Abstract
The enantiomeric excess of chiral reagents used in asymmetric syntheses directly affects the reaction selectivity and product purity. In this work, 84 of the more recently available chiral compounds were evaluated to determine their actual enantiomeric composition. These compounds are widely used in asymmetric syntheses as chiral synthons, catalysts, and auxiliaries. These include chiral alcohols, amines, amino alcohols, amides, carboxylic acids, epoxides, esters, ketones, and oxolanes among other classes of compounds. All enantiomeric test results were categorized within five purity levels (i.e. <0.01%, 0.01% to 0.1%, 0.1% to 1%, 1% to 10%, and >10%). The majority of the reagents tested were determined to have enantiomeric impurities over 0.01%, and two of them were found to contain enantiomeric impurities exceeding the 10% level. The most effective enantioselective analysis method was a GC approach using a Chiraldex GTA chiral stationary phase (CSP). This method worked exceedingly well with chiral amines and alcohols.
1. Introduction
Enantioselective reactions are of great importance to chemists involved in asymmetric synthesis. The enantiomeric purity of a product is affected by 3 factors: 1) the enantioselectivity of the reaction; 2) the enantiomeric excess of the starting material and/or the catalyst/auxiliary used; and 3) the susceptibility for the desired product to racemize, especially during work-up or storage. Previously, we found detectable amounts of enantiomeric impurities in 192 commercial chiral compounds.1, 2
These compounds are widely employed in asymmetric syntheses as chiral catalysts/catalyst ligands, synthons/synthetic building blocks, chiral auxiliaries and chiral resolving agents. New chiral compounds, catalysts, auxiliaries and synthons are continually being developed, the most useful of which are made available commercially. Herein, we examine new chiral compounds that have not been assayed previously and/or have been introduced after 1999, when the last comprehensive evaluation of commercial chiral compounds was reported.2 When enantiomerically impure compounds are employed in asymmetric synthesis, especially in the pharmaceutical industry, the underestimated contaminants will introduce various amounts of enantiomeric impurities in the “single-enantiomer” reaction and products. In biological processes, these undesired enantiomeric byproducts usually show different effects and/or different pharmacokinetics/pharmacodynamics and thus have different therapeutic values.3 Although the stereoselectivity of asymmetric synthetic processes continue to improve, an awareness of the enantiomeric composition of chiral reagents being used remains essential.
2. Results and Discussion
Of the 84 chiral compounds tested, 85 % of them were separated via enantioselective GC, and of these, 54 % were best separated on the Chiraldex GTA column. This is due to the fact that many of the compounds in this study were chiral amines and alcohols which, when trifluoroacetylated, gained distinct enantioselective interactions with the trifluoroacetylated chiral stationary phase of the GTA column.4 Also, the GTA chiral stationary phase showed impressive separating capabilities for ketones, epoxides, and halogenated acids. Examples of these include the separations of 3-methylcyclopentanone, epoxybutane, and chloropropionic acid, respectively.
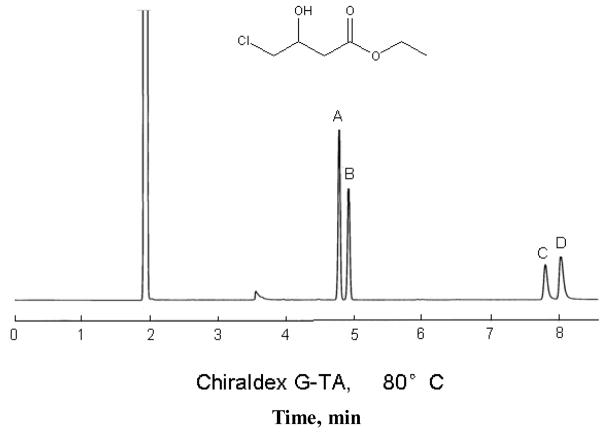
Other effects of the trifluoroacetylation of free alcohols and amines include altered enantioselectivity, increased analyte volatility, faster analysis time, improved peak shape, and increased efficiency. Figure 1 illustrates some of these properties in the GC enantiomeric separations of derivitized (Fig 1A and B) and native (Fig 1C and D) ethyl-4-chloro-3-hydroxybutyrate. Peaks A and D represent the (S)-(−) enantiomer while peaks B and C represent the (R)-(+) enantiomer. Both enantiomeric separations have comparable baseline separations with resolutions greater than two. However, a comparison of the separation of the native analyte versus its trifluoroacetyl derivative (Figure 1) shows that the analysis time for the derivative is shorter and the peaks are sharper (which makes the detection and quantitation of enantiomeric impurities much easier). Furthermore, the reversal of the elution order of the two enantiomers prior to, and after, derivatization indicates that the introduction of the trifluoroacetyl group alters the separation mechanism. It has also been reported that acetic anhydride, chloroacetic anhydride, dichloroacetic anhydride, and trichloroacetic anhydride may be used for this same purpose.5
Figure 1.
GC enantiomeric separations of derivatized (A and B) and native (C and D) ethyl-4-chloro-3-hydroxybutyrate. Peaks A and D represent the (S)-(−)-enantiomer and peaks B and C represent the (R)-(+) enantiomer. Helium carrier gas, G-TA column, 120°C, FID.
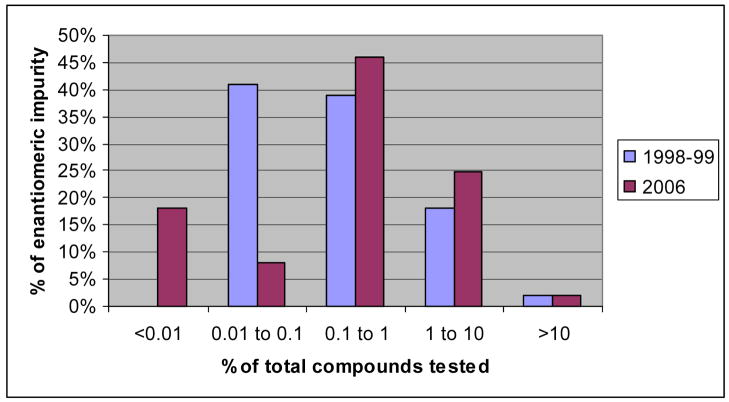
Table 3 lists all chiral compounds examined in this study, as well as, references describing their use in asymmetric syntheses. The separation conditions for each compound assayed are listed in Table 1 and Table 2. Also, Table 3 indicates the actual enantiomeric composition of each compound and the technique used to determine this composition. As shown in Figure 2, 82% of the compounds analyzed were found to contain enantiomeric impurities over 0.01%. Only 8% of the compounds contained enantiomeric impurities between 0.01%–0.1%, whereas, 46% of the samples had enantiomeric impurities in the level 0.1%–1%, and 25% of the samples displayed enantiomeric impurities in the range of 1%–10%. Two compounds were found with enantiomeric contaminants over 10%.
