Abstract
Since the introduction of in vitro fertilization (IVF) in 1978, over five million babies have been born worldwide using IVF. Contrary to the perception of many, IVF does not guarantee success. Almost 50% of couples that start IVF will remain childless, even if they undergo multiple IVF cycles. The decision to start or pursue with IVF is challenging due to the high cost, the burden of the treatment, and the uncertain outcome. In optimal counseling on chances of a pregnancy with IVF, prediction models may play a role, since doctors are not able to correctly predict pregnancy chances. There are three phases of prediction model development: model derivation, model validation, and impact analysis. This review provides an overview on predictive factors in IVF, the available prediction models in IVF and provides key principles that can be used to critically appraise the literature on prediction models in IVF. We will address these points by the three phases of model development.
Keywords: In vitro fertilization, Predictive factors, Prediction models, Pregnancy
Introduction
Since the birth of Louise Brown in 1978, over five million babies have been born worldwide using in vitro fertilization (IVF) [1]. The number of in vitro fertilization cycles has increased rapidly; in 2006, 458,759 cycles were reported in 32 European countries, 99,199 cycles in the USA and 50,275 cycles in Australia and New Zealand [2–4]. The number of cycles is increasing each year even further.
The increase in IVF cycles is not caused by a sudden epidemic of infertility, but by increased access to IVF, and by an expansion of the indications for IVF. Initially, IVF was performed in couples with bilateral tubal occlusion [5]. In 1992, intracytoplasmic sperm injection (ICSI) was first introduced and initiated in couples with severe male subfertility [6]. Later on, IVF/ICSI was also applied in couples without an absolute indication for IVF, such as unexplained subfertility, cervical hostility, failed ovulation induction, endometriosis, or unilateral tubal pathology [7,8]. The major difference between the original indication and the indications for which IVF is conducted nowadays is that the couples with bilateral tubal pathology or severe male subfertility have a zero chance of natural conception and completely depend on IVF/ICSI for a pregnancy, while couples with the newer indications are subfertile: they do have chances of natural conception, which may or may not be better than with IVF.
Despite the lack of evidence that IVF is effective in couples without an absolute IVF indication, IVF is often considered as a last resort for all subfertile couples regardless of the etiology of their subfertility [7–12]. Contrary to the perception of many, IVF does not guarantee success; almost 38–49% of couples that start IVF will remain childless, even if they undergo six IVF cycles [13]. Subfertile couples should therefore be well informed about the chances of success with IVF before starting their first or before continuing with a new IVF cycle. Based on a couple’s specific probability, one should decide whether the chances of success with IVF justify the burden, risks, and costs of the treatment. The threshold at which probability to start or to continue treatment may differ between different stakeholders, such as insurance companies, the tax payer, and the patients.
In optimal counseling on chances of a pregnancy after IVF, pregnancy prediction models may play a role, since doctors are not able to correctly predict pregnancy chances [14,15]. Predictions made by clinicians on the basis of clinical experience or “gut-feeling” have only slight to fair reproducibility, indicating that these predictions are likely to be inaccurate [15].
The efforts to develop prediction models for IVF reflect the need for such models in clinical practice. This need can be explained by the inability of diagnostic tests to detect factors that indicate subfertility with near 100% certainty in patients. Accurate diagnostic tests would allow treatment to focus on specific factors [16]. As IVF is currently used as an empirical treatment and not as a causal intervention for a specific disorder, there is a strong need to distinguish between couples with a good and a poor prognosis [16]. In the absence of randomized clinical trials, evaluating the effectiveness of IVF prediction models can be used to counsel couples.
The development of a prediction model can be divided into three phases: model derivation, model validation, and impact analysis [16,17] (Fig. 1). In the model derivation phase, predictors are identified, based on prior knowledge, and the weight of each predictor (regression coefficient) is calculated. In the model validation phase, the performance of the model, i.e. model’s ability to predict outcome is evaluated, and also the “generalizability” or “transportability” of the model is evaluated. The third and final phase consists of impact analysis. The impact analysis establishes whether the prediction model improves doctors’ decisions by evaluating the effect on patient outcome [16,17].
Fig. 1.
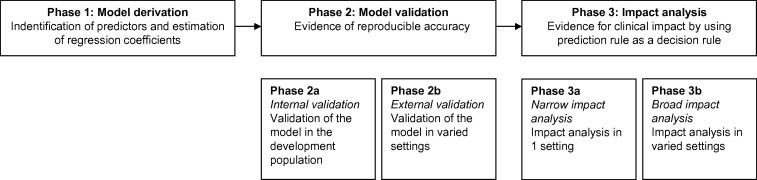
Three phases of model development.
