Abstract
The aim of this study was to develop and optimize Trimetazidine dihydrochloride (TM) controlled porosity osmotic pump (CPOP) tablets of directly compressed cores. A 23 full factorial design was used to study the influence of three factors namely: PEG400 (10% and 25% based on coating polymer weight), coating level (10% and 20% of tablet core weight) and hole diameter (0 “no hole” and 1 mm). Other variables such as tablet cores, coating mixture of ethylcellulose (4%) and dibutylphthalate (2%) in 95% ethanol and pan coating conditions were kept constant. The responses studied (Yi) were cumulative percentage released after 2 h (Q%2h), 6 h (Q%6h), 12 h (Q%12h) and regression coefficient of release data fitted to zero order equation (RSQzero), for Y1, Y2, Y3, and Y4, respectively. Polynomial equations were used to study the influence of different factors on each response individually. Response surface methodology and multiple response optimization were used to search for an optimized formula. Response variables for the optimized formula were restricted to 10% ⩽ Y1 ⩽ 20%, 40% ⩽ Y2 ⩽ 60%, 80% ⩽ Y3 ⩽ 100%, and Y4 > 0.9. The statistical analysis of the results revealed that PEG400 had positive effects on Q%2h, Q%6h and Q%12h, hole diameter had positive effects on all responses and coating level had positive effect on Q%6h, Q%12h and negative effect on RSQzero. Full three factor interaction (3FI) equations were used for representation of all responses except Q%2h which was represented by reduced (3FI) equation. Upon exploring the experimental space, no formula in the tested range could satisfy the required constraints. Thus, direct compression of TM cores was not suitable for formation of CPOP tablets. Preliminary trials of CPOP tablets with wet granulated cores were promising with an intact membrane for 12 h and high RSQzero. Further improvement of these formulations to optimize TM release will be done in further studies.
Keywords: Trimetazidine, Controlled porosity osmotic pump tablets, Factorial design, Response surface methodology
Introduction
Controlled drug delivery has taken an important position in pharmaceutical development due to improving the tolerability and patient compliance with prescribed dosing regimens [1]. Despite the extensive use of polymer-based systems, alternatives have been developed to decrease the influence of the different physiological factors affected by food intake and patient age [2]. Osmotic drug delivery systems use osmotic pressure as an energy source and driving force for delivery of drugs. Presence of food, pH, and other physiological factors may affect drug release from most controlled release systems (matrices and reservoirs), whereas drug release from oral osmotic systems is independent of these factors to a large extent [3].
The controlled porosity osmotic pump tablets (CPOP tablets) concept was developed by many researchers as an oral drug delivery system [4,5]. This CPOP tablet is a spray-coated tablet with a semipermeable membrane coat containing leachable pore former materials [6]. In this system, the drug, after dissolution in the core, is released from the osmotic pump tablet by hydrostatic pressure and diffusion through pores created by the dissolution of pore formers incorporated in the membrane. The hydrostatic pressure is created by an osmotic agent, the drug itself or a tablet component, after water is imbibed across the semipermeable membrane [7].
Trimetazidine dihydrochloride (TM) is a metabolic anti-ischemic drug which improves myocardial and muscles glucose utilization [8]. It is used in the prophylaxis against and management of angina pectoris, in cases of ischemia of neurosensorial tissues and also in Meniere’s disease [9]. It is rapidly absorbed, and its half-life is relatively short (t1/2 = 6.0 ± 1.4 h) [9]. Being a freely water soluble drug, it will be a challenging task to formulate it in a controlled release drug delivery system. The direct compression technique was used to prepare the tablet cores.
Direct Compression of tablets is the easiest way of processing tablets. It includes the main steps of powder blending, lubrication, and compaction. As there is no granulation step to improve the flow and compaction of ingredients, it is usually necessary to use excipients specifically designed for direct compression and engineered to provide the necessary flow and compaction properties [10].
23 factorial design was adopted in this study. Factorial designs are of the most efficient designs for experiments involving the study of the effects of two or more factors. By a factorial design, we mean that in each complete trial or replication of the experiment, all possible combinations of the levels of the factors are investigated [11]. Optimization technique based on a response surface methodology (RSM) using polynomial equations [12,13] enables the navigation of the experimental space and finding the optimized formula with predetermined constraints for multiple factors. This optimization technique will be used to search for the optimal TM zero order extended-release formulation for a period of 12 h.
