Abstract
Background
Children with chronic conditions often experience numerous symptoms, but few research studies examine patterns of symptoms and quality of life (QoL) indicators.
Objective
To examine if reliable latent classes of children with chronic medical conditions can be identified based on the clustering of symptoms and QoL indicators.
Methods
Structured interviews were conducted with children ages 9 to 21 living with chronic medical conditions (N = 90). Multiple symptoms (e.g., pain, sleep, fatigue, and depression) and QoL indicators (e.g., life satisfaction and social support) were measured. Physical health and emotional, social, and school functioning were measured using the Pediatric Quality of Life Inventory (PedsQL). Latent class analysis was used to classify each child into a latent class whose members report similar patterns of responses.
Results
A three-class solution had the best model fit. Class 1 (high-symptom group; n = 15, 16.7%) reported the most problems with symptoms and the lowest scores on the QoL indicators. Class 2 (moderate-symptom group; n = 39, 43.3%) reported moderate levels of both symptoms and QoL indicators. Class 3 (low-symptom group; n = 36, 40.0%) reported the lowest levels of symptoms and the highest scores on the QoL indicators.
Conclusions
The three latent classes identified in this study were distributed along the severity continuum. All symptoms and QoL indicators appeared to move in the same direction (e.g. worse symptoms with lower QoL). The PedsQL psychosocial health summary score (combining emotional, social, and school functioning scores) discriminated well between children with different levels of disease burden.
Keywords: Children with chronic medical conditions, Symptoms, Quality of life, Latent class analysis
Introduction
Approximately 43% of children under 17 years of age [n = 40,833 (out of 91,640)] are estimated to have one or more chronic health conditions associated with body function, activity, or participation (e.g., depression, anxiety problems, autism spectrum disorders, developmental delay, asthma, diabetes, epilepsy or seizure disorder, or brain injury) according to the 2007 National Survey of Children's Health.1 The children with chronic health conditions often experience pain, fatigue, and emotional distress and other problems that may affect their well-being.2-7 Quality of life (QoL) has been defined as an individual's perspective on satisfaction across many realms of one's life8, and health-related QoL includes aspects of overall quality of life that are directly related to health—either physical or mental.9-11
In addition to symptoms associated with their medical conditions, children with chronic medical conditions may have poorer QoL (e.g., worse physical and psychological functioning and lower satisfaction with life) due to reduced activity levels and restrictions in participation compared to their typically developing peers.11-14 Recently, more attention has been given to the measurement of health-related QoL in children and adolescents.15-19 For example, a study with children with moderate-to-severe plaque psoriasis found that health-related QoL is an important indicator of the burden of illness, and that these children had significantly lower QoL compared to their healthy peers.20 Previous studies also reported that adolescents with spina bifida (SB) had fewer positive experiences with family, peers, and schools (e.g., less participation in social groups, less social acceptance, and lower scholastic competence and academic grades), which may be one of the reasons for higher levels of depressive symptoms.21 Pain in children with SB has also been reported to have a negative impact on their QoL.6 Eddy and Cruz (2007) found that children (mostly with cancer) often reported fatigue and, as a result, lower QoL.22 One study also examined the contribution of recurrent musculoskeletal pain and mental health problems in children with cerebral palsy (CP) and found that pain and higher levels of mental health problems were associated with the reduced ability to accomplish daily activities and fulfill social roles.23
Several pediatric QoL measures have been frequently used to measure QoL of children with chronic disease. The Child Health and Illness Profile (CHIP) has been used in population-based assessment of the association between health care and school systems with the health and well-being of children.24-26 The Child Health Questionnaire (CHQ) examines a wide variety of physical and psychosocial domains.27 The Pediatric Quality of Life Inventory (PedsQL) has been used to assess children's perceptions of QoL, including the physical, psychological, and social functioning of the child, and it has been the most widely used pediatric QoL measure.28
Most studies investigate one specific symptom or QoL indicator; few research studies consider the pattern of symptoms such as pain, fatigue, depression, and QoL indicators in children and adolescents with chronic medical conditions. The purpose of this study was to investigate the presence of distinct latent classes of young individuals with chronic medical conditions that differ on their profiles of symptoms and QoL indicators. Latent class analysis (LCA) is a statistical approach that is used to investigate the presence of distinct latent classes of individuals based on similarities and differences in response patterns – in this case, symptoms and QOL indicators - and estimate for each respondent the probability of membership in a class. We refer to classes of individuals here, rather than to groups because classes are based on unobserved variability in a latent categorical variable represented by observed symptom clusters (e.g., patterns of symptoms), while groups are based on known characteristics (e.g., gender or age). In pediatric research, for example, one study examined the patterns of the relationship between bed sharing and breastfeeding and identified 4 classes (i.e., nonsharers, early bed sharers, late bed sharers, and constant bed sharers). The predominance of breastfeeding was significantly higher among the classes of early and constant bed sharers.29 LCA has also been used in pediatric research to identify multiple subtypes of maltreatment,30 to investigate latent classes with different levels of risk for Type 2 diabetes,31 to classify patterns of eating and physical activity,32 and to identify profiles of adolescents that differentiate depressive symptoms and aggressive behavior.33
Methods
Participants
Parents of children who were seen in outpatient rehabilitation clinics at two Pacific Northwest children's hospitals within the last three years were sent a letter inviting them and their child to participate in a longitudinal study of symptoms in children with chronic medical conditions. Families interested in participation were screened by research staff using the following eligibility criteria: the child (1) was diagnosed with a chronic condition associated with pain and/or fatigue such as SB, CP, neuromuscular disease (NMD), spinal cord injury (SCI), or limb deficiency (LD) [congenital or amputation (AMP)] (2) was between the ages of 8 and 21 years; (3) was able to understand and read English; (4) was able to understand and meaningfully respond (as assessed by parent report for children under 18 and direct report by children over 18); and (5) had previously reported at least some pain and/or fatigue. In addition to rehabilitation clinics participants were also recruited from local camps for children with chronic medical conditions.