Table 3.
The enantiomeric excess of the organic synthesis reagents
| Use in Asymmetric Synthesis and References |
Name and Structure of Chiral Compound |
Enantiomeric Composition | Method Numberb |
|
|---|---|---|---|---|
| %enantiomeric contaminant |
enantiomeric excess (e. e.)a |
|||
| Synthons or Chiral building blocks | ||||
2-Aminoheptane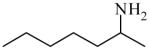
|
(S)=0.09 | *99.83 (R) | GC-1 | |
| (R)=0.35 | 99.31 (S) | |||
| 2-amino-3-thiazolidine derivatives as nitric oxide synthase inhibitors27, 28 | (S)=0.07 | *99.87 (R) | GC-2 | |
| (R)=0.09 | 99.82 (S) | |||
2-Amino-1-pentanol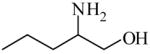
|
(S)<0.01 | *>99.99 (R) | GC-3 | |
| (R)<0.01 | >99.99 (S) | |||
2-Amino-1-phenyl-1, 3-propanediol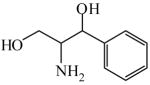
|
(S,S)=0.52 | *98.97 (R,R) | GC-4 | |
| (R,R)=0.06 | >99.89 (S,S) | |||
3-Amino-1, 2-propanediol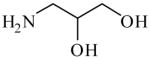
|
(S)=0.28 | 99.44 (R) | GC-5 | |
| (R)=0.93 | *98.14 (S) | |||
| Synthesis of antitumor agent35, 36 | 3-Aminopyrrolidine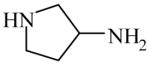
|
(S)=0.66 | 98.67 (R) | GC-5 |
| (R)=0.17 | *99.67 (S) | |||
| Potential human dopamine D4 antagonists synthesis37 | 1-Benzyl-3-aminopyrrolidine
|
(S)=0.17 | *99.67 (R) | GC-6 |
| (R)<0.01 | >99.99 (S) | |||
4-Benzyl-3-propionyl-2-oxazolidinone
|
(S)=0.37 | 99.26 (R) | GC-7 | |
| (R)<0.01 | *>99.99 (S) | |||
| Ring-closing metathesis to cyclic sulfamide peptidomimetics42 |
Bis (α-methylbenzyl)sulfamide |
(S)=6.49 | *87.03 (R,R) | GC-8 |
| (R)=3.29 | 93.43 (S,S) | |||
2-Bromo-α-methylbenzyl-alcohol
|
(S)=0.36 | *99.29 (R) | GC-9 | |
| (R)=0.14 | 99.73 (S) | |||
1-(4-Bromophenyl)ethylamine
|
(S)=0.24 | *99.53 (R) | GC-10 | |
| (R)=0.42 | 99.16 (S) | |||
1,2,4-Butanetriol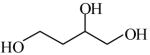
|
(S)=0.84 | *98.33 (R) | GC-5 | |
| (R)<0.01 | >99.99 (S) | |||
| Synthesis of 1,2-dihdroxyimino-3,7-di-aza-9,10- O-iso-propylidene decane, a vic-dioxime derivative for its metal complex study51 | 4-(Chloromethyl)-2,2-dimethyl-1,3-dioxolane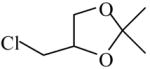
|
(S)=0.12 | 99.76 (R) | GC-11 |
| (R)=0.28 | *99.45 (S) | |||
|
3-Chloro-1-phenyl-1-propanol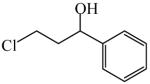
|
(S)=0.39 | *99.22 (R) | GC-12 |
| (R)=0.24 | 99.53 (S) | |||
Chloropropionic acid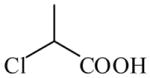
|
(S)=2.79 | *94.42 (R) | GC-5 | |
| (R)=0.38 | 99.25 (S) | |||
| β-Citronellol |
(S)=1.38 | *99.42 (R) | GC-13 | |
| (S)<0.01 | >99.99 (R) | |||
1-(2-Methoxybenzoyl)-2-Pyrolidinemethanol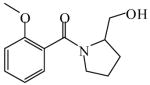
|
(S)<0.01 | >99.99 (R) | LC-1 | |
| (R)<0.01 | >*99.99 (S) | |||
2,3-O-Benzylidene-D-threitol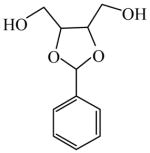
|
(−)=0.20 | *99.60 (+) | GC-14 | |
| (+)=7.42 | 85.16 (−) | |||
| Synthesis of lower alkyl 4-cyano-3-hydroxybutyrates71 | Ethyl-4-bromo-3-hydroxybutyrate |
(S)=0.37 | 99.26 (R) | GC-15 |
| (R)=0.36 | *99.29 (S) | |||
| Ethyl-4-chloro-3-hydroxybutyrate |
(S)=0.49 | *99.02 (R) | GC-15 | |
| (R)=0.04 | 99.93 (S) | |||
|
2-Heptanol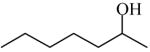
|
(S)=0.12 | *99.77 (R) | GC-16 |
| (R)=1.32 | 97.36 (S) | |||
| Synthesis of analogs of antitumor agents for action mechanism study of XK469 and SH8080, 81 | 2-(4-Hydroxyphenoxy) propionic acid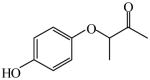
|
(S)<0.01 | >99.99 (R)c | LC-2 |
|
6-Hydroxy-2,5,7,8-tetramethyl- chroman-2-carboxylic acid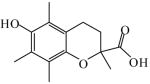
|
(S)<0.01 | >99.99 (R) | LC-3 |
| (R)<0.01 | >*99.99 (S) | |||
3-Methylcyclopentanone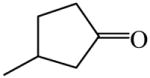
|