The aim of this study was to answer the question: Can TM release be optimized from CPOP tablets of directly compressed cores?
Material and methods
Materials
Trimetazidine dihydrochloride, Sharon Bio-Medicine, India; spray dried lactose, Molkerei MEGGLE Wasserburg GmbH & Co., KG, Germany; microcrystalline cellulose (avicel PH-102), F M C Biopolymer, Ireland and PEG400, BASF Fine Chemicals, Switzerland, were kind gift samples from Global Napi Pharmaceuticals Company. Magnesium stearate, Witco Corp, USA. Ethylcellulose, viscosity of 5% solution in toluene/ethanol 80:20 is 100 cP, extent of labeling: 49% ethoxyl, Sigma–Aldrich Chemie, Steinhiem, Germany. Dibutylphthalate, Sigma–Aldrich Company, St. Louis, USA. All other chemicals were of the analytical grade and used as received.
Experimental design for CPOP tablets of directly compressed cores
Three independent variables expected to have pronounced effects on the osmotic release of TM from CPOP tablets of directly compressed cores were investigated [14]. Each factor was studied at two levels; hence, a 23 full factorial design was applied [11]. The levels of these parameters vary widely in different researches of osmotic formulations. These specific levels were chosen based on the wide ranges used in other studies and on preliminary trials for formation of continuous coat. Other variables such as tablet cores, other coating components, and coating conditions were kept constant. The independent variables (factors) and their respective levels investigated together with the dependent variables (responses) and their constraints are shown in Table 1. These dependent variables constraints were used for obtaining a desirable drug release as described in literature [13,15]. These required constraints choice was based on the desired zero order release profile. The cumulative percentage of drug released was considered to be 0% at 0 h, and the ideal drug release was supposed to be 90% in 12 h. Therefore, the equation of zero order release is F(%) = 7.5 t where F(%) is the cumulative percentage released of drug, and t is the release time in hours [16]. Substitution with the required times for responses (2, 6, and 12 h) yielded the results of (15%, 45%, and 90%, respectively). The constraints were set by giving a range for each response around its calculated value. Setting the Y2 between 40% and 60% was to allow for about 50% of the drug to be released after half the release period. Y4 (which is the regression coefficient of release data fitted to zero order release equation) was chosen to be maximized to ensure fitting of the release data to zero order release kinetics.
Table 1.
Factors and respective levels investigated in the 23 design together with the responses and their constraints.
| Factors | Levels investigated |
|
|---|---|---|
| Low (−1) | High (+1) | |
| X1: PEG400 (%) | 10 | 25 |
| X2: Coating level (%) | 10 | 20 |
| X3: Hole diameter (mm) | 0 | 1 |
| Responses | Constraints | |
| Y1 = cumulative% drug released in 2 h (Q%2h) | 10% ⩽ Y1 ⩽ 20% | |
| Y2 = cumulative% drug released in 6 h (Q%6h) | 40% ⩽ Y2 ⩽ 60% | |
| Y3 = cumulative% drug released in 12 h (Q%12h) | 80% ⩽ Y3 ⩽ 100% | |
| Y4 = R2 (Regression coefficient of release data fitted to zero order equation) (RSQzero) | Maximize (>0.9) | |
The preparation of the tablets according to suggested trials as well as the release studies were done in random order. Each combination was performed twice in two separate replicates giving a total of sixteen runs. The trials listed in standard order [11] are shown in Table 2.
Table 2.
The composition and observed responses of the 23 factorial design with trials listed in the standard order of this design.
| Trial | Factors |
Responses |
|||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 (Q%2h) | Y2 (Q%6h) | Y3 (Q%12h) | Y4 (RSQzero) | |
| D1 | 10 | 10 | 0 | 0.5 | 1.6 | 2.8 | 0.975 |
| D2 | 25 | 10 | 0 | 0.5 | 68.5 | 100.0 | 0.864 |
| D3 | 10 | 20 | 0 | 0.6 | 1.8 | 54.6 | 0.560 |
| D4 | 25 | 20 | 0 | 0.8 | 70.7 | 100.0 | 0.880 |
| D5 | 10 | 10 | 1 | 1.4 | 4.9 | 18.6 | 0.857 |
| D6 | 25 | 10 | 1 | 31.4 | 87.9 | 100.0 | 0.761 |
| D7 | 10 | 20 | 1 | 0.5 | 77.0 | 100.0 | 0.904 |
| D8 | 25 | 20 | 1 | 26.5 | 82.7 | 99.5 | 0.831 |
where X1: PEG400 (%), X2: Coating level (%), and X3: Hole diameter (mm).
