Abstract
Organic agriculture as well as good agricultural practices (GAPs) intrigues the concern of both consumers and producers of agricultural commodities. Bio-preparates of various rhizospheric microorganisms (RMOs) are potential sources of biological inputs supporting plant nutrition and health. The response of open-field potatoes to the application of RMO bio-preparates, the biofertilizer “Biofertile” and the bioagent “Biocontrol”, were experimented over 5 successive years under N-hunger of north Sinai desert soils. Both vegetative and tuber yields of a number of tested cultivars were significantly improved due to rhizobacterial treatments. In the majority of cases, the biofertilizer “Biofertile” did successfully supply ca. 50% of plant N requirements, as the yield of full N-fertilized plants was comparable to those received 50% N simultaneously with bio-preparates treatment. The magnitude of inoculation was cultivar-dependent; cvs. Valor and Oceania were among the most responsive ones. Bio-preparate introduction to the plant–soil system was successful via soaking of tubers and/or spraying the plant canopy. The “Biocontrol” formulation was supportive in controlling plant pathogens and significantly increased the fruit yields. The cumulative effect of both bio-preparates resulted in tuber yield increases of ca. 25% over control.
Keywords: Potatoes, Organic farming, Rhizospheric microorganisms, Biofertilizers, Biocontrol, North Sinai
Introduction
Sustainable agriculture is a productive system that does not imply the rejection of conventional practices, but rather, the incorporation of innovations originated by scientists and farmers. The last two decades witnessed world-growing concern towards the quality, not only the quantity, of agricultural products. Varying agronomic practices, e.g. organic, biofarming, good agricultural practices (GAPs), are already introduced, monitored and regulated to secure good quality agricultural products, for both local and export markets. Europe (EU) is one of the major final destinations for products of various agricultural zones of the world, including north Africa. Fresh vegetable and fruit exports are having a significant market share. And to cope with EU regulations and standards (http://www.globalgap.org; http://ec.europa.eu/agriculture/organic/eu-policy/legislation_en), producing countries, including Egypt, are exploring every means to adopt environment-friendly approaches.
The beneficial plant–microbe interactions in the rhizosphere are determinant of plant health and soil fertility [1–5]. In the biogeochemical cycles of both organic and inorganic nutrients in soil and in maintenance of soil health and quality, soil microresidents are of special concern. They are active players in exploring the plant–soil system for major nutrients; mainly N. Mechanisms involved are carrying biological nitrogen fixation, producing plant growth promoting hormones, increasing availability and/or efficient plant uptake [6,7]. The use of environmental friendly microorganisms has proved useful not only for plant-growth promotion but also for disease control. Many investigators [8–10] averred that rhizobacterial inoculation is a promising agricultural approach that plays a vital role in crop protection, growth promotion and/or biological disease control.
The present publication reports on a number of field trials experimenting “Good Agricultural Practices, GAP” based on the rational use of N-fertilizers and intensive application of both bio-preparates and organic manure. The microorganisms entrapped in the experimented bio-propagates are multifunctional ones, e.g. N2-fixers (Azotobacter spp., Azospirillum spp., Enterobacter spp.), plant growth promoting rhizobacteria, PGPR (Azotobacter spp., Azospirillum spp., Enterobacter spp.) and fungi antagonists (Bacillus spp., Enterobacter spp) [6,7,11–15]. The major objective was to support nutrition and health of tested potatoes, being the world’s fourth largest food crop, under the rigorous desert conditions of north Sinai.
Material and methods
Experimental site
The field trials were executed at Rafah Experimental Farm of the Faculty of Agriculture, Cairo University. The site lies at 34°14−E and 31°18−N at altitude of 60 m above sea level in Rafah, north Sinai. The climate is characterized by (a) rainfall <20 mm a month, (b) average temperatures of 15 °C in winter and 30 °C in summer and (c) relative humidity of ca. 60% and 70% in winter and summer respectively. Detailed meteorological data and isohyets of precipitation in Sinai are reported in Refs. [12,14]). The soil is sand with pH, 8.34; saturation percentage (SP), 27%; electrical conductivity (EC), 0.29 dSm−1; 3% organic carbon (OC), 0.06%; total nitrogen (TN); available N, P and K 0. 203, 4.13 and 85.5 pap, respectively. For drip irrigation network, underground water of pH, 7.93 and EC, 0.77 dSm−1 was used.
