Abstract
Aims
Glucuronoxylomannan (GXM) is the major polysaccharide component of Cryptococcus neoformans. We evaluated in this study whether GXM fractions of different molecular masses were functionally distinct.
Materials & methods
GXM samples isolated from C. neoformans cultures were fractionated to generate polysaccharide preparations differing in molecular mass. These fractions were used in experiments focused on the association of GXM with cell wall components of C. neoformans, as well as on the interaction of the polysaccharide with host cells.
Results & conclusion
GXM fractions of variable molecular masses bound to the surface of a C. neoformans acapsular mutant in a punctate pattern that is in contrast to the usual annular pattern of surface coating observed when GXM samples containing the full molecular mass range were used. The polysaccharide samples were also significantly different in their ability to stimulate cytokine production by host cells. Our findings indicate that GXM fractions are functionally distinct depending on their mass.
Keywords: Cryptococcus neoformans, glucuronoxylomannan, host response, molecular mass, polysaccharides
Background
Polysaccharides are key regulators of virulence in a number of bacterial and fungal infections [1,2]. These molecules typically consist of complex carbohydrates and are composed of at least twenty monosaccharide units connected by glycosydic linkages [3]. In contrast to proteins and nucleic acids, the cellular synthesis of polysaccharides does not involve the typical scaffolds used in transcriptional and translational processes, and there is an absence of codon-like structures to regulate the termination of polysaccharide synthesis. Therefore, one key and general characteristic of microbial polysaccharides is their heterogeneity in molecular mass, which results in molecules with identical composition but variable dimensions [3,4]. Hence, by their very nature, polysaccharides are heterodisperse.
It is uncertain whether diversity in polysaccharide mass translates into functional variability. A recent study suggested that polysaccharide preparations with diverse molecular masses differed in function [5]. Chitin, a linear polysaccharide that is abundant in insect and fungal cells, stimulated cell-mediated immune responses through size-dependent mechanisms [5,6]. While chitin molecules of higher dimensions and, consequently, molecular masses, are immunologically inert, small polysaccharide fractions are efficient stimulators of IL-17 production by macrophages [5]. This observation provided the first precedent showing that polysaccharide molecules of identical composition but variable molecular mass might differ in function.
Polysaccharide synthesis is essential for the pathogenesis of cryptococcosis, a fungal lung infection that can evolve to meningitis in immunosup-pressed individuals [1]. Cryptococcosis is caused by the fungal pathogens Cryptococcus neoformans and Cryptococcus gattii [7]. C. neoformans preferentially causes disease in immunosuppressed patients, while C. gattii is a primary pathogen for immunocompetent individuals [8]. AIDS-associated cryptococcal meningitis caused by C. neoformans is the most fatal infection of the CNS in humans [8,9]. In fact, the global burden of C. neoformans-related disease is estimated to be approximately one million people per year, resulting in more than 500,000 annual deaths [9].
Cryptococcal virulence traits include a poly-saccharide capsule, which is mainly composed of glucuronoxylomannan (GXM) [1]. This polysaccharide consists of an α-1,3-linked mannan substituted with β-1,2- and/or β-1,4-linked glucuronyl and xylosyl units, which results in serological diversity [10,11]. Structural differences in polysaccharide structure translate into antigenic differences that have allowed the characterization of C. neoformans and C. gattii strains into four serotypes known as A–D. Serotypes A and D of GXM are associated with C. neoformans species, while the B and C serotypes are associated with C. gattii [1]. Capsule formation in cryptococci requires poly-saccharide export to the extracellular space [12], followed by cation-mediated aggregation of GXM molecules of variable dimensions at the cell surface [13,14]. It is well recognized that both extracellular and capsule-linked GXM are heterodisperse with regards to molecular mass and dimensions [1,13]. Little is known about the relationship between molecular masses and biological functions of GXM, but a previous study suggested an inverse correlation between the molecular dimensions of this polysaccharide and its ability to stimulate Toll-like receptor (TLR)-mediated responses and nitric oxide production [15]. These effects, however, were restricted to serotype B samples of C. gattii. The relationship between dimensions and functions in other GXM serotypes is unknown.
In this study, GXM samples isolated from C. neoformans cultures were fractionated to generate polysaccharide preparations differing in molecular mass. These fractions were used in experiments focused on the association of GXM with cell wall components of C. neoformans, as well as on the interaction of the polysaccharide with host cells. Our results demonstrated that GXM fractions of different molecular masses are functionally distinct, which supports the notion that monosaccharide composition and glycosyl conformation are not the only structural parameters defining the functions of polysaccharides [3]. These results also add considerable complexity to the interpretation of the functions of capsular components in Cryptococcus species.
Materials & methods
Fungal strains & polysaccharide fractionation
Cryptococcal strains used in this study included the standard serotype A isolate H99 [16] and the acapsular mutant cap67Δ [17]. For experiments using acapsular cells, the mutant was cultivated for 48 h at 25°C in a minimal medium composed of glucose (15 mM), MgSO4 (10 mM), KH2PO4 (29.4 mM), glycine (13 mM) and thiamine-HCl (3 μM); pH 5.5. GXM was obtained by ultra-filtration of culture supernatants of encapsulated cells [14]. Cryptococcus cells (4 × 109) were inoculated in 100 ml of the same minimal medium. After cultivation for 48 h at 25°C, each fungal suspension was used to inoculate 300 ml of minimal medium in a 1000-ml Erlenmeyer flask. Fungal cultures were then incubated for 4 days at 25°C, with shaking. Culture supernatants were obtained by sequential centrifugation (4000 × g, 15 min, 4°C; then 15,000 × g, 15 min, 4°C) to ensure removal of cells and debris. The resulting culture fluid was then ultrafiltered in an Amicon (Millipore, MA, USA) system (1-kDa cut-off;), as previously described [14,15]. The polysaccharide films formed over the ultrafiltration discs [14] were collected with a cell scraper for carbohydrate determination by the Dubois method [18]. Alternatively, GXM was fractionated on the basis of its great molecular mass diversity [13–15,19]. In these assays, the supernatant fractions were first passed through a 300-kDa membrane and the GXM film was collected with a cell scraper, as described above. The filtered supernatant was again concentrated using a 100-kDa filtration disc and the process was sequentially repeated using discs with 10- and 1-kDa cut-offs. The viscous layers were again collected, as detailed above, and used for quantitative and functional determinations. The procedure described above therefore resulted in GXM samples containing its full molecular mass range and fractions in molecular mass ranges of 1–10, 10–100, 100–300 and >300 kDa. GXM samples had their dimensions determined by dynamic light scattering in a 90Plus/BI-MAS Multi Angle Particle Sizing analyzer (Brookhaven Instruments Corp., NY, USA), as described [13]. The serological reactivity of GXM in the different fractions obtained by ultrafiltration was analyzed by ELISA and dot blot, as previously described [14,20,21].
