Summary
The hippocampus generates distinct neural codes to disambiguate similar experiences, a process thought to underlie episodic memory function. Entorhinal grid cells provide a prominent spatial signal to hippocampus, and changes in their firing pattern could thus generate a distinct spatial code in each context. We examined whether we would preclude the emergence of new spatial representations in a novel environment during muscimol inactivation of the medial septal area, a manipulation known to disrupt theta oscillations and grid cell firing. We found that new, highly distinct configurations of place fields emerged immediately and remained stable during the septal inactivation. The new place code persisted when theta oscillations had recovered. Theta rhythmicity and feedforward input from grid cell networks were thus not required to generate new spatial representations in the hippocampus.
Introduction
Hippocampal neurons generate distinct spatial firing patterns to disambiguate contexts (Muller and Kubie, 1987), which is thought to support episodic and spatial memory function (Burgess et al., 2001; Buzsaki and Moser, 2013). However, the neural mechanisms that establish and maintain distinct codes for separate locations are unclear. Many models predict that hippocampal representations of self-location emerge from two inputs, one from landmarks in the environment and the second from self-motion (Burgess and O’Keefe, 1996, 2011; O’Keefe, 1976; Rotenberg and Muller, 1997). It has been suggested that medial entorhinal cortex (MEC) grid cells provide the self-motion configured spatial framework (McNaughton et al., 2006) and that they are the primary source of spatial information onto place cells (Blair et al., 2008; Cheng and Frank, 2011; de Almeida et al., 2012; Hasselmo, 2009; Hayman and Jeffery, 2008; McNaughton et al., 2006; Molter and Yamaguchi, 2008; Monaco and Abbott, 2011; Rolls et al., 2006; Savelli and Knierim, 2010; Si and Treves, 2009; Solstad et al., 2006). This hypothesis is supported by the findings that grid cells project directly to the hippocampus (Zhang et al., 2013), that entorhinal lesions disrupt the precision and stability of hippocampal place fields (Brun et al., 2008; Miller and Best, 1980; Van Cauter et al., 2008), and that global remapping in the hippocampus co-occurs with grid field realignment (Barry et al., 2012; Fyhn et al., 2007). However, other studies suggest that entorhinal grid cells may not be required for generating place fields in particular conditions. In pre-weaned rat pups, stable hippocampal place fields are observed as soon as border cells emerge, which is 1–3 days prior to the emergence of spatially periodic grid fields (Bjerknes et al., 2014; Langston et al., 2010; Wills et al., 2010). In adult animals, inactivation of the medial septal area causes a disruption of the grid cell spatial firing pattern (Brandon et al., 2011; Koenig et al., 2011) while the spatial selectivity of hippocampal place fields is unaffected (Koenig et al., 2011).
Nonetheless, evaluation of the grid to place cell transfer hypothesis has been constrained by limitations of these data. Spatial tuning in pre-weaned rats is displayed by border cells and putative developing grid cells before their full grid regularity appears, and either cell type could provide input to place cells. The septal inactivation recordings that disrupted grid firing in adults were all conducted in highly familiar environments in which place fields were well established prior to the disruption of grid cells. This raises the possibility that entorhinal inputs other than grid cells, along with intrahippocampal connections, can sustain well-learned hippocampal firing patterns and that grid cells are primarily necessary to initially establish hippocampal place codes.
To test whether grid cells are required to form new place fields, an experimental design should examine whether place fields emerge in a novel room without grid cell input. However, selective experimental manipulations of grid cells by targeting the entire MEC are not feasible, as these neurons are anatomically interspersed with other cell types, such as boundary cells and head direction cells. Furthermore, cell-type specific genetic markers are not yet available to target grid cells for selective silencing. We therefore took advantage of the finding that septal inactivation selectively disrupts grid cells, whereas the spatial selectivity of boundary cells and the direction tuning of head-direction cells is largely retained (Brandon et al., 2011; Koenig et al., 2011). Using this manipulation, we examined whether place fields emerged in a completely novel room while the grid cell signal and theta oscillations were compromised.
Results
Local field potentials (LFPs) and single-units (n cells = 65) were recorded from the CA1 region of the hippocampus as animals (n = 4) explored an open field in a familiar (F) and novel (N) room before, during, and after inactivation of the medial septum with muscimol (Figures 1A–C; Figure S1). Recordings were from a series of ten-minute recording sessions during random foraging in familiar and novel environments. Each muscimol infusion experiment (n = 5) began with two baseline sessions in the familiar room (F1, F2). Muscimol was then infused into the septum, which was followed by a 30-minute interval to allow time for the drug to diffuse throughout the septal area. After the septal inactivation was fully effective, recordings were first conducted during an additional session in the familiar room (F3), then throughout three sessions in a novel room (N1, N2, N3), and next in a session back in the familiar room (F4). Recovery recordings in the familiar environment were then taken 6 hours after the infusion (F5) (Figure 1D). On the next day, an additional series of recordings (F6, N4, N5, F7) was performed without drug infusion. For comparison to the recordings after intraseptal muscimol infusions, identical control experiments (aCSF infusions) were performed (n = 33 cells in two animals).