Field experiments were executed for 5 consecutive seasons (2006/2011). The experimental area, ca 3 acres, was divided into 16 major plots; each includes 24 rows of 20 m long and 1 m apart. Prior to planting, soil was fertilized with P, K and S at rates of 6, 0.5 and 0.5 kg row−1 of single super phosphate (P2O5, 15%), potassium sulphate (K2O, 50%) and agricultural sulphur respectively. Chicken manure was incorporated into soil (10 m3 acre−1) as a slow release organic fertilizer. The chemical profile of the manure is as follows: pH, 7.8; EC, 2.12 dSm−1 ; OC, 28%; TN, 2.5%, available N, P and K are 0.2, 0.15 and 1.3% respectively.
Bio-preparates
Two microbial preparations, developed at the Environmental Studies and Research Unit (ESRU), Faculty of Agriculture, Cairo University, are composites of rhizobacterial strains supporting plant nutrition (Biofertile) and health (Biocontrol). The bacteria were previously isolated from the rhizosphere of desert and Nile Delta plants. Biofertile (Table 1) is a mixture of rhizobacterial isolates of diazotrophic nature, i.e. efficient in biological nitrogen fixation and production of auxins, mainly gibrillic acid [12,13]. Biocontrol (Table 2) is a bioagent antagonizing the pathogenic fungi Fusarium spp. (F. proliferatum, F. oxysporium and F. solani), Alternaria solani, Botrydiplodia spp., Rhizoctonia solani and Schlerotinia sclerotiorum [15].
Table 1.
Rhizobacterial strains composing the biofertilizer “Biofertile”.
Table 2.
Rhizobacterial strains composing the bio-control agent “Biocontrol”.
The bio-preparates were produced on pilot scale at the laboratory. Bacterial strains were maintained on the N-deficient combined carbon sources medium, CCM [16]. For biomass production, batches of the liquid CCM were inoculated with the individual strains (10%) and incubated in a rotary shaker (100 rpm) at 30 °C to reach a population density of ca. 108 cfu ml−1. For the formulation of bio-preparates, equal volumes of respected liquid batch cultures were simultaneously mixed. The resulting composite preparations were mixed with equal portions of 20% pero-dexin, which is a by-product of starch industry and used as a bio-carrier for cell stabilization. The final bacterial slurry prepared, ready for use as bacterial inoculum, is labeled as “Biofertile” or “Biocontrol” [11].
As a winter crop, sowing of potato was carried out during the first week of November. Both tested bio-propagates containing ca.108 cfu ml−1, were mixed together in equal portions and further diluted with irrigation water (1:8, v/v). They were applied to the field by soaking the tubers prior to planting and/or spraying the plant foliage. For soaking, tuber bags were rinsed 30–40 min. in the diluted preparate just prior to planting. The treated tubers (2–3) were manually sown 50 cm apart in rows 1 m apart; each raw was having 40 plants. For spraying, the diluted preparations were alternatively sprayed twice on the plant foliage 2 and 4 weeks post emergence.
The different inoculation and N-fertilization treatments were allocated in a split- split plot design with four replicates where soaking in bio-preparates was the main plot, spraying with the microbial formulations was the sub-plot and sub-sub plot assigned to N-fertilization level.
Potato cultivars
Five major potato winter cultivars were used, cvs. Spunta (2006/2007), Lady Balfour (2007/2008 and 2010/2011), Valor (2007/2008, 2008/2009, 2009/2010 and 2010/2011) as well as Oceania and Osprey (2010/2011). Tubers were kindly provided by AgroFood Co. Ltd., Dokki, Giza, Egypt.
Fertilization and pest management
In addition to the basic fertilization during field preparation, fertigation throughout the season included full N-treatment with ammonium nitrate (33% N) at the recommended levels of 200 kg acre−1 distributed respectively in 5 successive equal doses throughout the growing season. The effect of inoculation with bio-preparates was experimented in the presence of either full or rational (1/2) N doses. Supplementary P and K fertigation was applied for all treatments through the application of 80 kg of potassium sulphate (K2O, 50%) and 8 l of phosphoric acid (85%) per acre divided in 8 doses along the growing season.
For protection against fungal pathogens, di-cupper chloride trihydroxide (85%, 200 g 100 l−1) and micronite sulphur (80%, 250 g 100 l−1) were sprayed 3 times during the growing season.