Coating acapsular C. neoformans cells with GXM
The method used for incorporation of GXM by acapsular C. neoformans cells (strain cap67Δ) adapted from the model described by Reese and Doering [17]. Acapsular yeast cells (106) were suspended in GXM solutions of different molecular masses (100 μl phosphate-buffered saline [PBS], 10 μg/ml) and incubated for the extended period of 4 h at 25°C, followed by extensive washing with PBS. In some systems, the cells were sequentially incubated with fractions of increasing (1–10, 10–100, 100–300 and >300 kDa) or decreasing (>300, 100–300, 10–100 and 1–10 kDa) molecular masses, under similar conditions. Control systems consisted of cap67Δ cells incubated in minimal medium. The cells were washed with PBS and prepared for immunofluorescence using IgG and M to GXM, as follows. Monoclonal antibodies (mAbs) to GXM included 12A1 and 13F1, two clonally related IgMs that differ in fine specificity [22]. mAb 12A1 produces annular immuno-fluorescence on C. neoformans, while mAb 13F1 produces punctate annular immunofluorescence [15,22]. Another IgM used in these assays was mAb 2D10, which reacts with cell wall and capsule epitopes [23]. mAb 18B7 is a protective IgG1 that reacts with all GXM serotypes [24]. C. neoformans cells (106) were fixed with 4% paraformaldehyde. The cells were further blocked for 1 h in PBS containing 1% bovine serum albumin and incubated with the mAbs described above (10 μg/ml) for 1 h at room temperature, followed by Alexa Fluor® 488/568 Goat antimouse (IgG or IgM) secondary antibodies (Life Technologies, São Paulo, Brazil). Surface coating was finally analyzed with an Axioplan 2 fluorescence microscope using an AxioCam MRc digital camera and the AxioVision 4.8 software (Zeiss, Oberkochen, Germany). Controls consisted of similar systems, except for the replacement of GXM-binding mAbs by isotype-matched irrelevant antibodies.
Binding of GXM to macrophages
Bone marrow-derived macrophages were obtained from wild-type (WT), CD14−/− or Tlr2−/− mice (C57BL/6, gender matched, 6–10 weeks old), as described previously [25]. Mice were kindly donated by Drs Shizuo Akira (Osaka University, Japan) and Douglas Golenbock (University of Massachusetts, USA). The Animal Ethics Committee at the Federal University of Rio de Janeiro (Brazil) approved the animal protocols. Bone marrow was harvested from the tibias and femurs of mice and differentiated into bone marrow-derived macrophages [25]. The cells were suspended in Roswell Park Memorial Institute (RPMI) medium 1640 medium supplemented with 20% of fetal calf serum (FCS) and 30% of supernatant from L929 cells cultures to form a density of 5 × 106 cells/10 ml. The cells were maintained in a 5% CO2 atmosphere for 3 days and then fresh medium was added to the cultures. At day 6, 5 × 105 macrophages were added to the wells of 24-well plates containing RPMI 1640 medium with 10% of FCS. The cells were cultivated for 12 h before the experiments described below. Alternatively, the murine macrophage cell line RAW 264.7 (American Type Culture Collection, MD, USA) was used in GXM-binding assays. The phagocytes were grown to confluence in 24-well plates containing Dulbecco’s modified Eagle’s medium, supplemented with 10% fetal bovine serum, at 37°C in a 5% CO2 atmosphere. The medium was replaced by fresh Dulbecco’s modified Eagle’s medium supplemented with GXM (10 μg/ml), followed by incubation at 37°C for 5–60 min. Unbound GXM was removed by extensive washing with PBS. Cell suspensions were obtained after detaching monolayers from the plastic support with PBS containing 1 mM ethylenediaminetetraacetic acid. The cells were then fixed with 4% paraformaldehyde and incubated for 1 h at 37°C in the presence of PBS supplemented with 1 mg/ml bovine serum albumin. After washing with PBS, cell suspensions were incubated with mAb 18B7 (10 μg/ml) for 1 h at room temperature. The cells were again washed with PBS and incubated with a Alexa Fluor® 488 goat anti-mouse (IgG or IgM) antibody (Life Technologies). The cells were then analyzed in a FACS Calibur (BD Biosciences, CA, USA) flow cytometer and data were processed with FCS Express 4 Plus (DeNovo Software, CA, USA [26]). Controls consisted of similar preparations where the anti-GXM antibody was replaced by an isotype-matched irrelevant antibody.
Cytokine determination
The cytokine response to polysaccharide samples was evaluated in vitro and in vivo. For the in vitro analysis, peritoneal macrophages were obtained (C57BL/6, gender matched, 6–10 weeks old), as previously described [27].
The cells were stimulated with the GXM fractions (10 μg/ml) for 18 h in RPMI and then culture supernatants were collected for cytokine determination using commercially available kits from Peprotech (NJ, USA) and R&D (MN, USA) according to the manufacturers’ instructions. Positive controls (not shown) were stimulated with 100 ng ultrapure lipopolysaccharide (InvivoGen, CA, USA, tlrl-peklps) or 200 ng pam3csk4. Negative controls were stimulated with sterile PBS. In vivo cytokine analysis was performed on the basis of a proof-of-concept model that was previously established by our group [28]. In this model, the differential ability of polysaccharide fractions to induce certain cytokines was considered rather than its significance for the anti-GXM immune response. Before GXM administration, mice (female BALB/c, 4–8 weeks old, n = 5) were given ketamine (0.125 mg/g) and xylazine (0.01 mg/g) intraperitoneally for anesthesia. Each animal was then treated intranasally with GXM (50 μl, 500 μg/ml in PBS). GXM samples tested in vivo included the full molecular mass range sample and fractions in the molecular mass ranges of 10–100 and >300 kDa. After 24 h, the animals were sacrificed by cervical hyperextension. For cytokine determination, the lungs were excised and weighted for further normalization. The lungs were macerated in PBS and then the tissular suspension was clarified by centrifugation. IL-10 and TNF-α [28] were quantified using the DuoSet ELISA Development System kit (R&D System) following the manufacturer’s instructions. Lipopolysaccharide-free water and glassware were used in all experiments to avoid interference with the cytokine response.
Statistical analysis
Paired groups were statistically analyzed using the Student’s t test. Statistical tests of multiple groups required analysis of variance (two-way). The software used for statistical analysis was GraphPad Prism (version 6.0; GraphPad Software, CA, USA).
Results
Size determination of GXM & serological tests
The great diversity in GXM dimensions [1,13–15] allows fractionation of the polysaccharide into samples distributed in different ranges of molecular mass [15]. On the basis of this particular property, we obtained, by ultrafiltration [14], polysaccharide fractions distributed in the molecular mass ranges of 1–10, 10–100, 100–300, and >300 kDa. For a didactic purpose, we classified as low-mass samples the fractions with molecular masses below 100 kDa. Fractions with molecular masses greater than 100 kDa were classified as high-mass samples. Molecular dimensions of polysaccharides correlated positively with their masses [29]. Therefore, since the principal aim of this study was to investigate the relationship between the biological functions of GXM and the diversity in its molecular mass, we first evaluated the dimensions of all polysaccharide fractions used in this work. For this analysis, GXM dimension was determined by dynamic light scattering, using protocols that were recently established by our group [13].
