Abstract
The principal capsular component of Cryptococcus neoformans, glucuronoxylomannan (GXM), interacts with surface glycans, including chitin-like oligomers. Although the role of GXM in cryptococcal infection has been well explored, there is no information on how chitooligomers affect fungal pathogenesis. In this study, surface chitooligomers of C. neoformans were blocked through the use of the wheat germ lectin (WGA) and the effects on animal pathogenesis, interaction with host cells, fungal growth and capsule formation were analyzed. Treatment of C. neoformans cells with WGA followed by infection of mice delayed mortality relative to animals infected with untreated fungal cells. This observation was associated with reduced brain colonization by lectin-treated cryptococci. Blocking chitooligomers also rendered yeast cells less efficient in their ability to associate with phagocytes. WGA did not affect fungal viability, but inhibited GXM release to the extracellular space and capsule formation. In WGA-treated yeast cells, genes that are involved in capsule formation and GXM traffic had their transcription levels decreased in comparison with untreated cells. Our results suggest that cellular pathways required for capsule formation and pathogenic mechanisms are affected by blocking chitin-derived structures at the cell surface of C. neoformans. Targeting chitooligomers with specific ligands may reveal new therapeutic alternatives to control cryptococcosis.
Keywords: Cryptococcus neoformans, Virulence, Lectin binding, Capsule, Chitin oligosaccharides
1. Introduction
Cryptococcosis, the disease caused by the fungal pathogens Cryptococcus neoformans and Cryptococcus gattii, kills about 650,000 people per year around the world (Park et al., 2009). Treatment of human cryptococcosis is unsatisfactory in many cases (Roy and Chiller, 2011). In this context, efforts to understand how C. neoformans and C. gattii cause damage to the human host have been actively pursued during the last two decades (Albuquerque and Rodrigues, 2012) and therapeutic alternatives that neutralize cryptococcal virulence factors could be promising alternatives to standard antifungal therapy.
C. neoformans and C. gattii are encapsulated eukaryotic pathogens (Zaragoza et al., 2009). The cryptococcal capsule, which is a major determinant of virulence, has been studied in detail in the C. neoformans model. The capsular network consists of highly hydrated polysaccharides, including glucuronoxylomannan (GXM), the most abundant component of the capsule, and glucuronoxylomannogalactan (GXMGal), a minor capsular polysaccharide [reviewed in (Zaragoza et al., 2009)]. The complexity of the cell surface architecture of C. neoformans is further increased by the presence of other complex glycans, including mannoproteins, glucans and chitooligomers (Rodrigues and Nimrichter, 2012). While the functions of GXM have been extensively explored during the last two decades (Zaragoza et al., 2009), the knowledge on how GXMGal and mannoproteins impact cryptococcal pathogenesis has advanced more significantly only during last years. On the other hand, the roles of glucans and chitooligomers in the interaction of C. neoformans with host cells remain virtually unknown.
Chitooligomers or chitooligosaccharides are chitin-derived structures composed of three to twenty residues of β1,4 linked N-acetylglucosamine. In C. neoformans, they are embedded within the capsular network (Fonseca et al., 2009; Rodrigues et al., 2008) and interact with GXM to form complex glycans (Rodrigues et al., 2008). Chitin-derived oligomers were also shown to regulate capsular architecture in C. neoformans cells from both in vitro cultures and infected tissues (Fonseca et al., 2009), implying an indirect role in the cryptococcal pathogenesis. Chitooligomers have been detected at the capsular surface (Rodrigues et al., 2008), suggesting their potential for recognition by host receptors possibly affecting cryptococcal pathogenesis.
Fungal chitooligomers are recognized with high affinity by the wheat germ lectin (WGA), a reagent that is commonly used to study cellular distribution and functions of chitin and chitin-like molecules (Orgad et al., 1984). The addition of WGA to Fusarium sp cultures resulted in morphological alterations of the germ tubes, including vacuolation of the cellular content and lysis of cell walls, culminating in the prevention of spreading of the fungus within the host (Ciopraga et al., 1999). Indeed, chitin-derived oligomers have been implicated with cell division in different models (Chen and Contreras, 2004).
Considering the virtually unknown role of chitin-derived oligomers in the pathogenesis of fungal infections and their recently described functions in the physiology of C. neoformans (Fonseca et al., 2009; Rodrigues et al., 2008), we aimed at determining how these molecules affect pathogenic mechanisms of this fungus. Based on a model where chitooligomer exposure at the cell surface was blocked with WGA, we demonstrated that these glycans are involved in brain colonization of mice and interaction with phagocytes. Unexpectedly, blocking of chitooligomers with the lectin resulted in defective capsule formation and down regulation of genes required for synthesis, cellular traffic and signaling pathways controlling capsular components. These results reveal novel functions for surface glycans in C. neoformans.
2. Methods
2.1. Microorganism and growth conditions
The C. neoformans strain used in all experiments in this study was the standard serotype A isolate H99 (Loftus et al., 2005). The only exception was the use of the C. gattii standard serotype B strain R265 (Gillece et al., 2011) as a control in scanning electron microscopy procedures utilizing the lectin WGA as an inhibitor of capsule formation. Yeast cells were inoculated into 100 ml Erlenmeyer flasks containing 50 ml of minimal medium composed of glucose (15 mM), MgSO4 (10 mM), KH2PO4 (29.4 mM), glycine (13 mM), and thiamine-HCl (3 μM) (pH 5.5). Fungal cells were cultivated for 2 days at 30 °C, with shaking. Yeast cells were obtained by centrifugation, washed in phosphate-buffered saline (PBS) and counted in Neubauer chamber. All media were prepared with apyrogenic water, and glassware was rendered sterile and pyrogen free by heating to 190 °C for 4 h.
2.2. Macrophages
Bone marrow-derived macrophages (BMDM) were obtained from wild type (WT) or Tlr2−/− mice (C57BL/6, gender matched, 6–10 week old) (Marim et al., 2010). The animals were kept at 25 °C with free access to chow and water in a room with a 12-h light/dark cycle. The Animal Ethics Committee at the Federal University of Rio de Janeiro approved the animal protocols. Bone marrow was harvested from the tibias and femurs from mice and differentiated into BMDM as described previously (Marim et al., 2010). The cells were suspended at 5 × 106/10 ml in RPMI 1640 medium supplemented with 20% of FCS and 30% of supernatant from L929 cells cultures. After 3 days, fresh supplemented medium was added to the cell cultures. Macrophages were collected at day 6, and 5 × 105/well were plated in 24-well plates in RPMI 1640 medium with 10% of FCS. Cells were cultivated at least 12 h before further experimental procedures.