Microbiological parameters
Only for the first season (2006/2007), plants were sampled 45 days after planting for the determination of total bacterial load on both roots and shoots. Total rhizospheric microorganisms (RMO) in the different root spheres, i.e. rhizosphere soil, ecto-rhizosphere (rhizoplane) and endo-rhizosphere (endophytes), were determined by the surface-inoculated agar plate technique [12–14]. Agar plates of the standard N-free combined C-sources medium, CCM [16] as well as the ice plant juice (crude juice diluted 1:40 by distilled water, v/v, [17] were used. The rhizosphere soil was carefully shaken off the roots and aseptically transferred into sampling bottles containing the basal salt solution of CCM culture medium as diluent. Ecto-rhizosphere samples were prepared [12] by transferring sufficient portions of root systems with closely-adhering soil into sampling bottles containing sufficient volume of the diluent. For internal root colonists (endophytes), samples were prepared by careful washing of another set of roots with tap water, then with 95% ethanol for 5–10 s, followed by 3% sodium hypochlorite for 1.5 h. [18]. Surface sterilized roots were then thoroughly washed by sterile water and crushed for 5 min in Waring blender with adequate volume of basal salts of CCM medium. For the phyllosphere, sufficient plant materials representing the different parts of the plant shoot were cut into smaller pieces and transferred to bottles of the diluent. Bottles prepared for the entire three root spheres and the phyllosphere were vigorously shaken for 30 min., and further decimal dilutions were prepared. Suitable dilutions of each sphere were surface inoculated on agar plates prepared from the tested culture media. Plates were incubated at 30 °C for 2–7 days, and colony forming units (c.f.u) were counted. Dry weights for suspended roots (80 °C) and rhizosphere/ecto-rhizosphere soils (105 °C) were determined.
Agronomicl parameters
After 45 days of cultivation, plant samples were obtained to determine the shoot biomass. For harvest, the tuber yield per harvest row, 20–40 plants, was determined for 4 replicates representing various treatments. Distribution of tuber sizes as well as fresh plant biomass yield were reported for the harvest of season 2006/2007.
Statistical analysis
Data were statistically analyzed using STATISTICA [19]. Analysis of variance (ANOVA) was employed to examine the independent and interacted effects of bacterial inoculation, N-fertilization and/or plant cultivar.
Results
The first field trial (2006/2007) dealt with the effect of bio-preparate application, in presence of either full or rational dose of N-fertilizer, on the productivity of cultivar “Spunta”. This particular cultivar is cultivated to meet heavy demands of the local market. Harvest data indicated that full N fertilization resulted in the highest tuber yield. Intensive application of bio-preparates by both soaking of tubers and foliar spray positively interacted with the rational dose of N (1/2 N) yielding tuber harvest approaching to full N-fertilization (Fig. 1A). In other words, under N-stress, successful bacterial inoculation did biologically furnish potato plants with ca. 1/2 N dose. The presence of copious N did not allow the microorganisms of the bio-preparates to express their activities, encompassing nitrogen-fixation, production of plant hormones and/or efficient uptake of nutrients, to the benefits of potato plants. Not only the tuber yield but also the shoot biomass followed a similar trend. The highest biomass was reported for full N-fertilized plants as well as those received 1/2 N in combination with intensive inoculation (simultaneous soaking and foliar spraying) (Fig. 1B). It is evident that inoculation did furnish the plants with additional supplies of N. In general, inoculation with soaking was much better than spraying for both plant biomass and tuber yield. Plant growth and productivity were also expressed as indicated by tuber size (data not shown). Higher percentages of medium and big tuber sizes were reported for full N-fertilized plants. Such sizes were relatively inferior with the rational dose of N, but significantly improved with simultaneous inoculation reaching values comparable to full N fertilization.
Fig. 1.
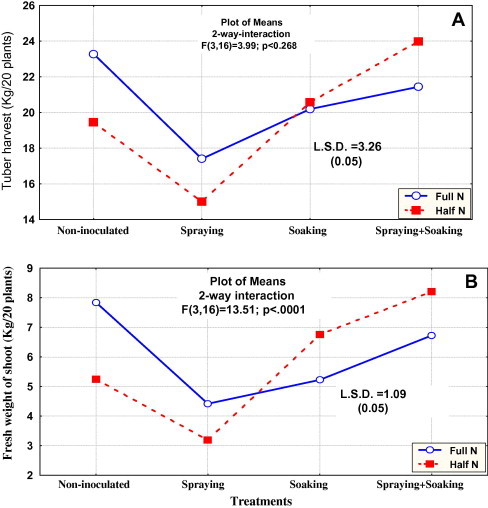
Interaction of mode of application of bio-preparates with both tuber harvest (A) and total fresh shoot biomass (B) of the cultivar Spunta. Results represent data of the first season (2006/2007).
Fig. 2 presents the bacterial load in various root spheres as well as on the phyllosphere of tested plants. The inoculation effect was not pronounced in the rhizosphere soil, as differences among treatments were not significant. Towards the plant root, enrichment of the bacterial load was significantly affected by inoculation. Spraying as such or in combination with soaking resulted in the highest bacterial colonization of the internal root tissues (endo-rhizosphere). This was also the case with phyllospheres, where introduced microorganisms significantly harboured the tested potato vegetative parts. The nature of the plating agar medium, either CCM or plant juice, did not significantly affect the recovery of culturable microorganisms associated to the roots and shoot spheres (data not shown).