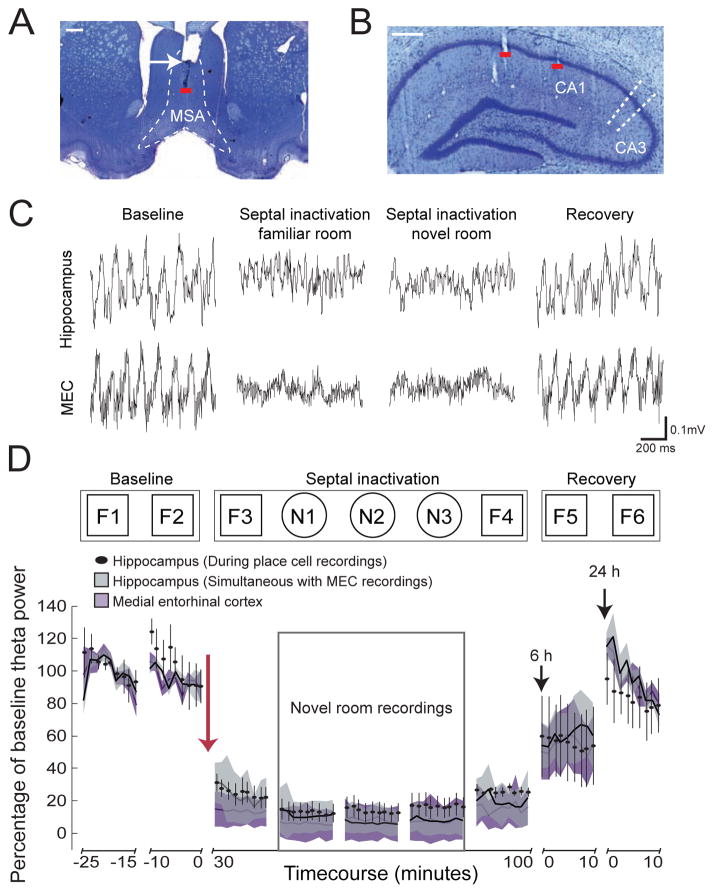
Figure 1.
Near-complete reduction of theta power in the familiar and novel room during septal inactivation. Coronal sections show (A) infusion cannula (white arrow) in the medial septal area (MSA, dashed line) and (B) tetrode tracks that terminated in hippocampal region CA1 (red lines). Scale bars, 0.5 mm. (C) LFP traces recorded simultaneously from the hippocampus (top row) and medial entorhinal cortex (MEC, bottom row). Theta oscillations are reduced during septal inactivation. All traces are from periods with running speeds > 20 cm/s. (D) (Top) Experimental timeline of familiar (F) and novel (N) room recordings during baseline (F1, F2), septal inactivation (F3, N1, N2, N3, F4), and recovery from inactivation (F5, F6). Bottom: Mean percentage of baseline theta (5–10 Hz) power after muscimol infusions into the septal area. The magnitude and timecourse of the reduction in theta power during hippocampal place cell recordings (mean ± 95% confidence intervals in black) was compared to the reduction in theta power in simultaneous LFP recordings from the hippocampus (in gray) and MEC (in purple). Recordings at 6 hours and at 24 hours after muscimol infusion (shown in intervals from 0 to 10 minutes) show recovery of theta oscillations. The timecourse of the reduction in theta power corresponded between the hippocampus and MEC. The theta reduction during place cell recordings closely matched the previously reported theta reduction during recordings in which grid cells were disrupted (Figure S2).
We found that septal inactivation reduced hippocampal theta power to levels below 20 % of baseline theta power, and that the reduction persisted throughout the series of place cell recordings in the familiar and novel rooms (F3, N1, N2, N3, F4) (Figure 1D). Was the extent of theta reduction sufficient to abolish grid firing in MEC? A prior study using corresponding intraseptal muscimol infusions showed that theta power reductions in MEC to less than 50% of baseline levels reliably disrupted all recorded grid cells (Brandon et al., 2011). To confirm that hippocampal theta reduction is a reliable indicator of the reduction of theta and of grid firing in MEC, we performed simultaneous recordings of hippocampal and MEC theta oscillations. The septal inactivation led to a decrease in the theta amplitudes in both brain regions, which were matched in their magnitude and in their time course [n = 4 simultaneous recordings in 3 rats, between groups: F(1, 480) = 0.98, n.s.; interaction between time and group, F(80,480) = 0.24, n.s., repeated measures ANOVA] (Figures 1C and 1D; Figure S3). These data confirm that reduced theta power in the hippocampus after septal inactivation is a reliable predictor of the theta power and grid cell activity in MEC and that the substantial reduction of theta oscillations during place cell recordings in the current dataset was sufficient to disrupt the firing patterns of MEC grid cells (Figure S2).
In each recording series, we first recorded the place cells in a familiar room before and after the intraseptal muscimol infusion. As expected from prior studies (Koenig et al., 2011; Mizumori et al., 1989), we found that the spatial selectivity was high in the familiar environment before the septal inactivation (F1 and F2) and remained high after the inactivation (F3) despite a substantial reduction in the theta rhythmicity of the neurons (Figure S4). The size of place fields even sharpened slightly during septal inactivation (n cells with fields = 43, F1:F3 p < 0.05, F2:F3, P < 0.01) probably owing to a small decrease in mean firing rate (n = 65, both P values < 0.001) (Figure S4B and Table S1A).