This review provides an overview on predictive factors in IVF, the available prediction models in IVF and provides key principles that can be used to critically appraise the literature on prediction models in IVF. We will address these points by the three phases of model development: model derivation, model validation, and impact analysis.
Phase 1: model derivation
Identification of predictors
Candidate predictors are variables that are chosen to be studied for their predictive performance. These can include subject demographics, clinical history, physical examination, disease characteristics, test results, and previous treatments [18]. The identification of candidate predictors is preferably based on subject knowledge, on pathophysiological mechanisms, or the results of previous studies. Studied predictors should be clearly defined, standardized, and reproducible to enhance generalizability and application of study results to practice [18]. Researchers frequently measure more predictors than can reasonably be analyzed. When the number of predictors is much larger than the number of outcome events, there is a risk of overestimating the predictive performance of the model. To reduce the risk of false positive findings (predictors), at least 10 individuals having (developed) the event of interest are needed per candidate variable/predictor to allow for reliable prediction modeling [19].
A recent systematic review and meta-analysis on predictive factors in IVF evaluated nine predictive factors: female age, duration of subfertility, type of subfertility, indication for IVF, basal follicle stimulating hormone (bFSH), fertilization method, number of oocytes, number of embryos transferred, and embryo quality [20].
Female age is one of the most important prediction factors for success with IVF. Increasing female age was associated with lower pregnancy chances in IVF (OR 0.95, 95% CI: 0.94–0.96) [20]. The decrease in fertility sets in after the age of 30 years, with a marked decline after 35 years for both spontaneous as IVF-induced pregnancies [20–23]. The biological explanation for the declining chances to conceive with increasing female age most likely lies in the diminished ovarian reserve: the decrease in both quantity and quality of oocytes [24]. Diminished ovarian reserve generally leads to a poor response to gonadotropin therapy and limits the possibility of a successful pregnancy [25].
Increasing duration of subfertility is known to be associated with a reduced possibility of natural conception [7,26–29] (adjusted hazard rate 0.83; 95% CI 0.78–0.88) [30]. In IVF, pregnancy rates were slightly lower in couples with a longer duration of subfertility (OR 0.99, 95% CI: 0.98–1.00) [20], even after adjustment for age [23,31–33].
Although the meta-analysis did not find a significant association between type of subfertility (primary versus secondary subfertility) and pregnancy with IVF (unadjusted OR 1.04 95% CI: 0.65–1.43) [20], two recent, large studies did find an association. A previous ongoing pregnancy or live birth, adjusted for factors such as age, substantially increases the likelihood of success with IVF [31,33].
Through the years, several studies have reported on the association between the indication for IVF and pregnancy with IVF without consistent results. These studies did not use the same reference categories making the interpretation of the data difficult. There is evidence for an association between tubal pathology and pregnancy with IVF. Women with tubal pathology alone had lower pregnancy chances compared to women with unexplained subfertility or other indications [23,31,34–36]. On the other hand, another study suggested that women with tubal pathology had higher pregnancy chances after IVF compared with couples with unexplained subfertility, though not significantly [37]. There is also evidence for an association between male subfertility and pregnancy with IVF. Although two studies (N = 2628 cycles) reported that couples with male subfertility have lower pregnancy chances than those with unexplained subfertility [34,35], a very large cohort study (N = 144,018 cycles) showed that couples with only male subfertility had increased pregnancy chances compared to couples with unexplained subfertility [31]. Since these studies use different reference categories and different number of categories, it is not possible to compare these results optimally. For future studies and the development for prediction models, it would be useful to report every indication for IVF as a separate variable instead of combining all indications into one factor, to be able to compare all studies [20].
Basal FSH is an indirect estimate of ovarian reserve. A higher bFSH value was associated with lower pregnancy rates after IVF (OR 0.94; 95% CI: 0.88–1.00) [20].
Increasing number of oocytes was associated with higher pregnancy chances with IVF (OR 1.04, 95% CI: 1.02–1.07) [20]. A recent large cohort study (N = 400,135) also showed a strong relationship between the number of oocytes and live birth rate with IVF. The association is not linear; the best chance of live birth is associated with approximately 15 oocytes [38].
Although the meta-analysis did not find a significant association between pregnancy chances with ICSI compared to IVF (OR 0.95, 95% CI: 0.79–1.14) [20], a more recent large cohort study (N = 144,018 cycles) reported higher chances with ICSI compared to IVF (OR 1.28, 95% CI: 1.25–1.31), even after adjusting for all relevant factors (OR 1.27, 95% CI: 1.23–1.31) [31].
The number of embryos transferred and embryo quality were associated with increased pregnancy chances [20].