2.3. Interaction of C. neoformans with mammalian cells
For interaction with host cells, control fungi or WGA-treated cryptococci were used. Treatment with WGA consisted of incubation of yeast cells (5 × 106 cells/ml) for 30 min at 37 °C with the lectin at 10 μg/ml in PBS. The cells were washed in PBS by centrifugation and then stained with 0.5 mg/ml fluorescein isothiocyanate (FITC; Sigma) in PBS (25 °C) for 10 min (Barbosa et al., 2006). Staining with FITC was monitored by flow cytometry and fluorescence microscopy, which revealed that both control and WGA-treated fungi had similar levels of fluorescence (data not shown). Fungal suspensions were prepared in DMEM at a ratio of 10 yeasts per host cell. Interactions between fungal and host cells occurred at 37 °C with 5% CO2 for 12 h. Incubation time was based on previous phagocytosis studies developed in our laboratory showing that 60–70% of the phagocytes become infected and efficiently internalize C. neoformans after overnight incubations in the absence of opsonins (Kmetzsch et al., 2011a,b,c, 2010). In some systems, the medium of interaction was supplemented with the trisaccharide (GlcNAc)3 at 10 μg/ml (Sigma; Richmond, VA). Cells were washed three times with PBS to remove non-adherent yeasts. After removal from the plastic surface with a cell scrapper, the cells were analyzed by flow cytometry as described previously (Barbosa et al., 2006). Control preparations were developed as described above by using uninfected cells and non-stained yeast (data not shown).
2.3.1. Animal infection
Before infection, fungal suspensions were treated for 1 h at 37 °C with PBS (control) or with the same buffer supplemented with WGA (10 μg/ml, final concentration). The cells were then washed with PBS to remove unbound WGA, collected by centrifugation and adjusted to the density of 106 yeast cells per 50 μl of PBS. Female BALB/c mice (6–8 weeks old, n = 10) were first submitted to anesthesia through intraperitoneal administration of ketamine (10 mg/kg) and xylazine (4 mg/kg). The fungal suspension was then inoculated intratracheally. Alternatively, mice were infected with C. neoformans as described above, but with no previous treatment with WGA. In these systems, mice were given WGA during infection in five doses with 48 h intervals. The first dose was administered 24 h after infection. Each dose corresponded to 50 μl PBS containing 10 μg of WGA, to mimic the lectin-cryptococci ratio (10 μg/106 cells) used in the in vitro macrophage assays. Control systems included infected mice given similar volumes of PBS. To evaluate whether WGA alone could induce acute toxicity, the lectin was also administered to uninfected mice in a similar fashion, with all animals remaining alive for at least 30 days (data not shown). Animals were monitored daily for mortality or, alternatively, sacrificed 5 days post-infection for counting colony forming units (CFUs). For this analysis, brains and lungs were excided, macerated in PBS and plated onto Sabouraud dextrose agar, as previously described (Rodrigues et al., 2007b). Histopathologic analysis was also performed in 5 days post-infection. In all experimental groups, the lung right upper lobe was removed and placed into formalin for histology. Formalin-fixed, paraffin-embedded tissues were examined with hematoxylin-eosin for evaluation of histopathology and fungal distribution. Local ethics committees at both Albert Einstein College of Medicine and University of São Paulo approved protocols for animal experimentation.
2.3.2. Susceptibility of WGA to the activity of peptidases
To evaluate the possibility of WGA degradation by host peptidases and loss of biological activity during infection, samples of TRITC-lectin (5 μg/ml) were incubated in PBS alone or in the same buffer supplemented with trypsin (100 μg/ml, pH 8.0) for 16 h at 37 °C. Alternatively, the lectin was incubated under the same conditions in human or mouse sera. These samples were then used to stain C. neoformans, followed by analysis by fluorescence microscopy as previously described (Rodrigues et al., 2008). Our parameter to analyze the integrity of the lectin and/or retaining of biological activity was the ability to produce the typical polarized pattern of staining of budding sites (Fonseca et al., 2009; Rodrigues et al., 2008).
2.4. Effects of WGA on polysaccharide extracellular release, capsule formation and fungal viability
C. neoformans cells were first cultivated for 24 h at room temperature in liquid Sabouraud medium, a condition at which capsule formation is impaired (Zaragoza and Casadevall, 2004). To stimulate capsule production, fungal cells (106 cells/ml) were transferred to minimal medium and cultivated for 48 h at room temperature. This assay included systems in which the medium was supplemented with WGA at concentrations varying from 10 to 1000 μg/ml. Fungal growth was monitored spectrophotometrically (absorbance at λ = 600 nm) during 48 h in 12 h-intervals. Cells were harvested by centrifugation and tested for capsule formation by counter-stained with India ink and immunofluorescence as previously described (Fonseca et al., 2009; Rodrigues et al., 2008). Alternatively, the cells were plated onto Sabouraud agar for CFU quantification. For scanning electron microscopy, fungal cells were fixed in 4% glutaraldehyde for 1 h at room temperature. The cells were then placed onto poly-L-lysine-coated coverslips and submitted to dehydration and critical point drying with CO2. Samples were mounted with gold–palladium and viewed with a Quanta 50 scanning electron microscope. Supernatants were collected for GXM determination by ELISA as described before (Casadevall et al., 1992). In both ELISA and immunofluorescence tests, the monoclonal antibody to GXM 18B7 (mAb 18B7) (Casadevall et al., 1998) was used. Importantly, serologic reactions of mAb 18B7 with cryptococcal GXM are not affected by the presence of varying concentrations of WGA (Fonseca et al., 2009).
2.5. Quantitative real time RT-PCR analysis
Two sets of RNA samples from independent experiments using control cryptococci or yeast cells were prepared using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. For RNA extraction, yeast cells (106 cells/ml) were incubated in minimal medium supplemented with WGA (500 μg/ml) for 48 h at room temperature, as described in the item above. After DNase treatment, RNA preparations were utilized for first-strand synthesis of cDNA using M-MLV reverse transcriptase (Promega). Real-time PCR reactions were performed in an Applied Biosystems StepOnePlus Real-Time PCR System. PCR thermal cycling conditions were an initial step at 95 °C for 5 min followed by 40 cycles at 95 °C for 15 s, and 60 °C for 1 min. Platinum SYBR Green qPCR SuperMix-UDG with ROX (Invitrogen) was used as reaction mix, supplemented with 4 pmol of each primer and 2 μl of the cDNA template in a final volume of 20 μl. All experiments were done in duplicate and each cDNA sample was analyzed in triplicate with each primer pair. Melting curve analysis was performed at the end of the reaction to confirm a single PCR product. Data was normalized to actin gene cDNA amplified in each set of PCR experiments. Relative expression was determined by the 2−ΔCT method (Livak and Schmittgen, 2001). The primers utilized in these experiments are listed in Table 1.
Table 1.