Interviewers were trained in strategies for interviewing people with speech disorders and other limitations. Response keys were used to make responding easier for children with speech problems. One hundred and thirteen children completed the baseline interview and were invited to complete additional interviews every three months for a total of five time points. Ninety completed the first three-month follow-up survey, which included relevant measures of quality of life (e.g., Children's Depression Inventory), and the current study is based on data from this survey time point (age range of 9-21). Approximately 30% of participants had mobility restrictions (used a mobility aid such as a wheelchair or walker) while moving around house and about 38% of participants had mobility restrictions while moving away from home. Parents could choose to be present during the child's interview, and this was the case for approximately 50% of the baseline interviews. However, we requested that parents not assist or contribute to their child's interview. The interview typically lasted about 30-45 minutes, and participants received $25 for completing each survey with an additional $25 bonus for completing all five surveys.
Children 18 years and older signed a consent form that described the study and the risks and benefits of participating. Participants younger than 18 years were asked to sign an assent form that described the study in age-appropriate language (one form was used for children aged 8–13 and a second for children aged 14–17), with parental consent required for participation. The study protocol was approved by the University of Washington Institutional Review Board, the Washington State University Institutional Review Board, and the institutional review boards of participating children's hospitals.
Data Collection
Interviews were conducted in person or over the telephone by trained research staff. A parent, guardian, or camp counselor was present if the child or adolescent participant so desired. Validated measures of pain, fatigue, social support, emotional health, and physical function were administered, including items developed as part of the National Institutes of Health Patient Reported Outcomes Measurement Information System Project (www.nihpromis.org).34,35
The presence of chronic pain was assessed with the following question: In the last three months have you had any pain that bothered you? The PedsQL Multidimensional Fatigue Scale (MFS);36,37 was used to assess fatigue. The 18-item MFS is composed of three subscales: general fatigue (6 items), sleep/rest fatigue (6 items), and cognitive fatigue (6 items). Higher scores on MFS indicate fewer fatigue symptoms. Good internal consistency has been reported for the MFS (alpha = 0.9).36
Depression was measured by the Children's Depression Inventory Short Version (CDI),10 which consists of 10 items. The CDI scores range from 0 to 20, with higher scores indicating more depressive symptoms. The raw score is converted to a T-score, which can be compared to scores of children of the same age range and gender. A T-score of 65 and above (1.5 SD above the mean) indicates clinically significant depressive symptoms.38 Various raw cut-off scores have also been used for CDI full and short versions.39 For this study, a raw total score ≥8 for both girls and boys age 7-12 years and a raw score ≥9 for boys age 13 and older were considered to be indicative of clinically significant depression, in accordance with the CDI technical manual.38 The CDI provides scores that are comparable to the longer 27-item version of the CDI (r = 0.9) and demonstrates an acceptable alpha reliability coefficient (alpha = 0.8).10
Physical health and emotional, social, and school functioning were assessed with the 23-item PedsQL subscales40 with response options expanded to include ‘unable to do’ to ensure appropriate response options for all respondents, but the scores were derived using the published scoring instructions. There is a distinct subscale for physical health (i.e. Physical Health Summary score). The Psychosocial Health Summary score is a mean of the emotional functioning, social functioning, and school functioning subscale scores. Scores on each subscale range from 0 to 100 with higher scores indicating better QoL. Good internal consistency has been demonstrated for the Physical Health Summary Score (alpha = 0.8) and the Psychosocial Health Summary Score (alpha = 0.8), and scores are moderately correlated with reports of morbidity and illness burden.37 The PedsQL is a user-friendly instrument often used in clinical or research settings. 41
Social support was assessed using the 12-item Multi-dimensional Scale of Perceived Social Support (MSPSS).42,43 The MSPSS assesses social support across three relevant domains of family, friends, and significant others, and higher scores represent greater perceived social support. The MSPSS was shown to have good internal (0.85-0.91)43 and test-retest reliability (0.72-0.85) as well as moderate construct validity.42,44,45 Higher MSPSS scores are associated with lower levels of depression and anxiety as measured by the Hopkins Symptom Checklist (HSCL).46 The response options range from 1=very strongly disagree to 7=very strongly agree. This response set was found problematic in our previous studies with adults, so we modified the response options to a 5-point scale (never to almost always) that are preferred and better understood by children and adults.2, 47 The revised response worked well and made the MSPSS more age-appropriate, as children had no difficulties using the revised responses options. For instance, in responses to questions such as “I can talk about my problems with my friends” and “My family really tries to help me,” children readily picked a response from the response set of “never true” to “almost always true”. Although the MSPSS was not specifically developed for the youth population, it has been successfully used in adolescents (with the original response categories).48,49
Children completed the 6-item Brief Multidimensional Student's Life Satisfaction Scale (BMSLSS)50 to assess perceived QoL. Scores can range from 0 to 24, and higher scores indicate better life satisfaction. The measure demonstrated adequate internal consistency (alpha = 0.8) and was strongly correlated with two other life satisfaction measures: the Multidimensional Students' Life Satisfaction Scale total score (r = 0.7) and the Students' Life Satisfaction Scale total score (r = 0.6).50
Sleep problems were assessed by the 26-item Children's Sleep Habits Questionnaire child self-report version.51 The CSHQ is comprised of three subscales (Bedtime, Sleep behavior, and Daytime sleepiness) and an overall sleep functioning score. A higher score indicates more sleep problems. The CSHQ showed adequate internal consistency for both a community sample of children with no sleep disorders (alpha = 0.7) and a clinical sample of children being evaluated for a sleep disorder (alpha = 0.8). Test-retest reliability was acceptable (range 0.6 to 0.8), and CSHQ scores were consistently higher for the clinical sample as compared with the community sample, supporting the validity of the CSHQ as a measure of children's sleep habits.51
In addition to clinical and demographic items, participants responded to the Wide Range Achievement Test 3–Reading Subtest (WRAT–3)52 as a proxy for literacy level. Assessment of literacy level is important in survey research and reading ability is a stronger and independent predictor of health outcomes than, for instance, years of education.53 The WRAT is a commonly used brief test of literacy in adult and child populations.54 It contains a reading subtest that is used to test reading ability independent of reading comprehension. The WRAT 3 reading subtest is provided in the “blue” and “tan” parallel forms. Each version contains 42 different words. According to the WRAT-3 Administration Manual, individuals are asked to pronounce the list of words in ascending order of difficulty and are instructed to stop once 10 consecutive pronunciation errors have been made. Both subtests also have 15 letters to be read aloud if the participant is not able to pronounce at least five words correctly prior to reaching the test discontinue point of 10 consecutive words mispronounced. Raw scores are based on a participant's overall response; final standardized scores are scaled on the norm to correspond to median performance for specific age groups.52 Each age group has a scale mean of 100 and a standard deviation of 15; higher scores indicate better literacy.