Preparation of tablet cores
Tablet cores of 300 mg each were prepared. Each tablet core contained: 35 mg TM, 131 mg spray dried lactose, 131 mg microcrystalline cellulose (avicel PH-102), and 3 mg magnesium stearate.
Aliquots corresponding to 125 g powder blend (passed through sieve #40) except magnesium stearate were geometrically mixed in a plastic bag. Finally, magnesium stearate was passed through sieve #60 and added to the previous blend just before tabletting.
The bulk density of the powder blend containing magnesium stearate was determined using a tapped density tester (Erweka Type: SVM202, Erweka, Germany). The lift height was kept at 3 mm. Carr’s compressibility index and Hausner ratio were calculated.
The powder blend was then directly compressed using a single punch tablet machine (Korsch XP1, Korsch, Germany) using 9 mm deep concave punch and die set.
Evaluation of tablet cores
The tablet cores were evaluated for the different physicochemical parameters, viz. appearance, weight variation, diameter, thickness, hardness, friability (Tablet friabilator, Digital test apparatus, Model DFI-1, Veego, Bombay, India) and drug content [14,17].
Coating and drilling
Tablet cores were coated using pan coating technique [18–20], with a flow of hot air to aid the coalescence of coating material droplets. The coating polymer of choice was ethylcellulose 4% w/w in ethyl alcohol (95%) [16,21]. Ethylcellulose was soaked in ethanol and stirred on a magnetic stirrer for 12 h. Dibutylphthalate (2% w/w) was then added. PEG400 was added as a pore former [18,22] in a specified amount (10% or 25%) based on polymer weight [16,21] for controlling membrane porosity. The coating was carried out by coating pan having a diameter of 20 cm attached to a drive motor. The rotating speed was kept at 12 rpm. The spraying mixture was sprayed using a spray gun fitted to an air compressor. The pan and tablet cores were preheated to 40 °C before spraying the coating mixture. Spraying of the coating mixture was continuously done at a rate of (1.5 ml/min) with a flow of hot air. Coating process was continued till tablets acquire the desired increase in weight [19,22]. Tablets were subjected to thermal after treatment (curing) for 2 h at 60 °C [14,17,23]. One hole of the desired size (1 mm) was mechanically drilled on one face of each tablet if needed [18,20]. This hole aimed at increasing the release rate from the early time of dissolution as the drug solution can exit through the hole as well as from the pores in the membrane [6].
In vitro release studies
The release of TM from the coated tablets was performed in USP paddle dissolution tester (USP Dissolution Tester, Apparatus II, Varian VK7000, Varian Inc., North Carolina, USA). Two-steps dissolution test [24] was carried out to simulate the physiological condition of GIT. In order to detect the drug release levels in the initial hours, the volume of release medium was reduced [14]. The release medium was 300 ml 0.1 N HCl for 2 h, then 100 ml of 0.2 M tribasic sodium phosphate solution were added to the dissolution medium and pH adjusted to 6.8 ± 0.05 with 2 N HCl or 2 N NaOH if needed [25], and the dissolution experiment was continued till a total period of 12 h (pH meter, Jenway 3510 pH meter, Barlworld scientific Ltd., UK). The temperature was maintained at 37.5 ± 0.5 °C, and the paddle speed was set at 100 rpm [14,24]. Aliquots of 3 ml from the dissolution medium were withdrawn at specified time points and filtered. The same volume of fresh medium was replaced after each sample withdrawal [22]. All release studies were done in duplicates. The amount of TM dissoluted was measured spectrophotometrically at λ = 231 nm (UV/VIS Spectrophotometer, UV-1601, Shimadzu, Japan) against the respective medium as blank. Although the maximum wavelength of trimetazidine is 269 nm, it has another absorption maximum at 231 nm. The wavelength of 231 nm was chosen in our study as trimetazidine spectrophotometric absorptivity is much higher at 231 nm than at 269 nm, to enable the detection of low trimetazidine concentrations.