Fig. 2.
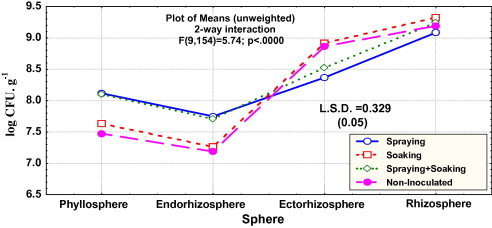
Population of culturable rhizobacteria in the root spheres (rhizosphere soil, ecto- and endo-rhizospheres) as well as phyllosphere, as affected by various treatments of potato plants (tuber soaking and/or spraying phylloplanes). Results represent data of the first season and the cultivar Spunta (2006/2007).
The second field trial of 2007/2008 season dealt mainly with the response of other 2 cultivars, Lady Balfour and Valor (Table 3), to bio-preparates application in presence of rational dose of N. These particular varieties are grown for exports to EU markets. Statistical analysis of harvest data demonstrated the significant single effects of cultivars; Valor being more productive than Lady Balfour; As to the mode of application of bio-preparates, spraying phylloplanes was superior to soaking tubers. Two-way interactions indicated the significant responses in the cases of Lady Balfour to spraying and Valor to simultaneous spraying and soaking. Significantly, the most productive cultivar was Valor, particular in the case of intensive application of bio-preparates by spraying and soaking. As to size of tubers, percentages of the medium size were highest for Valor and of the big size for Lady Balfour (data not shown).
Table 3.
Potato tuber harvest (kg/40 plants) for the season 2007/2008: response of cultivars to various mode of biofertilization application, in presence of a rational dose of N fertilization.
| Treatments | Lady Balfour | Valor |
|---|---|---|
| 1/2 N + Non-inoculated | 53.59CD | 57.13BC |
| 1/2 N + Spraying bio-preparates | 67.33A | 63.86AB |
| 1/2 N + Soaking in bio-preparates | 55.60C | 59.89BC |
| 1/2 N + Soaking and spraying | 45.55D | 69.78A |
| LSD (0.05) | 6.84 | |
Means followed by the same letter are not significants different (p<0.05).
Results of the two successive seasons of 2008/2009 and 2009/2010 confirmed the significant response of the cultivar Valor to the application of biopreparates (Fig. 3). Respective yield increases were in the averages of >6–18 and 18–35%. The effect of mode of action was not consistent.
Fig. 3.
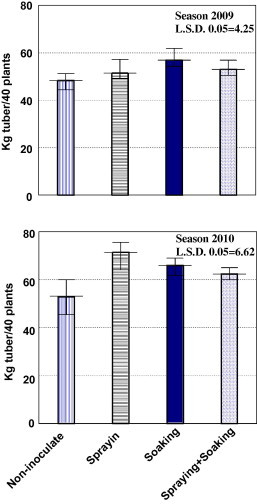
Effect of biofertilization on potato tuber harvest (kg/40 plants) for the cultivar “Valor” during the two successive seasons 2008/2009 and 2009/2010.
Combined statistical analysis was carried out for the harvest data obtained during the four consecutive seasons of field experimentation (2006/2010). The season effect (Fig. 4A) was demonstrated and productivity was the highest for the fourth season where soil biofertility was cumulatively built up. Results confirmed the significant yield responses of potato cultivars to the biopreparate application. The introduction of rhizobacteria to the soil–plant system was not affected by the mode of application, either soaking tuber or spraying the phylloplanes (Fig. 4B). The cultivar “Valor” was the most responsive one (Fig. 4C).
Fig. 4.
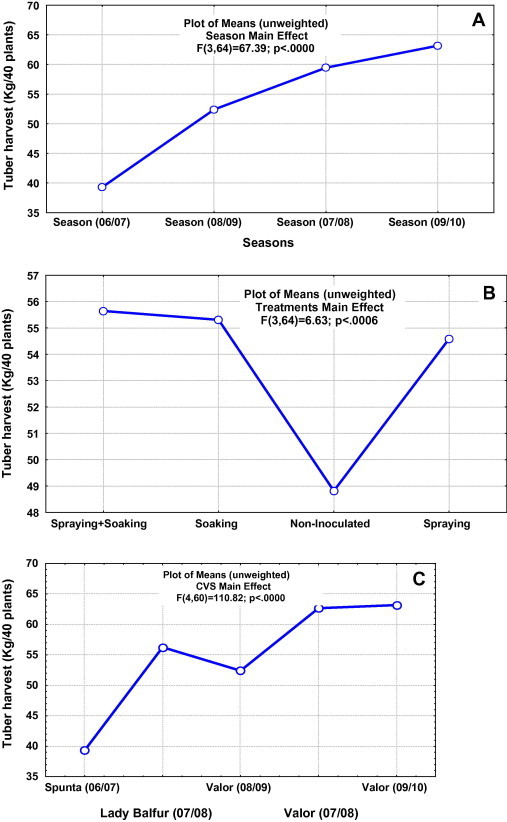
Combined statistical analysis of tuber harvest data obtained during the four consecutive seasons (2006/2010) of field experimentations. (A) Season effect, (B) biofertilization effect and (C) cultivar effect.