To determine whether theta oscillations and a functioning grid cell network were required for the generation of new hippocampal maps, we continued the recording series in a new environment during a series of three 10-minute exposures to a novel room (N1, N2, and N3). New place fields rapidly emerged in the novel room during septal inactivation (Figures 2A–B). Theta rhythmicity of spiking was decreased in comparison to the control condition (n muscimol cells = 39 with fields, n control cells with fields = 26, all P values < 0.001) (Figures 2C–D; Tables S1A–B). The mean firing rate and place field size showed a small increase in the novel room compared to the reduced rate and size in the familiar room after inactivation (mean firing rate, F3:N1, n cells = 65, P < 0.01; place field size, F3:N1, n cells with fields = 39, P < 0.01). However, field size and mean firing rates in the novel room were not different from pre-infusion baseline recordings in the familiar room (F1:N1 and F2:N1, all n.s.) (Figure 2D; Table S1A). The peak spatial firing rate and spatial information of the place fields did not change between any of the familiar to novel room comparisons within the muscimol condition (all n.s.) (Figure 2D; Table S1A). Place fields that newly formed after aCSF infusions displayed substantially increased mean firing rates and peak firing rates compared to place fields in the muscimol condition (mean firing rate, N1, P < 0.01, N2, and N3, P < 0.001; peak firing rate; N1 and N2, P < 0.05, N3, P < 0.01). The place field size in the control condition returned to baseline levels over the 30 minutes of recording in the novel room, as previously reported (Barry et al., 2012; Karlsson and Frank, 2008). Thus, place field size in controls was larger than after muscimol infusion only during the first 20 minutes (place field size, N1, P < 0.05, N2, P < 0.05, N3, n.s.) (Figure 2D; Tables S1A–B), although spatial information per spike did not differ between groups at any time period (all n.s.) (Figure 2D; Tables S1A–B). These results demonstrate that reduced grid cell and/or septal input precluded the transient increase in firing rate in the novel room, but that sharply tuned hippocampal place fields nonetheless emerged rapidly in a novel room, suggesting that mechanisms other than theta oscillations and stable grid cell inputs are sufficient for generating new hippocampal place fields.
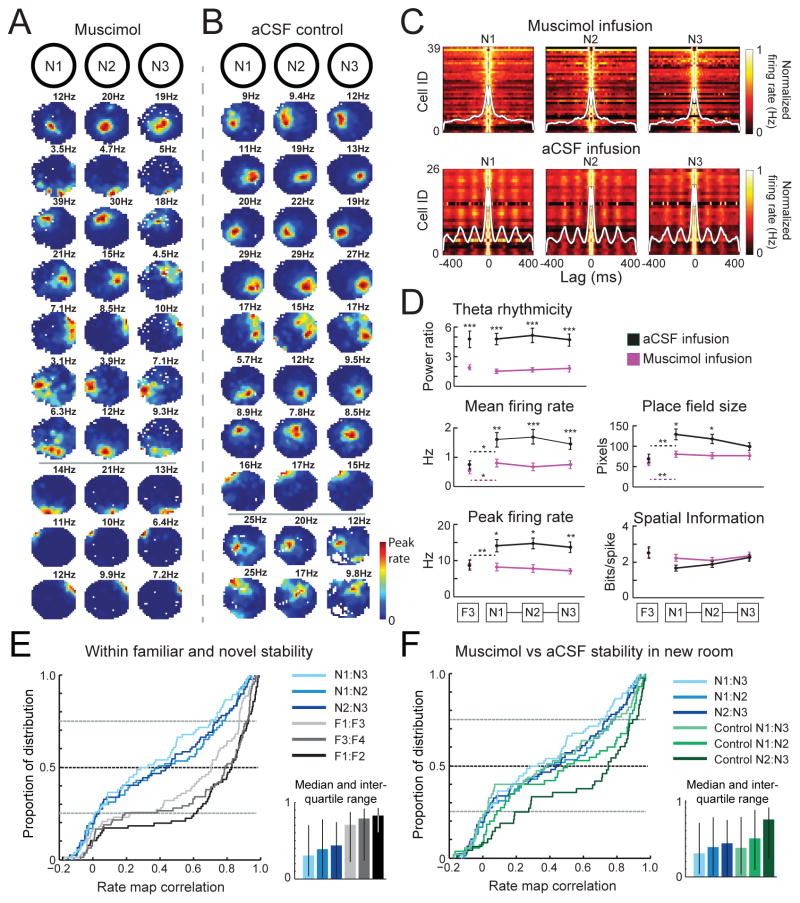
Figure 2.
Place fields emerge in a novel room during septal inactivation. (A) Firing rate maps of 10 representative hippocampal neurons from 2 representative rats (separated by horizontal line) in the novel room after intraseptal muscimol infusion. Each row is a cell. (B) Same as in (A), but from different cells in a novel room after intraseptal aCSF infusion. (C) Heat map of vertically stacked autocorrelograms from cells recorded in the novel room after muscimol (top) and aCSF (bottom) infusions. The average population autocorrelogram is overlaid in white. (D) Theta rhythmicity, mean firing rate, peak firing rate, place field size, and spatial information for cells recorded in F3, N1, N2, and N3 after intraseptal muscimol (purple) or aCSF (black) infusions. (E–F) Cumulative distribution functions (CDFs) were generated to compare distributions of rate map correlation scores between conditions. The median and inter-quartile ranges are shown as stippled line in the CDFs and are displayed to the right of each panel. (E) Comparisons are between pairs of novel room sessions after muscimol infusion (shades of blue) and between pairs of familiar room sessions (shades of gray; familiar room sessions include recordings before and after the muscimol infusion). All novel room comparisons differ from the familiar room comparisons. (F) Distributions in the novel room during muscimol are re-plotted from (E) and compared to rate map correlations in a novel room after control infusions.