The drug concentration values were corrected for progressive dilution to obtain cumulative amount permeated using the following equation [26].
where is the current cumulative mass of drug dissoluted at time t, Cn represents the current concentration in the dissolution medium, Σ Cm denotes the summed total of the previous measured concentrations [m = 1 to n − 1], Vr is the volume of the dissolution medium, Vs corresponds to the volume of the sample removed for analysis, and dose is the amount of drug per tablet (35 mg).
The in vitro release data obtained were plotted as the cumulative percentage drug released as a function of time (hour) [18,22].
Statistical analysis of the 23 factorial results
Design-Expert software (V. 7.0.0, Stat-Ease Inc., Minneapolis, USA) was used for the evaluation of the statistical experimental design. Means were compared by ANOVA-factorial. Significance level was set at α = 0.05. Suitable regression models were driven to enable navigation of the experimental space [27]. Response surface methodology and multiple response optimization were used to search for an optimized formula [13].
Results and discussion
Osmotic pumping is the primary mechanism of drug release from the oral osmotic pumps with simple diffusion playing a minor role [6]. The prepared CPOP tablets are spray-coated tablets with a semipermeable membrane coat containing leachable pore former material (PEG400). Upon contact with the release medium, this pore former leaches out leaving a microporous structure in the membrane. Water enters through the membrane, where it dissolves the water soluble components (TM and spray dried lactose). Hydrostatic pressure is created between the core of the tablet and the release medium. The lactose diluent as an osmogen helps in increasing this hydrostatic pressure. Most soluble sugars and salts function effectively for this purpose [19]. In this system, the drug after dissolution in the core is released from the CPOP tablets by hydrostatic pressure and diffusion through the pores created in the membrane and the drilled hole if any [7]. This drug release rate is usually controlled by the coat thickness, pore former percentage, core osmogen content, and hole size. In the present study, the cores composition was kept constant, as the drug is freely water soluble and the diluents contain 50% lactose, which together create enough osmotic pressure for water entrance. The studied variables were coat thickness, pore former percentage, and hole size.
Dibutylphthalate was used by Makhija and Vavia [14] to control membrane porosity in a concentration of 20% w/w of the used coating polymer (cellulose acetate). Garg et al. [28] used dibutylphthalate in a concentration of 0.6% of total coating mixture as a plasticizer for the cellulose acetate coat. In the present study, dibutylphthalate was added as a plasticizer in a concentration of 2% of total coating mixture (50% of the ethylcellulose polymer) as a constant variable to aid the membrane forming properties of ethylcellulose. This helped in forming continuous good adhering membranes even at low PEG400 concentration. Higher concentrations of dibutylphthalate were not used to avoid sticking between tablets during coating. Triethyl citrate and PEG1500 were tried in preliminary experiments of this study as alternative plasticizers, yet dibutylphthalate was chosen for its better film forming properties.
A thermal after treatment (curing) was applied to obtain sufficient polymer particle coalescence in the membrane [23,29]. If the polymer particles do not completely fuse during coating, further coalescence may occur during storage, resulting in denser and less permeable membranes. Consequently, the resulting drug release rate may significantly decrease on storage.
In vitro evaluation of tablet cores
Bulk density was found to be 0.494 g/ml, while the tap density = 0.635 g/ml. Carr’s compressibility index was found to be 22.2% and Hausner ratio was found to be 1.285, indicating passable flowability.
Tablet cores showed acceptable weight variation of 302.12 mg ± 8.8, acceptable drug content of 35.03 mg ± 0.33, acceptable friability of 0.048%, and hardness value of 4.74 kg ± 0.207. These parameters were suitable for further work [30].
In vitro release results for CPOP tablets of directly compressed cores
In general, an optimal extended release dosage form must have a minimal burst effect with most of the drug being released in a specific time period [12]. Therefore, the percentage of drug released after 2 h (Q%2h), 6 h (Q%6h), and 12 h (Q%12h) were selected as the response variables. These time points were used to detect the burst effect at an earlier stage and to ensure that most of the drug is released in a period of time suitable to the gastrointestinal residence time. RSQzero was used as the fourth response to ensure the zero order release pattern [3]. The constraints of these responses were specified to obtain a zero order release profile and percentage released of more than 80% in 12 h as shown in Table 1. Release profiles of the different prepared formulae are shown in Fig. 1. In Fig. 1, the wide variation indicated that the investigated factors and their studied levels resulted in different drug release rates.