After different classes were identified by the results of the LCA analyses, descriptive statistics of the average and worst pain intensity were calculated for each latent class separately for variables that were not included in the LCA model. These variables included pain questions as follows: (1) “In the past week, how bad was your worst pain?” (no pain [0] to very bad pain [3]); (2) “On a typical day, how much do you hurt?” (not at all [0] to a lot [3]). Descriptive statistics were also computed for the children's demographic characteristics, primary conditions across latent classes and PedsQL psychosocial health summary score, a composite of scores on emotional health, socializing, and school functioning were also computed by each class.
Statistical Analyses
LCA groups individuals into different latent classes based on unobserved variability in a latent categorical variable reflected in patterns or clusters of symptoms or characteristics. Individuals within a particular class share homogeneous characteristics and demonstrate heterogeneous characteristics across different classes.29 The important difference between the LCA and the analyses that compare known groups is that LCA defines the classes empirically, from the data, rather than using a measured variable (such as gender or age). LCA has been used extensively in health research.55-58
How well individuals have been classified into each latent group is evaluated by a statistic called entropy. Entropy has a range of 0 to 1, with higher values of entropy indicating a better classification of individuals. Entropy of .8 or greater indicates good classification.59 Identifying the model with the optimal number of latent classes is guided by comparative measures of model fit that include Bayesian information criteria (BIC),60,61 but interpretability of the latent classes is equally important when selecting a final model. The goal of the current study was to use LCA to identify distinct classes of children and adolescents with chronic medical conditions based on the patterns of symptoms and QoL indicators. Accordingly, the number of latent classes was guided by both BIC indices of comparative fit and entropy measures. Descriptive statistics were also calculated for the children's demographic characteristics and primary conditions. Subsequent exploratory analyses included analysis of variance to assess statistical significance of the differences between the latent classes on demographics and other characteristics that were not included in the statistical model (i.e. not used to derive the latent classes). Data preparation and descriptive summaries were carried out using SAS62 and SPSS63 and LCA was carried out using Mplus.64
Results
Participants
Table 1 summarizes the demographic characteristics of the sample. The average age of the sample was 14.8 (SD = 3.2; range = 9.4 – 21). Most of the children with chronic medical conditions were white (76.7%), and females (53.3%) slightly outnumbered males. The average standardized WRAT score was 97.4 (SD = 21.6), which was slightly lower than the mean of 100 for the general population. Parental education data were available for 59 of the 90 children participants (66%), as parental participation was voluntary. The majority of the parents who responded to the questionnaire were college graduates (51%). Table 1 also summarizes the primary medical condition or diagnosis of the children for the current study. CP (24.8%) and SB (17.8%) were more highly represented than other conditions. Nearly one-third (27.7%) of the participants, however, reported more than one condition (total endorsements: N = 101) such as diabetes, bone tumor, scoliosis, asthma, or Guillain-Barre Syndrome.
Table 1. Demographic and Clinical Characteristics of Sample.
| Mean (SD) | |
|---|---|
| Age | 14.8 (3.2) |
| Standardized WRAT scorea | 97.4 (21.6) |
| n (%) | |
|
| |
| Female | 48 (53.3%) |
| Non-Hispanic Whiteb | 69 (76.7%) |
| Education (parents)c | |
| High school degree/GED | 4 (6.8%) |
| Some college/technical degree/AA | 25 (42.4%) |
| College / advanced degree | 30 (50.9%) |
| Primary conditionsd | |
| Spina bifida | 18 (17.8%) |
| Cerebral palsy | 25 (24.8%) |
| Neuromuscular disease | 14 (13.9%) |
| Congenital limb deficiency | 6 (5.9%) |
| Amputation | 6 (5.9%) |
| Spinal cord injury | 4 (4.0%) |
| Other conditions | 28 (27.7%) |
Note.
The calculation is based on 79 participants.
The calculation is based on 82 participants.
The calculation is based on 59 parents.
Participants could endorse more than one condition and calculations are based on the total endorsement (total N =101).
WRAT = Wide Range Achievement Test
GED = General Educational Development
AA = Associate of Arts
While the child was being interviewed, parents were asked to complete a separate questionnaire about their child. The paired t tests compared the responses of children and parent Based on t tests, we did not see any difference in baseline mean scores of children and their parents for measures of friend and family support, school functioning, and depression (family support: t = -0.32, df = 96, p > 0.05; friend support: t = 0.44, df = 93, p > 0.05; school functioning: t = 1.36, df = 93, p > 0.05; and depression: t = -0.77, df = 59, p > 0.05).
Table 2 presents correlations between symptoms and QoL Indicators included in the latent class analysis. Correlations ranged in magnitude from 0.2 (physical health and social support) to 0.7 (fatigue and sleep problems, fatigue and school functioning, physical health and social functioning). The majority of correlations observed were moderate in magnitude, and all correlations were in the expected directions.