Estimation of the regression coefficient
After identifying all potential predictors, a multivariable model can be constructed by regression analysis (logistic regression or proportional hazard analysis). To evaluate the quantitative effect of each predictor, the weight of each predictor is calculated by estimating the corresponding regression coefficient in a linear model.
Currently, over 21 papers have reported on the development and or validation of models for the prediction of pregnancy with IVF (Table 1) [23,31–37,39–54].
Table 1.
Characteristics on prediction models for pregnancy after IVF and IVF-eSET.
| Author (year) | Inclusion of embryo characteristics | IVF-eSET | Outcome |
|---|---|---|---|
| Van Loendersloot et al. [33] | Yes | No | Ongoing pregnancy |
| Nelson and Lawlor [31] | No | No | Live birth |
| van Weert et al. [54] | No | No | Ongoing pregnancy |
| Lintsen et al. [23] | No | No | Ongoing pregnancy |
| Verberg et al. [55] | Yes | Yes | Ongoing pregnancy |
| Carrera-Rotllan et al. [40] | No | No | Pregnancy |
| Ottosen et al. [35] | Yes | Yes | Pregnancy |
| Ferlitsch et al. [42] | No | No | Pregnancy |
| Hunault et al. [37] | Yes | Yes | Ongoing pregnancy |
| Stolwijk et al. [52] | No | No | Ongoing pregnancy |
| Bancsi et al. [34] | No | No | Ongoing pregnancy |
| Minaretzis et al. [47] | Yes | No | Live birth |
| Commenges-Ducos et al. [41] | Globel model: No | No | Ongoing pregnancy |
| Model for implantation: Yes | |||
| Templeton et al. [32] | No | No | Live birth |
| Stolwijk et al. [50] | Model A: No | No | Ongoing pregnancy |
| Model B: Yes | |||
| Model C: Yes | |||
| Bouckaert et al. [39] | Yes | No | Pregnancy |
| Haan et al. [43] | No | No | Ongoing pregnancy |
| Hughes et al. [44] | No | No | Ongoing pregnancy |
| Nayudu et al. [48] | No | No | Ongoing pregnancy |
Phase 2: model validation
The second phase in the development of a prediction model is the evaluation of the model performance, i.e. model validation. The performance of the model can be evaluated by calculating its discriminative capacity and the degree of calibration. Discrimination relates to how well a model can distinguish between patients with and without the outcome, i.e. discriminate between women who achieved pregnancy and those who did not. Discriminative capacity can be expressed by the area under the receiver operating characteristic curve (AUC), also known as the c-statistic. A model with a c-statistic of 0.5 has no discriminative power at all, while 1.0 would reflect perfect discrimination. Calibration relates to the agreement between observed outcomes and calculated probabilities, i.e. if we calculate a 30% probability of a pregnancy with IVF, the observed relative frequency of pregnancy should be approximately 30 out of 100 women. Calibration can be assessed by the Hosmer and Lemeshow goodness-of-fit test statistic. A Hosmer–Lemeshow statistics with a p-value above 0.05 implies that there is no significant miscalibration. In addition, calibration can also be assessed by comparing the average calculated probabilities with the actual proportions in disjoint subgroups. The average calculated probabilities and actual proportions in each group can be plotted in a calibration plot. In case of perfect calibration, all points in a calibration plot are on the diagonal, the line of equality, and probabilities correspond perfectly to the actual proportions.
The validation phase can be subdivided in internal validation (phase 2a) and external validation (phase 2b). With internal validation, the model’s ability to predict the outcome in the group of patients in which it was developed is evaluated (reproducibility). Internal validation should be seen as validating the modeling process [56]. Of the 21 papers reporting on IVF prediction model development, only 11 are also internally validated [23,31–35,37,40,45,49–51,53–55].
Before being able to use prediction models for clinical decision making, it is not enough to demonstrate a reasonable or good performance after internal validation. Most models show too optimistic results, even after corrections from interval validation procedures. It is essential to confirm that any developed model also predicts well in a “similar but different” population outside the development set, i.e. external validation (generalizability). The more these populations differ from the development study, the stronger the test of generalizability of the model [57].
There are three different types of external validation, temporal validation, geographical validation, and domain validation. In temporal validation, the model is validated on new patients that are from the same center as the development set, but in a different time period [57,58]. In geographical external validation, the model is validated on new patients from a different center as the development set [57,58]. In domain validation, the model is validated on new patients that are very different from the patients from which the model was developed [57].
Of the 12 IVF models that went through internal validation, only four models have also been validated externally [32,33,37,45,49,51,53]. One model was validated temporally, the model calibrated well both in the development set and in a separate validation set [33]. Three models have been validated geographically [32,37,45,49–51,53], but only one model showed good calibration after validation [37,45]. So at this moment, there is only one model that is generalizable to other clinics [37,45]. All other models have to be geographically validated first before using the models in practice.