Although place field parameters appear normal or sharpened, place fields may nonetheless show instability in their firing location. We examined whether the cells’ firing was at a consistent location across the series of recording sessions. First, we measured the spatial stability in the familiar environment for each place cell between baseline conditions (Figure 2E, F1:F2), between baseline and muscimol conditions (F1:F3), and between muscimol conditions (F3:F4). There were no differences in stability between any of the comparisons in familiar environments (all n.s.) (Table S2). Next, we measured the stability across the three sessions in the novel room. In the muscimol condition we found that all novel room comparisons (Figure 2E, N1:N2, N1:N3, N2:N3) showed decreased stability compared to familiar room comparisons (all novel room stability comparisons to F1:F2, P < 0.001 and to F3:F4, P < 0.05) (Table S2). A similar reduction of stability in the novel room was initially also seen in the control condition (N1:N2 and N1:N3, n.s. from muscimol condition), but control cells gained stability over the 30 minutes in the novel room so that they fired at more consistent locations than in the muscimol condition when the second and third 10-min period were compared (N2:N3, P < 0.05) (Figure 2F; Figure S5 and Table S2). These data therefore show that stability across recording sessions in the novel room during the inactivation was initially not different from control levels, but also suggest that septal inactivation may preclude further stabilization to familiar room levels.
Exposure to a sufficiently distinct environment is known to recruit an orthogonal population of place cells to represent the second room, a process known as global remapping (Leutgeb et al., 2005; Muller and Kubie, 1987). To test whether global remapping occurred between rooms during septal inactivation we examined whether a distinct cell population was active in the familiar compared to the novel environment by calculating the overlap between mean firing rates (maximum rate divided by minimum rate) for each cell (Figures 3A–C). Across repeated experiences in the same room, most cells were consistently activated and thus had high overlap values (Figure 3A). For comparisons between the familiar and the novel room, we found low overlap values indicating that cells were selectively active in one compared to the other environment (Figure 3B). Examination of firing rate maps of the few cells with high firing in both rooms showed dissimilar spatial firing patterns (Figure 3B). Distributions of overlap values from between-environment comparisons were substantially lower than distributions of overlap values from within-environment comparisons (all P values < 0.001) (Figure 3C). To further test whether the spatial maps in the novel room might show similarity to maps in the familiar room despite the difference in firing rate, we examined the possibility that firing rate maps were rotated between environments. We computed the spatial correlation between maps in the familiar and novel room after rotating one of the maps by 0, 90, 180, and 270 degrees and selected the highest correlation value. Rate map correlations between rooms remained substantially lower than correlations between repeated sessions within the novel room and lower than correlations between repeated sessions within familiar rooms (Figure 3D) (all P values < 0.001). Furthermore, when comparing the between-environment firing rate overlap and the between-environment rate map correlations with corresponding randomly shuffled distributions we found no differences (all n.s.) (Figure 3C–D). Together, these data demonstrate that a randomly selected population of hippocampal cells generated an orthogonal spatial representation of the novel room during the septal inactivation.
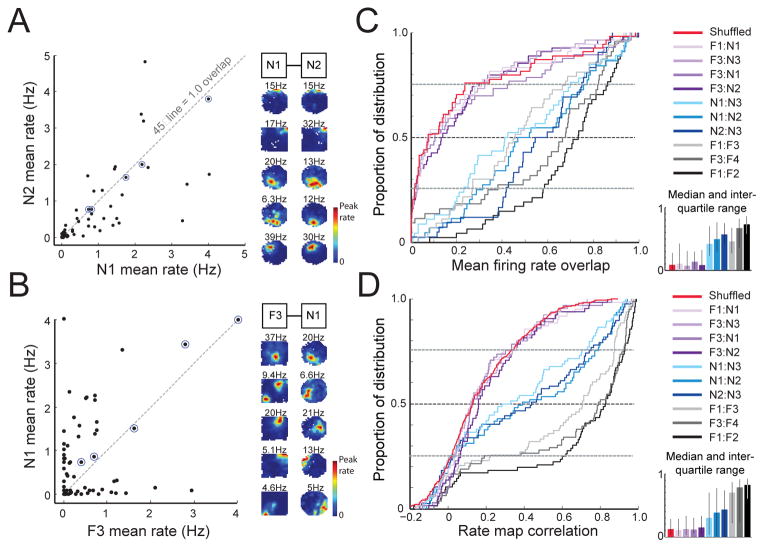
Figure 3.
Global remapping in the novel room during septal inactivation. (A–B) Scatterplots compare the mean firing rate of each cell between repeated sessions in the novel room (N1 and N2) and between the familiar and novel room (F3 and N1). Firing rate maps of the cells with the top five overlap scores (circled in blue) are displayed to the right of each scatterplot. Cells with high overlap scores between F3 and N1 show dissimilar spatial firing patterns. (C) CDFs of the rate overlap scores and (D) CDFs of rate map correlations. Comparisons are between repeated recordings in the familiar room (gray), between repeated recordings in the novel room (blue), and between familiar and novel room recordings (purple). The median and inter-quartile ranges are shown as stippled lines in the CDF and are displayed to the right of each panel. Red lines display shuffled distributions. Novel to familiar comparisons were not different from the shuffled distributions.