The fifth season (2010/2011) was devoted primarily to experiment 2 new cultivars, just introduced to the Egyptian agriculture, namely cvs. Oceania and Osprey. In comparison to cultivars of the former seasons, Valor and Lady Balfour, the cultivar Oceania was the highest in yield and the most responsive to biofertilization (Table 4). This particular cultivar is now approved by the agricultural authorities in Egypt as a recommended cultivar mainly for industrial purposes.
Table 4.
Potato tuber harvest (kg/20 m row) for the season 2010/2011: Response of various potato cultivars to biofertilization (ANOVA, 2-ways interaction).
| Cultivars | Treatments |
|
|---|---|---|
| Non-inoculated plants | Inoculated plants | |
| Valor | 29.70CD | 34.59BC |
| Lady Balfour | 37.15B | 35.47BC |
| Oceania | 28.65CD | 46.44A |
| Osprey | 24.23D | 24.91D |
| LSD 0.05 | 7.40 | |
Means followed by the same leter are not significants different (p<0.05).
Discussion
The agriculture of today and tomorrow are facing serious challenges. This is in respect of producing enough to feed escalating world population. By 2055, FAO predicted a rise of up to 10 Billion people and of 70% of global calorie demand. Concomitantly, awareness of consumers towards food quality is substantially mounting. The scientific community is of the belief that innovations are the heart and soul of agro-industry development, engaging in constant quest to secure more production, and to improve safety and efficacy of agro-products. Taking very much into consideration that technological progress is to merge with existing tradition of various world communities.
A truly sustainable agricultural system, as one of the eight millennium development goals identified by FAO, is a major approach to establish produce in harmony with the environment, communities and the economy. As to the plant–soil ecosystem, microorganisms and their potential functions are one of the key elements to establish sustainability. Of particular importance are those docking the root sphere, being named as rhizospheric microorganisms (RMOs) [20].
Rhizospheric microorganism (RMO) in the plant–soil system are principal players in environmentally friendly agricultural practices, referred to as organic farming (bio-farming, bio-dynamic) as well as good agricultural practices (GAPs). Such practices are carefully regulated and certified on governmental (EC 834 &889 regulations, http://ec.europa.eu/agriculture/organic/eu-policy/legislation_en).) or private (EurepGAP, now GLOBALG.A.P, http://www.globalgap.org) levels. Among various scenarios implemented in this respect is the in situ enrichment of the plant–soil system with crop residues, accompanied with no or minimum tillage, which results in booming RMO activities contributing to the bio-fertility of the plant–soil system. Under semi-arid conditions, it is estimated that as much as 60 kg N ha−1 are biologically gained post wheat and maize harvesting [21]. Another scenario is the direct introduction of selected potent RMO isolates (bio-preparates) to the plant rhizosphere. Such bio-preparates are of variable functions in relation to plant nutrition, e.g. dinitrogen fixation (diazotrophs), production of plant hormones (plant growth promoting rhizobacteria, PGPR), efficient uptake of nutrients through bioavailability of nutrients (P) and sequestration of iron [2,22–24]. The bio-preparate “Biofertile” used in this study is a composite preparation of representatives of Azospirillum brasilense, Azotobacter chroococcum, Bacillus polymyxa, Enterobacter agglomerans and Pseudomonad putida. Each of the individual strains included in such mixture is of different modes of action efficiently fix N2 and/or produce plant hormones, antagonist of fungal pathogens [12,15], but synergism is the type of interaction among such interacting individuals [1]. The present results of field trials showed that ca. 50% of N-requirements of potato plants were biologically secured via the application, by tuber soaking and/or canopy spray, of such bio-preparate (Fig. 1). The effect was cultivar dependent where Valor and Oceania were the most responsive (Fig. 3). An effect that was earlier reported for potatoes treated with pure strains of A. chroococcum [25] as well as other vegetable crops [26].
