Abstract
The present work aims to study the in vitro properties of nano-hydroxyapatite/chitosan–gelatin composite materials. In vitro behavior was performed in simulated body fluid (SBF) to verify the formation of apatite layer onto the composite surfaces. The in vitro data proved the deposition of calcium and phosphorus ions onto hydroxyapatite /polymeric composite surfaces especially those containing high concentrations of polymer content. The degradation of the composites decreased with increase in the polymeric matrix content and highly decreased in the presence of citric acid (CA), especially these composites which contain 30% polymeric content. The water absorption of the composites increased with increase in the polymeric content and highly increased with CA addition. The Fourier transformed infrared reflectance (FT-IR) and scanning electron microscope (SEM) for the composites confirmed the formation of bone-like apatite layer on the composite surfaces, especially those containing high content of polymers (30%) with 0.2 M of CA. These promising composites have suitable properties for bio-applications such as bone grafting and bone tissue engineering applications in the future.
Keywords: Hydroxyapatite, Chitosan, Composites, In vitro, SEM
Introduction
Hydroxyapatite (HA) is derived either from natural sources or from synthetic sources and regarded as bioactive substance, since it forms a strong chemical bond with host bone tissue, and hence, it is recognized as a good bone graft material. HA is not only bioactive but also osteoconductive, non-toxic and non-immunogenic, and its structure is crystallographically similar to that of bone mineral [1]. Li et al. reported that nano-hydroxyapatite (nHA) precipitates may have higher solubility and therefore affect the biological responses [2]. It has been shown that HA and its composites are suitable for attachment, proliferation, and differentiation of mesenchymal stem cells (MSCs), owing to their structure and chemical compositions [3]. Kong et al. [4] reported that the apatite-coated chitosan/nano-hydroxyapatite composite scaffolds cells presented better proliferation than on apatite-coated chitosan scaffolds. Therefore, the addition of nano-hydroxyapatite improved the bioactivity of chitosan/nano-hydroxyapatite composite scaffolds. Also, Xianmiao et al. [5] reported that the morphology and behavior of bone marrow stem cell (BMSC) cultured in vitro with the n-HA/chitosan (CS) composite membranes are observed under phase-contrast microscope. In chitosan–nHA scaffolds, pre-osteoblasts have high affinity to the surface of chitosan–nHA composite, which is attributed to its increase in surface area and composition [6].
For the increase in bioactivity and mechanical property, some composites of polymer and bioactive ceramics have been developed for bone tissue engineering. Among these composites, HAp/polymer composites have attracted much attention since such composites may have osteoconductivity due to the presence of HAp. Thus, HA/polymer composite scaffolds are of interest for biomedical applications [7]. HA–collagen composite is similar microstructure to native bone and showed osteoclastic resorption and good osteoconductivity [8] but the high cost of collagen, which limits its clinical application in healing bone defect to its insouciant formability and flexibility.
Polymers such as chitosan have a higher degradation rate than bioceramics; chitosan is a unique polysaccharide based biopolymer that shares a number of chemical and structural similarities with collagen [9]. Recently, chitosan (CS) being a natural biodegradable cationic polymer with good biocompatibility, much attention has been paid to chitosan-based biomedical materials [10]. Therefore, incorporation of HA into a chitosan polymer matrix has been shown to increase osteoconductivity and biodegradability with significant enhancement of mechanical strength [11]. Another example for natural polymer is gelatin, which is a biodegradable polymer with many attractive properties, such as excellent biocompatibility, non-antigenicity, plasticity and adhesiveness, and it is widely used in biomedical and pharmaceutical fields. Thus, gelatin was selected as a suitable candidate blended with chitosan [12]. Also, gelatin is blended with chitosan to improve the biological activity since (i) gelatin promotes cell adhesion and migration and (ii) forms a polyelectrolyte complex [13]. Citric acid found in bone in the form of citrate in 0.9 wt% [14]. The three carboxyl of citric acid provide more nucleation sites to formation of ultra fine nano-sized carbonate apatite and the increase in citric acid benefit the bone resorption and ossification through the formation of dissociated calcium citrate complexes in the surrounding body fluid [15].