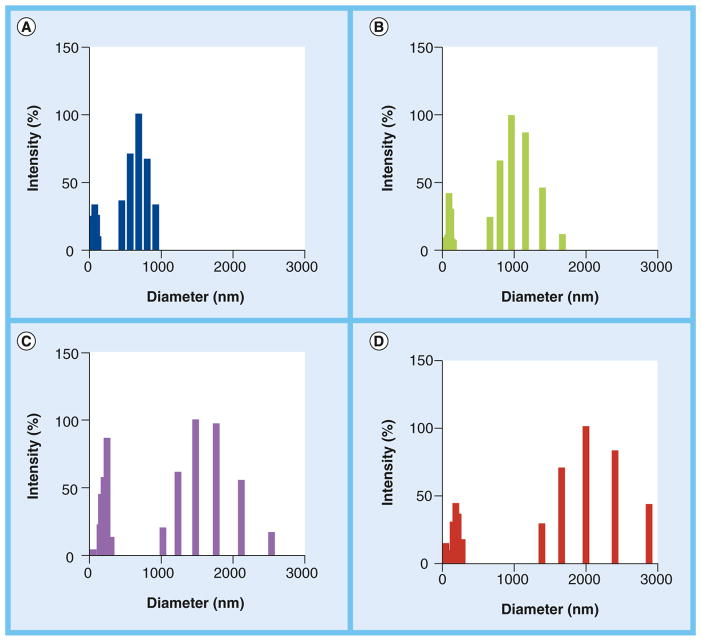
The distinct molecular mass ranges resulted in clear differences in polysaccharide dimensions, as concluded from the dynamic light scattering analysis of polysaccharide fractions (Figure 1). Polysaccharide molecules of 1–10 kDa were distributed in the 500–1000-nm diameter range, while the 10–100-kDa fraction gave a profile of diameter distribution between 800 and 1800 nm. Higher mass samples were distributed into the diameter ranges of 1000–2500 nm (100–300kDa) and 1500–3000 nm (>300 kDa). All samples contained smaller molecules in the range of 0–20 nm. The nature of these molecules is still unknown, but we speculate that they may correspond to low-mass polysaccharides dissociated from larger GXM aggregates formed through noncovalent interactions [14]. Since these molecules were common to all fractions, we assumed that they did not influence the biological parameters investigated in this study. In summary, we concluded that GXM was successfully fractionated into samples that differed in molecular mass and, consequently, dimensions.
Figure 1. Dynamic light-scattering analysis of glucuronoxylomannan fractions obtained by ultrafiltration.
Polysaccharide dimensions were proportional to the molecular mass cut-off used for fractionation, as concluded from the analysis of glucuronoxylomannan samples in the ranges of (A) 1–10, (B) 10–100, (C) 100–300 and (D) >300 kDa.
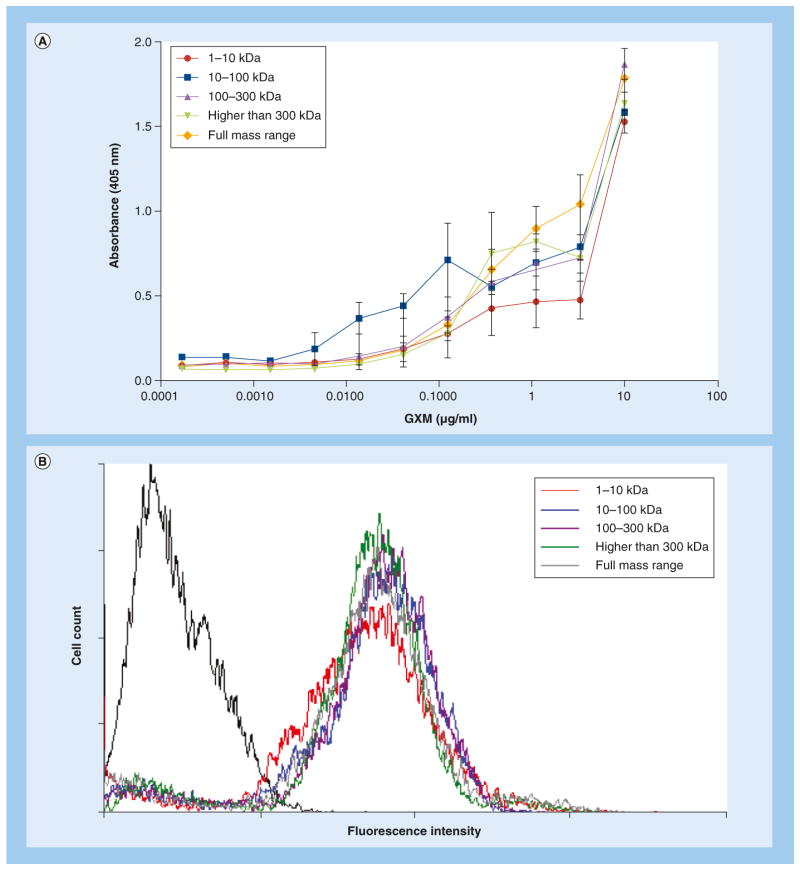
Most of the experiments in this study required protocols of polysaccharide detection by serologic approaches using mAb 18B7, an antibody to GXM that has been consistently used as a tool for polysaccharide analysis in the C. neoformans model [24,30]. Since differences in the serological properties of the GXM fractions used in this study could lead to misinterpretation of data, we evaluated the reactivity of all polysaccharide samples with mAb 18B7. The antibody recognized all polysaccharide fractions in a similar fashion (Figure 2A). The results obtained by ELISA were similar to those obtained by dot blot analysis of polysaccharides with mAb 18B7 and other antibodies (data not shown). Since some of our functional studies also required GXM incorporation by cap67Δ cells, which lack a detectable capsule but retain the ability to incorporate exogenous GXM [17], the polysaccharide fractions were tested for their ability to coat acapsular cells. The reactivity of GXM-coated cells with mAb 18B7 was analyzed by flow cytometry, which revealed similar levels of antibody-derived fluorescent reactions independently of the polysaccharide fraction used (Figure 2B). In conclusion, these results validated mAb 18B7 as a tool for the analysis of the different GXM fractions used in this study.
Figure 2. Serologic properties of glucuronoxylomannan fractions of different molecular masses.
(A) Reactivity of polysaccharide fractions distributed in different molecular mass ranges with monoclonal antibody 18B7. The dose-dependent profile of serologic reactivity was similar for all fractions. (B) Incorporation of the different GXM fractions by acapsular Cryptococcus neoformans cells for further serological detection by flow cytometry with monoclonal antibody 18B7. Similar levels of fluorescent intensity were observed for all fractions. The isolated histogram on the left (black line) represents the background fluorescence levels of cap67Δ cells that were not coated with GXM fractions.
GXM: Glucuronoxylomannan.