A prediction model often performs less well in a new group of patients than in the study group in which it was developed. This can be caused by differences in the case-mix between the development and validation population or by true differences between populations [58]. Instead of simply rejecting the prediction model and develop or fit a new one, a better alternative is to update existing prediction models and adjust or recalibrate it to the local circumstances or setting of the validation set [57,58]. As a result, the updated model is adjusted to the characteristics of new individuals. Several methods for updating prediction models are possible. Most often, differences are seen in the outcome frequency between the development and new validation set. This results in poor calibration of the model; predicted probabilities are systematically too high or too low. By adjusting the intercept (baseline risk) of the original model, calibration can be improved. Additional updating methods vary from adjustment of all predictor regression coefficients, adjustment of regression coefficients for particular predictor weight, to the addition of a completely new predictor or marker to the existing model [57,58].
As patient populations may shift during the years, the group of patients used for the development and validation of the prediction model may differ from the current patient population. Reproductive techniques may evolve during the years, new biomarkers with predictive value may become available, and the prediction model should be regularly updated and adapted to the new setting, so that predictions for future patients remain valid and may even improve [58]. IVF centers should therefore consider collecting their own data in electronic databases, so that with accumulation of the number of IVF cycles over time, they can update the model with their own data.
Phase 3: impact analysis
The third and final phase in the evaluation of models is impact analysis; it establishes whether the prediction model improves decisions, in terms of quality or cost-effectiveness of patient care [17,57,58]. This can be evaluated in one setting (phase 3a) or in varied settings (phase 3b). Different study designs to evaluate the impact of a prediction model are possible, such as comparing the outcomes between patients randomly assigned to receive management guided by the prediction model and patients managed without the prediction model (care-as-usual). A less valid alternative is to ask fertility specialists to document therapeutic management decisions before and after being “exposed” to a model’s predictions. None of the existing IVF prediction models has reached the impact analysis phase yet.
Discussion
As IVF can be stressful physically and emotionally and is not without health risks, subfertile couples should thus be well informed about the chances for success with IVF before each cycle. Unfortunately at this point, there are no randomized controlled clinical trials comparing IVF with natural conception. Thus, the only way to counsel couples properly is by model-based prognosis.
Over 21 articles have reported on the development and/or validation of prediction models in IVF. Of these 21 articles, only two models had a good performance after external validation. Impact analyses have not yet been performed for any of these models. Future research should focus more on updating existing prediction models and adjust or recalibrate them to the local circumstances or setting rather than developing new prediction models. This way prediction models may strengthen evidence-based, individualized decision-making and can contribute to a rational use of scarce resources.
Conflict of interest
The authors have declared no conflict of interest.
Biographies

Laura van Loendersloot graduated from medical school at the University of Amsterdam, The Netherlands. She worked as fertility doctor and studied for her PhD at the Center of Reproductive Medicine at the Academic Medical Center, University of Amsterdam. She obtained her PhD in 2013, her thesis was titled ‘Predicting IVF outcome’. She is currently a resident in Obstetrics and Gynaecology at Sint Lucas Andreas Hospital in Amsterdam.

Sjoerd Repping (1974) obtained his Master’s degree in Medical Biology cum laude from the University of Amsterdam (UvA) specialising in genetics and immunology. He was trained as a clinical embryologist at the Academic Medical Center and was a visiting scientist at the Whitehead Institute in the US in 2001. He obtained his PhD cum laude in 2003 with a thesis describing the role of the human Y-chromsome in male infertility. In 2009 he became full professor of Human Reproductive Biology at the UvA. Currently, he heads the Center for Reproductive Medicine at the Academic Medical Center of the UvA and is chair of the Dutch Society of Clinical Embryology.

Patrick M.M. Bossuyt is the professor of Clinical Epidemiology at the University of Amsterdam, and dean of the School of Public Health in his university.
Dr Bossuyt leads the Biomarker and Test Evaluation Program, a line of research to appraise and develop methods for evaluating medical tests and biomarkers, and to apply these methods in relevant clinical studies.

Fulco van der Veen MD, PhD, is a professor of Reproductive Medicine at the Center for Reproductive Medicine of the University of Amsterdam. His research interests include evaluation research on diverse topics like prediction models in reproductive medicine, preimplantation genetic screening, ectopic pregnancy, male infertility and polycystic ovary syndrome, and translational research on the Y chromosome and human spermatogonial stem cells. Since 2008, professor Van der Veen has been awarded 11 grants (4 as principal investigator and 11 as co-applicant) worth a total of $ 2,962,658.