The current work is aimed of the evaluation of bioactivity for the prepared nano-HA/chitosan–gelatin composites in the presence and absence of citric acid via in vitro study in the simulated body fluid (SBF). The in vitro study includes two assessments: the first is the measurement of calcium (Ca2+) and phosphate ions in SBF after withdrawal of the composite. The second is the degradation behavior, water absorption ability, FT-IR, and SEM-EDX analyses for these composites to confirm the bone-like apatite layer formation on their surfaces.
Experimental
Preparation of the biocomposites
The biocomposites were prepared according to Mohamed et al. [16]. Table 1 shows compositions for preparation of HA/chitosan–gelatin composites (HACG composites). Preparation of HA80CG20 composites with weight ratio of composition [(HA/chitosan–gelatin) (80:20)]. The chitosan–gelatin solution with a concentration of 4% was prepared by dissolving chitosan (2 g) into 2% acetic acid (50 ml) with stirring for 5 h to get a perfectly transparent solution and dissolving gelatin (2 g) into distilled water (50 ml). The chitosan and gelatin solutions were mixed together and then mixed with 9.37% solution of H3PO4. This solution was dropped slowly into 11.81% ethanol solution of Ca (OH)2 with vigorous stirring. The pH of the mixture was adjusted with NaOH solution up to 10. The stirring was kept for 24 h after dropping and then the paste obtained was aged for another 24 h. Finally, the precipitate was filtered and washed with distilled water to remove the excess NaOH and dried in a vacuum oven at 70 °C. Preparation of HA/chitosan–gelatin composites in the presence of citric acid (CA) (HACGCA composites) was also performed according to Table 1. Dissolve each of chitosan and gelatin polymers in 0.2 M of CA solution. The above steps for preparation of HACGCA composites were repeated to obtain different HACGCA composites with various weight ratios of HA and chitosan–gelatin mixture, respectively.
Table 1.
Notations and compositions of HA/chitosan–gelatin composite materials (HACG composites).
| Notations | HA/chitosan–gelatin (%) | Ca(OH)2 (g) | H3PO4 (g) | Chitosan (g) | Gelatin (g) | Citric acid (CA) (%) |
|---|---|---|---|---|---|---|
| HA | 100% HA | 14.76 | 11.71 | – | – | – |
| HA80CG20 comp. | 80% HA:20% CG | 11.81 | 9.37 | 2.00 | 2.00 | – |
| HA80CG20CA comp. | 80% HA:20% CG/CA | 11.81 | 9.37 | 2.00 | 2.00 | 3.84 |
| HA70CG30 comp. | 70% HA:30% CG | 10.33 | 8.20 | 3.00 | 3.00 | – |
| HA70CG30CA comp. | 70% HA:30% CG/CA | 10.33 | 8.20 | 3.00 | 3.00 | 3.84 |
| HA60CG40 | 60% HA:40% CG | 8.86 | 7.30 | 4.00 | 4.00 | – |
| HA60CG40CA comp. | 60% HA:40% CG/CA | 8.86 | 7.30 | 4.00 | 4.00 | 3.84 |
NB: The ratio between chitosan (C) and gelatin (G) is 1:1 in all composites.
In vitro behavior
Solution analysis
The SBF solution and solid composites were assessed pre- and post-immersion for different periods. The notations and compositions of HA/chitosan–gelatin composites (HACG composites) were shown in Table 1. To study the bioactivity, the composites were soaked in SBF which is proposed by Kokubo and Takadama [17] at body temperature (37 °C) and pH: 7.4 for different periods up to 28 days. The SBF has a composition similar to human blood plasma and has been extensively used for in vitro test. After the immersion periods, the solutions were analyzed by spectrophotometer to detect the calcium ions at λ = 570 nm and phosphorus ions concentration at λ = 675 nm [18]. Each test was repeated three times, and the average value was taken to confirm the results.