For color image please see online at www.futuremedicine.com/doi/full/10.2217/FMB.13.163
Different molecular masses of GXM are required for full coating of the C. neoformans surface
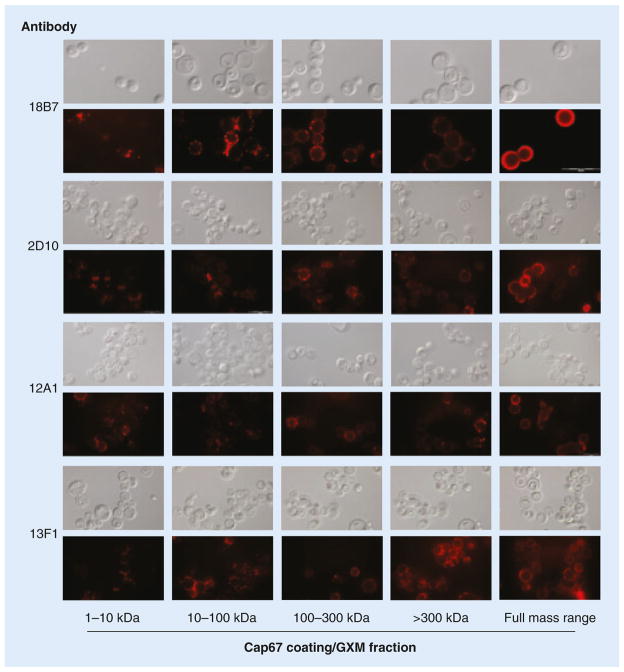
The morphological aspects of GXM incorporation by acapsular cells were evaluated by fluorescence microscopy (Figure 3), which revealed important differences between distinct GXM fractions. We observed that samples of GXM at the lower end of the molecular mass range (1–10 and 10–100 kDa) preferably accumulated at cell division sites. By contrast, higher-mass fractions (100–300 and >300 kDa) generated a punctate pattern of GXM binding that was not restricted to any specific area of the cell surface. Most interestingly, the typical annular pattern of polysaccharide incorporation into the cell surface of cap67Δ cells was only observed when the cells were exposed to fractions containing the full molecular mass diversity of GXM. The results described above were observed after incubation of GXM-coated cells with mAb 18B7. To avoid the possibility that these observations were artifacts derived from the use of this specific antibody, the experiments were repeated using three other mAbs, with similar results (Figure 3).
Figure 3. Surface distribution of GXM in acapsular (cap67Δ) Cryptococcus neoformans cells after incubation of cells with different fractions of the polysaccharide.
GXM detection was based on the serologic reactivity of yeast cells with four different antibodies to the polysaccharide, listed on the left. Molecular mass ranges are indicated on the bottom. Cryptococcus neoformans cells are shown under the differential interferential contrast and red fluorescence modes (gray and black panels, respectively). Scale bar in bottom-right 18B7 panel represents 20 μm.
GXM: Glucuronoxylomannan.
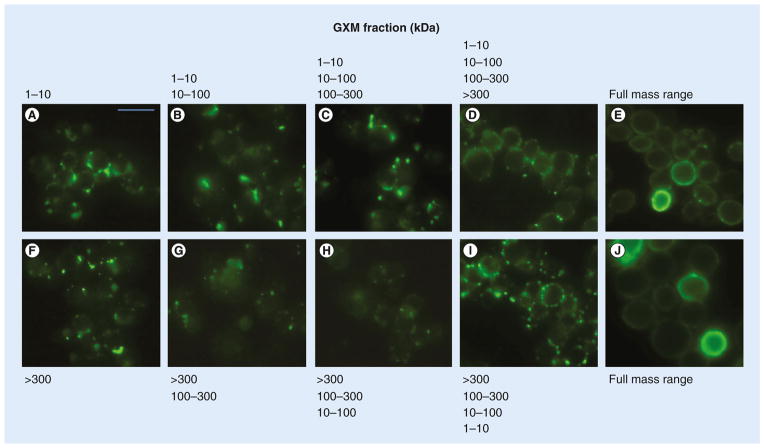
On the basis of the results described above, we could not rule out the possibility that partial combinations of GXM fractions would be sufficient for complete surface coating. Therefore, to gain a better understanding of how GXM fractions with different molecular masses interacted with the cell wall of C. neoformans, we performed sequential incubations of cap67Δcells with the GXM samples obtained by ultrafiltration. These experimental systems included assays where acapsular cells were sequentially incubated with fractions of increasing (1–10, 10–100, 100–300 and >300 kDa) or decreasing (>300, 100–300, 10–100 and 1–10 kDa) molecular masses. Once again, full surface coating with GXM was achieved only when all fractions were combined for incubation with cap67Δ cells (Figure 4). This observation is in agreement with the supposition that multiple molecular masses of GXM are required for a more homogeneous surface coating of C. neoformans (Figure 3).
Figure 4. Sequential incubations of acapsular Cryptococcus neoformans cells with different polysaccharide fractions.
Cryptococcus neoformans cells were sequentially coated with fractions of increasing or decreasing molecular masses. (A–D) represent sequential incubations of cap67Δ cells with fractions of increasing molecular masses, starting with (A) the 1–10 kDa sample alone, followed by (B–D) samples of higher masses and (E & J) the full molecular mass range. (F–I) show sequential incubations of acapsular cells with fractions with decreasing molecular masses, starting with (F) the fraction >300 kDa alone, followed by (G–I) samples of lower masses. Scale bar represents 20 μm.
GXM: Glucuronoxylomannan.