List of primers used in qRT-PCR.
| Gene | Accession numbera | Primer | Sequence (5′–3′) |
|---|---|---|---|
| CAP59 | CNAG_00721 | CnCAP59F | AACCCGAACGAAGAAACCTC |
| CnCAP59R | ACCCCAGCACCACACATACTC | ||
| CAP64 | CNAG_02885 | CnCAP64F | ACAAGGAAAGGGCATTCAGAG |
| CnCAP64R | TATCACTGGGACGGTCAGAAAG | ||
| CAP10 | CNAG_07554 | CnCAP10F | TCTTCTTCCGTCATTCGTTC |
| CnCAP10R | TTCTCCCGTACCTCTTCTTG | ||
| PKA1 | CNAG_00396 | CnPKA1F | CTGAACGAGAGATGTTGGTAC |
| CnPKA1R | GCTGACTTTTACGAAGAAGG | ||
| MAN1 | CNAG_04312 | CnMAN1F | TTGATGAGAAGCCTGATGTC |
| CnMAN1R | TAGGGATTCGGTGAGAGAAG | ||
| GPA1 | CNAG_04505 | CnGPA1F | GATGAGGCTGATGTTCTGAG |
| CnGPA1R | CTTCTTTCTCTCGCTTCTCTG | ||
| CMT1 | CNAG_03158 | CnCMT1F | CTCGTACAGCCTATTCCAAC |
| CnCMT1R | GACATCACCTCTCCTGAAAC | ||
| GRASP | CNAG_03291 | CnGRASPF | GAGGGAGAGGGAATAGTAGTG |
| CnGRASPR | CTTTTGACGCTTCTTCTGAC | ||
| CAP60 | CNAG_00600 | CnCAP60F | CGCATATTACCCCCTTGAAG |
| CnCAP60R | CGGCATTGTTGAGTTGACTG | ||
| ACT1 | CNAG_00483 | CnACTF | CCTTGCTCCTTCTTCTAT |
| CnACTR | CTCGTCGTATTCGCTCTT | ||
| TUB | CNAG_01840 | CnTUBF | TGTCCTCTACCTTCATTTGC |
| CnTUBR | CCATACCCTCTCCAGTGTAC |
Cryptococcus neoformans H99 Genome Database at Broad Institute – http://www.broadinstitute.org/annotation/genome/cryptococcus_neoformans/MultiHome.html. Gene products are phosphomannose isomerase 1 (MAN1), a-1,3-mannosyltransferase (CMT1), Golgi reassembly and stacking protein (GRASP), capsule-related (CAP) proteins 59, 10, 64 and 60, G-protein alpha subunit 1 (GPA1), cyclic AMP (cAMP)-dependent protein kinase catalytic subunit 1 (PKA1) and actin (ACT1).
2.6. Statistical analysis
Student’s t test was used for comparison of two different groups and one-way ANOVA was used for comparison of several groups. Mice survival was statistically analyzed through the use of the Kaplan–Meier test. Statistical tests were performed with GraphPad Prism (version 5.0).
3. Results
3.1. Chitooligomers participate in C. neoformans animal pathogenesis
The possibility that chitin-like structures might contribute to the pathogenesis of C. neoformans (Fonseca et al., 2009; Ramos et al., 2012; Rodrigues et al., 2008; Rodrigues and Nimrichter, 2012) led us to evaluate whether blocking the formation of these molecules would influence the course of animal cryptococcosis. We therefore aimed at interfering with the exposure of chitooligomers by blocking C. neoformans cells with WGA before infection of mice. In these experiments, mice infected with untreated C. neoformans displayed regular symptoms of murine pulmonary cryptococcosis, characterized by partial loss of appetite and apparent depression. At 10 days post-infection, an increase in the respiration rate was noted and within 15–20 days, symptoms gradually advanced to significant anorexia, lethargy, loss of hair arrangement and tachypnea. Within a 20–30 days interval, animals became moribund and expired. The WGA-treated group displayed similar symptoms, although they were apparent with a 3–5 days delay in comparison with control mice. These observations were in agreement with the fact that pretreatment of yeast cells with the chitooligomer-binding lectin attenuated the ability of C. neoformans to kill mice (Fig. 1A). This observation in association with the delayed manifestation of systemic symptoms led us to suppose that infection with WGA-treated C. neoformans could have altered kinetics of dissemination. Indeed, WGA-treatment was associated with a severe deficiency in the capacity of yeast cells to colonize the brain of animals at day 5 post-infection (Fig. 1B and C), suggesting that the structures blocked by WGA could be involved with fungal dissemination.
Fig. 1.
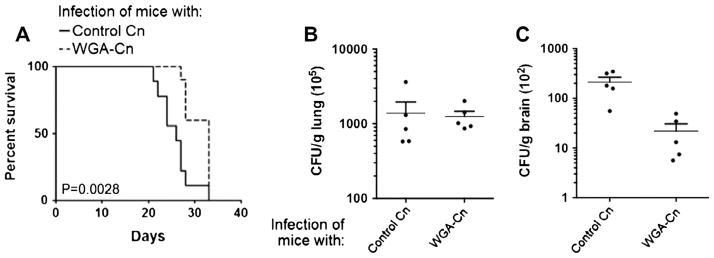
Blocking chitooligomers at the surface of C. neoformans altered the course of animal cryptoccocosis in favor of the host. (A) Comparison of mortality rates between animals infected with control cryptococci or with WGA-treated yeast cells revealed that the previous exposure of fungal cells to the lectin caused attenuation in the ability of C. neoformans to kill mice (n = 10). Determination of fungal burden in lungs (B) and brains (C) of infected animals (n = 5) indicated that lectin binding resulted in decreased ability to colonize the central nervous system (P = 0.0164), but not the lung (P = 0.8274). Data in A–C are representative of two independent experiments with similar results.
The fact that WGA-treated cryptococci were initially contained in the lung raised several hypotheses to explain the inefficiency of these cells to reach the brain at early stages of infection. We first considered the possibility that cell wall-bound WGA could stimulate cellular inflammatory responses in the lung resulting in control of dissemination, based on the fact that pulmonary inflammatory processes are crucial for the local control of cryptococcosis (Goldman et al., 1994). Histopathological analysis of animals that were infected with C. neoformans or with WGA-treated yeast cells revealed that both systems produced similar profiles (Fig. 2A), implying that wall-associated WGA did not affect the cellular inflammatory response in the lungs of infected animals. These initial observations strongly suggested that blocking of C. neoformans chitooligomers would affect only the initial interactions of the fungus with host cells, since the cryptococcal progeny would not carry WGA. To evaluate this hypothesis, WGA was administered during mouse infection instead of being used to coat C. neoformans before animal inoculation. In contrast to what has been observed when the fungus was treated with WGA before inoculation, administration of WGA during animal cryptococcosis did not interfere with the course of infection (Fig. 2B). One possible explanation for this finding would be related to the degradation of soluble WGA by host’s proteases with consequent loss of capacity to recognize chitooligomers. To rule out this possibility, TRITC-WGA was incubated with PBS (control), trypsin, human or mouse fresh sera. In all cases, the lectin retained the ability to produce staining profiles of C. neoformans that were similar to those produced after incubation under control conditions (Fig. 2C). This observation suggested that even if WGA was partially degraded by host’s peptidases, the properties related to chitooligomer binding were retained. These results also ruled out the possibility that WGA was acting as an immune modulator in favor of the host, since no protective effect was observed when the lectin was used to treat cryptococcal infection.
Fig. 2.