(S)=0.10 | 99.80 (R)c | GC-17 | |
2-Methylpiperazine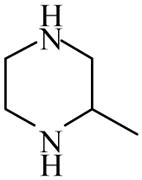
|
(S)=0.13 | *99.75 (R) | GC-6 | |
| (R)=0.15 | 99.71 (S) | |||
| Synthesis of 10-azaprostaglandinE192 | 5-Oxo-3-pyrrolidinecarboxylic acid ethyl ester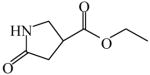
|
(S)<0.01 | *>99.99 (R) | GC-18 |
| (R)=0.62 | 98.76 (S) | |||
| Efficient Synthesis of Cyclopentenones from Enynyl Acetates93 | 1-Octyn-3-ol |
(S)=0.20 | *99.61 (R) | GC-19 |
| (R)=0.17 | 99.77 S) | |||
4-Penten-2-ol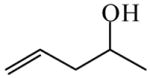
|
(S)=1.11 | *97.79 (R) | GC-13 | |
| (R)=0.39 | 99.22 (S) | |||
3-Pyrrolidinol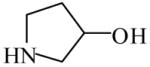
|
(S)=0.04 | 99.92 (R) | GC-20 | |
| (R)=0.54 | *98.92 (S) | |||
| Used in the study of enantioselective palladium(II)-catalyzed aerobic Alcohol oxidations with(−)-sparteine98, 99 | α-(Trifluoromethyl) benzyl alcohol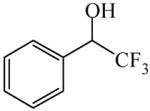
|
(S)=1.06 | 97.88 (R) | GC-2 |
| (R)=1.32 | *97.36 (S) | |||
| Important synthetic component of ferroelectric liquid crystals100 | 1,1,1-Trifluorooctan-2-ol |
(S)=0.90 | 98.20 (R) | GC-21 |
| (R)=0.56 | *98.88 (S) | |||
| Catalyst/catalyst ligands | ||||
| Solid support catalysis for hydrosilylation of ketones101 | 2-Amino-1, 2-diphenylethanol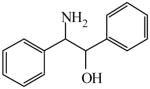
|
(1S,2R)<0.01 | *>99.99 (1R,2S) | GC-22 |
| (1R,2S)<0.01 | 99.60 (1S,2R) | |||
| Solid support catalysis for hydrosilylation of ketones101 | 2-Amino-3-methyl-1-butanol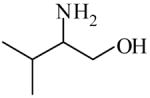
|
(S)=0.23 | *99.54 (R) | GC-3 |
| (R)=0.74 | 98.52 (S) | |||
| Solid support catalysis for hydrosilylation of ketones101 | 2-Amino-1-phenylethanol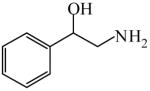
|
(S)=2.15 | *95.70 (R) | GC-20 |
| (R)=3.17 | 93.66 (S) | |||
Bis (α-methylbenzyl)amine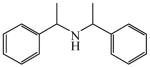
|
(S)<0.01 | *>99.99 (R) | LC-4 | |
| (S)=0.61 | 98.79 (S) | |||
Epoxybutane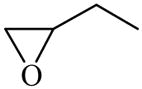
|
(S)=0.13 | 99.74 (R) | GC-23 | |
| (R)=0.01 | *99.98 (S) | |||
| Accelerators for hydrosilation of unsaturated organic compounds108 | 1-Phenyl-2-propyn-1-ol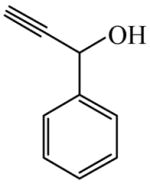
|
(S)=2.02 | 95.96 (R) | GC-24 |
| (R)=0.15 | *99.70 (S) | |||
| Chiral auxiliaries | ||||
| Asymmetric alkynylation of aromatic ketones109 | 2-Amino-1,1-diphenyl-1-propanol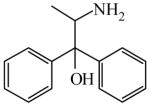
|
(S)=1.26 | *97.49 (R) | LC-5 |
| R<0.01 | >99.99 (S) | |||
2-(Anilinomethyl)pyrrolidine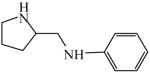
|
(S)=9.18 | *81.64 (R) | LC-6 | |
| (R)=4.98 | 90.04 (S) | |||
| Scholkopf chiral auxiliary 113–117 | 2,5-Dihydro-3,6-dimethoxy-2-iso propylpyrazine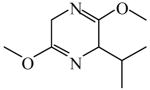
|
(S)=4.59 | *90.83 (R) | GC-11 |
| (R)=2.37 | 95.26 (S) | |||
2,2-Dimethyl-1,3-dioxolane-4-methanol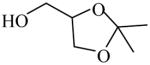
|
(S)=2.23 | 95.55 (R) | GC-25 | |
| (R)=2.40 | *95.21 (S) | |||
| NMR configuration assignment of α-carboxylic acids120 | 1-Indanol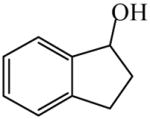
|
(S)=13.8 | *72.50 (S) | LC-7 |
| (R)=11.1 | 77.80 (R) | |||
| Dynamic kinetic resolution of α bromoesters121 | Lactamide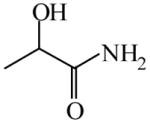
|
(S)=1.10 | *97.80 (R) | GC-15 |
| (R)=1.12 | 97.77 (S) | |||
The first eluted peaks are indicated with the sign *.
GC=gas chromatography, LC=liquid chromatography (here high pressure chromatography). Each method is taken from Table 1 and Table 2 where the specific condition for each separation is listed
For these two compounds, only one enantiomer is tested because the other enantiomer is not commercially available.
Table 1.