Fig. 1.
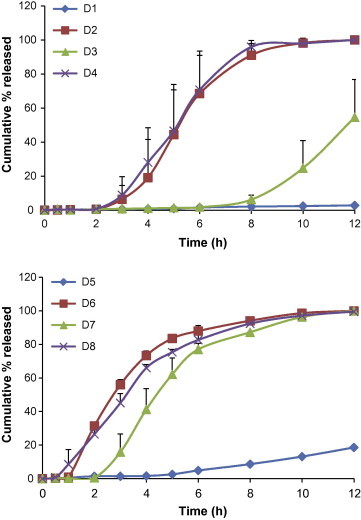
Release profile of TM from different trials, (n = 2), mean + σ.
The composition of different trials of the 23 factorial design and their respective observed responses listed in the standard order of this design are shown in Table 2.
Release studies for different trials were performed randomly.
Statistical analysis results of the 23 design
Each response was analyzed individually. Full factorial 3 factor interaction (3FI) model was used to describe the relation between the response under question and the variables studied. The general 3FI polynomial equation is as follows:
where Yi is the response under question, Xi’s (for i = 1–3) are the factors, XiXj (for i, j = 1–3, i < j) are the 2 factor interactions, XiXjXk (for i, j, k = 1–3, i < j < k) are the 3 factor interactions, b0 the intercept term, bi’s (for i = 1–3) are the linear effects coefficients, bij’s (for i, j = 1–3, i < j) are the interaction coefficients between the ith and jth variables, and bijk (for i, j, k = 1–3, i < j < k) are the interaction coefficients between the ith, jth and kth variables.
Model reduction [20] was adopted by removing nonsignificant model terms not required to support hierarchy [31]. For evaluation of different models, several R2 statistics are calculated, namely, ordinary R2, adjusted R2, and predicted R2 [13]. The model chosen either full or reduced was the one with the highest prediction R2 and the lowest prediction error sum of squares (PRESS) [12]. Summary of R2 statistics and PRESS for models of different responses is shown in Table 3. For Y1, a certain reduced model showed higher prediction R2 and lower PRESS than the full factorial model. This reduced model was the model of choice for this response. For the other responses, all the possible reduced models did not produce any improvement. The full factorial models were chosen for these responses. Table 4 shows the coefficient estimates for different model terms appearing in the final equation for each response and their significance levels.
Table 3.
R-squared values and PRESS for models of different responses.
| R2 | Adjusted R2 | Predicted R2 | PRESS | ||
|---|---|---|---|---|---|
| Y1 | Full factorial model | 0.9858 | 0.9733 | 0.9431 | 139.64 |
| Y1 | Reduced model (chosen) | 0.9754 | 0.9693 | 0.9563 | 107.14 |
| Y2 | Full factorial model | 0.9518 | 0.9097 | 0.8073 | 4337.19 |
| Y3 | Full factorial model | 0.9795 | 0.9616 | 0.9181 | 1978.92 |
| Y4 | Full factorial model | 0.9912 | 0.9835 | 0.9649 | 0.01 |
Table 4.
Coefficient estimates for different model terms appearing in the final equation for each response and their significance levels.
|
Y1 |
Y2 |
Y3 |
Y4 |
|||||
|---|---|---|---|---|---|---|---|---|
| CE | p-Value | CE | p-Value | CE | p-Value | CE | p-Value | |
| b0 | 7.79 | <0.0001⁎ | 49.38 | <0.0001⁎ | 71.93 | <0.0001⁎ | 0.829 | <0.0001⁎ |
| b1 | 7.03 | <0.0001⁎ | 28.07 | <0.0001⁎ | 27.95 | <0.0001⁎ | 0.005 | 0.2184 |
| b2 | – | – | 8.66 | 0.0177⁎ | 16.59 | <0.0001⁎ | −0.035 | <0.0001⁎ |
| b3 | 7.18 | <0.0001⁎ | 13.73 | 0.0015⁎ | 7.59 | 0.0048⁎ | 0.009 | 0.0432⁎ |
| b12 | – | – | −9.44 | 0.0118⁎ | −16.71 | <0.0001⁎ | 0.057 | <0.0001⁎ |
| b13 | 6.97 | <0.0001⁎ | −5.9 | 0.0774 | −7.71 | 0.0044⁎ | −0.047 | <0.0001⁎ |
| b23 | – | – | 8.06 | 0.0244⁎ | 3.64 | 0.1011 | 0.065 | <0.0001⁎ |
| b123 | – | – | −9.92 | 0.0092⁎ | −3.76 | 0.0922⁎ | −0.051 | <0.0001⁎ |
CE: Coefficient estimate.