Assessment of the composites
Weight loss (%)
The weight loss of the composites was carried out in vitro by incubating the composite in SBF at pH: 7.4 and 37 °C for different periods (1, 3, 7, 15, 21 and 28 days). At interval time, the composites were taken from the medium and dried at 50 °C over night. The weight loss was calculated by the following equation [18].
where Wo denotes the original weight of the composite, while Wt is the weight at time (t). Each experiment was carried out for three samples and the average value was taken to insure the results.
Water absorption (%)
For water uptake measurements, all the specimens were weighted before being immersed in distilled water at 37 °C. After immersion for different periods, the samples were carefully removed from the media and gently pressed in-between two filters papers to remove excess water and finally weighted using a sensitive balance. The water absorption ability (%) is calculated by the following equation [19].
where Wi is the initial weight of the sample, and Wf is the sample weight after immersion. The test was carried out for three samples, and the average value was taken to insure the data.
FT-IR analysis
The FT-IR analysis was used to assess the composite post-immersion in SBF for 7 and 28 days. The range of the wave number used was 4000–400 cm−1 with normal slit. The available computerized infrared spectrometer (Jasco, FT/IR 300E, Fourier Transform Infrared Spectrometer, Serial No. 4140109, and Japan) was used.
Surface analysis
The surface morphology of the samples was studied using SEM, JXA 840A Electron Probe Microanalyzer (JEOL, Japan). For SEM, the substrates were mounted on metal stubs and coated with gold before being examined. Also, X-ray elemental analysis (EDAX) was carried out for the samples post-immersion to monitor the ratio of calcium and phosphorus elements and to prove the formation of apatite layer on the surface.
Results and discussion
In vitro behavior
The SBF solution and composites were assessed post-immersion in the SBF media for several periods.
Solution analysis
Calcium ions (Ca2+)
Fig. 1 shows that the concentration of calcium ions recorded low values for HA sample, HA80CG20 and HA70CG30 composites compared to that of control proving the deposition of Ca2+ ions as a result of interaction of Ca2+ with functional groups of both chitosan and gelatin polymers. For HA60CG40 composite, the Ca2+ ions concentration recorded high in the surrounding media compared to control. The degradation rate had increased because of their high chitosan and gelatin contents in all composites. In this domain, as a hydrophilic polymer, gelatin macromolecule chain hydrolyzes quickly due to the existence of water, so the degradation rate of the composites with high gelatin content in phosphate buffer saline (PBS) is quicker [20]. Fig. 2 shows that the concentration of calcium ions recorded lower values for HA sample and all HACGCA composites compared to control proved the deposition of Ca2+ ions due to the presence of CA which enhanced the interaction with Ca2+ ions forming calcium citrate complex [21]. Comparing Figs. 1 and 2, it is noted that the calcium ion adsorption increased with the presence of CA into the HACG composites except for HA70CG30CA composite. This behavior is important for chitosan–gelatin polymer network that indicated the osteogentic differentiation [22].
Fig. 1.
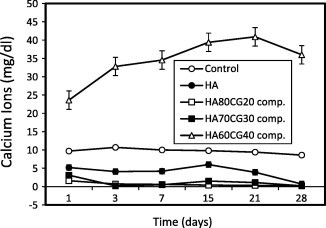
Concentration of calcium ions in SBF post-immersion of the HACG composites compared to control and HA sample. Standard error ± 0.384 (n = 3).
Fig. 2.
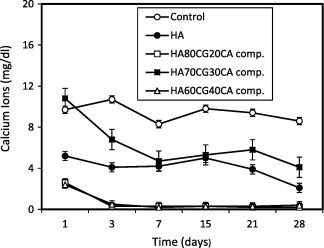
Concentration of calcium ions in SBF post-immersion of the HACGCA composites compared to control and HA sample. Standard error ± 0.336 (n = 3).