GXM fractions with distinct masses manifest different profiles of interaction with host cells
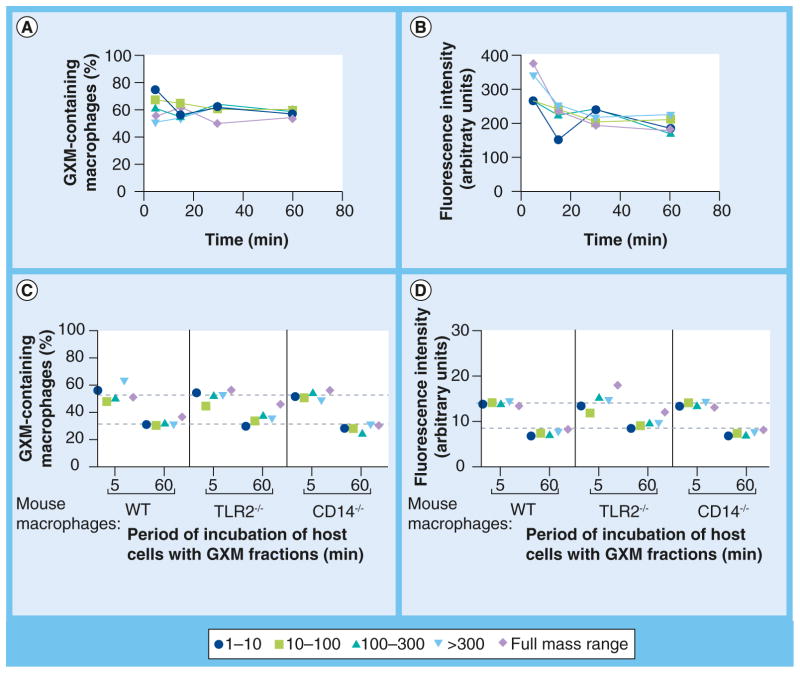
The results obtained with acapsular C. neoformans cells indicated that GXM fractions of different molecular masses have diverse patterns of interaction with surface fungal ligands (Figures 3 & 4). The supposition that the interaction of GXM with cell wall components of C. neoformans might require polysaccharide molecules of similar composition but different dimensions raised the possibility that similar mechanisms could occur with host cell molecules recognizing GXM. To test this possibility, we incubated murine macrophages with polysaccharide fractions of variable molecular masses for further GXM detection with mAb 18B7. At initial stages of incubation, the percentage of GXM-containing macrophages slightly varied according to the sample used, but all GXM fractions produced similar levels of polysaccharide-containing phagocytes after 60 min (Figure 5A). Similar results were obtained when the intensity of the fluorescent reactions was analyzed (Figure 5B). We also evaluated the binding profiles of the different polysaccharide fractions to macrophages lacking the well-characterized GXM receptors TLR2 [15,31] and CD14 [31,32], in comparison with WT phagocytes. Macrophages obtained from WT, Tlr2−/− or CD14−/− mice were incubated with the different GXM fractions for 5 or 60 min. In all systems, both the percentage of GXM-containing macrophages (Figure 5C) and the indices of fluorescence intensity (Figure 5D) were higher after the 5-min incubation, which was consistent with the kinetics vdescribed in Figure 5A & B and with the previously characterized early internalization of GXM by mouse macrophages [33]. All polysaccharide fractions were similar in their ability to bind to macrophages from WT and knockout mice. These results strongly suggest that the molecular mass of GXM does not influence its interaction with host receptors.
Figure 5. Binding of glucuronoxylomannan fractions with variable molecular masses to murine macrophages.
The phagocytes were exposed to the different molecular mass samples of GXM and then incubated with monoclonal antibody 18B7 for flow cytometry analysis. The percentage of GXM-containing macrophages as a function of time is shown in (A), while the intensity of fluorescent reactions is shown in (B). Polysaccharide-binding was also tested in macrophages obtained from WT mice and knockout animals lacking the GXM receptors TLR2 and CD14. (C) Analyses of the percentage of GXM-containing macrophages and (D) fluorescence levels of the phagocytes indicate similar profiles of GXM binding to all phagocytes, independently of the molecular mass of the polysaccharide. In all cases, binding efficacy was higher after the 5-min period of incubation. Data are representative of two independent experiments producing similar results.
GXM: Glucuronoxylomannan; TLR: Toll-like receptor; WT: Wild-type.
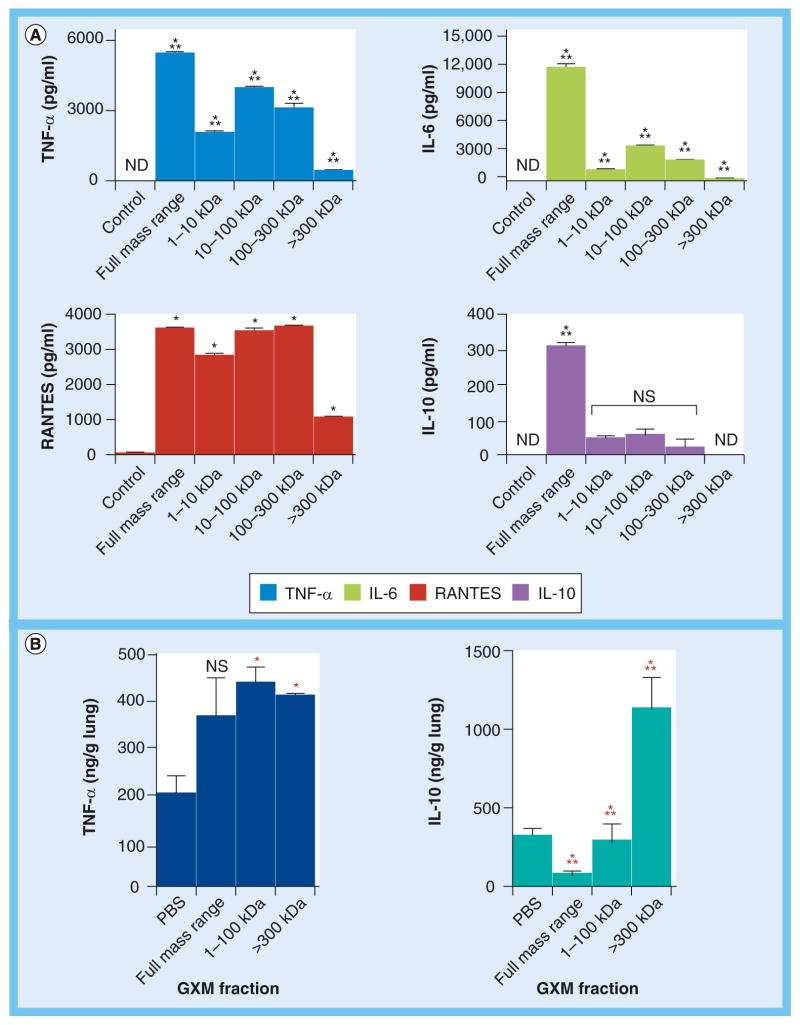
The results described above suggested that GXM molecules of variable dimensions were recognized by host cells with similar efficiency. However, they do not discriminate eventually different cellular responses. To assess this possibility, we used a proof-of-concept model testing cytokines that are usually produced in response to the activation of GXM-binding receptors [15,31,34–36]. Mouse peritoneal macrophages were treated in vitro with the different GXM fractions, and four cytokines (TNF-α, RANTES, IL-10 and IL-6) were quantified in culture supernatants (Figure 6A). For all cytokines tested in vitro, the fraction with molecular mass >300 kDa manifested the lowest effectiveness in stimulating cellular responses in comparison with the other polysaccharide samples. Fractions containing the full molecular mass range of GXM were the most efficient stimulators of all cytokines. Most importantly, in all cases, the GXM fractions differed considerably in their ability to stimulate cytokines, confirming the supposition that differences in molecular mass result in functional diversity. To support this hypothesis, we used an in vivo model of lung cytokine stimulation that was previously established by our group to test functional properties of fungal poly-saccharides [28]. These experiments consisted of measurements of lung cytokines after intranasal stimulation of mice with the polysaccharide fractions. To minimize animal experimentation, only representative GXM fractions and cytokines were used in vivo. Polysaccharide fractions in the range of 10–100 kDa were selected as the prototypes representing lower-molecular-mass samples, while fractions with masses greater than 300 kDa were selected to represent large-mass samples. These polysaccharide preparations were compared with GXM samples containing the full molecular mass range. The cytokine prototypes for lung quantification were IL-10 and TNF-α, which corresponded to the less intense and most efficient macrophage responses in vitro, respectively. The in vivo profiles of cytokine production were considerably different from those determined in vitro (Figure 6B), which is consistent with the high diversity of cytokine-producing cells in the lung tissue in contrast to the pure macrophage cultures tested in vitro. In the in vivo system, the full molecular mass sample tended to induce an increase in the production of TNF-α, although no significant differences between control systems (stimulated with PBS) and GXM-treated animals were observed in this case. The comparison between PBS-treated animals with mice exposed to samples in the molecular mass range of 10–100 kDa or >300 kDa revealed that both fractions efficiently stimulated lung TNF-α. The most pronounced differences between the experimental systems that we analyzed, however, were observed when IL-10 was assessed. While the full molecular mass range sample caused a reduction in the basal levels of lung IL-10, the 10–100-kDa fraction had no effect on the levels of this cytokine in comparison with PBS-treated animals. The high molecular sample, however, induced markedly increased levels of IL-10 production in the lung in comparison to animals that were stimulated with PBS, 10–100 kDa fractions or the full molecular mass sample. These results clearly demonstrate that GXM samples with different molecular masses can have distinct immunological activities.