Table 2. Correlation among Symptom and Quality of Life Indicators for Latent Class Model.
| Measure | Sleep problems | Fatigue | Social support | Physical health | Emotional functioning | Social functioning | School functioning | Student's life satisfaction |
|---|---|---|---|---|---|---|---|---|
| Sleep problems | ||||||||
| Fatigue | -0.7 | |||||||
| Social support | -0.3 | 0.4 | ||||||
| Physical health | -0.6 | 0.6 | 0.2 | |||||
| Emotional functioning | -0.6 | 0.6 | 0.4 | 0.6 | ||||
| Social functioning | -0.6 | 0.6 | 0.4 | 0.7 | 0.6 | |||
| School functioning | -0.5 | 0.7 | 0.3 | 0.6 | 0.6 | 0.5 | ||
| Student's life satisfaction | -0.6 | 0.5 | 0.3 | 0.4 | 0.4 | 0.5 | 0.3 | |
| Depression | 0.3 | -0.5 | -0.5 | -0.3 | -0.6 | -0.5 | -0.3 | -0.5 |
Latent Class Analysis
Multiple latent class models were constructed with 1 through 6 latent classes, and BIC comparative model fit criteria suggested that the 3 or 4 class solutions were optimal. (Table 3) The three class solution was selected as the best model because the 4, 5, and 6 class solutions included a class comprised of a single participant. Entropy statistics for each model were all > 0.90 suggesting more than adequate classification.
Table 3. Latent Class Model Fit Criteria.
| Number of classes | BIC | Entropy |
|---|---|---|
| 1 | 5765.3 | - |
| 2 | 5533.1 | 0.91 |
| 3 | 5479.8 | 0.92 |
| 4 | 5478.4 | 0.94 |
| 5 | 5488.8 | 0.95 |
| 6 | 5494.2 | 0.95 |
Table 4 summarizes the symptoms and QoL indices used to derive the latent classes as well as the demographic and clinical descriptors of each latent class. As expected due to the study eligibility criteria, all three classes reported substantial chronic pain in the last three months. Class 1 (n =15), the high-symptom group, had an average age of 14.4 (SD = 3.3) and was predominantly female (60%) and white (76.9%). The average standardized WRAT score was 96.1 (SD = 18.0). SB and NMD were the most frequently endorsed single conditions in this class (23.5%). This group reported the most problems with symptoms (i.e., sleep, fatigue, and depression) as measured by the children's sleep habits, MFS, and CDI. 26.7% of participants were identified with clinically significant depression based on CDI cutoff criterion. 80% of participants reported bothersome pain in the last three months. Average scores on the QoL indicators such as social support and satisfaction with life were the lowest of all three classes. This class also reported the highest mean scores on two pain items (i.e., “How bad was your worst pain?” and “On a typical day, how much do you hurt?”) and had the lowest average psychosocial health summary scores.
Table 4. Symptom and Quality of Life Indicators and Demographic Descriptors of Latent Classes.
| Latent Class 1 (High-symptom) |
Latent Class 2 (Moderate-symptom) |
Latent Class 3 (Low-symptom) |
|
|---|---|---|---|
|
| |||
| n= 15 (16.7%) | n = 39 (43.3%) | n = 36 (40.0%) | |
|
| |||
| Symptom and Quality of life indicators for latent class model | |||
|
|
|||
| Mean (SE) | Mean (SE) | Mean (SE) | |
|
|
|||
| Sleep problems | 42.7 (1.4) | 37.2 (0.9) | 30.4 (0.7) |
| Fatigue | 44.1 (3.3) | 60.5 (2.5) | 83.1 (2.0) |
| Social support | 3.0 (0.1) | 3.3 (0.1) | 3.7 (0.1) |
| Physical health | 34.9 (6.5) | 61.1 (2.9) | 83.6 (3.3) |
| Emotional functioning | 33.4 (4.2) | 59.6 (3.3) | 82.7 (2.7) |
| Social functioning | 35.4 (4.2) | 62.8 (2.6) | 86.3 (3.6) |
| School functioning | 42.7 (4.7) | 58.8 (3.1) | 82.1 (2.7) |
| Student's life satisfaction | 17.2 (0.9) | 19.4 (0.6) | 22.8 (0.4) |
| Depression | 6.1 (1.0) | 2.1 (0.3) | 0.80 (0.2) |
|
|
|||
| n (%) | n (%) | n (%) | |
|
|
|||
| Clinically significant depression (from depression scores) | 4 (26.7%) | 1 (2.6%) | 0 (0%) |
| Bothersome pain (In the last three months, have you had any pain that bothered you?) | 12 (80%) | 36 (92.3%) | 27 (75%) |
| Demographic and clinical descriptors of latent classes | |||
|
|
|||
| Mean (SD) | Mean (SD) | Mean (SD) | |
|
|
|||
| Age (SD) | 14.4 (3.3) | 15.1 (3.0) | 14.8 (3.5) |
| Standardized WRAT score (SD)a | 96.1 (18.0) | 95.7 (24.0) | 100.0 (20.9) |
| How bad was your worst pain?b | 1.8 (0.9) | 1.2 (0.7) | 1.2 (0.9) |
| On a typical day, how much do you hurt?c | 1.3 (1.0) | 1.0 (0.6) | 0.7 (0.7) |
| PedsQL psychosocial health summary score | 36.8 (11.8) | 60.2 (8.2) | 83.5 (8.5) |
|
|
|||
| n (%) | n (%) | n (%) | |
|
|
|||
| Female | 9 (60.0%) | 19 (48.7%) | 20 (55.6%) |
| Non-Hispanic Whited | 10 (76.9%) | 31 (86.1)% | 28 (84.8%) |
| Education (parents)e | n = 10 | n = 25 | n = 24 |
| High school degree/GED | 0 (0.0%) | 2 (8.0%) | 2 (8.3%) |
| Some college/technical degree/AA | 3 (30.0%) | 21(44.0%) | 11 (45.8%) |
| College / advanced degree | 7 (70.0%) | 2 (48.0%) | 11 (45.8%) |
| Primary conditionsf | |||
| Spina bifida | 4 (23.5%) | 9 (20.0%) | 5 (12.8%) |
| Cerebral palsy | 2 (11.8%) | 9 (20.0%) | 14 (35.9%) |
| Neuromuscular disease | 4 (23.5%) | 7 (15.6%) | 3 (7.7%) |
| Congenital limb deficiency | 0 (0.0%) | 2 (4.4%) | 4 (10.3%) |
| Amputation-upper extremity | 0 (0.0%) | 5 (11.1%) | 1 (2.6%) |
| Spinal cord injury | 1 (5.9%) | 2 (4.4%) | 1 (2.6%) |
| Other conditions | 6 (35.3%) | 11 (24.4%) | 11 (28.2%) |
Note.