Enantioselective methods by gas chromatography (GC)
| GC Method Number a | Column b | Length (m) | Temperature (°C) | flow rate (ml/min) |
|---|---|---|---|---|
| GC-1 | Chiraldex G-PN | 20 | 110 | 1 |
| GC-2 | Chiraldex G-TA | 30 | 110 | 1 |
| GC-3 | Chiraldex G-PN | 20 | 100 | 1 |
| GC-4 | Chiraldex G-TA | 30 | 115 | 1 |
| GC-5 | Chiraldex G-TA | 30 | 100 | 1 |
| GC-6 | Chiraldex G-TA | 30 | 130 | 1 |
| GC-7 | Chiraldex B-DM | 20 | 130 | 1 |
| GC-8 | Chiraldex G-TA | 30 | 110 | 1 |
| GC-9 | Chiraldex B-DM | 20 | 150 | 1 |
| GC-10 | Chiraldex G-BP | 20 | 135 | 1 |
| GC-11 | Chiraldex B-DM | 20 | 90 | 1 |
| GC-12 | Chiraldex B-DM | 20 | 140 | 1 |
| GC-13 | Chiraldex G-TA | 30 | 60 | 1 |
| GC-14 | Chiraldex G-TA | 30 | 140 | 1 |
| GC-15 | Chiraldex G-TA | 30 | 120 | 1 |
| GC-16 | Chiraldex G-TA | 30 | 65 | 1 |
| GC-17 | Chiraldex G-TA | 30 | 50 | 1 |
| GC-18 | Chiraldex G-TA | 30 | 160 | 1 |
| GC-19 | Chiraldex B-DM | 20 | 80 | 1 |
| GC-20 | Chiraldex G-TA | 30 | 150 | 1 |
| GC-21 | Chiraldex B-TA | 20 | 100 | 1 |
| GC-22 | Chiraldex G-BP | 20 | 140 | 1 |
| GC-23 | Chiraldex G-TA | 30 | 35 | 1 |
| GC-24 | Chiraldex B-DM | 20 | 125 | 1 |
| GC-25 | Chiraldex G-TA | 30 | 80 | 1 |
Is used to identify the Seperation techniques in Table 3. Every analyte with amino or hydroxyl functional groups was derivatized with trifluoroacetic anhydride to help with the selectivity of separation and the volatility of analytes (see experimental section)
Is the abbreviation for the GC columns used. All the full names can be looked up in the experimental section.
Table 2.
Enantioselective methods by gas chromatography (GC)
| HPLC Method Number a | Column b | Mobile Phase c (%, v/v) | Flow Rate (ml/min) |
|---|---|---|---|
| LC-1 | Chirobiotic T2 | H2O:TEAA=100:0.1, pH 4.1 | 0.25 |
| LC-2 | Chirobiotic T2 | ACN: HOAc: TEA=100:0.15:0.15 | 1 |
| LC-3 | Poly-DPEDA | Heptane: IPA: TFA=95:5:0.1 | 0.25 |
| LC-4 | Cyclobond I 2000 AC | H2O:TEAA=100:0.1, pH 4.1 | 1 |
| LC-5 | Cyclobond I 2000 AC | H2O:TEAA=100:0.1, pH 4.1 | 1 |
| LC-6 | Cyclobond I 2000 AC | H2O:TEAA =100:0.3, pH 4.1 (0 °C) | 0.25 |
| LC-7 | Cyclobond I 2000 DM | H2O:TEAA:CH3OH=97:0.1:3, PH=7.1 | 1 |
is notation is used to identify the separation techniques in Table 3
is the abbreviation for the HPLC columns used. All the full names can be looked up in the experimental section.
Mobile phase: ACN=acetonenitrile; TEAA=triethylamine acetate; HOAc=acetic acid
Figure 2.
Comparison of results obtained in this study (2006) and results obtained in prior work (1998–99)
Figure 2 also shows a direct comparison of the level of enantiomeric contaminants found in the chiral compounds assayed in this study with those that were tested in 1998–99. In both studies, 2% of the chiral compounds tested contained enantiomeric impurities greater than 10%. However, the greatest enantiomeric impurity for any chiral compound was found in the 1998 analysis of (R)-tert-butyl-4-formyl-2, 2-dimethyl-3-oxazolidine, which was determined to contain 15.11% of the (S)-enantiomer. This is just slightly higher than the 13.80% maximum enantiomeric impurity found in (R)-1-indanol during this study. Figure 2 also shows that within the enantiomeric impurity ranges of 1–10% and 0.1–1%, the results of the two studies are fairly comparable with the abundances in this study being just slightly higher. The major difference in reagent purity in the studies is in the 0.01–1% and <0.01% ranges. The number of very high enantiomeric excess compounds found in this study approached 20%, whereas few, if any, chiral compounds of these purities were available prior to 1998–99. However, there were a higher percentage of compounds in the 0.01–1% range in previous studies.
It was also observed that two enantiomeric compounds will not necessarily contain comparable amounts of enantiomeric impurities. This trend is best observed with the assay of 2, 3-O-benzylidene-D-threitol. The (+)-enantiomer had an enantiomeric excess of 85.16%, while the much more pure (−)-enantiomer had an enantiomeric excess of 99.60%. This determination is consistent with findings in other studies.1, 2
Given the results of this and previous studies, it is apparent that further improvements in the enantiomeric purities of reagents used in asymmetric synthesis would be beneficial. This can be achieved through further refinements in the manufacture and purification of most of these chiral reagents. Since novel chiral compounds are constantly being developed and added to the repertoire of synthetic organic chemists, 6–21 some knowledge as to their enantiomeric composition and the availability of facile methods for their analysis will remain important.
3. Experimental
3.1 Materials
All HPLC columns (25 cm × 4.6 mm i. d.) and GC columns (10 m × 0.25 mm, 20 m × 0.25 mm, 30 m × 0.25 mm) were obtained from Advanced Separation Technologies, Inc. (Whippany, NJ). The LC columns used were Cyclobond I 2000 AC (acetylated-β-cyclodextrin), Cyclobond I 2000 DM (dimethylated-β-cyclodextrin), Chirobiotic T2 (teicoplanin), and Poly-DPEDA (poly N,N′-[(1R, 2R)-1,2-diphenyl-1,2-ethanediyl] bis-2-propenamide).22 GC analysis was performed using Chiraldex B-DM (di-O-methyl-β-cyclodextrin), Chiraldex G-PN (2, 6-di-O-pentyl-3-propionyl-γ-cyclodextrin), Chiraldex G-BP (2, 6-di-O-pentyl- 3-butyryl-γ-cyclodextrin), Chiraldex G-TA (2, 6-di-O-pentyl-3-trifluoroacetyl-γ-cyclodextrin) columns and Chiraldex B-TA(2, 6-di-O-pentyl-3-trifluoroacetyl- β-cyclodextrin).
Trifluoroacetic anhydride (99+ %) was from Aldrich (Milwaukee, WI). Trifluoroacetic acid was obtained from Fisher Scientific (St Louis, MO). The water used, was deionized and purified with a Synery 185, Millipore filter. All the mobile phases were degassed with a VWR Model 250HT sonicator before HPLC analyses. All the chiral compounds examined in this paper were obtained from Aldrich.