It is possible that grid cells are not necessary to establish new maps, but that the input from grid cells is nonetheless predominant in determining distinct spatial firing patterns in normal conditions. If this were the case, the place fields that had appeared in the novel room without grid cells can be expected to be substantially altered when intact grid cell input remerged. To test this possibility, we identified cells (n = 20) that were reliably tracked between the day of muscimol infusion and the following day, when theta amplitude and grid cell firing had recovered from the inactivation (Figure 4). Neurons that formed place fields in the novel room during the inactivation maintained their place field in the novel room on the day after the infusion (Figure 4A), similar to the stability of place fields in the familiar room (n novel cells = 12, n familiar cells = 11, n.s.). Across the two recording days, rate map stability within the familiar and within the novel environment across days was substantially higher than a shuffled distribution (both P values < 0.001). In addition, neurons that only had fields in the familiar room but not in the novel room during inactivation remained silent in the novel room 24 hours after infusion (n cells = 8, Figure 4B) with the exception of one neuron that was silent during inactivation but formed a field after theta oscillations had recovered (Figure 4C). These data show that the spatial representation that was generated in the novel room during septal inactivation was preserved after recovery from muscimol inactivation.
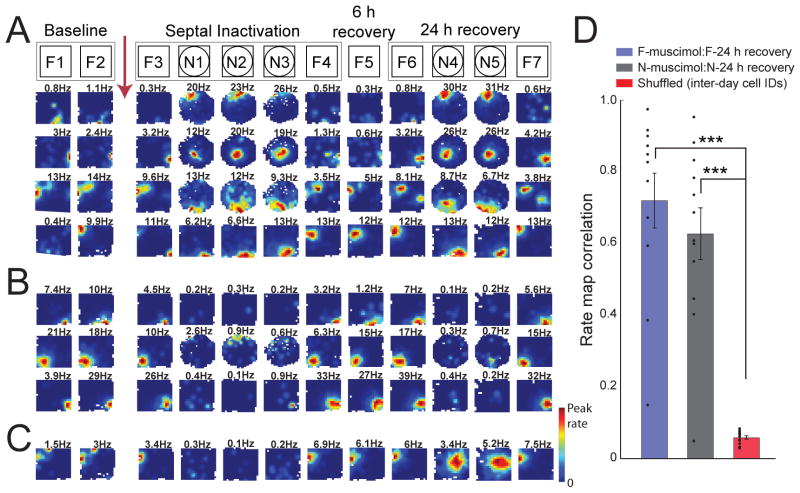
Figure 4.
Newly formed place fields are retained after recovery from septal inactivation. Novel room recordings were performed in either a circle or square environment in different experiments. (A) Firing rate maps show that the place fields that were formed in the novel room during septal inactivation (N1, N2, N3) are retained during retesting in the same room 24 hours after the infusion (N4, N5). In the familiar room, spatial firing patterns also match the patterns from the previous day. (B) Examples of place cells that were active in only the familiar room, but not in the novel room during any of the sessions. (C) Example of the only neuron (of twenty) that was silent during septal inactivation but generated a place field after recovery from septal inactivation. (D) Mean (± SEM) of cells’ rate map correlations between the inactivation and the recovery day. For the novel room, the averaged rate map during septal inactivation (N1, N2, N3) was compared to the average during recovery (N4, N5) (brown bar). For the familiar room, the average rate map of F1, F2, and F3 was compared to the average of the 24 hours maps (F6, F7). After 24 hours, fields that had emerged in the novel room during the inactivation on the previous day were as stable as those in a familiar room, and 24-hour stability within each of the rooms was substantially higher than for shuffled place field locations.
Discussion
We used muscimol inactivation of the medial septum to reduce the power of theta oscillations to levels known to reliably disrupt the spatial firing patterns of grid cells (Brandon et al., 2011). At the same level of theta reduction, we found that precise place fields emerged during the first recording session in a novel environment and that the fields remained stable across three 10-min sessions in the novel environment. The newly formed place fields conveyed the same amount of spatial information as those in the familiar room and as those that formed after control infusions. Moreover, the active set of neurons in the novel room and their spatial firing locations were entirely distinct from those in a familiar room. Finally, the fields that were generated during septal inactivation were retained after recovery from the inactivation. These results present a challenge to computational models that rely solely or predominantly on spatial inputs from grid cells to update spatial maps in the hippocampus.
The discovery of grid cells has led to the widespread notions that the hippocampus depends on inputs from grid cells to form place cells (Blair et al., 2008; Cheng and Frank, 2011; de Almeida et al., 2012; Hasselmo, 2009; Hayman and Jeffery, 2008; McNaughton et al., 2006; Molter and Yamaguchi, 2008; Monaco and Abbott, 2011; Rolls et al., 2006; Savelli and Knierim, 2010; Si and Treves, 2009; Solstad et al., 2006) and that the generation of new orthogonal hippocampal maps arises from the realignment of grid cell inputs (Barry et al., 2012; Fyhn et al., 2007; Hayman and Jeffery, 2008; Monaco and Abbott, 2011; Stensola et al., 2012). Several lines of evidence nonetheless suggest that it is premature to conclude that the flow of spatial information within the entorhino-hippocampal circuit is predominantly from grid cells to place cells (Brandon et al., 2014; Bush et al., 2014; Poucet et al., 2014). (1) The considerable diversity of place cell firing and entorhinal firing in double rotation experiments compared to the much more coherent response of grid cells and head direction cells suggest that place cells are not exclusively activated by a coherent grid cell input (Knierim et al., 2014). (2) During postnatal development, the emergence of hippocampal place cells precedes the emergence of completely regular grid cells (Langston et al., 2010; Wills et al., 2010). (3) Grid cells can be abolished by inactivating the medial septal area while hippocampal place fields retain their spatial specificity in familiar environments (Koenig et al., 2011). It is therefore possible that place cells can be reliably activated by inputs other than grid cells. However, the previous data allow for the interpretation that the complete loss of the grid cell fraction of entorhinal inputs to hippocampus may only be without consequence on place cells if the spatial firing patterns of place cells are well established. This aligns with theories of pattern completion, which suggest that the complete remembered information can be retrieved from partial inputs (Marr, 1971; Mcnaughton and Morris, 1987; Treves and Rolls, 1994).