Professor Van der Veen has supervised 40 PhD students during his career until now. Professor Van der Veen has over 300 publications to his credit in top leading journals such as Fertility and Sterility, Human Reproduction, Human Reproduction Update, JAMA, Lancet, NEJM and Nature Genetics.
The h-index is 38. The sum of times cited is 5,584. The median impact factor of his publications is 11,91 (average citations per item). Median impact factors for his own field(s) such as Obstetrics & Gynaecology are 1.804 for median impact factor and 2.326 for aggregate impact factor.
In Reproductive Biology the median impact factor is 2.385 and the aggregate impact factor is 3.041.
Among his relevant experience and professional memberships, those deserving mention are,
- He worked as Associate Editor Human Reproduction from 1-1-2001-1-1-2004.
- He made notable contributions as the Chairman of the Foundation named GynaecologischeEndocrinologie en Kunstmatige Humane Voortplanting.
- He was a senior member of the pre-review group “Human Reproduction”.
- His contributions as a member of the committee for Preimplantation diagnostics and screening of the Health Council from 2005 -2006 were remarkable.
- He made a mark as associate Editor Human Reproduction from 1-1-2008 – 1-1-2012.
- He was selected as Chairman of the Local Organizing Committee for the 25th Annual Meeting of ESHRE from 28 -6 2009 – 1-7 2009.
- He was a member of the Advisory Board for the Journal of advanced Research.
- He worked as Principal Investigator in 2013.
- His presence on the Editorial Board for the Journal of Reproduction and Infertility (JRI) in 2013 was noteworthy.

Dr van Wely is a clinical epidemiologist specialized in human reproduction. She completed his PhD in 2004 at the University of Amsterdam on optimal treatment of women with polycystic ovary syndrome. After obtaining her PhD she continued to work at Center for Reproductive Medicine and at the Dutch Obstetrics and Gynecology Consortium. She has been involved as a methodologist in many randomized trials conducted within the Dutch Obstetrics and Gynecology Consortium (www.studies-obsgyn.nl), and as such, assisted other investigators and performed the statistical analyses of the studies. She has participated in several succesfull grant applications.
She is a registered reviewer and ad hoc reviewer for scientific journals and is an editor for the Cochrane Menstrual Disorders and Subfertility Group (MDSG) and is Deputy Editor of Human Reproduction Update.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.European Society of Human Reproduction and Embryology (ESHRE). ART fact sheet, <http://www.eshre.eu/Guidelines-and-Legal/ART-fact-sheet.aspx>; 2013.
- 2.Australian Institute of Health and Welfare. Assisted reproductive technology in Australia and New Zealand, <http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=6442458968>; 2006.
- 3.Centers for Disease Control and Prevention (CDC). Assisted reproductive technology report,. <http://www.cdc.gov/art/ART2006/PDF/2006ART.pdf>; 2006.
- 4.de Mouzon J., Goossens V., Bhattacharya S., Castilla J.A., Ferraretti A.P., Korsak V. Assisted reproductive technology in Europe, 2006: results generated from European registers by ESHRE. Hum Reprod. 2010;25:1851–1862. doi: 10.1093/humrep/deq124. [DOI] [PubMed] [Google Scholar]
- 5.Edwards R.G. Maturation in vitro of human ovarian oocytes. Lancet. 1965;2:926–929. doi: 10.1016/s0140-6736(65)92903-x. [DOI] [PubMed] [Google Scholar]
- 6.Hamberger L., Lundin K., Sjogren A., Soderlund B. Indications for intracytoplasmic sperm injection. Hum Reprod. 1998;13(Suppl. 1):128–133. doi: 10.1093/humrep/13.suppl_1.128. [DOI] [PubMed] [Google Scholar]
- 7.Hull M.G., Glazener C.M., Kelly N.J., Conway D.I., Foster P.A., Hinton R.A. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291:1693–1697. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Clinical Excellence (NICE). National collaborating centre for women’s and children’s health. Fertility guideline: fertility guideline: assessment and treatment for people with fertility problems, <http://www.nice.org.uk/nicemedia/pdf/CG011niceguideline.pdf>; 2004.