Calculations are based on 15 participants for class 1, 34 for class 2, and 30 for class 3.
Calculations are based on 12 participants for class 1, 36 for class 2, and 26 for class 3.
Calculations are based on 12 participants for class 1, 36 for class 2, and 27 for class 3.
Calculations are based on 13 participants for class 1, 36 for class 2, and 33 for class 3.
Calculations are based on 10 participants for class 1, 25 for class 2, and 24 for class 3.
Participants could endorse more than one condition.
Calculations are based on the total endorsement (total N = 101; class 1: n = 17; class 2: n = 45; class 3: n = 39).
SE = Standard error; SD = Standard deviation; WRAT = Wide Range Achievement Test; GED = General Educational Development; AA = Associate of Arts.
Class 2, the moderate-symptom group, had an average age of 15.1 (SD = 3.0), was predominantly white (86.1%), and was nearly evenly distributed with respect to gender (48.7% female). The average standardized WRAT score was 95.7 (SD = 24.0), and 36 (92.3%) participants endorsed the item indicating bothersome pain in the last three months. Only one (2.6%) participant was identified with clinically significant depression based on CDI cutoff criterion. SB and CP were most commonly endorsed primary conditions (20.0%). 92.3% of participants reported bothersome pain in the last three months. Members of this class reported moderate levels of most of symptoms and QoL indicators.
Class 3, the low-symptom group, had an average age of 14.8 (SD = 3.5), was predominantly white (84.8%), and the majority were female (55.6%). The average standardized WRAT score in this class was 100.0 (SD = 20.9). This class included the highest proportion of CP among diagnostic conditions (35.9%) and reported the lowest levels of fatigue, sleep, and depression. 75% of participants reported bothersome pain in the last three months. In addition, this class reported the highest average scores on life satisfaction, social support, and physical health, as well as emotional, social, and school functioning measured by the MSPSS, SWLS, BMSLSS, and PedsQL. No one was identified with clinically significant depression. 75% of participants reported bothersome pain in the last three months. This class also reported the lowest mean scores on two pain items. Class 3 also had the highest average score on PedsQL psychosocial health summary and the lowest averages on two pain items.
Figure 1 illustrates the distribution of scores on the PedsQL indicators, which are on the same scale (i.e., one summary score and four subscales). Lower scores represent more problems with emotional, social, and school functioning and physical health. As clearly shown, Class 3 had the highest scores on all of the PedsQL indicators, whereas Class 1 had the lowest scores.
Figure 1. Averages of PedsQL Indicators across Latent Classes.
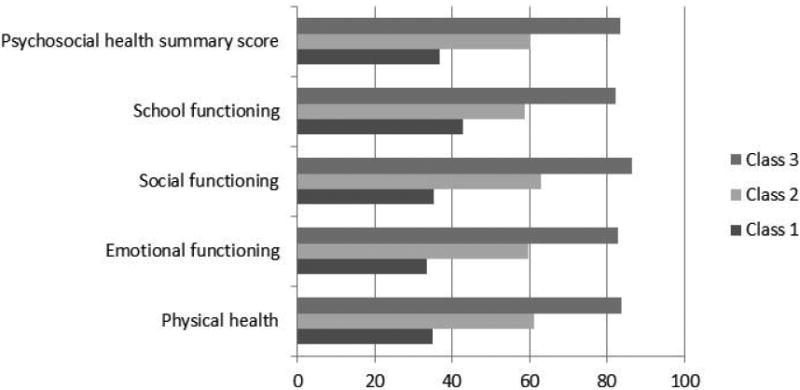
Between class differences
While the classes were similar on age, there was some separation among the classes on gender (high symptom class had a higher proportion of females), ethnicity (the moderate symptom class had the highest proportion of white children), and literacy (the low symptom class had the highest mean reading score), although these differences were not statistically significant. The presence of chronic pain was the only symptom that was higher for the moderate-symptom class than for the high-symptom class, but the ratings of worst and average pain followed the pattern of the variables included in the model (i.e., the highest level of pain was reported by the high-symptom class and the lowest by the low-symptom class). This suggests that pain intensity is distributed similarly to the other symptoms and QoL indicators. Average pain intensity was statistically significantly different between the high and low symptom classes, but was not different between the high and moderate and moderate and low symptom classes. The three latent classes were statistically significantly different on the PedsQL psychosocial functioning summary score with mean scores of 84, 60, and 37 (F = 156.3, df = 89, p < 0.001).
Discussion
The LCA results identified three latent classes that differed according to the level of symptoms and QoL indicators. The classes separated along the continuum of disease burden with all the symptoms and QoL indicators moving in the same direction. The higher the level of symptoms (such as sleep problems and fatigue) children report, the lower the social support, social and school functioning, and student's life satisfaction.
One of the limitations of the current study is potential bias from recall and self-report. Other limitations also include small sample size in the overall sample and, by extension, in the latent classes. Because questionnaires were voluntary, the information about parent education is incomplete. In addition, the sample was composed of the children with many different chronic medical conditions, and that makes it difficult to generalize findings to specific diagnostic groups. Future studies are needed to replicate this analysis with larger samples of children with greater racial/ethnic and socioeconomic diversity both within and across chronic medical conditions before generalizing these findings to the broader population of children with chronic medical conditions.