Significant at p < 0.05.
Inference of the statistical analysis of the different responses in the 23 design
The coefficient estimate of each term is half the effect of that term, whether this term is a main effect or a 2 factor interaction. Thus, the main effect of each factor on different responses will be discussed. Also, each 2 factor interaction for different responses will be discussed [27].
Effect of PEG400%
Percentage of PEG400 had a significant positive effect on the Q%2h, Q%6h, and Q%12h. That is the higher the PEG400% the higher the percentage of the drug released after 2 h, 6 h, and 12 h, respectively. This could be explained by the water soluble nature of this pore former. Since PEG400 is a hydrophilic plasticizer, the higher the PEG400% the more the void space formed in the membrane after PEG400 leaching which results in higher permeability of the membrane allowing higher influxes of water and solubilization of TM then exit of TM solution to the release medium. This is in accordance with the results found in many other researches. Xu et al. [22] stated that the salvianolic acid release rate increased from a microporous cellulose acetate membrane as the pore forming substance (PEG400) increased. Lu et al. [18] stated that the increase in PEG400 level led to an increase in naproxen release rate from controlled porosity osmotic pump coated with PEG400 plasticized cellulose acetate membrane. Makhija and Vavia [14] stated that as the amount of PEG 400 in the cellulose acetate polymeric coat increased, pseudoephedrine release rate also increased.
Effect of coating level%
Coating level% had a significant positive effect on Q%6h and Q%12h. That is the higher the value of coating level% the higher the values of Q%6h and Q%12h. This is in contrary to the results found in many other studies. Xu et al. [22] stated that the salvianolic acid release rate from a microporous membrane was affected by and was inversely proportional to overall coating weight. Makhija and Vavia [14] stated that as the polymer loading (coating level) of cellulose acetate polymeric coat increased, pseudoephedrine release rate decreased. They explained this by the fact that the fluid had to penetrate the polymer coat of greater thickness as the polymer loading increased. Liu and Xu [21] found that nifedipine release rate decreased as the PEG400 plasticized ethylcellulose membrane thickness increased. They reported that the increase in thickness led to increase in the membrane resistance to water penetration, resulting in a decrease in the drug release rate. Lu et al. [18] stated that the increase in membrane thickness had no significant difference on naproxen release rate from controlled porosity osmotic pump coated with PEG400 plasticized cellulose acetate membranes. The difference between the thicknesses of membranes used in that research was explained by them to be not enough to significantly change the release profiles.
In the present work, we suggest that the elasticity of the membrane coat decreased as its thickness increased. On entrance of water into the tablet core, the avicel present in the core imbibes water and tends to increase in volume. In the presence of a thicker coat, the coat cracked, leading to a higher release of TM solution through the cracks formed resulting into higher Q%6h and Q%12h. This explanation is also supported by the nonsignificant effect of coating level for the response Q%2h. That is because there had not been any cracks yet in the membrane to allow higher fluxes of TM solution from the core. These cracks were large enough to be observed with naked eye.
Coating level% had a significant negative effect on RSQzero. That is as the coat thickness increases, the release profile becomes less fitting to zero order release mechanism. This may be due to the higher possibility of cracks formation on thicker membranes which disturbed zero order release profile.
Effect of hole diameter
The two levels studied for hole diameter were 0 that is no hole and 1 mm. Generally, hole presence in CPOP tablets is not a must due to the in situ formed micro-pores after the leaching of pore forming material. Yet, a lag time usually appears in the release profile due to time taken for coat hydration and PEG400 leaching. Presence of an extra hole was thought to overcome this delay in drug release initiation.
Hole diameter had a significant positive effect on Q%2h, Q%6h, and Q%12h. This could be explained by the presence of an opening for direct water entrance. This allows higher influx of water and solubilization then exit of TM solution to the release medium in higher amounts through this opening. This is in accordance with the results found by Prabakaran et al. [6].
The presence of the hole had also a significant positive effect on RSQzero as it resulted in direct water entrance and less lag time before acquiring a near zero order drug release profile.