- 9.American Society for Reproductive Medicine (ASRM) Endometriosis and infertility. Fertil Steril. 2006;86:S156–S160. doi: 10.1016/j.fertnstert.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 10.American Society for Reproductive Medicine (ASRM) Aging and infertility in women. Fertil Steril. 2006;86:S248–S252. doi: 10.1016/j.fertnstert.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 11.American Society for Reproductive Medicine (ASRM) Effectiveness and treatment for unexplained infertility. Fertil Steril. 2006;86:S111–S114. doi: 10.1016/j.fertnstert.2006.07.1475. [DOI] [PubMed] [Google Scholar]
- 12.European Society of Human Reproduction and Embryology (ESHRE). Good clinical treatment in assisted reproduction – an ESHRE position paper, <http://www.eshre.eu/binarydata.aspx?type=doc&sessionId=yygglojxjtmhjv5555k2peat/Good_Clinical_treatment_in_Assisted_Reproduction_ENGLISH_new.pdf>; 2008.
- 13.Malizia B.A., Hacker M.R., Penzias A.S. Cumulative live-birth rates after in vitro fertilization. New Engl J Med. 2009;360:236–243. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 14.van der Steeg J.W., Steures P., Eijkemans M.J., Habbema J.D., Bossuyt P.M., Hompes P.G. Do clinical prediction models improve concordance of treatment decisions in reproductive medicine? BJOG. 2006;113:825–831. doi: 10.1111/j.1471-0528.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- 15.Wiegerinck M.A., Bongers M.Y., Mol B.W., Heineman M.J. How concordant are the estimated rates of natural conception and in-vitro fertilization/embryo transfer success? Hum Reprod. 1999;14:689–693. doi: 10.1093/humrep/14.3.689. [DOI] [PubMed] [Google Scholar]
- 16.Mol B.W., van Wely M., Steyerberg E.W. Using prognostic models in clinical infertility. Hum Fertil. 2000;3:199–202. doi: 10.1080/1464727002000198981. [DOI] [PubMed] [Google Scholar]
- 17.Reilly B.M., Evans A.T. Translating clinical research into clinical practice: impact of using prediction rules to make decisions. Ann Intern Med. 2006;144:201–209. doi: 10.7326/0003-4819-144-3-200602070-00009. [DOI] [PubMed] [Google Scholar]
- 18.Moons K.G., Kengne A.P., Woodward M., Royston P., Vergouwe Y., Altman D.G. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart. 2012;98:683–690. doi: 10.1136/heartjnl-2011-301246. [DOI] [PubMed] [Google Scholar]
- 19.Moons K.G., Royston P., Vergouwe Y., Grobbee D.E., Altman D.G. Prognosis and prognostic research: what, why, and how? BMJ. 2009;338:b375. doi: 10.1136/bmj.b375. [DOI] [PubMed] [Google Scholar]
- 20.van Loendersloot L.L., van Wely M., Limpens J., Bossuyt P.M., Repping S., van der Veen F. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update. 2010;16:577–589. doi: 10.1093/humupd/dmq015. [DOI] [PubMed] [Google Scholar]
- 21.Baird D.T., Collins J., Egozcue J., Evers L.H., Gianaroli L., Leridon H. Fertility and ageing. Hum Reprod Update. 2005;11:261–276. doi: 10.1093/humupd/dmi006. [DOI] [PubMed] [Google Scholar]
- 22.Faddy M.J., Gosden R.G., Gougeon A., Richardson S.J., Nelson J.F. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 23.Lintsen A.M., Eijkemans M.J., Hunault C.C., Bouwmans C.A., Hakkaart L., Habbema J.D. Predicting ongoing pregnancy chances after IVF and ICSI: a national prospective study. Hum Reprod. 2007;22:2455–2462. doi: 10.1093/humrep/dem183. [DOI] [PubMed] [Google Scholar]
- 24.Broekmans F.J., Knauff E.A., te Velde E.R., Macklon N.S., Fauser B.C. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab. 2007;18:58–65. doi: 10.1016/j.tem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Ulug U., Ben-Shlomo I., Turan E., Erden H.F., Akman M.A., Bahceci M. Conception rates following assisted reproduction in poor responder patients: a retrospective study in 300 consecutive cycles. Reprod Biomed Online. 2003;6:439–443. doi: 10.1016/s1472-6483(10)62164-5. [DOI] [PubMed] [Google Scholar]
- 26.Collins J.A., Burrows E.A., Wilan A.R. The prognosis for live birth among untreated infertile couples. Fertil Steril. 1995;64:22–28. [PubMed] [Google Scholar]
- 27.Eimers J.M., te Velde E.R., Gerritse R., Vogelzang E.T., Looman C.W., Habbema J.D. The prediction of the chance to conceive in subfertile couples. Fertil Steril. 1994;61:44–52. doi: 10.1016/s0015-0282(16)56451-6. [DOI] [PubMed] [Google Scholar]