The PedsQL is the most widely used QoL instrument65,66 used in studies with children with migraine,67 cancer-related pain and emotional distress,40 juvenile idiopathic arthritis68 as well as cardiology, orthopedic, and rheumatology outpatient settings.69 Additional research is always warranted with empirically derived classes, but in our study this PedsQL seems the best predictor of class membership. These preliminary results suggest that a PedsQL psychosocial health summary score under 40 likely indicates a child with high symptoms and low QoL, a score between 40 and 70 indicates moderate symptoms and QoL, and the score over 70 suggests low symptoms and higher QoL. Our results are similar to Varni at al. (2003)70 in that the score that best discriminates between moderate and low symptoms is between 40 and 70 (i.e., child self-report score of 66.03 and parent proxy-report score of 64.38). The result of our study added a cut-off point of 40 that indicates a child with high symptoms and low QoL. This finding is important since it is often not practical to administer a large battery of test to determine classes. Thus, this indicator can be used to predict class membership in clinical practice or future research.
Acknowledgments
The project described was funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant 5U01AR052171-03 to University of Washington (Amtmann, PI). Information on the “Dynamic Assessment of Patient-Reported Chronic Disease Outcomes” can be found at http://nihroadmap.nih.gov/clinicalresearch/index.asp
Footnotes
This research has not been presented at any meeting.
The authors have no financial interests to disclose directly or indirectly related to the research in the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jiseon Kim, Email: jiseonk@u.washington.edu, Department of Rehabilitation Medicine, University of Washington, Box 359612, Seattle, WA 98104 USA, Tel: 512-299-5991, Fax: 206-685-9224.
Hyewon Chung, Department of Education, College of Education, Chungnam National University (CNU), 99 Daehangno, Yuseong-gu, Daejeon, South Korea, 305-764.
Dagmar Amtmann, Department of Rehabilitation Medicine, University of Washington, Box 354237, Seattle, WA 98195 USA.
Rana Salem, Department of Rehabilitation Medicine, University of Washington, Box 354237, Seattle, WA 98195 USA.
Ryoungsun Park, Educational Psychology, University of Texas at Austin, 1 University Station D5800, Austin, TX 78712 USA.
Robert L. Askew, Department of Rehabilitation Medicine, University of Washington, Box 354237, Seattle, WA 98195 USA.
References
- 1.National Survey of Children's Health. NSCH 2007. [Accessed June 14, 2013];Data query from the Child and Adolescent Health Measurement Initiative, Data Resource Center for Child and Adolescent Health website. www.childhealthdata.org.
- 2.Eddy L, Khastou L, Cook KF, Amtmann D. Item selection in self-report measures for children and adolescents with disabilities: Lessons from cognitive interviews. Journal of Pediatric Nursing. 2011;26:559–65. doi: 10.1016/j.pedn.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engel JM, Jensen MP, Schwartz L. Coping with chronic pain associated with cerebral palsy. Occupational Therapy International. 2006;13:224–33. doi: 10.1002/oti.219. [DOI] [PubMed] [Google Scholar]
- 4.Engel JM, Kartin D, Jaffe KM. Exploring chronic pain in youths with Duchenne Muscular Dystrophy: A model for pediatric neuromuscular disease. Physical Medicine and Rehabilitation Clinics of North America. 2005;16:1113–24. doi: 10.1016/j.pmr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Jan FK, Wilson PE. A survey of chronic pain in the pediatric spinal cord injury population. Journal of Spinal Cord Medicine. 2004;27:S50–3. doi: 10.1080/10790268.2004.11753785. [DOI] [PubMed] [Google Scholar]
- 6.Oddson BE, Clancy CA, McGrath PJ. The role of pain in reduced quality of life and depressive symptomology in children with spina bifida. The Clinical Journal of Pain. 2006;22:784–9. doi: 10.1097/01.ajp.0000210929.43192.5d. [DOI] [PubMed] [Google Scholar]
- 7.Wilkins KL, McGrath PJ, Finley GA, Katz J. Prospective diary study of nonpainful and painful phantom sensations in a preselected sample of child and adolescent amputees reporting phantom limbs. The Clinical Journal of Pain. 2004;20:293–301. doi: 10.1097/00002508-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bjornson K, McLaughlin J. The measurement of health-related quality of life (HRQOL) in children with cerebral palsy. European Journal of Neurology. 2006;8:183–93. doi: 10.1046/j.1468-1331.2001.00051.x. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Measuring healthy days: Population assessment of health-related quality of life. Atlanta, GA: Centers for Disease Control and Prevention; 2000. [Google Scholar]
- 10.Kovaks M, Beck AT. An empirical-clinical approach toward a definition of childhood depression. In: Schultenbrandt JG, Raskin A, editors. Depression in childhood: Diagnosis, treatment, and conceptual models. New York: Raven Press; 1977. [Google Scholar]
- 11.Vetter TR. The epidemiology of pediatric chronic pain. In: McClain BCC, Suresh S, editors. Handbook of pediatric chronic pain: current science and integrative practice (perspectives on pain in psychology) New York: Springer; 2011. [Google Scholar]
- 12.Tuzun EH, Eker L, Daskapan A. An assessment of the impact of cerebral palsy on children's quality of life. Fizyoterapi Rehabilitasyon. 2004;15:3–8. [Google Scholar]
- 13.Vargus-Adams J. Health-related quality of life in childhood cerebral palsy. Archives of Physical Medical and Rehabilitation. 2005;86:940–5. doi: 10.1016/j.apmr.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 14.Varni J, Burwinkle T, Sherman S, et al. Health related quality of life of children and adolescents with cerebral palsy: Hearing the voices of the children. Developmental Medicine and Child Neurology. 2005;47:592–7. [PubMed] [Google Scholar]