Phosphate ions
The concentration of ions post-immersion in SBF recorded lower values for the HA60CG40 composite compared to the control proving deposition of ions on the surface of the material. It is noted that the addition of 40% chitosan–gelatin into the HA60CG40 composite had improved the deposition of ions. The high concentration of gelatin and chitosan may increase the amino groups that bound with phosphorous ions in SBF through hydrogen bonding (Fig. 3). Also, the concentration of ions post-immersion recorded lower values for HA sample and HA70CG30CA composites compared to control, especially after longer time of immersion proving the deposition of ions on their surfaces (Fig. 4). By comparing, it is noted that the presence of CA in the HACG composites especially in HA70CG30CA composite accelerated the apatite formation as a result of the deposition of and Ca2+ ions onto the composite surface as shown in Figs. 3 and 4. This deposition behavior is due to the fact that concentration of and Ca2+ ions was reduced for HA70CG30CA composite compared to control.
Fig. 3.
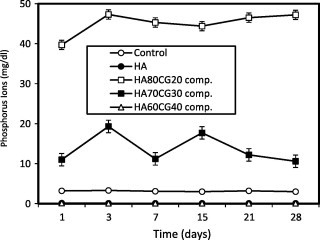
Concentration of phosphorus ions in SBF post-immersion of the HACG composites compared to control and HA sample. Standard error ± 0.214 (n = 3).
Fig. 4.
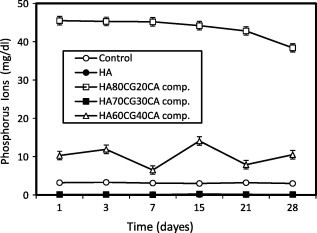
Concentration of phosphorus ions in SBF post-immersion of the HACGCA composites compared to control and HA sample. Standard error ± 0.199 (n = 3).
Assessment of the composites
Weight loss
Fig. 5 shows the weight loss behavior of the HACG and the HACGCA composites compared to HA sample. With gradual increase in chitosan–gelatin polymeric matrix content in the composites, the weight loss for HA80CG20 composite had increased compared to HA70CG30 and HA60CG40 composites because HA80CG20 composite has high content of HA and low content of polymeric matrix, which resulted in high degradation of the composite. In the presence of CA, the weight loss of the all HACGCA composites decreased comparing with the HACG composites proving that the CA increased the interaction between HA particles and chitosan and gelatin molecules via their functional groups resulting in low degradation and high stability of the composites [15].
Fig. 5a.
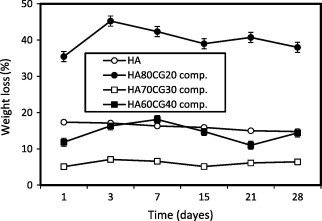
The weight loss% of the HACG composites compared to HA sample. Standard error ± 0.370 (n = 3).
Fig. 5b.
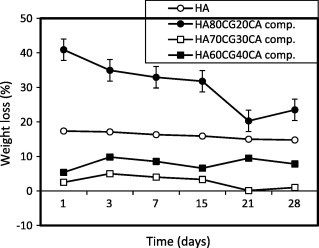
The weight loss% of the HACGCA composites compared to HA sample. Standard error ± 0.370 (n = 3).