- 28.Evers J.L. Female subfertility. Lancet. 2002;360:151–159. doi: 10.1016/S0140-6736(02)09417-5. [DOI] [PubMed] [Google Scholar]
- 29.Snick H.K., Snick T.S., Evers J.L., Collins J.A. The spontaneous pregnancy prognosis in untreated subfertile couples: the Walcheren primary care study. Hum Reprod. 1997;12:1582–1588. doi: 10.1093/humrep/12.7.1582. [DOI] [PubMed] [Google Scholar]
- 30.Hunault C.C., Habbema J.D., Eijkemans M.J., Collins J.A., Evers J.L., te Velde E.R. Two new prediction rules for spontaneous pregnancy leading to live birth among subfertile couples, based on the synthesis of three previous models. Hum Reprod. 2004;19:2019–2026. doi: 10.1093/humrep/deh365. [DOI] [PubMed] [Google Scholar]
- 31.Nelson S.M., Lawlor D.A. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilisation: a prospective study of 144,018 treatment cycles. PLoS Med. 2011;8:e1000386. doi: 10.1371/journal.pmed.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Templeton A., Morris J.K., Parslow W. Factors that affect outcome of in-vitro fertilisation treatment. Lancet. 1996;348:1402–1406. doi: 10.1016/S0140-6736(96)05291-9. [DOI] [PubMed] [Google Scholar]
- 33.van Loendersloot LL, van Wely M, Repping S, Bossuyt PMM, van der Veen F. Individualized decision-making in IVF: calculating the chances of pregnancy. Hum Reprod; 2013 [Epub ahead of print]. [DOI] [PubMed]
- 34.Bancsi L.F., Huijs A.M., den Ouden C.T., Broekmans F.J., Looman C.W., Blankenstein M.A. Basal follicle-stimulating hormone levels are of limited value in predicting ongoing pregnancy rates after in vitro fertilization. Fertility & Sterility. 2000;73:552–557. doi: 10.1016/s0015-0282(99)00552-x. [DOI] [PubMed] [Google Scholar]
- 35.Ottosen L.D., Kesmodel U., Hindkjaer J., Ingerslev H.J. Pregnancy prediction models and eSET criteria for IVF patients – do we need more information? J Assist Reprod Gen. 2007;24:29–36. doi: 10.1007/s10815-006-9082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strandell A., Bergh C., Lundin K. Selection of patients suitable for one-embryo transfer may reduce the rate of multiple births by half without impairment of overall birth rates. Hum Reprod. 2000;15:2520–2525. doi: 10.1093/humrep/15.12.2520. [DOI] [PubMed] [Google Scholar]
- 37.Hunault C.C., Eijkemans M.J., Pieters M.H., te Velde E.R., Habbema J.D., Fauser B.C. A prediction model for selecting patients undergoing in vitro fertilization for elective single embryo transfer. Fertility & Sterility. 2002;77:725–732. doi: 10.1016/s0015-0282(01)03243-5. [DOI] [PubMed] [Google Scholar]
- 38.Sunkara S.K., Rittenberg V., Raine-Fenning N., Bhattacharya S., Zamora J., Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26:1768–1774. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
- 39.Bouckaert A., Psalti I., Loumaye E., de Cooman S., Thomas K. The probability of a successful treatment of infertility by in-vitro fertilization. Hum Reprod. 1994;9:448–455. doi: 10.1093/oxfordjournals.humrep.a138526. [DOI] [PubMed] [Google Scholar]
- 40.Carrera-Rotllan J., Estrada-Garcia L., Sarquella-Ventura J. Prediction of pregnancy in IVF cycles on the fourth day of ovarian stimulation. J Assist Reprod Gen. 2007;24:387–394. doi: 10.1007/s10815-007-9144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Commenges-Ducos M., Tricaud S., Papaxanthos-Roche A., Dallay D., Horovitz J., Commenges D. Modelling of the probability of success of the stages of in-vitro fertilization and embryo transfer: stimulation, fertilization and implantation. Hum Reprod. 1998;13:78–83. doi: 10.1093/humrep/13.1.78. [DOI] [PubMed] [Google Scholar]
- 42.Ferlitsch K., Sator M.O., Gruber D.M., Rucklinger E., Gruber C.J., Huber J.C. Body mass index, follicle-stimulating hormone and their predictive value in in vitro fertilization. J Assist Reprod Gen. 2004;21:431–436. doi: 10.1007/s10815-004-8759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haan G., Bernardus R.E., Hollanders J.M., Leerentveld R.A., Prak F.M., Naaktgeboren N. Results of IVF from a prospective multicentre study. Hum Reprod. 1991;6:805–810. doi: 10.1093/oxfordjournals.humrep.a137432. [DOI] [PubMed] [Google Scholar]