- 15.Clarke SA, Eiser C. The measurement of health-related quality of life (QOL) in paediatric clinical trials: A systematic review. Health and Quality of Life Outcomes. 2004;2:66. doi: 10.1186/1477-7525-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cremeens J, Eiser C, Blades M. Characteristics of health-related self-report measures for children aged three to eight years: A review of the literature. Quality of Life Research. 2006;15:739–54. doi: 10.1007/s11136-005-4184-x. [DOI] [PubMed] [Google Scholar]
- 17.Davis E, Waters E, Mackinnon A, et al. Paediatic quality of life instruments: A review of the impact of the conceptual framework on outcomes. Developmental Medicine and Child Neurology. 1996;48:311–8. doi: 10.1017/S0012162206000673. [DOI] [PubMed] [Google Scholar]
- 18.Eiser C, Morse R. The measurement of quality of life in children: Past and future perspectives. Journal of Developmental and Behavioral Pediatrics. 2001;22:248–56. doi: 10.1097/00004703-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Matza LS, Swensen AR, Flood EM, Secnik K, Leidy NK. Assessment of health-related quality of life in children: A review of conceptual, methodological, and regulatory issues. Value in Health. 2004;7:79–92. doi: 10.1111/j.1524-4733.2004.71273.x. [DOI] [PubMed] [Google Scholar]
- 20.Varni JW, Globe DR, Gandra SR, et al. Health-related quality of life of pediatric patients with moderate to severe plaque psoriasis: comparisons to four common chronic diseases. European Journal of Pediatric. 2012;171:1587–2. doi: 10.1007/s00431-011-1587-2. [DOI] [PubMed] [Google Scholar]
- 21.Essner BS, Grayson NH. The impact of family, peer, and school contexts on depressive symptoms in adolescents with spina bifida. Rehabilitation Psychology. 2010;55:340–50. doi: 10.1037/a0021664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eddy L, Cruz M. The relationship between fatigue and quality of life in children with special needs: a systematic review. Journal for Specialist in Pediatric Nursing. 2007;12:105–14. doi: 10.1111/j.1744-6155.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- 23.Ramstad K, Jahnsen R, Skjeldal OH, Diseth TH. Parent-reported participation in children with cerebral palsy: the contribution of recurrent musculoskeletal pain and child mental health problems. Developmental Medicine and Child Neurology. 2012;54:829–35. doi: 10.1111/j.1469-8749.2012.04341.x. [DOI] [PubMed] [Google Scholar]
- 24.Riley AW, Forrest CB, Starfield B, et al. Reliability and validity of the adolescent health profile-types. Medical Care. 1998;36:1237–48. doi: 10.1097/00005650-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Riley AW, Forrest CB, Rebok GW, et al. The child report form of the CHIP-child edition: Reliability and validity. Medical Care. 2004;42:221–31. doi: 10.1097/01.mlr.0000114910.46921.73. [DOI] [PubMed] [Google Scholar]
- 26.Riley AW, Forrest CB, Starfield B, et al. The parent report form of the CHIP-child edition: Reliability and validity. Medical Care. 2004;42:210–20. doi: 10.1097/01.mlr.0000114909.33878.ca. [DOI] [PubMed] [Google Scholar]
- 27.Landgraf JM, Abetz L, Ware JE. The CHQ: A user's manual. Boston: HealthAct; 1999. [Google Scholar]
- 28.Vetter TR. A primer on health-related quality of life in chronic pain medicine. Anesthesia and Analgesia. 2007;104:703–18. doi: 10.1213/01.ane.0000255290.64837.61. [DOI] [PubMed] [Google Scholar]
- 29.Blair PS, Heron J, Fleming PJ. Relationship between bed sharing and breastfeeding: longitudinal, population-based analysis. Pediatrics. 2010;126:e1119–26. doi: 10.1542/peds.2010-1277. [DOI] [PubMed] [Google Scholar]
- 30.Petrenko CL, Friend A, Garrido EF, Taussig HN, Culhane SE. Does subtype matter? Assessing the effects of maltreatment on functioning in preadolescent youth in out-of-home care. Child Abuse & Neglect. 2012;36:633–44. doi: 10.1016/j.chiabu.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greig F, Hyman S, Wallach E, Hildebrandt T, Rappaport R. Which obese youth are at increased risk for type 2 diabetes? Latent class analysis and comparison with diabetic youth. Pediatric Diabetes. 2011;13:181–8. doi: 10.1111/j.1399-5448.2011.00792.x. [DOI] [PubMed] [Google Scholar]
- 32.Huh J, Riggs NR, Spruijt-Metz D, et al. Identifying patterns of eating and physical activity in children: a latent class analysis of obesity risk. Obesity (Silver Spring) 2010;19:652–8. doi: 10.1038/oby.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copeland-Linder N, Lambert SF, Ialongo NS. Community violence, protective factors, and adolescent mental health: a profile analysis. Journal of Clinical Child and Adolescent Psychology. 2010;39:176–86. doi: 10.1080/15374410903532601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irwin DE, Stucky BD, Thissen D, et al. Sampling plan and patient characteristics of the PROMIS pediatrics large-scale survey. Quality of Life Research. 2010;19:585–94. doi: 10.1007/s11136-010-9618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varni JW, Stucky BD, Thissen D, et al. PROMIS pediatric pain interference scale: An item response theory analysis of the pediatric pain item bank. Journal of Pain. 2010;11:1109–19. doi: 10.1016/j.jpain.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varni JW, Burwinkle TM, Szer IS. The PedsQL Multideminsional Fatigue Scale in pediatric rheumatology: reliability and validity. Journal of Rheumatology. 2004;31:2494–500. [PubMed] [Google Scholar]
- 37.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL™ in pediatric cancer: Reliability and validity of the Pediatric Quality of Life Inventory™ Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94:2090–106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 38.Kovaks M, Multi-Health Systems, Inc. Staff . Technical Manual Update. North Tonawanda, NY: Multi-Health Systems; 2003. Children's Depression Inventory. [Google Scholar]