Water absorption (%)
To evaluate the biomaterial for tissue engineering, hydrophilicity of the chitosan–gelatin polymer matrix is one of the most critical features and it is important for the absorption of body fluid and transfer of cell nutrients and metabolites [23]. Water absorption ability ratio constantly increased in parallel with increasing the concentration of CG polymer matrix, especially the HA60CG40 composite which contains 40% chitosan and gelatin matrix. The observed results were due to the ability of CG polymeric matrix to form reversible gel; its increasing pressure in mixture of HACG lowers the weight fractions of HA in it. In this way, a lower degree of crystallinity enhanced water absorption ability % of the HA60CG40 composites (Fig. 6). In this text, Zhuang et al. proved that gelatin had positive effects on cell adhesion, viability, and growth. Moreover, the incorporation of gelatin with chitosan improved the hydrophilicity of chitosan membranes and the hydrophilic surface is more suitable for cell attachment and proliferation [20]. After the addition of CA, the water absorption ability (%) also increased with increasing content of CG matrix, especially the HA70CG30CA and HA60CG40CA composites containing 30% and 40% chitosan–gelatin polymeric matrix, respectively, so the addition of CA increased the OH groups in the composite that enhanced the hydrophilicity of the HACG composites. These results coincided with Saito et al. [24] who proved that the addition of citric acid derivative (CAD) into gelatin gels after immersion in phosphate buffer saline (PBS) up to 70 mM resulted in gel water absorption ability. Additionally, the great difference in water absorption ability between HA70CG30 and HA70CG30CA composite groups is due to the OH groups in HA70CG30CA composite is higher than in HA70CG30 composite because of CA has OH groups in its structure, so the HA70CG30CA composite had higher affinity to water molecules.
Fig. 6.
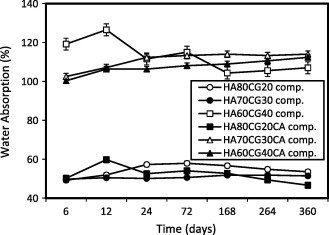
Water absorption ability % of the HACG and the HACGCA composites in distilled water. Standard error ± 0.391 (n = 3).
FT-IR analysis
FT-IR of HA shows that the intensity of HA bands such as phosphate, carbonate and OH groups in HA sample decreased post-immersion for 28 days compared to 7 days denoting high solubility properties of the precipitated HA (Fig. 7a). For HA70CG30 composite, the intensity of HA bands increased post-immersion for 28 days compared to 7 days proving that chitosan–gelatin polymeric matrix increased the bioactivity of HA due to the enhancement of ions deposition on the composite surface (Fig. 7b). For HA70CG30CA composites, the FT-IR results proved the enhancement in the intensity of the HA bands at 7 days post-immersion for the HA70CG30CA composite compared to 28 days confirming early the deposition of ions on its surface due to effect of CA (Fig. 7c). The increment in the intensity of the phosphate, carbonate, and OH groups at 7 and 28 days for HA70CG30CA composite compared to HA70CG30 composite indicates the enhancement of apatite formation in the presence of CA.
Fig. 7a.
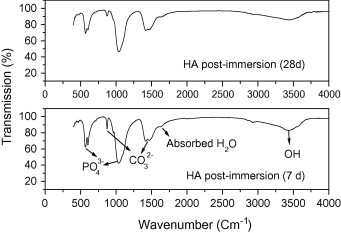
The FT-IR of HA sample post-immersion in SBF.
Fig. 7b.
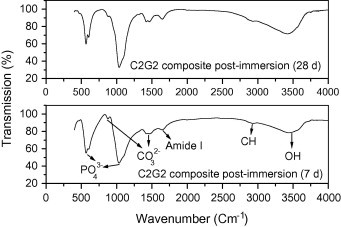
The FT-IR of HA70CG30 composite post-immersion in SBF.
Fig. 7c.
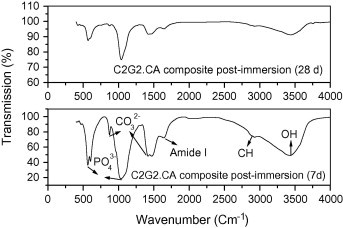
The FT-IR for HA70CG30CA composite post-immersion in SBF.
Surface morphology
HA sample
Fig. 8 shows the SEM images of HA sample pre- and post-immersion for 7 days and 28 days in SBF. SEM of pre-immersed samples shows many white rods and few needle shapes on the surface, thus proving the formation of CHA (Fig. 8a). For 7 days post-immersion, HA surface has many spherical particles with many pores and some nucleation of apatite (Fig. 8b). On the other hand, after 28 days post-immersion, more and more apatite particles were deposited, concentrated, and covered the surface with many minute pores proving effect of immersion (Fig. 8c).
Fig. 8.