3.2 Apparatus and methods
All HPLC separations were performed on the following Shimadzu (Columbia, MD) instrumentation: two LC-6A pumps; a SPD-6A UV spectrophotometric detector; a SCL-10A system controller; and a CR 601 Chromatopac integrator. All compounds were dissolved in acetonitrile and the wavelength of detection was 254 nm. Most of the compounds were tested with a flow rate of 1 ml/min at ambient temperature (25°C). Lower flow rate was used for 2-(Anilinomethyl) pyrrolidine, 6-hydroxy-2, 5, 7, 8- tetramethyl-chroman-2-carboxylic acid and 1-(2-methoxybenzoyl)-2-pyrrolidinemethanol to increase peak efficiency through decreasing the band broadening effect of mass transfer of analyte. Also, 2-(anilinomethyl) pyrrolidine was chromatographed at 0°C in order to optimize the selectivity of separation.
The GC equipment used was a Shimadzu (Columbia, MD) model GC-17A gas chromatograph equipped with a flame ionization detector and EZStart 7.2.1 SP1 data acquisition software. All analyses were performed with a helium carrier gas flow rate of 1 ml/min and a split ratio of 100/1. The injector and detector temperature was set at 250°C and 280°C, respectively. In all GC analyses, chiral compounds with amino and/or hydroxyl groups were derivatized with excess trifluoroacetic anhydride, an achiral reagent that does not induce any change of analyte configuration. 23
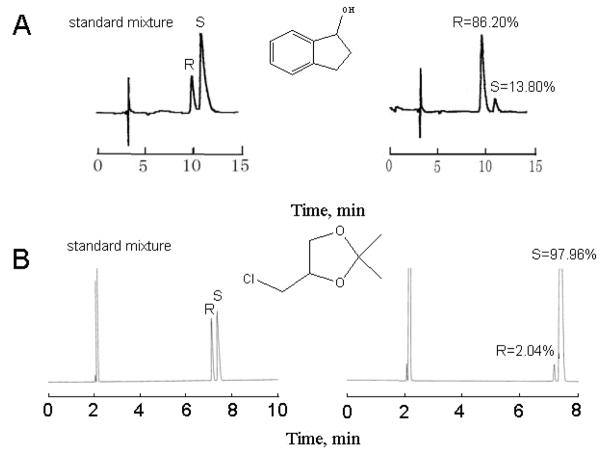
Typical enantiomeric separations on HPLC and GC are shown in Figure 3. All the results were calculated from at least 3 parallel measurements of sample with different concentrations. Each pair of enantiomers were separated with resolution greater than 1.5, so that when an excessive amount of a single enantiomer was injected, its broadening baseline width would not cause a co-elution of the single enantiomer being tested and the enantiomeric impurity.
Figure 3.
Chromatograms A show the enantiomeric separation of 1-indanol on HPLC and the impurity tested in the (R)-enantiomer purchased from Aldrich. Chromatograms B show the enantiomeric separation of 4-(chloromethyl)-2, 2-dimethyl-1, 3-dioxolane on GC and the assay of the (S)-enantiomer obtained from Aldrich. The separation methods for A and B are listed in Table 3 as LC-7 and GC-11, respectively.
Acknowledgments
Support of this work by the National Institute of Health (GM053825-11) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armstrong DW, Lee JT, Chang LW. Tetrahedron: Asymmetry. 1998;9:2043–2064. [Google Scholar]
- 2.Armstrong DW, He LF, Yu T, Lee JT, Liu YS. Tetrahedron: Asymmetry. 1999;10:37–60. [Google Scholar]
- 3.Jenkins AL, Hedgepeth WA. Chirality. 2005:S24–S29. doi: 10.1002/chir.20104. [DOI] [PubMed] [Google Scholar]
- 4.Berthod A, Li W, Armstrong DW. Anal Chem. 1992;64:873–879. doi: 10.1021/ac00028a014. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DW, Li W, Pitha J Anal Chem. 1990;62:214–217. doi: 10.1021/ac00201a023. [DOI] [PubMed] [Google Scholar]
- 6.Moreno RM, Rosol M, Moyano A. Tetrahedron: Asymmetry. 2006;17:1089–1103. [Google Scholar]
- 7.Dimitrov V, Kostova K. Lett Org Chem. 2006;3:176–182. [Google Scholar]
- 8.Cho YH, Fayol A, Lautens M. Tetrahedron: Asymmetry. 2006;17:416–427. [Google Scholar]
- 9.Tokuda R, Matsunaga H, Ishizuka T, Nakajima M, Kunieda T. Heterocycles. 2005;66:135–141. [Google Scholar]
- 10.Hayashi T. Shokubai. 2005;47:533–538. [Google Scholar]
- 11.Sedlak M, Drabina P, Cisarova I, Ruzicka A, Hanusek J, Machacek V. Tetrahedron Lett. 2004;45:7723–7726. [Google Scholar]
- 12.Nesterrov VV, Kolodiazhnyi OI. Tetrahedron: Asymmetry. 2006;17:1023–1026. [Google Scholar]
- 13.Enders D, Tedeschi L, Foerster D. Synthesis. 2006;9:1447–1460. [Google Scholar]
- 14.Wu YT, Hayama T, Baldridge KK, Linden A, Siegel JS. J Am Chem Soc. 2006;128:6870–6884. doi: 10.1021/ja058391a. [DOI] [PubMed] [Google Scholar]
- 15.Mateus N, Routaboul L, Daran JC, Manoury E. J Organometallic Chem. 2006;691:2297–2310. [Google Scholar]
- 16.Lin G, Lu W, Chen P. Faming Zhuanli Shenqin Gongkai Shuomingshu. 2006:1743308. [Google Scholar]
- 17.Ichikawa Y, Matsukawa Y, Isobe M. J Am Chem Soc. 2006;128:3934–3938. doi: 10.1021/ja056253f. [DOI] [PubMed] [Google Scholar]
- 18.Penhoat M, Bohn P, Dupas G, Papamicael C, Marsais F, Levacher V. Tetrahedron: Asymmetry. 2006;17:281–286. [Google Scholar]
- 19.Larrosa I, Romea P, Urpi F. Org Lett. 2006;8:527–530. doi: 10.1021/ol052900w. [DOI] [PubMed] [Google Scholar]
- 20.Du ZY, Xiao RT. Yingyong Huaxue. 2005;22:1372–1374. [Google Scholar]