Figure 6. Glucuronoxylomannan fractions of variable molecular masses differentially stimulate cytokine responses in host cells.
(A) In vitro cytokine production by mouse peritoneal macrophages stimulated with the different polysaccharide fractions. Control systems were stimulated with PBS. The cytokine response to GXM fractions varied considerably depending on the polysaccharide molecular mass. (B) Analysis of mice lung (n = 5) levels of TNF-α and IL-10 in response to lower- (10–100 kDa) or higher- (>300 kDa) mass GXM fractions, as well as to PBS or the full-mass-range GXM sample, revealed that the polysaccharide preparations of different molecular masses are distinct in their ability to induce cytokines.
*Statistical significance in comparison with control (PBS-stimulated) systems; ***Statistical differences between GXM-treated systems; Black asterisks: p < 0.001; Red asterisks: p < 0.01.
ND: Not detected; NS: Not significant; PBS: Phosphate-buffered saline.
For color image please see online at www.futuremedicine.com/doi/full/10.2217/FMB.13.163
Discussion
In recent years, it has become evident that the relationship between structural properties and biological functions of fungal polysaccharides is highly complex [3]. For instance, in the C. neoformans model, it was demonstrated that capsule-associated and soluble, extracellular GXM differed in physical, chemical and functional properties, including carbohydrate composition, molecular mass, effective diameter, charge, viscosity and serological reactivity [19]. GXM also associates with other molecules to form hybrid aggregates [37,38] that differ from the isolated polysaccharide in their ability to induce cytokines in mice [28]. The choice of methodological approaches to isolate GXM is also a determinant for the analysis of its general properties, as concluded from the fact that polysaccharide fractions vary in effective diameter and serologic reactivity depending on the purification method [14]. Samples of GXM with similar monosaccharide composition but variable dimensions are also different in their ability to stimulate TLR activation and nitric oxide production by host cells [15]. This enormous diversity in function and structure makes clear the complexity of understanding how the polysaccharide impacts the physiology of host cells. Considering that immunological studies with GXM have been classically performed with polysaccharide preparations that most likely included mixtures of variable molecular masses, it seems reasonable to suppose that the functional diversity of GXM has been underestimated [39].
Chitin efficiently illustrates the complex relationship between molecular dimensions and functions of polysaccharides [5,6,40]. Relatively large chitin fractions were demonstrated to be inert [5]. Fractions with intermediate and small dimensions, however, stimulated TNF-α elaboration by macrophages. Intermediate fractions required TLR2 and NF-κB to induce TNF-α, but smaller fractions principally required dectin-1 and Syk to stimulate production of this cytokine. Finally, only smaller fractions were able to induce IL-10 production, in processes at which dectin-1 and Syk played major and TLR2 and NF-κB minor roles in cytokine stimulation. As pointed out by Da Silva and colleagues [5], these findings demonstrate that different-sized chitin fragments interact with diverse innate immune receptors to activate distinct intracellular signaling pathways. This observation may be related to results described with a smaller size fraction of C. gattii GXM, which was more efficient than larger polysaccharides in its ability to activate cellular responses mediated by TLR1–TLR6 and TLR2–TLR6 dimers in vitro [15].
Our current results are in agreement with the notion that GXM molecules of different sizes differ in function with regards to both fungal physiology and modulation of the cytokine response of host cells in vivo. The first evidence supporting this view was obtained from surface-coating experiments demonstrating that GXM fractions of different molecular masses interact with distinct sites of the fungal cell wall. These results are consistent with the existence of specific cell wall ligands for different GXM molecules, which might impact the understanding of how the C. neoformans capsule is anchored at the fungal surface. Chitin [38] and glucans [17,41] have been reported to interact with GXM at the cell wall, but additional ligands may exist that have not been identified. Therefore, the putative existence of differential interactions between GXM molecules of variable masses and specific cell wall regions suggests that capsular anchors might be more numerous than initially thought. Another interesting aspect of the interaction between GXM and cell wall components in our study was that full surface coating was only achieved when samples containing the full molecular mass ranges were incubated with acapsular C. neoformans cells. Furthermore, the annular pattern of full surface coating of the acapsular cells was not achieved in any of the sequential incubations performed in this study. This result might suggest that the efficient interaction of GXM with C. neoformans cell wall components includes simultaneous interactions with polysaccharides of variable molecular dimensions. Taken together, these results suggest that both large and small GXM fibers are necessary for capsular assembly. Similar assumptions were made before in models of capsule enlargement describing that molecules of higher dimensions are essential for capsule growth [13]. It is noteworthy, however, that these studies were focused on GXM–GXM interactions, but not on capsule–cell wall connections. On the basis of the results herein described, we cannot rule out the possibility that, in models of sequential coating incubations, the initial GXM–cell wall interactions would then be followed by GXM–GXM interactions.
It remains unknown whether the molecular mass of GXM is correlated with its well-known ability to protect C. neoformans against phagocytosis [1]. Experiments performed in our laboratory using GXM-coated cap67Δ cells and mouse macrophages suggest no clear correlation between molecular mass, antiphagocytic potential and fungal survival [Albuquerque PC, Rodrigues ML, Unpublished Data].. This model, however, is probably minimalist, since GXM fibers of different dimensions [13] coexist with proteins and pigments that, individually, have been shown to protect the C. neoformans against phagocytosis [42–45]. The relative contribution of each of these molecular classes to antiphagocytic protection is unknown.