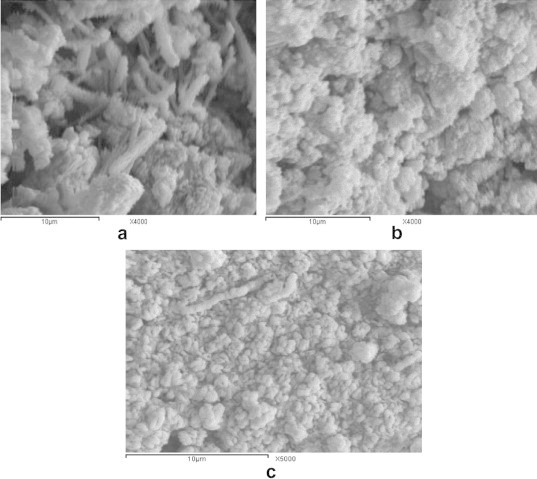
The SEM images of HA sample (a) pre- and (b) post-immersion for 7 days and (c) 28 days in SBF.
HA70CG30 composite
Fig. 9 shows the surface morphology of HA70CG30 composite pre- and post-immersion in SBF and its EDAX analysis. For pre-immersion, SEM indicates the presence of rough surface with embedded particles into the composite proving coating and formation of the composite (Fig. 9a). For 7 days post-immersion, SEM reveals the presence of some aggregated spherical particles and small particles on the surface with bright color and few pores denoting effect of mineralization of calcium phosphate ions and immersion (Fig. 9b). After 28 days post-immersion, SEM shows a plenty of the spherical particles formed on the surface denoting the growth of apatite crystals with increase in immersion time. Also, the number and size of the pores which are important for bone growth had increased due to increase in the immersion time (Fig. 9c).
Fig. 9.
The SEM images of the HA70CG30 composite (a) pre- and post-immersion for 7 days (b), 28 days (c), and its EDX analysis (d).
HA70CG30CA composite
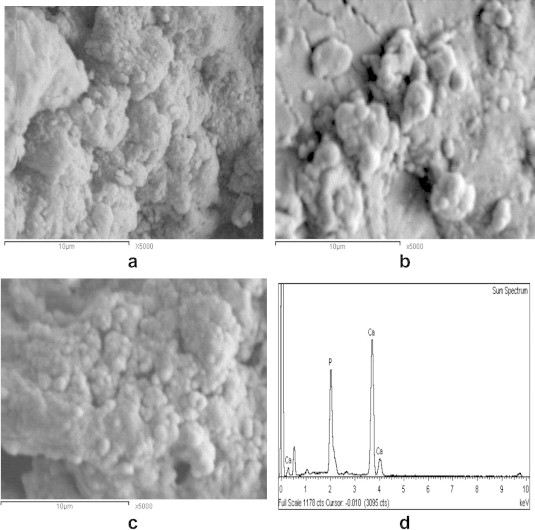
Fig. 10a shows the SEM images of the HA70CG30CA pre-immersion; it reveals good spherical geometry and high dispersion of particles on the surface of the composite due to effect of CA that enhanced the formation of HA [15]. For 7 days post-immersion, SEM shows accumulated and concentrated spherulites with number of tiny crystals which deposited on the surface and many pores between the particles as a result of immersion effect (Fig. 10b). For 28 days post-immersion, SEM indicates increase in the number of deposited particles forming spongy structure compared to 7 days post-immersion proving effect of CA (Fig. 10c). In addition, EDAX of HA70CG30CA composite 28 days post-immersion recorded high Ca/P ratio (1.71) compared to HA70CG30 composite (1.45) proving the role of CA in improving the calcium phosphate precipitation and its growth (Figs. 9d and 10d). This result coincided with Mohamed et al. who reported the important role of CA addition into chitosan/hydroxyapatite composites for HA formation [25].
Fig. 10.