- 39.Allgaier AK, Frühe B, Pietsch K, et al. Is the Children's Depression Inventory Short version a valid screening tool in pediatric care? A comparison to its full-length version. Journal of Psychosom Research. 2012;73:369–74. doi: 10.1016/j.jpsychores.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Varni JW, Burwinkle TM, Katz ER. The PedsQL in pediatric cancer pain. A prospective longitudinal analysis of pain and emotional distress. Journal of Developmental and Behavioral Pediatrics. 2004;25:239–46. doi: 10.1097/00004703-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Vetter TR. A clinical profile of a cohort of patients referred to an anesthesiology-based pediatric chronic pain medicine program. Anesthesia and Analgesia. 2008;106:786–94. doi: 10.1213/ane.0b013e3181609483. [DOI] [PubMed] [Google Scholar]
- 42.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment. 1988;52:30–41. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 43.Dahlem NW, Zimet GD, Walker RR. The Multidimensional Scale of Perceived Social Support - a Confirmation Study. Journal of Clinical Psychology. 1991;47:756–61. doi: 10.1002/1097-4679(199111)47:6<756::aid-jclp2270470605>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 44.Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment. 1990;55:610–7. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 45.Blumenthal JA, Burg MM, Barefoot J, et al. Social support, type A behavior, and coronary artery disease. Psychosomatic Medicine. 1987;49:331–40. doi: 10.1097/00006842-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): A self-report symptom inventory. Behavioral Science. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 47.Amtmann D, Cook KF, Johnson KL, Cella D. The PROMIS initiative: Examples of applications in rehabiliation. Archives of Physical Medicine Rehabilitation. 2011;10(Suppl):S12–19. doi: 10.1016/j.apmr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canty-Mitchell J, Zimet GD. Psychometric properties of the Multidimensional Scale of Perceived Social Support in urban adolescents. American Journal of Community Psychology. 2000;28:391–400. doi: 10.1023/A:1005109522457. [DOI] [PubMed] [Google Scholar]
- 49.Bruwer B, Emsley R, Kidd M, Lochner C. Psychometric properties of the Multidimensional Scale of Perceived Social Support in youth. Comparative Psychiatry. 2008;49:195–201. doi: 10.1016/j.comppsych.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Seligson JL, Huebner ES, Valois RF. Preliminary validation of the Brief Multidimensional Students' Life Satisfaction Scale (BMSLSS) Social Indicators Research. 2003;61:121–45. [Google Scholar]
- 51.Owens J, Nobile C, McGuinn M, Spirito A. The Children's Sleep Habits Questionnaire: Construction and validation of a sleep survey for school-aged children. Sleep. 2000;23:1043–51. [PubMed] [Google Scholar]
- 52.Wilkinson GS. Wide range achievement test 3 administration manual. Lutz, FL: Psychological Assessment Resource; 1993. WRAT-3. [Google Scholar]
- 53.Dewalt DA, Berkman ND, Sheridan S, et al. Literacy and health outcomes: a systematic review of the literature. Journal of General Internal Medicine. 2004;19:1228–39. doi: 10.1111/j.1525-1497.2004.40153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parker G. WRAT-3: Wide Range Assessment Test. Journal of Occupational Psychology, Employment and Disability. 2002;5:61–6. [Google Scholar]
- 55.Zwemmer JNP, Berkhof J, Castelijns JA, et al. Classification of multiple sclerosis patients by latent class analysis of magnetic resonance imaging characteristics. Multiple Sclerosis. 2006;12:565–72. doi: 10.1177/1352458506070759. [DOI] [PubMed] [Google Scholar]
- 56.Neumann M, Wirtz M, Ernstmann N, et al. Identifying and predicting subgroups of information needs among cancer patients: an initial study using latent class analysis. Supportive Care in Cancer. 2011;19:1197–209. doi: 10.1007/s00520-010-0939-1. [DOI] [PubMed] [Google Scholar]
- 57.Soothill K, Francis B, Awwad F, et al. Grouping Cancer Patients by Psychosocial Needs. Journal of Psychosocial Oncology. 2004;22:90–109. [Google Scholar]
- 58.Joshua MT, Carolyn TT, Korey AK, et al. Patterns of perceived barriers to medical care in older adults: a latent class analysis. BMC Health Services Research. 2011;11:181. doi: 10.1186/1472-6963-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification. 1996;13:195–212. [Google Scholar]
- 60.Karen L, Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–69. [Google Scholar]
- 61.Clark SL, Muthén B. [Accessed November 15, 2012];Relating latent class analysis results to variables not included in the analysis. http://statmodel2.com/download/relatinglca.pdf.
- 62.SAS Institute Inc. SAS 9.3. Help and Documentation. Cary, NC: SAS institute Inc; 2012–2013. [Google Scholar]
- 63.SPSS Inc. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc; 2009. [Google Scholar]
- 64.Muthén LK, Muthén BO. Mplus User's Guide. Sixth. Los Angeles, CA: Muthén & Muthén; 1998-2010. [Google Scholar]
- 65.Eiser C, Morse R. Quality-of-life measures in chronic diseases of childhood. Health Technol Assess. 2001;5:1–157. doi: 10.3310/hta5040. [DOI] [PubMed] [Google Scholar]
- 66.Eiser C, Vance Y, Horne B, Glaser A, Galvin H. The value of the PedsQL™ in assessing quality of life in survivors of childhood cancer. Child: Care, Health, and Development. 2003;29:95–102. doi: 10.1046/j.1365-2214.2003.00318.x. [DOI] [PubMed] [Google Scholar]
- 67.Powers SW, Patton SR, Hommel KA, Hershey AD. Quality of life in paediatric migraine: Characterization of age-related effects using PedsQL 4.0. Cephalalgia. 2004;24:120–7. doi: 10.1111/j.1468-2982.2004.00652.x. [DOI] [PubMed] [Google Scholar]
- 68.Sawyer MG, Whitham JN, Roberton DM, et al. The relationship between health-related quality of life, pain and coping strategies in juvenile idiopathic arthritis. Rheumatology. 2004;43:325–30. doi: 10.1093/rheumatology/keh030. [DOI] [PubMed] [Google Scholar]
- 69.Varni JW, Seid M, Knight TS, Uzark K, Szer IS. The PedsQL 4.0 Generic Core Scales: Sensitivity, responsiveness, and impact on clinical decision-making. Journal of Behavioral Medicine. 2002;25:175–93. doi: 10.1023/a:1014836921812. [DOI] [PubMed] [Google Scholar]
- 70.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambulatory Pediatrics. 2003;3:329–41. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]