Although all GXM fractions used in the current study efficiently bound to murine macrophages, the response of host cells to the GXM fractions varied according to molecular mass. In vitro, the polysaccharide fraction containing the full molecular mass range of GXM was always the most effective cytokine-inducing sample. The relative contribution of each molecular mass range to cytokine responses, however, was highly variable, which supports the hypothesis that they are functionally distinct. In vivo, high- and low-mass polysaccharide samples also differed in their ability to stimulate host cytokines. The most prominent diversity in our model was observed for IL-10 in in vivo determinations, where the high molecular mass sample was significantly more effective than all others in inducing the lung production of this cytokine. These results were in agreement with a previous report by our group using GXM–chitin hybrids [28]. In fact, the association between chitin fragments and GXM generates larger polysaccharides [37] that are more efficient than the isolated molecules in inducing IL-10 and IL-17 in vivo [28]. The similarity between the profile of cytokine induction by large GXM–chitin hybrids demonstrated by Ramos and colleagues [28] and our current in vivo results suggests that polysaccharide dimensions are essential for cytokine induction. Other possibilities, however, cannot be discarded at this time. For example, IL-10 levels in vivo were reduced when mice were given the full mass range of GXM. The 1–100-kDa fraction, on the other hand, had no effect on IL-10 production, but the fraction of molecular mass >300 kDa was highly effective in inducing this cytokine. This observation suggests a nonlinear correlation between molecular mass and functions of GXM. It was clear that in vitro and in vivo results were considerably different, which is probably due to the enormous differences in the cellular complexity of each system. Although this diversity probably explains the apparently conflicting results observed in our study, the conclusion that GXM fractions of different molecular masses are different in their immunological activity was supported by these findings.
During infection, C. neoformans supposedly produces GXM molecules with distinct dimensions and, consequently, variable masses. Indirect evidence supporting this assumption comes from studies demonstrating that capsular dimensions are highly variable in vivo depending on the organ colonized by pathogenic cryptococci [46]. The currently available techniques for polysaccharide production in vivo do not discriminate GXM molecules with different masses. Therefore, it is impossible to predict, in quantitative terms, which form of GXM predominates in vivo. On the basis of the results presented in this manuscript and in previous studies with chitin and GXM [5,6,28,40], we propose that cryptococcal pathogenesis is influenced by its major capsular polysaccharide in multiple ways, which makes the understanding of how the polysaccharide impacts the development of cryptococcosis much more complex.
Future perspective
Our results demonstrate that biological properties of polysaccharides are greatly influenced by their molecular masses, implying that previously unexplored structural parameters need to be included in functional studies of these glycans. A remarkable complexity in the functional analysis of C. neoformans GXM as a function of its physical properties was suggested, indicating that poly-saccharide fractions may induce different host responses depending on their mass. Our findings suggest that attempts to understand how capsular polysaccharide impacts cryptococcal pathogenesis must consider the form of polysaccharide used in biological studies. The results also argue against the general assumption that polysaccharides are poor immunogens, since most of the studies available in the literature did not consider the possibility that polysaccharide fractions differing in molecular mass might be functionally different.
EXECUTIVE SUMMARY.
Rationale
Polysaccharides with identical composition but variable molecular masses have different biological functions in other systems. It is unclear whether the functions of Cryptococcus neoformans glucuronoxylomannan (GXM) vary depending on molecular mass.
Size determination of GXM & serological tests
The molecular mass of C. neoformans GXM is correlated with polysaccharide size. These parameters were not important for reactivity with monoclonal antibodies raised to the polysaccharide.
Different molecular masses of GXM are required for full coating of the C. neoformans surface
Acapsular C. neoformans cells efficiently incorporate GXM fractions of variable molecular dimensions.
Multiple molecular masses of GXM are required for a homogeneous surface coating of C. neoformans.
GXM fractions with distinct masses manifest different profiles of interaction with host cells
GXM fractions with different masses/dimensions bind to macrophages with similar efficiencies.
The cytokine response to GXM is influenced by the molecular mass of GXM.
Conclusion
Physiological and pathogenic functions of GXM varied depending on molecular mass. This observation suggests that the functional analysis of GXM in the C. neoformans model must consider the form of polysaccharide used in biological studies.
Acknowledgments
The authors thank Allan Guimaraes for help with animal experimentation, Leonardo Nimrichter for suggestions and Debora Foguel for the use of the light scattering device.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
ML Rodrigues, MT Bozza, FF Dutra, S Frases, PC Albuquerque and FL Fonseca are supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FA PERJ, Brazil). ML Rodrigues, PC Albuquerque and FL Fonseca were also supported by the National Institute for Science and Technology on Innovation on Neglected Diseases (INCT/IDN, CNPq). A Casadevall is supported by NIH Grants AI033142, AI033774, AI052733 and HL059842, and the Center for AIDS Research at Einstein. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol. 2009;68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor CM, Roberts IS. Capsular polysaccharides and their role in virulence. Contrib Microbiol. 2005;12:55–66. doi: 10.1159/000081689. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues ML, Nimrichter L, Cordero RJ, Casadevall A. Fungal polysaccharides: biological activity beyond the usual structural properties. Front Microbiol. 2011;2:171. doi: 10.3389/fmicb.2011.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo H, Yi W, Song JK, Wang PG. Current understanding on biosynthesis of microbial polysaccharides. Curr Top Med Chem. 2008;8(2):141–151. doi: 10.2174/156802608783378873. [DOI] [PubMed] [Google Scholar]
- 5••.Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG, Elias JA. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J Immunol. 2009;182(6):3573–3582. doi: 10.4049/jimmunol.0802113. First evidence in the literature showing that polysaccharide dimensions correlated with immunological activity. [DOI] [PubMed] [Google Scholar]
- 6.Lee CG, Da Silva CA, Lee JY, Hartl D, Elias JA. Chitin regulation of immune responses: an old molecule with new roles. Curr Opin Immunol. 2008;20(6):684–689. doi: 10.1016/j.coi.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues ML, Alviano CS, Travassos LR. Pathogenicity of Cryptococcus neoformans: virulence factors and immunological mechanisms. Microbes Infect. 1999;1(4):293–301. doi: 10.1016/s1286-4579(99)80025-2. [DOI] [PubMed] [Google Scholar]
- 8.Lester SJ, Malik R, Bartlett KH, Duncan CG. Cryptococcosis: update and emergence of Cryptococcus gattii. Vet Clin Pathol. 2011;40(1):4–17. doi: 10.1111/j.1939-165X.2010.00281.x. [DOI] [PubMed] [Google Scholar]
- 9.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 10.Kumar P, Yang M, Haynes BC, Skowyra ML, Doering TL. Emerging themes in cryptococcal capsule synthesis. Curr Opin Struct Biol. 2011;21(5):597–602. doi: 10.1016/j.sbi.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doering TL. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Ann Rev Microbiol. 2009;63:223–247. doi: 10.1146/annurev.micro.62.081307.162753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues ML, Nimrichter L, Oliveira DL, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007;6(1):48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Frases S, Pontes B, Nimrichter L, Viana NB, Rodrigues ML, Casadevall A. Capsule of Cryptococcus neoformans grows by enlargement of polysaccharide molecules. Proc Natl Acad SciUSA. 2009;106(4):1228–1233. doi: 10.1073/pnas.0808995106. Demonstration, by biophysical approaches, that polysaccharide dimensions are determinant for capsule enlargement in Cryptococcus neoformans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nimrichter L, Frases S, Cinelli LP, et al. Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot Cell. 2007;6(8):1400–1410. doi: 10.1128/EC.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Fonseca FL, Nohara LL, Cordero RJ, et al. Immunomodulatory effects of serotype B glucuronoxylomannan from Cryptococcus gattii correlate with polysaccharide diameter. Infect Immun. 2010;78(9):3861–3870. doi: 10.1128/IAI.00111-10. First demonstration that the immunological functions of glucuronoxylomannan are affected by its dimensions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broad Institute of Health and Massachusetts Institute of Technology. Cryptococcus neoformansvar. grubii H99 Sequencing Project. www.broadinstitute.org.