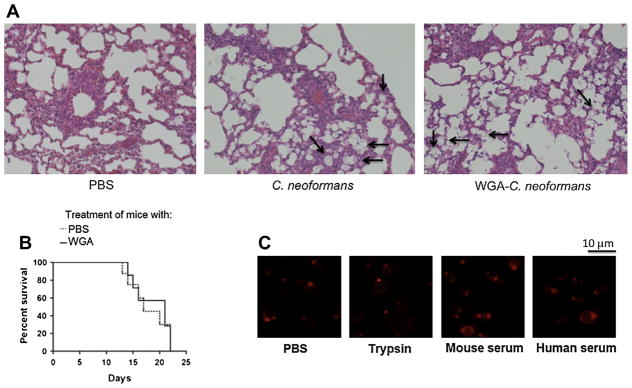
Influence of WGA on pathophysiologic aspects of mice infected with C. neoformans. (A) Histology of lungs 5 days after intratracheal infection with C. neoformans. Similar pulmonary histologic profiles of mice receiving PBS, C. neoformans and WGA-treated cryptococci are shown. Some of the fungal cells identified in the images are highlighted with arrows. Images are representative of similar sections from mice in each group (n = 10). Original magnification 100×. (B) Treatment of lethally infected mice with WGA did not influence the course of animal cryptococcosis. Mice given PBS or WGA had similar mortality rates (n = 10, P = 0.7124). (C) Incubation of the lectin in the presence of PBS (control), trypsin or serum did not result in loss of its ability to recognize C. neoformans, as concluded by the similar profiles of fluorescence staining in all systems. The patterns of lectin staining in all cases corresponded to those described in previous studies (Fonseca et al., 2009; Rodrigues et al., 2008).
3.2. Chitooligomers are involved in the recognition of C. neoformans by animal macrophages
Recently, the ability of macrophage TLR2 to interact with chitin fragments was characterized (Da Silva et al., 2009), suggesting that this could also be the receptor for the cryptococcal chitin-like molecules. In addition, macrophages are supposed to play central roles in initial interactions of host cells with C. neoformans (Del Poeta, 2004) and in dissemination to the brain (Kechichian et al., 2007). We then analyzed the levels of association between yeast cells and macrophages obtained from wild type (WT) or Tlr2−/− mice. Approximately 75% of the WT macrophages were infected with C. neoformans (Fig. 3). This value, which was in agreement with previous studies using similar models (Kmetzsch et al., 2011a,b,c, 2010), was about threefold higher than the percentage of Tlr2−/− macrophages infected by C. neoformans. Blocking of fungal chitooligomers with WGA before incubation with BMDM caused a decrease of approximately 50% in the efficacy of association of C. neoformans with macrophages obtained from WT mice. In systems using macrophages from Tlr2−/− mice, WGA did not exert any inhibitory effect. The lectin did not influence phagocytosis levels of C. neoformans by WT and Tlr2−/− macrophages in systems where it was used to supplement the interaction medium instead of coating yeast cells (data not shown). Finally, the levels of association of cryptococci with macrophages in the presence of a soluble chitooligomer, (GlcNAc)3 were evaluated. The efficacy of C. neoformans to associate with WT macrophages was decreased in approximately 25% in the presence of the chitooligomer. However, in accordance with results obtained with WGA-treated fungi, (GlcNAc)3 did not cause any inhibition in the association of Tlr2−/− macrophages with C. neoformans.. Taken together, these results suggest that C. neoformans chitooligomers are recognized by host macrophages through surface TLR2.
Fig. 3.
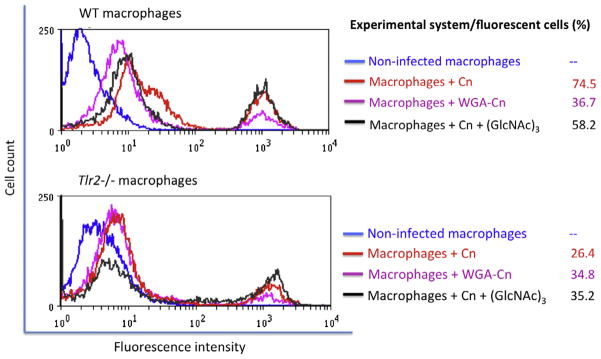
Chitooligomers are required for association of C. neoformans with phagocytes. The efficacy of association of C. neoformans (Cn) with macrophages obtained from WT animals (upper panel) was much higher than that observed when macrophages from Tlr2−/− mice were used (lower panel). Treatment of C. neoformans cells with WGA before exposure to host cells affected the interaction of fungi with macrophages from WT animals, but not with the phagocytes obtained from Tlr2−/− mice. Incubation of fungal cells with macrophages in the presence of the chitooligomer (GlcNAc)3 resulted in decreased levels of infection of WT macrophages, but not of the Tlr2−/− phagocytes. Results illustrated in this figure are representative of three independent experiments.
3.3. Blocking of C. neoformans chitooligomers with WGA affects extracellular release of GXM, but not fungal viability
Capsule formation is fundamental for the outcome of the interaction of C. neoformans with macrophages and for fungal virulence (Zaragoza et al., 2009). The fact that GXM associates with chitooligomers (Fonseca et al., 2009; Rodrigues et al., 2008) and the key contribution of this polysaccharide to fungal virulence (Zaragoza et al., 2009) led us to analyze the effect of WGA binding on capsule formation and polysaccharide release to the extracellular space.
WGA did not induce a general failure in C. neoformans secretory processes, since protein secretion was not inhibited by treatment of yeast cells with WGA (Fonseca, unpublished data). After exposure to the lectin, however, C. neoformans cells manifested clear defects to produce extracellular GXM (Fig. 4). Decreased extracellular GXM release could also be related to lectin-induced cell death (Ciopraga et al., 1999; Mirelman et al., 1975). Determination of fungal viability after treatment with WGA, however, revealed that the lectin had no effect on cryptococcal viability or growth. As the polysaccharide quantification was based on ELISA we had to exclude the possibility that false-negative results were obtained due to structural modifications of the polysaccharide and consequent loss of serological reactivity. Therefore, C. neoformans cells were stained with a monoclonal antibody to GXM after incubation with WGA showing that the polysaccharide was regularly recognized by the antibody after incubation of fungal cells with the lectin (Fig. 4). Lectin-treated cells, however, had an apparent reduction in capsular dimensions. This observation and the fact that extracellular release of GXM is mandatory for capsule enlargement (Zaragoza et al., 2006) led us to evaluate the effects of chitooligomer blocking on capsule formation.
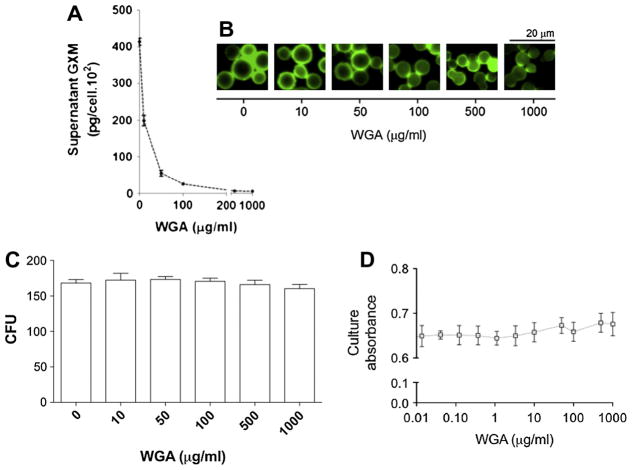
Fig. 4.
Treatment of C. neoformans with WGA affects polysaccharide secretion, but not cell viability or serologic properties of GXM. (A) Incubation of cryptococci with WGA followed by GXM determination in supernatants revealed a dose-dependent inhibition of polysaccharide extracellular release. (B) Immunofluorescence microscopy demonstrating that exposure of C. neoformans to WGA did not affect the recognition of GXM by a monoclonal antibody raised to the polysaccharide. Analysis of fungal proliferation by CFU counting (C) or culture spectrophotometry (D) revealed that growth rates were similar in control systems (CT, incubated in the absence of the lectin) or in cultures that were supplemented with WGA.