- 21.Shi H. Syn Commun. 2006;36:237–248. [Google Scholar]
- 22.Han X, He L, Zhong Q, Beesley TE, Armstrong DW. Chromatographia. 2006;63:13–23. [Google Scholar]
- 23.Armstrong DW, Jin HL. J Chromatogr. 1990;502:154–159. [Google Scholar]
- 24.Ding YL, Habib Q, Shaw SZ, Li DY, Abt JW, Hong Z, An HY. J Comb Chem. 2003;5:851–859. doi: 10.1021/cc0300199. [DOI] [PubMed] [Google Scholar]
- 25.Thomas BF, Francisco MEY, Seltzman HH, Thomas JB, Fix SE, Schulz AK, Gilliam AF, Pertwee RG, Stevenson LA. Bioorg Med Chem. 2005;13:5463–5474. doi: 10.1016/j.bmc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Karapire C, Zafer C, Icli S. Synth Met. 2004;145:51–60. [Google Scholar]
- 27.Ueda S, Terauchi H, Yano A, Matsumoto M, Kubo T, Kyoya Y, Suzuki K, Ido M, Kawasaki M. Bioorg Med Chem. 2004;12:4101–4116. doi: 10.1016/j.bmc.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Shankaran K, Donnelly KL, Shah SK, Guthikonda RN, MacCoss M, Humes JL, Packolok SG, Grant SK, Kelly TM. Bioorg Med Chem. 2004;14:4539–4544. doi: 10.1016/j.bmcl.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez S, Brieva R, Rebolledo F, Gotor V. J Chem Soc Perkin Trans. 1;1992:2885–2889. [Google Scholar]
- 30.Ueda S, Terauchi H, Yano A, Ido M, Matsumoto M, Kawasaki M. Bioorg Med Chem. 2004:313–316. doi: 10.1016/j.bmcl.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Brown HC, Prasad JVNV. J Org Chem. 1986;51:4526–4530. [Google Scholar]
- 32.Raboisson P, Mekonnen B, Peet NP. Tetrahedron Lett. 2003;44:2919–2921. [Google Scholar]
- 33.Gerster JF, Lindstrom KJ, Miller RL, Tomai MA, Birmachu W, Bomersine SN, Gibson SJ, Imbertson LM, Jacobson JR, Knafla RT, Maye PV, Nikolaides N, Oneyemi FY, Parkhurst GJ, Pecore SE, Reiter MJ, Scribner LS, Testerman TL, Thompson NJ, Wagner TL, Weeks CE, Andre JD, Lagain D, Bastard Y, Lupu M. J Med Chem. 2005;48:3481–3491. doi: 10.1021/jm049211v. [DOI] [PubMed] [Google Scholar]
- 34.Zubin EM, Stetsenko DA, Zatsepin TS, Gait MJ, Oretskaya TS. Bioorg Med Chem. 2005;13:4912–4920. doi: 10.1016/j.bmc.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 35.Tomita K, Tsuzuki Y, Shibamori KI, Tashima M, Kajikawa F, Sato Y, Kashimoto S, Chiba K, Hino K. J Med Chem. 2002;45:5564–5575. doi: 10.1021/jm010057b. [DOI] [PubMed] [Google Scholar]
- 36.Tsuzuki Y, Tomita K, Shibamori KI, Sato Y, Kashimoto S, Chiba K. J Med Chem. 2004;47:2097–2109. doi: 10.1021/jm0304966. [DOI] [PubMed] [Google Scholar]
- 37.Egle I, Barriault N, Bordeleau M, Drage J, Dube L, Peragine J, Mazzocco L, Arora J, Jarvie K, Tehim A. Bio & Med Chem Lett. 2004;14:4847–4850. doi: 10.1016/j.bmcl.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 38.Neri C, Williams JM. J Adv Synth Catal. 2003;345:835–848. [Google Scholar]
- 39.Neri C, Williams JMJ. Tetrahedron Lett. 2002;43:4257–4260. [Google Scholar]
- 40.Aldrich CC, Venkatraman L, Sherman DH, Fecik RA. J Am Chem Soc. 2005;127:8910–8911. doi: 10.1021/ja0504340. [DOI] [PubMed] [Google Scholar]
- 41.Gaul C, Njardarson JT, Shan D, Dorn DC, Wu KD, Tong WP, Huang XY, Moore MAS, Danishefsky SJ. J Am Chem Soc. 2004;126:11326–11337. doi: 10.1021/ja048779q. [DOI] [PubMed] [Google Scholar]
- 42.Dougherty JM, Probst DA, Robinson RE, Moore JD, Klein TA, Snelgrove KA, Hanson PR. Tetrahedron. 2000;56:9781–9790. [Google Scholar]
- 43.Arink AM, Kronenburg MP, Jastrzebski JTBH, Lutz M, Spek AL, Gossage RA, Koten GV. J Am Chem Soc. 2004;126:16249–16258. doi: 10.1021/ja045914q. [DOI] [PubMed] [Google Scholar]
- 44.Richardson TI, Ornstein PL, Briner K, Fisher MJ, Backer RT, Biggers K. J Med Chem. 2004;47:744–755. doi: 10.1021/jm0304109. [DOI] [PubMed] [Google Scholar]
- 45.Wu X, Mahalingam AK, Wan Y, Alterman M. Tetrahedron Lett. 2004;43:4635–4638. [Google Scholar]
- 46.Feng DM, Wai JM, Kuduk SD, Ng C, Murphy KL, Ransom RW, Duane R, Chang RS, Harrell CM, MacNeil T, Tang C, Prueksaritanont T, Freidinger RM, Pettibone DJ, Bock MG. Bioorg Med Chem Lett. 2005;15:2385–2388. doi: 10.1016/j.bmcl.2005.02.077. [DOI] [PubMed] [Google Scholar]
- 47.Yamagishi T, Okumura Y, Nukui S, Nakao K. Int Appl. 2005;209:WO 2005021508. [Google Scholar]
- 48.Kim S, Powell WS, Lawson JA, Jacobo SH, Pratico D, FitzGerald GA, Maxey K, Rokach J Bioorg Med Chem Lett. 2005;15:1613–1617. doi: 10.1016/j.bmcl.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki K, Suzuki N, Yamaura M. J Carbohydr Chem. 2005;24:73–84. [Google Scholar]
- 50.Aepkers M, Wuensch B. Synthesis. 2004:1033–1036. [Google Scholar]
- 51.Canpolat E, Kaya M. J Coord Chem. 2002;55:961–968. [Google Scholar]