As to plant health, it is reported that the introduction of specific groups of rhizobacteria to the plant–soil system are able to antagonize a number of fungal pathogens through antibiotic production, reduction of iron availability, synthesis of fungal cell wall lysing-enzymes and spatial competition with pathogens on plant roots [15]. Taking into consideration that prevailing fungal pathogens poses a continual threat to potato as well as other vegetable crops [27] and that combating such diseases are heavily depending on the application of pesticides [28]. Avoiding the overuse of such agrochemicals, for the sake of environment and human beings health, there is a continuing search for other means to secure economic production [8]. The use of resistant cultivars is still limited, especially with fruit and vegetable crops [29–32], including genetically modified crop resistance for potatoes [33]. One attractive possibility to suppress soil borne plant pathogens is to aim at the activity of microorganisms in the root sphere [7,34]. Plant pathogens common in the open-fields of the area under investigations are early blight (Alternaria solani), late blight (Phytophthora infestans) and black scurf (Rhizoctonia solani) [15]. The prevailing pathogens were actually isolated and in vitro tested for the antagonistic effect of a group of rhizobacterial isolates [15]. Bioassay on potato dextrose agar plates discriminated representatives of Bacillus circulans, Bacillus macerans and Bacillus polymyxa able to suppress >25–66% of the fungal growth. Therefore, such rhizobacterial isolates were included into the present bio-preparate “Biocontrol”. Observations, along the five successive growing seasons, showed very sporadic infection of potato plants and tubers, especially those sprayed with the tested bio-preparates. Taking into consideration that the cultivated soil is virgin not cultivated before and potatoes are the first standing crop. Further evidence for positive response of potato to inoculation with rhizobacteria was presented by other investigators [10]. They demonstrated cultivar dependent suppression of fungal pathogens (Phytophthora infestans) by pure strains of rhizobacteria (Pseudomonas putida). PCR-DGGE fingerprints indicated that P. putida was an avid colonizer to potato plants and competing with microbial populations indigenous to the potato phytosphere. The positive response of potato growth was not confined to rhizobacteria (Pseudomonas fluorescens) but extended to the combined action of mycorrhizal fungi [35].
The positive effect of bio-preparates application on potato growth and yield was consistent during the successive field trials. The steady increase in productivity, along the years (Fig. 4), is a strong evident on the sustainability of the system and the cumulative build up of soil biofertility. However, being not as specific as the symbiotic rhizobia-plant system, the rhizobacteria of the sort PGPR, diazotrophs and bioagents are generally lacking comparative studies between crop types and different species and/ or strains of rhizobacteria. As reported earliar [36] when Pseudomonas putida GR12-12 was introduced to various crops, there were dissimilarities in plant stimulation between monocot and dicot plants. There are also significant differences in yield between summer versus winter crops following inoculation with Azospirillum brasilense Cd [37]. Nevertheless, the positive effects of various rhizobacterial types on many economically imported crops is a valid phenomenon, and results obtained by various research groups can act as a basis for the effective utilization of these microorganisms in a variety of applications [6,3,11].What is needed for the future is to have a better understanding of how different bacterial strains work together, in a composite, for the synergistic promotion of plant growth. In addition, the inoculant strains should be labeled, so they can be easily detected and followed in the environment after being introduced.
Conclusion
The application of GAP practices through rationalizing inputs of N-fertilizers and pesticides together with the application of bio-preparates, did significantly support good productivity of potatoes. The productivity obtained during the five seasons of the presented small-scale farming experiments is averaging 14–18 ton acre−1, an acreage that is not very much inferior to intensive conventional production applying heavy fertilizers and pesticides. This encouraged a number of farm operators of potential grower/exporters in Egypt to experiment this particular practice. Bio-preparates were supplied to the winter potatoes of 2008–2010 (AgroFood Company, Egypt) and 2010/2011 (Daltex Company, Egypt), and applied by mixing them in the water tank of the planter for mechanical seeding. Field observations indicated significant recovery of biologically-fixed nitrogen and lower incidence of fungal pathogens, soil-borne as well as early and late blight, which supported good productivity.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgments
The present work represents data obtained during the successive phases (2005–2012) of the project “Agrotecnologies based on biological nitrogen fixation for the development of agriculture in north Sina” kindly funded by the Egyptian Ministry of Agriculture and Land Reclamation. We appreciate the technical support of Eng. Mahmoud Abd-el Hamid and his co-workers at the Experimental Farm of the Center of Research and Training for Agro-biotechnologies, Faculty of Agriculture, Cairo University, Rafah, north Sinai. Potato seeds were kindly provided by Agrofood Company, Giza, Egypt.
Footnotes
Peer review under responsibility of Cairo University.