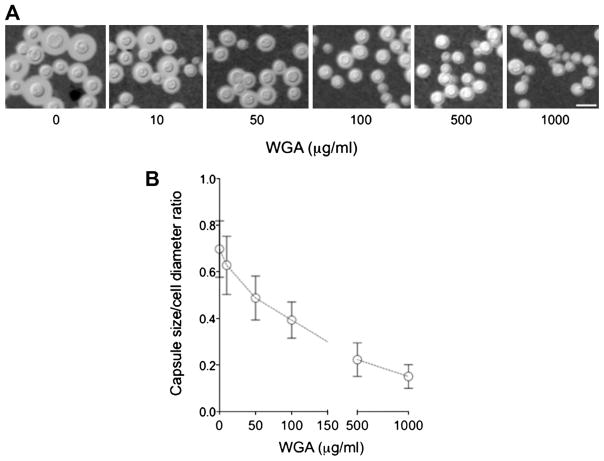
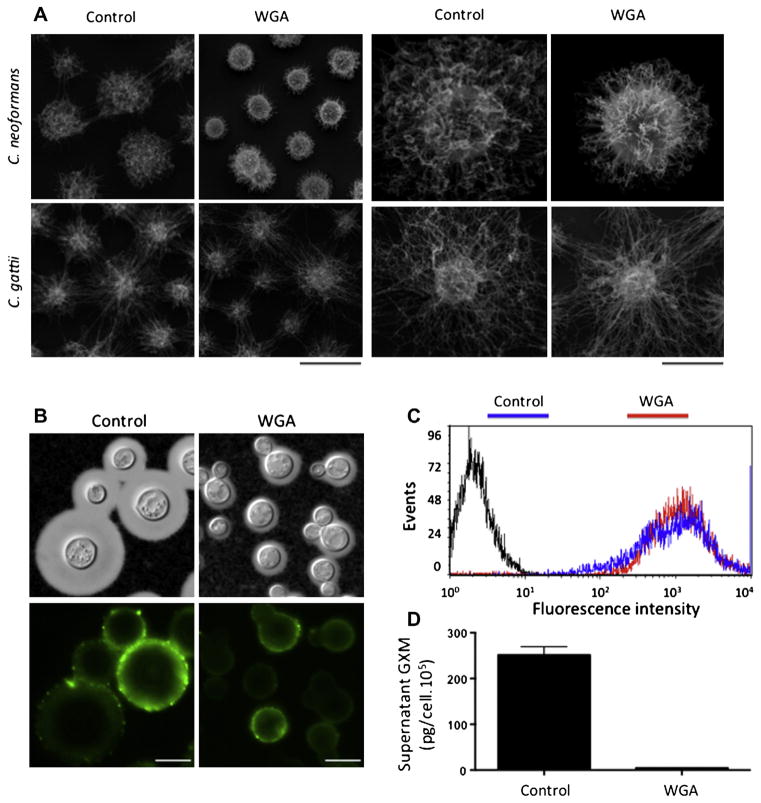
Both visual and quantitative determinations demonstrated that WGA caused a clear dose-dependent inhibition of capsule formation (Fig. 5). Since the mechanisms by which carbohydrate-binding proteins interfere with capsule formation are unknown, we selected conditions that combined maximal values of fungal viability with clear alterations in capsule formation for further investigation. Scanning electron microscopy analysis revealed that exposure of C. neoformans to WGA at 500 μg/ml induced a clear inhibition of capsule formation (Fig. 6A). This effect was exclusively observed when C. neoformans was exposed to the lectin, since the capsule of C. gattii was not affected by treatment with WGA at the same concentration. Under these conditions, the serological properties of surface GXM were preserved (Fig. 6B and C). Extracellular GXM was markedly decreased after exposure of C. neoformans to the lectin at this concentration (Fig. 6D). Based on these results, we selected the 500 μg/ml lectin concentration for evaluation of transcription levels of capsule-related genes.
Fig. 5.
WGA affects capsule formation in C. neoformans. Capsular morphology and dimensions were analyzed visually after counter-staining of yeast cells with India ink as illustrated in A for microscopic determination of capsule size (B). Scale bar corresponds to 10 μm.
Fig. 6.
Phenotypic analysis of capsule formation in C. neoformans after exposure of the fungus to WGA at 500 μg/ml. Scanning electron microscopy (A) demonstrated that capsular dimensions of C. neoformans, but not of C. gattii, were affected by treatment with WGA. Capsule morphology is shown for samples of C. neoformans and C. gattii populations (left panels; scale bar, 5 μm) and for individual cells (right panels; scale bar, 2 μm). SEM observations of C. neoformans correlate with the phenotype observed by India ink counter staining (B, upper panels) and with the regular recognition of surface polysaccharides by a monoclonal antibody to GXM, as demonstrated by immunofluorescence microcopy (B, lower panels) and flow cytometry (C). Under this condition, the concentration of extracellular GXM is significantly smaller in culture supernatants of WGA-treated cells (D, P < 0.00001).
3.4. Binding of C. neoformans chitooligomers to WGA reduces the transcription levels of genes involved with capsule formation
Capsule formation requires the synthesis of the basic GXM components (Klutts et al., 2006), the transport of newly formed polysaccharides to the extracellular space (Casadevall et al., 2009; Kmetzsch et al., 2011a; Rodrigues et al., 2007a; Yoneda and Doering, 2006), and their assembly at the fungal cell surface (Doering, 2009; Zaragoza et al., 2009). These events were the basis for selection of genes whose products were previously demonstrated to regulate capsule formation. These genes, which included GPA1 (Alspaugh et al., 1997), PKA1 (D’Souza et al., 2001), CAP10 (Chang and Kwon-Chung, 1999), CAP60 (Chang and Kwon-Chung, 1998), CAP59 (Chang et al., 1995; Garcia-Rivera et al., 2004), CAP64 (Chang et al., 1996), GRASP (Kmetzsch et al., 2011a), CMT1 (Sommer et al., 2003), and MAN1 (Wills et al., 2001), had their transcription levels tested after binding of WGA to C. neoformans cells. The relative expression of β-tubulin was evaluated as a control, to rule out the possibility that WGA treatment would cause a non-specific global reduction in gene expression. In accordance with viability data (Fig. 4), the transcription levels of β-tubulin were not affected in lectin-treated cells (Fig. 7; P = 0.8201 after comparison with untreated yeast cells).
Fig. 7.
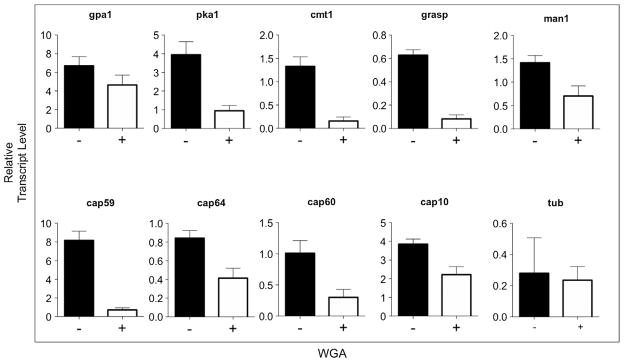
Treatment of C. neoformans with WGA affects the transcript levels of genes required for capsule formation. The relative expression of β-tubulin (TUB), phosphomannose isomerase 1 (MAN1), α-1,3-mannosyltransferase (CMT1), Golgi reassembly and stacking protein (GRASP), capsule-related (CAP) proteins 59, 10, 64 and 60, G-protein alpha subunit 1 (GPA1) and cyclic AMP (cAMP)-dependent protein kinase catalytic subunit 1 (PKA1) was quantified by qRT-PCR. RNA amounts in each sample were normalized using the threshold cycle (CT) values obtained for the actin gene.