- 52.Isaksson D, Lindmark-Henriksson M, Manoranjan T, Sjodin K, Hogberg HE. J Mol Catal B: Enzyme. 2004;31:31–37. [Google Scholar]
- 53.Handlon AL, Guo Y. Synth Lett. 2005;1:111–114. [Google Scholar]
- 54.Palin R, Barn DR, Clark JK, Cottney JE, Cowley PM. Bioorg Med Chem Lett. 2005;15:589–593. doi: 10.1016/j.bmcl.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 55.Silvestri R, Artico M, Regina GL, Pasquali AD, Martino GD, D’Auria FD, Nencioni L, Palamara AT. J Med Chem. 2004;47:3924–3926. doi: 10.1021/jm049856v. [DOI] [PubMed] [Google Scholar]
- 56.Zhao G, Yu T, Wang R, Wang X, Jing Y. Bioorg Med Chem. 2005;13:4056–4062. doi: 10.1016/j.bmc.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 57.Aikins JA, Haurez M, Rizzo JR, Van Hoeck JP, Brione W, Kestemont JP, Stevens C, Lemair X, Stephenson GA, Marlot E, Forst M, Houpis IN. J Org Chem. 2005;70:4695–4705. doi: 10.1021/jo050268e. [DOI] [PubMed] [Google Scholar]
- 58.Queron E, Lett R. Tetrahedron Lett. 2004;43:4527–4531. [Google Scholar]
- 59.Stage C, Le Bras J, Henin F, Muzart J Tetrahedron. 2005;61:8405–8409. [Google Scholar]
- 60.Mangion IK, MacMillan DWC. J Am, Chem, Soc. 2005;127:3696–3697. doi: 10.1021/ja050064f. [DOI] [PubMed] [Google Scholar]
- 61.Romeril SP, Lee V, Baldwin JE. Tetrahedron Lett. 2004;43:3273–3277. [Google Scholar]
- 62.Burke LT, Dixon DJ, Ley SV, Rodriguez F. Org & Biomole Chem. 2005;3:274–280. doi: 10.1039/b411350k. [DOI] [PubMed] [Google Scholar]
- 63.Prashad M, Lu Y, Kim HY, Hu B, Repic O, Blacklock TJ. Synth Commun. 1999;29:2937–2942. [Google Scholar]
- 64.Besson M, Gallezot P, Neto S, Pinel C. Tetrahedron: Asymmetry. 2000;11:1809–1818. [Google Scholar]
- 65.Schultz AG, Macielag M, Sundararaman P, Taveras AG, Welch M. J Am Chem Soc. 1988;110:7828–7841. [Google Scholar]
- 66.Gerencser J, Bathori N, Czugler M, Huszthy P, Nogradi M. Tetrahedron: Asymmetry. 2003;14:2803–2811. [Google Scholar]
- 67.Lobach AV, Leus ON, Titova NY, Luk’yanenko NG. Rus J Org Chem. 2003;39:1037–1041. [Google Scholar]
- 68.Luk’yanenko NG, Lobach AV, Leus ON, Titova NY. Rus J Org Chem. 2002;38:895–899. [Google Scholar]
- 69.Meyer O, Grosdemange-Billiard C, Tritsch D, Rohmer M. Org & Biomole Chem. 2003;1:4367–4372. doi: 10.1039/b312193c. [DOI] [PubMed] [Google Scholar]
- 70.Gryko DT, Piatek P, Salanski P, Jurczak J. Tetrahedron: Asymmetry. 1998;9:1771–1778. [Google Scholar]
- 71.Kwon T, Gu C, Yang S. Int Appl. 2004;11:WO 2004031131. [Google Scholar]
- 72.Urdiales EG, Rebolledo F, Gotor V. Tetrahedron: Asymmetry. 1999;10:721–726. [Google Scholar]
- 73.Eckert M, Rodefeld L, Brackemeyer T. Int Appl. 2005;20:WO 2005005375. [Google Scholar]
- 74.Wakita R. Jpn Kokai Tokkyo Koho. 2003:2003042267. [Google Scholar]
- 75.Nemoto H, Tsutsumi H, Yuzawa S, Peng X, Zhong W, Xie J, Miyoshi N, Suzuki I, Shibuya M. Tetrahedron Lett. 2004;45:1667–1670. [Google Scholar]
- 76.Cho CS, Kim BT, Choi HJ, Kim TJ, Shim SC. Tetrahedron Lett. 2003;59:7997–8002. [Google Scholar]
- 77.Blaser HU, Diggelmann M, Meier H, Naud F, Scheppach E, Schnyder A, Studer M. J Org Che. 2003;68:3725–3728. doi: 10.1021/jo034112v. [DOI] [PubMed] [Google Scholar]
- 78.Cho CS, Lim DK, Kim TJ, Shim SC. J Chem Res Synop. 2002:550–551. [Google Scholar]
- 79.Kadi N, Belloy L, Chalier P, Crouzet JC. J Agric Food Chem. 2002;50:5552–5557. doi: 10.1021/jf020382n. [DOI] [PubMed] [Google Scholar]
- 80.Hazeldine ST, Polin L, Kushner J, White K, Corbett TH, Biehl J, Horwitz JP. Bioorg Med Chem. 2005;13:1069–1081. doi: 10.1016/j.bmc.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 81.Hazeldine ST, Polin L, Kushner J, White K, Bouregeois NM, Crantz B, Palomino E, Corbett TH, Horwitz JP. J Med Chem. 2002;45:3130–3137. doi: 10.1021/jm0200097. [DOI] [PubMed] [Google Scholar]
- 82.Cena C, Boschi D, Tron GC, Chegaev K, Lazzarato L, Stilo AD, Aragno M, Fruttero R, Gasco A. Bioorg Med Chem. 2004;14:5971–5974. doi: 10.1016/j.bmcl.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 83.Koufaki M, Calogeropoulou T, detsi A, Roditis A, Kourounakis AP, Papazafiri P, Tsiakitzis K, Gaitanaki C, Beis I, Kourounakis PN. J Med Chem. 2001;44:4300–4303. doi: 10.1021/jm010962w. [DOI] [PubMed] [Google Scholar]
- 84.Tobe M, Isobe Y, Goto Y, Obara F, Tsuchiya M, Matsui J, Hirota K, Hayashi H. Bioorg Med Chem. 2000;8:2037–2047. doi: 10.1016/s0968-0896(00)00126-7. [DOI] [PubMed] [Google Scholar]
- 85.Yoda H, Takabe K. Chem Lett. 1989;3:465–466. [Google Scholar]
- 86.Wang S, Kayser MM. J Org Chem. 2003;68:6222–6228. doi: 10.1021/jo026605q. [DOI] [PubMed] [Google Scholar]