References
- 1.Hamza M.A., Youssef H., Helmy A., Amin G.A., Fayez M., Higazy A. Mixed cultivation and inoculation of various genera of associative diazotrophs. In: Hegazi N.A., Fayez M., Monib M., editors. Nitrogen fixation with non-legumes. The American University in Cairo Press; Cairo, Egypt: 1994. pp. 319–326. [Google Scholar]
- 2.Glick B.R. The enhancement of plant growth by free-living bacteria. Can J Microbiol. 1995;41:109–117. [Google Scholar]
- 3.Hegazi N.A., Fayez M. Biological nitrogen fixation to maximize productivity of intercropped legumes and non-legumes: ten years of field experimentations in semi-arid desert of Egypt. Arch Agron and Soil Sci. 2001;47:103–131. [Google Scholar]
- 4.Hegazi N.A., Fayez M. Biodiversity and endophytic nature of diazotrophs other than rhizobia associated to non-leguminous plants of semi-arid environments. Arch Agron Soil Sci. 2003;49:213–235. [Google Scholar]
- 5.Jeffries S., Gianinazzi S., Perotto S., Turnau K., Barea J.M. The contribution of Arbuscular mucorhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fertil Soils. 2003;37:1–16. [Google Scholar]
- 6.Okon Y., Labandera-Gonzalez C.A. Agronomic applications of Azospirillum: an evaluation of 20 years worldwide field inoculation. Soil Biol Biochem. 1994;26:1591–1601. [Google Scholar]
- 7.Vessey J.K. Plant growth-promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. [Google Scholar]
- 8.Ghorbani R., Wilcockson S., Leifert C. Alternative treatments for late blight control in organic potato: antagonistic micro-organisms and compost extracts for activity against Phytophthora infestans. Potato Res. 2005;48:181–189. [Google Scholar]
- 9.Dilantha F, Nakkeeran S, Yilan Z. Biosynthesis of antibiotics by PGPR and its relation in biocontrol of plant diseases. PGPR Biocontrol Biofert; 2006. p. 67–109.
- 10.Dini Andreote F., de Araújo W.L., de Azevedo J.L., van Elsas J.D., da Rocha U.N., van Overbeek L.S. Endophytic colonization of potato (Solanum tuberosum L.) by a novel competent bacterial endophyte, Pseudomonas putida strain P9, and its effect on associated bacterial communities. Appl Environ Microbiol. 2009;l 75(11):3396–3406. doi: 10.1128/AEM.00491-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali M.S., Hamza M.A., Amin G., Fayez M., EL-Tahan M., Monib M. Production of biofertilizers using baker’s yeast effluent and their application to wheat and barley grown in north Sinai deserts. Arch Agron and Soil Sci. 2005;51:589–604. [Google Scholar]
- 12.Othman A.A., Amer W.M., Fayez M., Monib M., Hegazi N.A. Biodiversity of diazotrophs associated to the plant cover of north Sinai deserts. Arch Agron Soil Sci. 2003;49:683–705. [Google Scholar]
- 13.Othman A.A., Amer W.M., Fayez M., Hegazi N.A. Rhizosheath of Sinai desert plants is a potential repository for associative diazotrophs. Microbiol Res. 2004;159:285–293. doi: 10.1016/j.micres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Othman AA. Biodiversity of diazotrophs associated to natural vegetation of Sinai and their contribution to soil biofertility. MSc Thesis, Fac Agric, Cairo Univ, Egypt; 2000.
- 15.Fayez M, Youssef H, Mounier B, Shaltout AM, El-Sherif EM, Hamza MA, et al. Biomanagement of vegetable fungal diseases by multifunctional rhizobacteria. In: Proceedings of the 4th conference on recent technologies in agriculture “Challenges in Agricultural Modernization”, Faculty of Agriculture, Cairo University, November, 3–5, Giza, Egypt; 2009. p. 934–45.
- 16.Hegazi N.A., Hamza M.A., Osman A., Ali S., Sedik M.Z., Fayez M. Modified combined carbon N-deficient medium for isolation, enumeration and biomass production of diazotrophs. In: Malik K.A., Mirza M.S., Ladha J.K., editors. Proceedings of the 7th International symposium on Nitrogen Fixation With Non-Legumes. Kluwer Academic Publishers; Dordrecht: 1998. pp. 247–253. [Google Scholar]
- 17.Hegazi NA, Ali SM, Nour E, Fayez M, Monib M. The reuse of olive oil mill wastewater (Alpechin) as a culture medium for bacterial growth and biomass production required for the preparation of biofertilizers. In: Proceedings of the 4th conference on recent technologies in agriculture “Challenges in Agricultural Modernization”, Faculty of Agriculture, Cairo University, November, 3–5, Giza, Egypt; 2009. p. 906–17.
- 18.Youssef H.H., Fayez M., Monib M., Hegazi N.A. Gluconacetobacter diazotrophicus: a natural endophytic diazotroph of Nile delta sugarcane capable of establishing an endophytic association with wheat. Biol Fertil Soils. 2004;39:391–397. [Google Scholar]
- 19.STATISICA, Statsoft, Inc. Tusla, USA, Version 6.0 <http://www.statsoft.com>; 1997.