WGA binding negatively modulated the transcription of nine different genes required for capsule formation (Fig. 7). MAN1 and CMT1, which are both essential for mannose metabolism (Sommer et al., 2003; Wills et al., 2001), had their transcription significantly reduced in WGA-treated cells (P < 0.0003 and 0.0001, respectively). GRASP and CAP59 were described to be required for the traffic of GXM (Garcia-Rivera et al., 2004; Kmetzsch et al., 2011a). Both genes had their transcription reduced in lectin-treated cells (P < 0.0001 for both systems). Other CAP genes, including CAP10, CAP60 and CAP64 (Chang et al., 1997, 1996, 1995; Chang and Kwon-Chung, 1998, 1999), were also negatively affected by lectin treatment (P < 0.0001 for CAP10 and CAP64 and 0.0006 for CAP60). The transcription levels of PKA1 and GPA1, which are key regulators of signaling pathways required for capsule formation (Alspaugh et al., 1997; D’Souza et al., 2001), were also evaluated. While exposure to WGA strongly affected the transcription of PKA1 (P < 0.0001), the effect of the lectin over the expression of GPA1 was discrete, although statistically significant (P < 0.006). Together, these results indicate that binding of WGA to C. neoformans cells efficiently attenuates the expression of genes involved in capsule formation.
4. Discussion
A number of studies have consistently demonstrated that GXM is the most important surface component of C. neoformans [reviewed in (Zaragoza et al., 2009)]. However, it is now clear that other glycans contribute to the architecture and functions of the capsule. Therefore, a realistic perspective is that many aspects related to the biological roles of surface components in C. neoformans require clarification.
Chitooligomers have been detected within the capsular matrix in both C. neoformans and C. gattii (Rodrigues et al., 2008). These oligosaccharides interact with GXM forming hybrid molecules that are apparently relevant for the architecture of the capsule, cellular responses and possibly interaction with the host (Fonseca et al., 2009; Ramos et al., 2012; Rodrigues et al., 2008; Rodrigues and Nimrichter, 2012). Chitooligosaccharides were also detected in outer layers of the capsule (Rodrigues et al., 2008), but their functions during the interaction of C. neoformans with host cells have not been explored thus far. Fungal chitin is normally not accessible to the immune system, but its smaller fragments have been demonstrated to be efficient immune modulators (Da Silva et al., 2009), in addition to being exposed at the surface of C. neoformans (Rodrigues et al., 2008).
Here we demonstrated that the pathogenesis of mice cryptococcosis was altered when infection was performed with WGA-treated C. neoformans cells. Lectin treatment was associated to an attenuated ability of C. neoformans to kill mice and failure in early colonization of the central nervous system. Noteworthy, the totality of animals infected with WGA-treated yeast cells died 34 days after infection. We believe this observation is in agreement with the fact that, in our model, the cryptococcal progeny generated during infection will not carry surface-bound WGA, limiting the eventual effects of the lectin to early pathogenic mechanisms. Therefore, one would indeed expect that infection of mice with lectin-treated cells results in virulence attenuation rather than in a dramatic alteration of the course of mice cryptococcosis in favor of the host. This supposition would be in agreement with the observation that WGA did not affect fungal proliferation, which could result in the recovery of the pathogenic potential by C. neoformans after a few rounds of replication.
Early steps of entrance of cryptococci into the central nervous system involves multiple mechanisms that may include breakage of alveolar barriers, direct invasion of brain endothelial cells, urease-dependent passage through the brain microvasculature and “Trojan horse” pathways whereby cryptococci enter the brain after macrophage ingestion (Casadevall, 2010; Charlier et al., 2009; Kechichian et al., 2007). Considering that phagocytosis is a key event in the pathogenesis of C. neoformans (Del Poeta, 2004) and that intracellular macrophage parasitism is believed to be advantageous to the fungus (Feldmesser et al., 2001), we speculate that blocking chitooligomers could be linked to a decreased efficacy in intracellular parasitism and to faulty dissemination to the brain by “Trojan horse”-related mechanisms. The fact that WGA-treated cryptococci were initially contained in the lung, however, raised several hypotheses to explain the reduced efficiency of these cells to reach the brain. Since pulmonary inflammatory processes are crucial for the local control of cryptococcosis (Goldman et al., 1994), cell wall-bound WGA could stimulate cellular inflammatory responses in the lung resulting in control of dissemination. However, the histopathological features of the lungs of animals that were infected with untreated C. neoformans or with WGA-treated yeast cells were similar, suggesting that wall-associated lectin did not affect the local inflammatory response. Administration of WGA during mouse infection instead of using the lectin to coat C. neoformans before animal inoculation did not interfere with the course of infection, although WGA retained its primary biological activity (chitooligomer binding) even after exposure to host’s proteases. Therefore, we considered unlikely the possibility that WGA was acting as an immune modulator in favor of the host.
It has been recently demonstrated that the efficacy of the interaction of TLR2 with chitin is inversely related to the dimensions of the polysaccharide (Da Silva et al., 2008). This size-dependent recognition of chitin fragments by TLR2 stimulates IL-17 production (Da Silva et al., 2008). In the C. neoformans model, the influence of TLR2 on the efficacy of the immune response is controversial. Nakamura and colleagues suggested that TLRs 2 and 4 would have a limited contribution to the host’s anti-cryptococcal response (Nakamura et al., 2006), but independent studies demonstrated that IL-17 (Wozniak et al., 2011) and TLR2 (Levitz, 2002, 2004; Yauch et al., 2005, 2004) are potentially involved in the anti-cryptococcal pulmonary defense. The latter reports suggest that blocking small chitin fragments or chitooligomers might affect the interaction of C. neoformans with host cells by interfering with fungal surface ligands that would be potentially recognized by host cell receptors. Indeed, we observed that TLR2 was involved in the chitooligomer-mediated phagocytosis of C. neoformans by macrophages. Blocking of the glycans with WGA also decreased the ability of yeast cells to associate with the phagocytes. It is unlikely that the effects of WGA on capsular assembly influenced this result, because the concentration used to block chitooligomers in phagocytosis assays was much smaller than that affecting the cryptococcal capsule. The observation that macrophages use TLR2 to interact with C. neoformans is in agreement with previous reports on the role of TLRs as mediators of processes of interaction of fungal molecules with phagocytes (Bittencourt et al., 2006; Figueiredo et al., 2011, 2010). The global contribution of the TLR2-mediated recognition of chitooligomers to the immunity to C. neoformans is uncertain, but our results indicate that these glycans can at least participate in the process of recognition of the pathogen by immune cells.