- 87.Bernini R, Coratti A, Fabrizi G, Goggiamani A. Tetrahedron, Lett. 2003;44:8991–8994. [Google Scholar]
- 88.Zhang W, Henry Y. Syn Lett. 2001;7:1129–1130. [Google Scholar]
- 89.Cappelli A, Giuliani G, Gallelli A, Valenti S, Anzini M, Mennuni L, Makovec F, Cupello A, Vomero S. Bioorg Med Chem. 2005;13:3455–3460. doi: 10.1016/j.bmc.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 90.Butts CP, Jazdzyk MDS. Org Biomole Chem. 2005;3:1209–1216. doi: 10.1039/b413173h. [DOI] [PubMed] [Google Scholar]
- 91.Fringuelli R, Schiaffella F, Utrilla Navarro MP, Milanese L, Santini C, Rapucci M, Marchetti C, Riccardi C. Bioorg Med Chem. 2003;11:3245–3254. doi: 10.1016/s0968-0896(03)00296-7. [DOI] [PubMed] [Google Scholar]
- 92.Zoretic PA, Barcelon F. Tetrahedron Lett. 1977;6:529–532. [Google Scholar]
- 93.Zhang L, Wang S. J Am Chem Soc. 2006;128:1442–1443. doi: 10.1021/ja057327q. [DOI] [PubMed] [Google Scholar]
- 94.Dunne KS, Bisaro F, Odell B, Paris JM, Gouverneur V. J Org Chem. 2005;70:10803–10809. doi: 10.1021/jo0518708. [DOI] [PubMed] [Google Scholar]
- 95.Ohno H, Okumura M, Maeda S, Iwasaki H, Wakayama R, Tanaka T. J Org Chem. 2003;68:7722–7732. doi: 10.1021/jo034767w. [DOI] [PubMed] [Google Scholar]
- 96.Tucci FC, Zhu YF, Guo Z, Gross TD, Connors PJ, Struthers RS, Reinhart GJ, Saunders J, Chen D. Bioorg Med Chem Lett. 2003;13:3317–3322. doi: 10.1016/s0960-894x(03)00619-x. [DOI] [PubMed] [Google Scholar]
- 97.Bridgeman E, Cavill JL, Schofield DJ, Wilkins DS, Tomkinson NCO. Tetrahedron Lett. 2005;46:8521–8524. [Google Scholar]
- 98.Mandal SK, Jensen DR, Pugsley JS, Sigman MS. J Org Chem. 2003;68:4600–4603. doi: 10.1021/jo0269161. [DOI] [PubMed] [Google Scholar]
- 99.Trend RM, Stoltz BM. J Am Chem Soc. 2004;126:4482–4483. doi: 10.1021/ja039551q. [DOI] [PubMed] [Google Scholar]
- 100.Hamada H, Shiromoto M, Funahashi M, Itoh T, Nakamura K. J Org Chem. 1996;61:2332–2336. [Google Scholar]
- 101.Lavastre O, Morken JP. New J Chem. 2002;26:745–749. [Google Scholar]
- 102.Sortais JB, Ritleng V, Voelklin A, Holuigue A, Smail H, Barloy L, Sirlin C, Verzijl GKM, Boogers JAF, Vries AHM, Vries JG, Pfeffer M. Org Lett. 2005;7:1247–1250. doi: 10.1021/ol047353d. [DOI] [PubMed] [Google Scholar]
- 103.Koide H, Hata T, Uemura M. J Org Chem. 2002;67:1929–1935. doi: 10.1021/jo011052p. [DOI] [PubMed] [Google Scholar]
- 104.Yamada H, Kawate T, Nishida A, Nakagawa M. J Org Chem. 1999;64:8821–8828. doi: 10.1021/jo9908602. [DOI] [PubMed] [Google Scholar]
- 105.Aggarwal VK, Humphries PS, Fenwick A. Angew Chen, Int Ed. 1999;38:1985–1986. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1985::AID-ANIE1985>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 106.Itoh K, Yamada H, Sera A. Bull Chem Soc Jpn. 1984;57:2140–2143. [Google Scholar]
- 107.Malievskii AD, Gorbunova OI. Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya. 1981;10:230702309. [Google Scholar]
- 108.Bank HM, Decher G T U S. 1995;7:US 5449802. [Google Scholar]
- 109.Liu L, Kang YF, Wang R, Zhou YF, Cheng C, Ni M, Gong MZ. Tetrahedron: Asymmetry. 2004;15:3757–3761. [Google Scholar]
- 110.Sakito Y, Mukaiyama T. Chem Lett. 1979;8:1027–1028. [Google Scholar]
- 111.Sakito Y, Tanaka S, Asami M, Mukaiyama T. Chem Lett. 1980;10:1223–1226. [Google Scholar]
- 112.Takayanagi H, Kitano Y, Morinaka Y. J Org Chem. 1994;59:2700–2706. [Google Scholar]
- 113.Chen J, Corbin SP, Holman NJ. Org Process Res Dev. 2005;9:185–187. [Google Scholar]
- 114.Cremonesi G, DallaCroce P, La Rosa C, Pizzatti E. Heterocycles. 2003;61:563–567. [Google Scholar]
- 115.Moller B, Undheim K. Tetrahedron. 1998;54:5789–5804. [Google Scholar]
- 116.Hammer K, Undhei K. Tetrahedron. 1997;53:10603–10614. [Google Scholar]
- 117.Kotha S, Sreenivasachary N, Llalder S. Bioorg Med Chem Lett. 1999;9:2565–2568. doi: 10.1016/s0960-894x(99)00437-0. [DOI] [PubMed] [Google Scholar]
- 118.Gilmore J, Prowse W, Steggles D, Urquhart M, Olkowski J. J Am Chem Soc, Perkin Trans: Org Bioorg Chem. 1996;23:2845–2850. [Google Scholar]
- 119.Jiang J, Li A, Jang S, Chang L, Melman N, Moro S, Ji X, Lobkovsky EB, Clardy JC, Jacobson KA. J Med Chem. 1999;42:3055–3065. doi: 10.1021/jm980688e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferreiro MJ, Latypov SK, Quinoa E, Riguera R. J Org Chem. 2000;65:2658–2666. doi: 10.1021/jo9916838. [DOI] [PubMed] [Google Scholar]
- 121.Ammazzalorso A, Amoroso R, Bettoni G, De Filippis B, Giampietro L, Maccallini C, Tricca M. ARKIVOC. 2004;5:375–381. [Google Scholar]