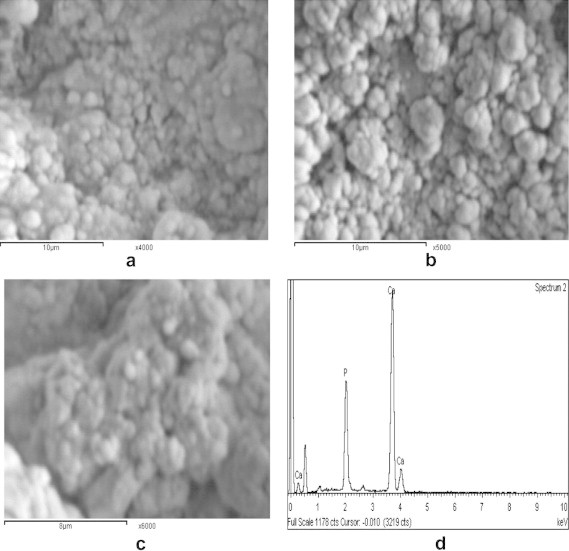
The SEM images of HA70CG30CA composite (a) pre- and (b) post-immersion for 7 days, (c) 28 days in SBF, and (d) its EDX analysis.
Conclusions
The in vitro analysis proved the mineralization of calcium and phosphorus ions onto nHA/chitosan–gelatin composite surfaces containing higher content of polymers, especially after longer time of immersion. The degradation of HA decreased within chitosan–gelatin matrix and highly decreased with CA addition. The water absorption ability of the composites increased with increase in chitosan–gelatin content and more increased with CA addition. The FT-IR and SEM-EDX analyses proved the formation of bone-like apatite layer on the surface of the composites especially those containing CA. Finally, the deposition of HA particles on the composite surface increased with addition of chitosan–gelatin matrix and more increased more with CA addition into the composites. Therefore, the HA70CG30 and HA70CG30CA nano-composites could be applied as suitable materials for bone substitutes and bio-applications in the future.
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Murugan R., Ramakrishna S. Nano-structured biomaterials. In: Nalwa H.S., editor. Encyclopedia of nanoscience and nanotechnology, vol. 7. American Scientific Publishers; California: 2004. p. 595. [Google Scholar]
- 2.Li J., Pin C.Y., Yuji Y., Yao F., Yao K. Modulation of nano-hydroxyapatite size via formation on chitosan–gelatin network film in situ. Biomaterials. 2007;28:781–790. doi: 10.1016/j.biomaterials.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 3.Jennifer L.M., Hockin H.K.X. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate–chitosan composite scaffold. Biomaterials. 2009;30(14):2675. doi: 10.1016/j.biomaterials.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong L., Gao Y., Lu G., Gong Y., Zhao N., Zhang X. A study on the bioactivity of chitosan/nanohydroxyapatite composite scaffolds for bone tissue engineering. Eur Polym J. 2006;42:3171–3179. [Google Scholar]
- 5.Xianmiao C., Yubao L., Yi Z., Li Z., Jidong L., Huanan W. Properties and in vitro biological evaluation of nano-hydroxyapatite/chitosan membranes for bone guided regeneration. Mater Sci Eng C. 2009;29:29–35. [Google Scholar]
- 6.Thein-Han W.W., Misra R.D.K. Biomimetic chitosan–nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009;5(4):1182–1197. doi: 10.1016/j.actbio.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Jin H.H., Lee C.H., Lee W.K., Lee J.K., Park H.C., Yoon S.Y. In-situ formation of the hydroxyapatite/chitosan–alginate composite scaffolds. Mater Lett. 2008;62:1630–1633. [Google Scholar]
- 8.Kikuchi M., Matsumoto H.N., Yamada T., Koyama Y., Takakuda K., Tanaka J. Glutaraldhyde cross-linked hydroxyapatite/collagen self-organization nanocomposites. Biomaterials. 2004;25:63–69. doi: 10.1016/s0142-9612(03)00472-1. [DOI] [PubMed] [Google Scholar]
- 9.Wilson O.C., Hull J.R. Surface modification of nanophase hydroxyapatite with chitosan. Mater Sci Eng C. 2008;28:434. [Google Scholar]
- 10.Jiang L.Y., Li Y., Zhang L., Wang X. Preparation and characterization of a novel composite containing carboxymethyl cellulose used for bone repair. Mater Sci Eng C. 2009;29:193. [Google Scholar]