In our study, WGA exerted important biological activities in variable concentrations. We have previously demonstrated that virtually 100% of the C. neoformans population stains positively for WGA at concentrations lower than 10 μg/ml, without affecting capsular dimensions (Fonseca et al., 2009; Rodrigues et al., 2008). Since capsular dimensions are determinant for interaction of C. neoformans with host cells [reviewed in (Zaragoza et al., 2009)], experiments aiming at evaluating the pathogenesis-related functions of chitooligomers were developed under conditions at which the capsule was not affected (10 μg/ml). On the other hand, in experiments focused on the ability of WGA to interfere with capsular architecture and gene expression, an experimental condition combining maximal values of fungal viability with unquestionable alterations in capsule formation was required (500 μg/ml). In this context, treatment of C. neoformans with WGA could interfere with polysaccharide synthesis, promoting a general attenuation in GXM metabolism, or simply block polysaccharide secretion. It has been described that binding of lectins to the surface of eukaryotic cells can profoundly modify intracellular signaling cascades and regulate gene expression (O’Brien et al., 2012; Zou et al., 2012). In fact, we observed that WGA caused an effective ablation of transcription levels of eight genes directly or indirectly involved in capsule formation and a discrete attenuation in the transcription of the gene encoding the subunit α of protein G, an important mediator of signaling pathways in C. neoformans (Alspaugh et al., 1997; D’Souza et al., 2001). Some of the functional products of the genes evaluated in this study are not physiologically connected, which may suggest that WGA could simply promote a general attenuation in gene expression. However, the facts that the fungal viability was not affected by the lectin and that the expression of a capsule-unrelated gene was not influenced by WGA argue against this hypothesis. The diminished transcription levels of the genes encoding Gpa1p and Pka1p, in addition, could explain the global effects observed in our study. Down-regulation of the expression of GPA1 and PKA1 could affect the intracellular signaling cascades that regulate capsule formation, as proposed in previous studies (Alspaugh et al., 1997; D’Souza et al., 2001). These events and related phenomena in C. gattii are under investigation in our laboratory.
Mechanisms connecting binding of external ligands to capsular glycomolecules with activation of intracellular signaling pathways are virtually unknown. However, WGA binds to some areas of the capsule and antibody binding to the capsule is known to trigger gene expression changes in C. neoformans (McClelland et al., 2010). Recently antibody binding has been shown to alter the mechanical properties of the polysaccharide capsule (Cordero et al., 2013) and it is possible that chitin oligosaccharides mediate similar phenomena that are sensed by the fungal cell resulting in a disruption in capsule synthesis. Our current results add chitooligomers to the list of surface components of C. neoformans interfering with fungal pathogenesis. Thus, it seems plausible to suppose that molecules with the ability to specifically block surface exposure of chitooligosaccharides in C. neoformans could have therapeutic potential. In this context, the production of chitooligomer-binding peptides and monoclonal antibodies raised to these glycans could represent promising strategies aiming at controlling cryptococcosis.
Acknowledgments
We thank Radames Cordero, Julian Muñoz, Antonio Nakouzi and Lorena Derengowski for suggestions and help with animal experimentation. M.L.R., A.S., C.C.S., M.H.V., F.L.F., C.P.T., A.J.G., M.T.B., C.P.T., and L.N. are supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP, Brazil) and Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ, Brazil). A.C. is supported by NIH Grants AI033142, AI033774, AI052733, and HL059842 and the Center for AIDS Research at Einstein. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors declare no reported conflicts of interest.
References
- Albuquerque PC, Rodrigues ML. Research trends on pathogenic Cryptococcus species in the last 20 years: a global analysis with focus on Brazil. Future Microbiol. 2012;7:319–329. doi: 10.2217/fmb.11.162. [DOI] [PubMed] [Google Scholar]
- Alspaugh JA, et al. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–3217. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FM, et al. Glucuronoxylomannan-mediated interaction of Cryptococcus neoformans with human alveolar cells results in fungal internalization and host cell damage. Microb Infect. 2006;8:493–502. doi: 10.1016/j.micinf.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Bittencourt VC, et al. An alpha-glucan of Pseudallescheria boydii is involved in fungal phagocytosis and Toll-like receptor activation. J Biol Chem. 2006;281:22614–22623. doi: 10.1074/jbc.M511417200. [DOI] [PubMed] [Google Scholar]
- Casadevall A. Cryptococci at the brain gate: break and enter or use a Trojan horse? J Clin Invest. 2010;120:1389–1392. doi: 10.1172/JCI42949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998;42:1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, et al. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J Immunol Methods. 1992;154:27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- Casadevall A, et al. Vesicular transport across the fungal cell wall. Trends Microbiol. 2009;17:158–162. doi: 10.1016/j.tim.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, et al. Structure and biological activities of acapsular Cryptococcus neoformans 602 complemented with the CAP64 gene. Infect Immun. 1997;65:1584–1592. doi: 10.1128/iai.65.5.1584-1592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Kwon-Chung KJ. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect Immun. 1998;66:2230–2236. doi: 10.1128/iai.66.5.2230-2236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Kwon-Chung KJ. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J Bacteriol. 1999;181:5636–5643. doi: 10.1128/jb.181.18.5636-5643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, et al. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect Immun. 1996;64:1977–1983. doi: 10.1128/iai.64.6.1977-1983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, et al. Further analysis of the CAP59 locus of Cryptococcus neoformans: structure defined by forced expression and description of a new ribosomal protein-encoding gene. Gene. 1995;167:179–183. doi: 10.1016/0378-1119(95)00640-0. [DOI] [PubMed] [Google Scholar]
- Charlier C, et al. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77:120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Contreras R. The bud scar-based screening system for hunting human genes extending life span. Ann NY Acad Sci. 2004;1019:355–359. doi: 10.1196/annals.1297.061. [DOI] [PubMed] [Google Scholar]
- Ciopraga J, et al. Fusarium. sp growth inhibition by wheat germ agglutinin. Biochim Biophys Acta. 1999;1428:424–432. doi: 10.1016/s0304-4165(99)00085-9. [DOI] [PubMed] [Google Scholar]
- Cordero RJ, et al. Antibody binding to Cryptococcus neoformans impairs budding by altering capsular mechanical properties. J Immunol. 2013;190:317–323. doi: 10.4049/jimmunol.1202324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza CA, et al. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol Cell Biol. 2001;21:3179–3191. doi: 10.1128/MCB.21.9.3179-3191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva CA, et al. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J Immunol. 2009;182:3573–3582. doi: 10.4049/jimmunol.0802113. [DOI] [PubMed] [Google Scholar]
- Da Silva CA, et al. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008;181:4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Poeta M. Role of phagocytosis in the virulence of Cryptococcus neoformans. Eukaryot Cell. 2004;3:1067–1075. doi: 10.1128/EC.3.5.1067-1075.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering TL. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu Rev Microbiol. 2009;63:223–247. doi: 10.1146/annurev.micro.62.081307.162753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmesser M, et al. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 2001;9:273–278. doi: 10.1016/s0966-842x(01)02035-2. [DOI] [PubMed] [Google Scholar]