If grid cells are not required for retaining well-established place fields, they may be predominantly required when new maps are first formed in novel environments such that their distinct inputs to hippocampus result in new configurations of hippocampal place cells (‘remapping’). Here we nonetheless found that remapping is intact at levels of theta reduction that selectively disrupted grid cells, as demonstrated by the activation of a new subpopulation of hippocampal cells with a unique configuration of place fields that was completely distinct from the familiar room (see Figure 3). These results are not consistent with models that propose that feedforward input from entorhinal grid cells generates new place cell configurations and that shifts between grid modules are necessary for global place cell remapping (Buzsaki and Moser, 2013; Fyhn et al., 2007; Monaco and Abbott, 2011; Rowland and Moser, 2014).
Navigation strategies can be grouped into two categories. First, a dead reckoning system can integrate translational movement by tracking running speed and movement direction. Several groups have hypothesized that grid cells perform this dead reckoning or path integration function (Burak and Fiete, 2009; McNaughton et al., 2006). Alternatively, a piloting system can use the perceived distance to and angle between landmarks to support navigation. This strategy may be supported by boundary cells that signal the distance from prominent landmarks (Lever et al., 2009; Solstad et al., 2008). Hippocampal place cells have been proposed to be jointly controlled by both navigation systems by integrating inputs from the grid cell system and the boundary cell system (Burgess and O’Keefe, 1996, 2011). Our finding that the hippocampus generates and maintains a stable representation of space during septal inactivation, when boundary cells are affected to a much lesser degree than grid cells (Brandon et al., 2011; Koenig et al., 2011) is consistent with models that predominantly rely on the representation of the distance to landmarks or boundaries to generate spatial maps (O’Keefe and Burgess, 1996; Shapiro and Hetherington, 1993; Zipser, 1985). However, it has also been suggested that place fields are primarily governed by path integration which sets up a pre-configured network with which other environmental cues are associated (McNaughton et al., 1996; Samsonovich and McNaughton, 1997). The question therefore arises whether grid cells, which may provide the path integration framework, would gain influence over place cells when they come back online. We show that spatial maps that emerged during septal inactivation were not substantially altered after recovery from septal inactivation, which suggests that the reemergence of grid cells did not overrule a landmark-based spatial representation. However, this does not exclude the possibility that grid cells may nonetheless gain joint control over place fields through a mechanism in which place cell information is used to sustain and align grid cells (Bonnevie et al., 2013; Kropff and Treves, 2008). Once both inputs are aligned, grid cells and boundary cells can provide redundant parallel spatial information, perhaps to support a self-localization system that is robust across environmental conditions and navigation strategies (Burgess and O’Keefe, 2011).
The reduction of theta oscillations has effects on spatial memory performance that are as deleterious as the effects of hippocampal lesions (O’Keefe and Nadel, 1978; Winson, 1978). This suggests that theta oscillations are necessary to temporally coordinate cell assemblies to support memory or that the effect is indirectly mediated by disrupting grid cells that participate in memory function (Buzsaki and Moser, 2013; Hasselmo, 2009). While our experimental approach is currently not able to distinguish between the relative importance of theta oscillations and grid cells for memory, we found that the generation of orthogonal spatial maps can proceed without either of these neural mechanisms. The results therefore challenge models that require theta oscillations for the spatial modulation of place cells (Lengyel et al., 2003; O’Keefe and Recce, 1993; Welday et al., 2011) and suggest that the generation of spatial maps is a basic function of the brain that can perhaps be supported solely by processing local stimuli (Miller and Best, 1980) without additional specialized computations that require the medial septal area. However, further stabilization of spatial maps and readout of spatial maps for memory functions may depend on additional mechanisms such as reciprocal network interactions between grid cells and place cells or the temporal coordination of these networks by the theta rhythm.
Experimental Procedures
Surgical and behavioral procedures
Six male Long-Evans rats (3–6 month old, 425–550 g) were used for single-unit and/or LFP recordings in this study. Prior to surgery, animals were trained daily for one week to forage for food reward. Animals were then implanted with a multi-electrode drive (‘hyperdrive’) aimed at the hippocampus and/or the entorhinal cortex and a drug infusion cannula aimed at the medial septal area. Following recovery from surgery, training continued in the same room as before surgery (i.e., the familiar room). Tetrode tips were positioned in the CA1 pyramidal cell layer, and septal infusion experiments began when single-units were well-isolated and stable. During each infusion experiment, single-units and LFPs were recorded in a series of 10-min sessions with 5-minute intersession intervals except for a longer interval after the muscimol (0.5 μl, 0.5 μg/μl in aCSF) or vehicle infusion into the medial septal area to allow for diffusion of the solution.
Data analysis
Tetrode locations were confirmed in histological material to terminate in or near the principal cell layer of region CA1. Methods for cluster cutting and cell tracking were the same as described previously (Brandon et al., 2011; Koenig et al., 2011), and only cells that could be reliably tracked were included in the analysis. We also compared the relative theta power before, during, and after inactivation of the septal area. In addition, theta rhythmicity of spike trains was quantified by determining the theta power in the spike time autocorrelation. The averaged population autocorrelation for each condition was calculated by averaging the autocorrelation for all cells in the condition.
