Syst. 2008;83:361–372. doi: 10.1266/ggs.83.361. [DOI] [PubMed] [Google Scholar]
- 27.Marti TMT, Kunz CC, Fleck OO. Repair of damaged and mismatched DNA by the XPC homologues Rhp41 and Rhp42 of fission yeast. Genetics. 2003;164:457–467. doi: 10.1093/genetics/164.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pai CC, Garcia I, Wang SW, et al. GINS inactivation phenotypes reveal two pathways for chromatin association of replicative and DNA polymerases in fission yeast. Mol Biol Cell. 2009;20:1213–1222. doi: 10.1091/mbc.E08-04-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strunnikov AV, Hogan E, Koshland D. SMC2, a Saccharomyces cerevisiae gene essential for chromosome segregation and condensation, defines a subgroup within the SMC family. Gen Dev. 1995;9:587–599. doi: 10.1101/gad.9.5.587. [DOI] [PubMed] [Google Scholar]
- 30.Bochman ML, Schwacha A. The Mcm Complex: Unwinding the mechanism of a replicative helicase. Microbiol Mol Biol Rev. 2009;73:652–683. doi: 10.1128/MMBR.00019-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torija MJ, Novo M, Lemassu A, et al. Glycogen synthesis in the absence of glycogenin in the yeast Saccharomyces cerevisiae. FEBS Lett. 2005;579:3999–4004. doi: 10.1016/j.febslet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Michelin M, Peixoto-Nogueira SC, Silva TM, et al. A novel xylan degrading β-D-xylosidase: purification and biochemical characterization. World J Microbiol Biotechnol. 2012;28:3179–3186. doi: 10.1007/s11274-012-1128-9. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues ML, Nakayasu ES, Almeida IC, Nimrichter L. The impact of proteomics on the understanding of functions and biogenesis of fungal extracellular vesicles. J Proteomics. 2013 Apr 10;:S1874–3919. doi: 10.1016/j.jprot.2013.04.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oda K, Kakizono D, Yamada O, et al. Proteomic analysis of extracellular proteins from Aspergillus oryzae grown under submerged and solid-state culture conditions. Appl Environ Microbiol. 2006;72:3448–3457. doi: 10.1128/AEM.72.5.3448-3457.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katsuya SS, Kaneko II, Owaki MM, et al. Circular DNA plasmid in the phytopathogenic fungus Alternaria alternata: its temperature-dependent curing and association with pathogenicity. Genetics. 1997;146:111–120. doi: 10.1093/genetics/146.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontaine TT, Delangle AA, Simenel CC, et al. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. 2011;7:e1002372. doi: 10.1371/journal.ppat.1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo PCYP, Lam CWC, Tam EWTE, et al. First discovery of two polyketide synthase genes for mitorubrinic acid and mitorubrinol yellow pigment biosynthesis and implications in virulence of Penicillium marneffei. PLoS Negl Trop Dis. 2012;6:e1871. doi: 10.1371/journal.pntd.0001871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franzen AJ, Cunha MM, Miranda K, et al. Ultrastructural characterization of melanosomes of the human pathogenic fungus Fonsecaea pedrosoi. J Struct Biol. 2008;162:75–84. doi: 10.1016/j.jsb.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Staerkel C, Boenisch MJ, Kröger C, et al. CbCTB2, an O-methyltransferase is essential for biosynthesis of the phytotoxin cercosporin and infection of sugar beet by Cercospora beticola. BMC Plant Biol. 2013;13:50. doi: 10.1186/1471-2229-13-50. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guimarães AJA, Frases SS, Pontes BB, et al. Agglutination of Histoplasma capsulatum by IgG monoclonal antibodies against Hsp60 impacts macrophage effector functions. Infect Immun. 2011;79:918–927. doi: 10.1128/IAI.00673-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zala DD, Hinckelmann MVM, Yu HH, et al. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 42.Barbosa MSM, Báo SNS, Andreotti PFP, et al. Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect Immun. 2006;74:382–389. doi: 10.1128/IAI.74.1.382-389.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodrigues ML, Franzen AJ, Nimrichter L, Miranda K. Vesicular mechanisms of traffic of fungal molecules to the extracellular space. Curr Opin Microbiol. 2013;16:414–420. doi: 10.1016/j.mib.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Gehrmann U, Qazi KR, Johansson C, et al. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses—novel mechanisms for host-microbe interactions in atopic eczema. PloS ONE. 2011;6:e21480. doi: 10.1371/journal.pone.0021480. [DOI] [PMC free article] [PubMed] [Google Scholar]