- 20.Hawkes C.V., DeAngelis M., Firestone M.K. Root interactions with soil microbial communities and processes. In: Carden Z.G., Whitbeek J.L., editors. The rhizosphere: an ecological perspective. Elsevier Inc.; Amsterdam: 2007. pp. 1–29. [Google Scholar]
- 21.Hegazi N.A., Khawas H.M., Farag R.S., Monib M. Effect of incorporation of crop residues on development of diazotrophs and patterns of acetylene-reducing activity in Nile Valley soils. Plant Soil. 1986;90:383–389. [Google Scholar]
- 22.Glick B.R., Patten C.L., Holguin G., Penrose D.M. Imperial College Press; London: 1999. Biochemical and genetic mechanisms used by plant growth-promoting bacteria. [Google Scholar]
- 23.Dilfuza E. Plant growth promoting properties of rhizobacterial isolates from wheat and peas grown in loamy sand soil. Turk J Biol. 2008;32:9–15. [Google Scholar]
- 24.Malboobi M.A., Behbahani M., Madani H., Owlia P., Deljou A., Yakhchali B. Performance evaluation of potent phosphate solubilizing bacteria in potato rhizosphere. World J Microb Biotech. 2009;25:1479–1484. [Google Scholar]
- 25.Imam M.K., Badawy F.H. Response of three potato cultivars to inoculation with Azotobacter. Potato Res. 1978;21:1–8. [Google Scholar]
- 26.Barassi C.A., Sueldo R.J., Creus C.M., Carrozzi L.E., Casanovas E.M., Pereyra M.A. Azospirillum spp., a dynamic soil bacterium favourable to vegetable crop production. Dynamic Soil, Dynamic Plant. 2007;1(2):68–82. [Google Scholar]
- 27.Velásquez V.R., Victoriano L.F. Presencia de patógenos en almácigos y semilla de chile (Capsicum annuum L.) en Aguascalientes y Zacatecas, México. Rev Mex Fitopatol. 2007;25:75–79. [Google Scholar]
- 28.Pérez ML, Durán OLJ, Ramírez MR, Sánchez PJR, Olalde PV. Sensibilidad in vitro de aislados del hongo Phytophthora capsici a funguicidas. Memorias Primera Convención Mundial del Chile. León, Guanajuato, México. Resumen; 2004. p. 144–50.
- 29.Frusciantei L., Barone A., Carputo D., Ranalli P. Breeding and physiological aspects of potato cultivation in the Mediterranean region. Potato Res. 1999;42:265–277. [Google Scholar]
- 30.Schneider M., Droz E., Malnoe P., Chatot C., Bonnel E., Metraux J.R. Transgenic potato plants expressing oxalate oxidase have increased resistance to oomycete and bacterial pathogens. Potato Res. 2002;45:177–185. [Google Scholar]
- 31.Meiyalaghan S., Jacobs J.M.E., Butler R.C., Wratten S.D., Conner A.J. Transgenic potato lines expressing cry1Ba1 or cry1Ca5 genes are resistant to potato tuber moth. Potato Res. 2006;49:203–216. [Google Scholar]
- 32.Vassilev N., Vassileva M., Nikolaeva I. Simultaneous P-solubilizing and biocontrol activity of microorganisms: potentials and future trends. Appl Microbiol Biotech. 2006;71:137–144. doi: 10.1007/s00253-006-0380-z. [DOI] [PubMed] [Google Scholar]
- 33.Haverkort A.J., Struik P.C., Visser R.G.F., Jacobsen E. Applied biotechnology to combat late blight in potato caused by Phytophthora infestans. Potato Res. 2009;52:249–264. [Google Scholar]
- 34.Sirii’ M.I., Villanueva P., Pianzzola M.J., Franco Fraguas L., Galvan G., Acosta M. In vitro antimicrobial activity of different accessions of Solanum commersonii Dun. from Uruguay. Potato Res. 2004;47:127–138. [Google Scholar]
- 35.Duffy E.M., Hurley E.M., Cassells A.C. Weaning performance of potato microplants following bacterization and mycorrhization. Potato Res. 1999;42:521–527. [Google Scholar]
- 36.Hall J.A., Peirson D., Ghosh S., Glick B.R. Root elongation in various agronomic crops by the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Isr J Plant Sci. 1996;44:37–42. [Google Scholar]
- 37.Okon Y, Kapulnik Y, Sarig S. Field inoculation studies with Azospirillium in Israel. In: Subba Rao NS, editor. Biological Nitrogen Fixation Recent Developments. Oxford and IBH Publishing Co. New Delhi, India; 1988. p. 175–95.