Firing rate maps of cell spiking in the open field were constructed for 4 cm × 4 cm bins of position data and were smoothed with a pseudo-Gaussian kernel with a standard deviation of one pixel. The peak firing rate and the spatial information (bits/spike) were calculated from the smoothed rate maps of each 10-min session, and place field size was calculated for each cell with a minimum peak rate of 5 Hz as the number of adjacent pixels that contained equal to or greater than 20% of the firing rate of the pixel with the highest firing rate. The spatial correlation was calculated between 10-min sessions to determine similarity in spatial firing.
Statistical Analysis
Wilcoxon’s paired, two-sided sign rank test was used when comparing spatial firing properties across repeated exposures to same room, and the Wilcoxon’s two-sided rank sum test was used when comparing spatial properties between place cell populations that were active in the muscimol and control infusions. A two-sample Kolmogorov-Smirnov test was used to compare cumulative distribution functions for firing rate overlap and firing rate map correlation comparisons. For comparisons of the reduction in theta power, we used a two-sample repeated measure ANOVA.
Approvals
All surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of California, San Diego and conducted according to NIH guidelines.
Supplementary Material
Highlights.
Septal inactivation reduced theta power in hippocampus and entorhinal cortex
Theta power was reduced to levels that are known to disrupt entorhinal grid cells
Intact hippocampal place fields appear in a new room during the septal inactivation
The newly established place fields are retained after recovery from inactivation
Acknowledgments
We thank L. Debs, S. Danesh, B. Styble, and M. Wong for technical assistance. This research was supported by a NIH postdoctoral NRSA (FMH096531A) to M.P.B and grants from the Whitehall Foundation (#2012-0685), NSF/BMBF German-US collaboration (CRCNS-IIS-1010463), the Ellison Medical Foundation (AG-NS-0724-10), NIMH (1 R21 MH100354-01), and NINDS (1 R01 NS086947-01) to S.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barry C, Ginzberg LL, O’Keefe J, Burgess N. Grid cell firing patterns signal environmental novelty by expansion. Proc Natl Acad Sci U S A. 2012;109:17687–17692. doi: 10.1073/pnas.1209918109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes TL, Moser EI, Moser MB. Representation of Geometric Borders in the Developing Rat. Neuron. 2014 doi: 10.1016/j.neuron.2014.02.014. http://dx.doi.org/10.1016/j.neuron.2014.02.014. [DOI] [PubMed]
- Blair HT, Gupta K, Zhang K. Conversion of a phase- to a rate-coded position signal by a three-stage model of theta cells, grid cells, and place cells. Hippocampus. 2008;18:1239–1255. doi: 10.1002/hipo.20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnevie T, Dunn B, Fyhn M, Hafting T, Derdikman D, Kubie JL, Roudi Y, Moser EI, Moser MB. Grid cells require excitatory drive from the hippocampus. Nat Neurosci. 2013;16:309–317. doi: 10.1038/nn.3311. [DOI] [PubMed] [Google Scholar]
- Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, Hasselmo ME. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science. 2011;332:595–599. doi: 10.1126/science.1201652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MP, Koenig J, Leutgeb S. Parallel and convergent processing in grid cell, head-direction cell, boundary cell, and place cell networks. WIREs: Cogn Sci. 2014;5:207–219. doi: 10.1002/wcs.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun VH, Leutgeb S, Wu HQ, Schwarcz R, Witter MP, Moser EI, Moser MB. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57:290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Burak Y, Fiete IR. Accurate path integration in continuous attractor network models of grid cells. PLoS Comput Biol. 2009;5:e1000291. doi: 10.1371/journal.pcbi.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Becker S, King JA, O’Keefe J. Memory for events and their spatial context: models and experiments. Philos Trans R Soc Lond B Biol Sci. 2001;356:1493–1503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, O’Keefe J. Neuronal computations underlying the firing of place cells and their role in navigation. Hippocampus. 1996;6:749–762. doi: 10.1002/(SICI)1098-1063(1996)6:6<749::AID-HIPO16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Burgess N, O’Keefe J. Models of place and grid cell firing and theta rhythmicity. Curr Opin Neurobiol. 2011;21:734–744. doi: 10.1016/j.conb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D, Barry C, Burgess N. What do grid cells contribute to place cell firing? Trends Neurosci. 2014;37(3):136–145. doi: 10.1016/j.tins.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Frank LM. The structure of networks that produce the transformation from grid cells to place cells. Neuroscience. 2011;197:293–306. doi: 10.1016/j.neuroscience.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida L, Idiart M, Lisman JE. The single place fields of CA3 cells: a two-stage transformation from grid cells. Hippocampus. 2012;22:200–208. doi: 10.1002/hipo.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. A model of episodic memory: mental time travel along encoded trajectories using grid cells. Neurobiol Learn Mem. 2009;92:559–573. doi: 10.1016/j.nlm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman RM, Jeffery KJ. How heterogeneous place cell responding arises from homogeneous grids--a contextual gating hypothesis. Hippocampus. 2008;18:1301–1313. doi: 10.1002/hipo.20513. [DOI] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. Network dynamics underlying the formation of sparse, informative representations in the hippocampus. J Neurosci. 2008;28:14271–14281. doi: 10.1523/JNEUROSCI.4261-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Neunuebel JP, Deshmukh SS. Functional correlates of the lateral and medial entorhinal cortex: objects, path integration and local-global reference frames. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130369. doi: 10.1098/rstb.2013.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J, Linder AN, Leutgeb JK, Leutgeb S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science. 2011;332:592–595. doi: 10.1126/science.1201685. [DOI] [PubMed] [Google Scholar]