- Figueiredo RT, et al. Fungal surface and innate immune recognition of filamentous fungi. Front Microbiol. 2011;2:248. doi: 10.3389/fmicb.2011.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo RT, et al. TLR4 recognizes Pseudallescheria boydii conidia and purified rhamnomannans. J Biol Chem. 2010;285:40714–40723. doi: 10.1074/jbc.M110.181255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca FL, et al. Role for chitin and chitooligomers in the capsular architecture of Cryptococcus neoformans. Eukaryot Cell. 2009;8:1543–1553. doi: 10.1128/EC.00142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rivera J, et al. Cryptococcus neoformans CAP59 (or Cap59p) is involved in the extracellular trafficking of capsular glucuronoxylomannan. Eukaryot Cell. 2004;3:385–392. doi: 10.1128/EC.3.2.385-392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillece JD, et al. Whole genome sequence analysis of Cryptococcus gattii from the Pacific Northwest reveals unexpected diversity. PLoS One. 2011;6:e28550. doi: 10.1371/journal.pone.0028550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, et al. Pathogenesis of pulmonary Cryptococcus neoformans infection in the rat. Infect Immun. 1994;62:4755–4761. doi: 10.1128/iai.62.11.4755-4761.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechichian TB, et al. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun. 2007;75:4792–4798. doi: 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klutts JS, et al. Glycosyltransferases and their products: cryptococcal variations on fungal themes. FEMS Yeast Res. 2006;6:499–512. doi: 10.1111/j.1567-1364.2006.00054.x. [DOI] [PubMed] [Google Scholar]
- Kmetzsch L, et al. Role for Golgi reassembly and stacking protein (GRASP) in polysaccharide secretion and fungal virulence. Mol Microbiol. 2011a;81:206–218. doi: 10.1111/j.1365-2958.2011.07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmetzsch L, et al. Calcium signaling components in the human pathogen: Cryptococcus neoformans. Commun Integr Biol. 2011b;4:186–187. doi: 10.4161/cib.4.2.14271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmetzsch L, et al. The vacuolar Ca(2)(+) exchanger Vcx1 is involved in calcineurin-dependent Ca(2)(+) tolerance and virulence in Cryptococcus neoformans. Eukaryot Cell. 2010;9:1798–1805. doi: 10.1128/EC.00114-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmetzsch L, et al. The GATA-type transcriptional activator Gat1 regulates nitrogen uptake and metabolism in the human pathogen Cryptococcus neoformans. Fungal Genet Biol. 2011c;48:192–199. doi: 10.1016/j.fgb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Levitz SM. Receptor-mediated recognition of Cryptococcus neoformans. Nihon Ishinkin Gakkai Zasshi. 2002;43:133–136. doi: 10.3314/jjmm.43.133. [DOI] [PubMed] [Google Scholar]
- Levitz SM. Interactions of Toll-like receptors with fungi. Microb Infect. 2004;6:1351–1355. doi: 10.1016/j.micinf.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loftus BJ, et al. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science. 2005;307:1321–1324. doi: 10.1126/science.1103773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marim FM, et al. A method for generation of bone marrow-derived macrophages from cryopreserved mouse bone marrow cells. PLoS One. 2010;5:e15263. doi: 10.1371/journal.pone.0015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland EE, et al. Ab binding alters gene expression in Cryptococcus neoformans and directly modulates fungal metabolism. J Clin Invest. 2010;120:1355–1361. doi: 10.1172/JCI38322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D, et al. Inhibition of fungal growth by wheat germ agglutinin. Nature. 1975;256:414–416. doi: 10.1038/256414a0. [DOI] [PubMed] [Google Scholar]
- Nakamura K, et al. Limited contribution of Toll-like receptor 2 and 4 to the host response to a fungal infectious pathogen, Cryptococcus neoformans. FEMS Immunol Med Microbiol. 2006;47:148–154. doi: 10.1111/j.1574-695X.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- O’Brien XM, et al. Lectin site ligation of CR3 induces conformational changes and signaling. J Biol Chem. 2012;287:3337–3348. doi: 10.1074/jbc.M111.298307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgad U, et al. Histochemical studies of epithelial cell glycoconjugates in atrophic, metaplastic, hyperplastic, and neoplastic canine prostate. Lab Invest. 1984;50:294–302. [PubMed] [Google Scholar]
- Park BJ, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Ramos CL, et al. Chitin-like molecules associate with Cryptococcus neoformans glucuronoxylomannan to form a glycan complex with previously unknown properties. Eukaryot Cell. 2012;11:1086–1094. doi: 10.1128/EC.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, et al. Binding of the wheat germ lectin to Cryptococcus neoformans suggests an association of chitinlike structures with yeast budding and capsular glucuronoxylomannan. Eukaryot Cell. 2008;7:602–609. doi: 10.1128/EC.00307-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, Nimrichter L. In good company: association between fungal glycans generates molecular complexes with unique functions. Front Microbiol. 2012;3:249. doi: 10.3389/fmicb.2012.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007a;6:48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, et al. Monoclonal antibody to fungal glucosylceramide protects mice against lethal Cryptococcus neoformans infection. Clin Vaccine Immunol. 2007b;14:1372–1376. doi: 10.1128/CVI.00202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Chiller T. Preventing deaths from cryptococcal meningitis: from bench to bedside. Exp Rev Anti Infect Ther. 2011;9:715–717. doi: 10.1586/eri.11.86. [DOI] [PubMed] [Google Scholar]
- Sommer U, et al. An alpha-1,3-mannosyltransferase of Cryptococcus neoformans. J Biol Chem. 2003;278:47724–47730. doi: 10.1074/jbc.M307223200. [DOI] [PubMed] [Google Scholar]
- Wills EA, et al. Identification and characterization of the Cryptococcus neoformans phosphomannose isomerase-encoding gene, MAN1, and its impact on pathogenicity. Mol Microbiol. 2001;40:610–620. doi: 10.1046/j.1365-2958.2001.02401.x. [DOI] [PubMed] [Google Scholar]
- Wozniak KL, et al. Role of IL-17A on resolution of pulmonary C. neoformans infection. PLoS One. 2011;6:e17204. doi: 10.1371/journal.pone.0017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch LE, et al. Receptor-mediated clearance of Cryptococcus neoformans capsular polysaccharide in vivo. Infect Immun. 2005;73:8429–8432. doi: 10.1128/IAI.73.12.8429-8432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch LE, et al. Involvement of CD14, toll-like receptors 2 and 4, and MyD88 in the host response to the fungal pathogen Cryptococcus neoformans in vivo. Infect Immun. 2004;72:5373–5382. doi: 10.1128/IAI.72.9.5373-5382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A, Doering TL. A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol Biol Cell. 2006;17:5131–5140. doi: 10.1091/mbc.E06-08-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, Casadevall A. Experimental modulation of capsule size in Cryptococcus neoformans. Biol Proced Online. 2004;6:10–15. doi: 10.1251/bpo68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, et al. The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol. 2009;68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, et al. The polysaccharide capsule of the pathogenic fungus Cryptococcus neoformans enlarges by distal growth and is rearranged during budding. Mol Microbiol. 2006;59:67–83. doi: 10.1111/j.1365-2958.2005.04928.x. [DOI] [PubMed] [Google Scholar]
- Zou C, et al. Murine hyperglycemic vasculopathy and cardiomyopathy: whole-genome gene expression analysis predicts cellular targets and regulatory networks influenced by mannose binding lectin. Front Immunol. 2012;3 doi: 10.3389/fimmu.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]