- 11.Yamaguchi I., Tokuchi K., Fukuzaki H., Koyama Y., Takakuda K., Monma H. Preparation and microstructure analysis of chitosan/hydroxyapatite nanocomposites. J Biomed Mater Res. 2001;55:20–27. doi: 10.1002/1097-4636(200104)55:1<20::aid-jbm30>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Cheng M., Deng J., Yang F., Gong Y., Zhao N., Zhang X. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials. 2003;24:2871. doi: 10.1016/s0142-9612(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 13.Chiono V., Pulieri E., Vozzi G., Ciardelli G., Ahluwalia A., Giusti P. Genipin-crosslinked chitosan/gelatin blends for biomedical applications. J Mater Sci Mater Med. 2008;19:889. doi: 10.1007/s10856-007-3212-5. [DOI] [PubMed] [Google Scholar]
- 14.Murugan R., Ramakrishna S. Crystallographic study of hydroxyapatite bioceramics derived from various sources. Cryst Growth. 2005;5:111. [Google Scholar]
- 15.Shen X., Tong H., Jiang T., Zhu Z., Wan P. Homogeneous chitosan/carbonate apatite/citric acid nanocomposites prepared through a novel in situ precipitation method. Compos Sci Technol. 2007;67:2238. [Google Scholar]
- 16.Mohamed K.R., El-Rashidy Z.M., Salama A.A. Preparation and characterization of nano hydroxyapatite/polymeric composites materials. Mater Chem Phys. 2011;130:561. [Google Scholar]
- 17.Kokubo T., Takadama H. How useful is SBF in predicting in vivo bone bioactivity. Biomaterials. 2006;27:2907. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed R.K., El- Bassyouni G.T., Al- Beheri H.H. Chitosan graft copolymer–HA/DBM biocomposites: preparation, characterization and in-vitro evaluation. J Appl Polym Sci. 2007;105:2553. [Google Scholar]
- 19.Oliveira A.L., Malafaya P.B., Reis R.L. Sodium silicate gel as a precursor for the in vitro nucleation and growth of a bone-like apatite coating in compact and porous polymeric structures. J Biomater. 2003;24(15):2575. doi: 10.1016/s0142-9612(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhuang H., Zheng J., Cao H., Yao K. In vitro biodegradation and biocompatibility of gelatin/montmorillonite–chitosan intercalated nanocomposite. J Mater Sci Mater Med. 2007;18:951. doi: 10.1007/s10856-006-0093-y. [DOI] [PubMed] [Google Scholar]
- 21.Li C., Meng F. Nano-crystalline hydroxyapatite synthesized by neutralization with the assist of citric acid. Mater Lett. 2008;62:932. [Google Scholar]
- 22.Zhao H., He P., Ping P., Qing Y. Properties of a calcium phosphate cement synergistically reinforced by chitosan fiber and gelatin. J Polym Res. 2006;13:323. [Google Scholar]
- 23.Hu Q., Li B., Wang M., Shen J. Preparation and characterization of biodegradable chitosan hydroxyapatite nano-composites rods via in situ hybridization. Biomaterials. 2004;25:779. doi: 10.1016/s0142-9612(03)00582-9. [DOI] [PubMed] [Google Scholar]
- 24.Saito H., Taguchi T., Kobayashi H., Kataoka K., Tanaka J., Shun M. Physicochemical properties of gelatin gels prepared using citric acid derivative. Mater Sci Eng C. 2004;24:781–785. [Google Scholar]
- 25.Mohamed K.R., El-Rashidy Z.M., Salama A.A. In vitro properties of nano-hydroxyapatite/chitosan biocomposites. J Ceram Int. 2011;37:3265. [Google Scholar]