- Kropff E, Treves A. The emergence of grid cells: Intelligent design or just adaptation? Hippocampus. 2008;18:1256–1269. doi: 10.1002/hipo.20520. [DOI] [PubMed] [Google Scholar]
- Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser EI, Moser MB. Development of the spatial representation system in the rat. Science. 2010;328:1576–1580. doi: 10.1126/science.1188210. [DOI] [PubMed] [Google Scholar]
- Lengyel M, Szatmary Z, Erdi P. Dynamically detuned oscillations account for the coupled rate and temporal code of place cell firing. Hippocampus. 2003;13:700–714. doi: 10.1002/hipo.10116. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, Jeewajee A, O’Keefe J, Burgess N. Boundary vector cells in the subiculum of the hippocampal formation. J Neurosci. 2009;29:9771–9777. doi: 10.1523/JNEUROSCI.1319-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, et al. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Mcnaughton BL, Morris RGM. Hippocampal Synaptic Enhancement and Information-Storage within a Distributed Memory System. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- Miller VM, Best PJ. Spatial correlates of hippocampal unit activity are altered by lesions of the fornix and endorhinal cortex. Brain Res. 1980;194:311–323. doi: 10.1016/0006-8993(80)91214-7. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, McNaughton BL, Barnes CA, Fox KB. Preserved spatial coding in hippocampal CA1 pyramidal cells during reversible suppression of CA3c output: evidence for pattern completion in hippocampus. J Neurosci. 1989;9:3915–3928. doi: 10.1523/JNEUROSCI.09-11-03915.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molter C, Yamaguchi Y. Impact of temporal coding of presynaptic entorhinal cortex grid cells on the formation of hippocampal place fields. Neural Netw. 2008;21:303–310. doi: 10.1016/j.neunet.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Monaco JD, Abbott LF. Modular realignment of entorhinal grid cell activity as a basis for hippocampal remapping. J Neurosci. 2011;31:9414–9425. doi: 10.1523/JNEUROSCI.1433-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; 1978. [Google Scholar]
- O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Poucet B, Sargolini F, Song EY, Hangya B, Fox S, Muller RU. Independence of landmark and self-motion-guided navigation: a different role for grid cells. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130370. doi: 10.1098/rstb.2013.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Stringer SM, Elliot T. Entorhinal cortex grid cells can map to hippocampal place cells by competitive learning. Network. 2006;17:447–465. doi: 10.1080/09548980601064846. [DOI] [PubMed] [Google Scholar]
- Rotenberg A, Muller RU. Variable place-cell coupling to a continuously viewed stimulus: evidence that the hippocampus acts as a perceptual system. Philos Trans R Soc Lond B Biol Sci. 1997;352:1505–1513. doi: 10.1098/rstb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland DC, Moser MB. From cortical modules to memories. Curr Opin Neurobiol. 2014;24C:22–27. doi: 10.1016/j.conb.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Samsonovich A, McNaughton BL. Path integration and cognitive mapping in a continuous attractor neural network model. J Neurosci. 1997;17:5900–5920. doi: 10.1523/JNEUROSCI.17-15-05900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelli F, Knierim JJ. Hebbian analysis of the transformation of medial entorhinal grid-cell inputs to hippocampal place fields. J Neurophysiol. 2010;103:3167–3183. doi: 10.1152/jn.00932.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, Hetherington PA. A simple network model simulates hippocampal place fields: parametric analyses and physiological predictions. Behav Neurosci. 1993;107:34–50. doi: 10.1037//0735-7044.107.1.34. [DOI] [PubMed] [Google Scholar]
- Si B, Treves A. The role of competitive learning in the generation of DG fields from EC inputs. Cogn Neurodyn. 2009;3:177–187. doi: 10.1007/s11571-009-9079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- Solstad T, Moser EI, Einevoll GT. From grid cells to place cells: a mathematical model. Hippocampus. 2006;16:1026–1031. doi: 10.1002/hipo.20244. [DOI] [PubMed] [Google Scholar]
- Stensola H, Stensola T, Solstad T, Froland K, Moser MB, Moser EI. The entorhinal grid map is discretized. Nature. 2012;492:72–78. doi: 10.1038/nature11649. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Van Cauter T, Poucet B, Save E. Unstable CA1 place cell representation in rats with entorhinal cortex lesions. Eur J Neurosci. 2008;27:1933–1946. doi: 10.1111/j.1460-9568.2008.06158.x. [DOI] [PubMed] [Google Scholar]
- Welday AC, Shlifer IG, Bloom ML, Zhang K, Blair HT. Cosine directional tuning of theta cell burst frequencies: evidence for spatial coding by oscillatory interference. J Neurosci. 2011;31:16157–16176. doi: 10.1523/JNEUROSCI.0712-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Cacucci F, Burgess N, O’Keefe J. Development of the hippocampal cognitive map in preweanling rats. Science. 2010;328:1573–1576. doi: 10.1126/science.1188224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Ye J, Miao C, Tsao A, Cerniauskas I, Ledergerber D, Moser MB, Moser EI. Optogenetic dissection of entorhinal-hippocampal functional connectivity. Science. 2013;340:1232627. doi: 10.1126/science.1232627. [DOI] [PubMed] [Google Scholar]
- Zipser D. A computational model of hippocampal place fields. Behav Neurosci. 1985;99:1006–1018. doi: 10.1037//0735-7044.99.5.1006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.