- 44.Hughes E.G., King C., Wood E.C. A prospective study of prognostic factors in in vitro fertilization and embryo transfer. Fertil Steril. 1989;51:838–844. doi: 10.1016/s0015-0282(16)60676-3. [DOI] [PubMed] [Google Scholar]
- 45.Hunault C.C., te Velde E.R., Weima S.M., Macklon N.S., Eijkemans M.J., Klinkert E.R. A case study of the applicability of a prediction model for the selection of patients undergoing in vitro fertilization for single embryo transfer in another center. Fertility & Sterility. 2007;87:1314–1321. doi: 10.1016/j.fertnstert.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 46.Leushuis E., van der Steeg J.W., Steures P., Bossuyt P.M., Eijkemans M.J., van der Veen F. Prediction models in reproductive medicine: a critical appraisal. Hum. Reprod. Update. 2009;15:537–552. doi: 10.1093/humupd/dmp013. [DOI] [PubMed] [Google Scholar]
- 47.Minaretzis D., Harris D., Alper M.M., Mortola J.F., Berger M.J., Power D. Multivariate analysis of factors predictive of successful live births in in vitro fertilization (IVF) suggests strategies to improve IVF outcome. J Assist Reprod Gen. 1998;15:365–371. doi: 10.1023/A:1022528915761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nayudu P.L., Gook D.A., Hepworth G., Lopata A., Johnston W.I. Prediction of outcome in human in vitro fertilization based on follicular and stimulation response variables. Fertil Steril. 1989;51:117–125. doi: 10.1016/s0015-0282(16)60439-9. [DOI] [PubMed] [Google Scholar]
- 49.Smeenk J.M., Stolwijk A.M., Kremer J.A., Braat D.D. External validation of the Templeton model for predicting success after IVF. Hum Reprod. 2000;15:1065–1068. doi: 10.1093/humrep/15.5.1065. [DOI] [PubMed] [Google Scholar]
- 50.Stolwijk A.M., Zielhuis G.A., Hamilton C.J., Straatman H., Hollanders J.M., Goverde H.J. Prognostic models for the probability of achieving an ongoing pregnancy after in-vitro fertilization and the importance of testing their predictive value. Human Reproduction. 1996;11:2298–2303. doi: 10.1093/oxfordjournals.humrep.a019092. [DOI] [PubMed] [Google Scholar]
- 51.Stolwijk A.M., Straatman H., Zielhuis G.A., Jansen C.A., Braat D.D., van Dop P.A. External validation of prognostic models for ongoing pregnancy after in-vitro fertilization. Human Reproduction. 1998;13:3542–3549. doi: 10.1093/humrep/13.12.3542. [DOI] [PubMed] [Google Scholar]
- 52.Stolwijk A.M., Wetzels A.M., Braat D.D. Cumulative probability of achieving an ongoing pregnancy after in-vitro fertilization and intracytoplasmic sperm injection according to a woman’s age, subfertility diagnosis and primary or secondary subfertility. Hum Reprod. 2000;15:203–209. doi: 10.1093/humrep/15.1.203. [DOI] [PubMed] [Google Scholar]
- 53.van Loendersloot L.L., van Wely M., Repping S., van der Veen F., Bossuyt P.M. The templeton prediction model underestimates IVF success in an external validation. Reprod Biomed Online. 2011;22:59–602. doi: 10.1016/j.rbmo.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 54.van Weert J.M., Repping S., van der Steeg J.W., Steures P., van der Veen F., Mol B.W. A prediction model for ongoing pregnancy after in vitro fertilization in couples with male subfertility. J Reprod Med. 2008;53:250–256. [PubMed] [Google Scholar]
- 55.Verberg M.F., Eijkemans M.J., Macklon N.S., Heijnen E.M., Fauser B.C., Broekmans F.J. Predictors of ongoing pregnancy after single-embryo transfer following mild ovarian stimulation for IVF. Fertil Steril. 2008;89:1159–1165. doi: 10.1016/j.fertnstert.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 56.Steyerberg E.W., Eijkemans M.J., Harrell F.E., Jr., Habbema J.D. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Making. 2001;21:45–56. doi: 10.1177/0272989X0102100106. [DOI] [PubMed] [Google Scholar]
- 57.Moons K.G., Kengne A.P., Grobbee D.E., Royston P., Vergouwe Y., Altman D.G. Risk prediction models: II. External validation, model updating, and impact assessment. Heart. 2012;9:691–698. doi: 10.1136/heartjnl-2011-301247. [DOI] [PubMed] [Google Scholar]
- 58.Steyerberg E.W. Clinical prediction models. 1st ed. Springer Science + Business Media, LCC; New York, USA: 2009. (A practical approach to development, validation and updating). [Google Scholar]