- 17.Reese AJ, Doering TL. Cell wall alpha-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol Microbiol. 2003;50(4):1401–1409. doi: 10.1046/j.1365-2958.2003.03780.x. [DOI] [PubMed] [Google Scholar]
- 18.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168(4265):167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 19.Frases S, Nimrichter L, Viana NB, Nakouzi A, Casadevall A. Cryptococcus neoformans capsular polysaccharide and exopolysaccharide fractions manifest physical, chemical, and antigenic differences. Eukaryot Cell. 2008;7(2):319–327. doi: 10.1128/EC.00378-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casadevall A, Mukherjee J, Scharff MD. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J Immunol Meth. 1992;154(1):27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- 21.Albuquerque PC, Cordero RJ, Fonseca FL, et al. A Paracoccidioides brasiliensis glycan shares serologic and functional properties with cryptococcal glucuronoxylomannan. Fung Genet Biol. 2012;49(11):943–954. doi: 10.1016/j.fgb.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleare W, Casadevall A. The different binding patterns of two immunoglobulin M monoclonal antibodies to Cryptococcus neoformans serotype A and D strains correlate with serotype classification and differences in functional assays. Clin Diagn Lab Immunol. 1998;5(2):125–129. doi: 10.1128/cdli.5.2.125-129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nussbaum G, Cleare W, Casadevall A, Scharff MD, Valadon P. Epitope location in the Cryptococcus neoformans capsule is a determinant of antibody efficacy. J Exp Med. 1997;185(4):685–694. doi: 10.1084/jem.185.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casadevall A, Cleare W, Feldmesser M, et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998;42(6):1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marim FM, Silveira TN, Lima DS, Jr, Zamboni DS. A method for generation of bone marrow-derived macrophages from cryopreserved mouse bone marrow cells. PLoS ONE. 2010;5(12):e15263. doi: 10.1371/journal.pone.0015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeNovo Software. www.denovosoftware.com.
- 27.Rodrigues ML, Rozental S, Couceiro JN, Angluster J, Alviano CS, Travassos LR. Identificationof N-acetylneuraminic acid and its 9-O-acetylated derivative on the cell surface of Cryptococcus neoformans: influence on fungal phagocytosis. Infect Immun. 1997;65(12):4937–4942. doi: 10.1128/iai.65.12.4937-4942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos CL, Fonseca FL, Rodrigues J, et al. Chitin-like molecules associate with Cryptococcus neoformans glucuronoxylomannan to form a glycan complex with previously unknown properties. Eukaryot Cell. 2012;11(9):1086–1094. doi: 10.1128/EC.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frases S, Viana NB, Casadevall A. Biophysical methods for the study of microbial surfaces. Front Microbiol. 2011;2:207. doi: 10.3389/fmicb.2011.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen RA, Pappas PG, Perfect J, et al. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob Agents Chemother. 2005;49(3):952–958. doi: 10.1128/AAC.49.3.952-958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yauch LE, Mansour MK, Shoham S, Rottman JB, Levitz SM. Involvement of CD14, Toll-like receptors 2 and 4, and MyD88 in the host response to the fungal pathogen Cryptococcus neoformans in vivo. Infect Immun. 2004;72(9):5373–5382. doi: 10.1128/IAI.72.9.5373-5382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbosa FM, Fonseca FL, Figueiredo RT, et al. Binding of glucuronoxylomannan to the CD14 receptor in human A549 alveolar cells induces interleukin-8 production. Clin Vaccine Immunol. 2007;14(1):94–98. doi: 10.1128/CVI.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang ZL, Netski D, Thorkildson P, Kozel TR. Binding and internalization of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans, by murine peritoneal macrophages. Infect Immun. 2006;74(1):144–151. doi: 10.1128/IAI.74.1.144-151.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura K, Miyagi K, Koguchi Y, et al. Limited contribution of Toll-like receptor 2 and 4 to the host response to a fungal infectious pathogen, Cryptococcus neoformans. FEMS Immunol Med Microbiol. 2006;47(1):148–154. doi: 10.1111/j.1574-695X.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- 35.Yauch LE, Mansour MK, Levitz SM. Receptor-mediated clearance of Cryptococcus neoformans capsular polysaccharide in vivo. Infect Immun. 2005;73(12):8429–8432. doi: 10.1128/IAI.73.12.8429-8432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitz SM. Interactions of Toll-like receptors with fungi. Microbes Infect. 2004;6(15):1351–1355. doi: 10.1016/j.micinf.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Fonseca FL, Nimrichter L, Cordero RJ, et al. Role for chitin and chitooligomers in the capsular architecture of Cryptococcus neoformans. Eukaryot Cell. 2009;8(10):1543–1553. doi: 10.1128/EC.00142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues ML, Alvarez M, Fonseca FL, Casadevall A. Binding of the wheat germ lectin to Cryptococcus neoformans suggests an association of chitinlike structures with yeast budding and capsular glucuronoxylomannan. Eukaryot Cell. 2008;7(4):602–609. doi: 10.1128/EC.00307-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodrigues ML, Fonseca FL, Frases S, Casadevall A, Nimrichter L. The still obscure attributes of cryptococcal glucuronoxylomannan. Med Mycol. 2009;47(8):783–788. doi: 10.3109/13693780902788621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008;181(6):4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reese AJ, Yoneda A, Breger JA, et al. Loss of cell wall alpha(1-3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol Microbiol. 2007;63(5):1385–1398. doi: 10.1111/j.1365-2958.2006.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stano P, Williams V, Villani M, et al. App1: an antiphagocytic protein that binds to complement receptors 3 and 2. J Immunol. 2009;182(1):84–91. doi: 10.4049/jimmunol.182.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luberto C, Martinez-Marino B, Taraskiewicz D, et al. Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J Clin Invest. 2003;112(7):1080–1094. doi: 10.1172/JCI18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chun CD, Brown JC, Madhani HD. A major role for capsule-independent phagocytosis-inhibitory mechanisms in mammalian infection by Cryptococcus neoformans. Cell Host Microbe. 2011;9(3):243–251. doi: 10.1016/j.chom.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nosanchuk JD, Casadevall A. Cellular charge of Cryptococcus neoformans: contributions from the capsular polysaccharide, melanin, and monoclonal antibody binding. Infect Immun. 1997;65(5):1836–1841. doi: 10.1128/iai.65.5.1836-1841.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivera J, Feldmesser M, Cammer M, Casadevall A. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect Immun. 1998;66(10):5027–5030. doi: 10.1128/iai.66.10.5027-5030.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]