Interaction of PEG400% and coating level%
Response surface plots for the effects of PEG400% and coating level% on different responses are shown in Fig. 2. A negative significant interaction occurred between PEG400% and coating level% for the responses Q%6h and Q%12h. This may be due to the cracks formed in the membrane at higher coating level which resulted in higher release even with lower PEG400%. This explanation is also supported by the absence of this interaction for the response Q%2h as no cracks in the membrane were yet formed.
Fig. 2.
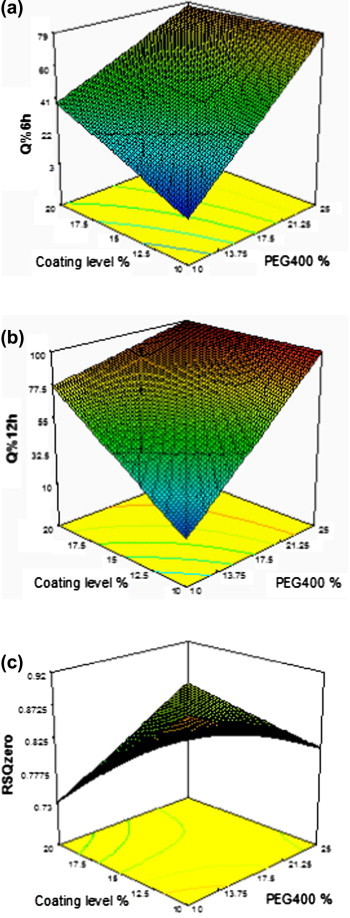
Response surface plot for the effect of PEG400% and coating level% on different responses: (a) Q%6h, (b) Q%12h, and (c) RSQzero.
A positive significant interaction occurred between PEG400% and coating level% for the response RSQzero. At higher PEG400% level, higher influxes of water were achieved from the beginning of release process, which compensated for the cracks formed after some time in the release, which resulted in increase in the value of this response. That is this positive interaction led to a more fitting of the release profile to the zero order release mechanism.
Interaction of PEG400% and hole diameter
Response surface plots for the effects of PEG400% and hole diameter on different responses are shown in Fig. 3. A positive significant interaction occurred between PEG400% and hole diameter for the response Q%2h. That is the effect of PEG400% on Q%2h increased by increasing the hole diameter. This could be explained by the increased water entry through the hole in the early stages of release resulting into an increase in the amount of TM diffused after 2 h.
Fig. 3.
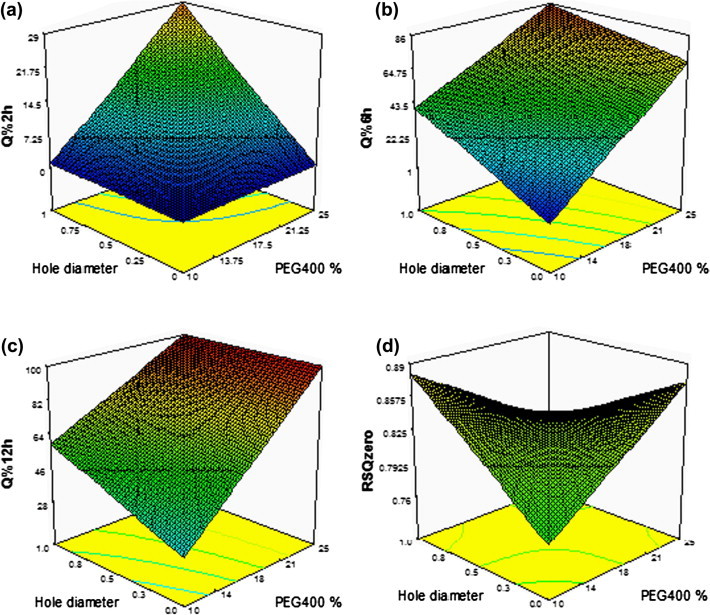
Response surface plot for the effect of PEG400% and hole diameter on different responses: (a) Q%2h, (b) Q%6h, (c) Q%12h, and (d) RSQzero.
On the other hand, a negative significant interaction occurred between PEG400% and hole diameter for the response Q%12h. After 12 h, PEG400 had completely dissolved and enough openings had formed and the presence of the hole did not have a pronounced effect on release.
Also, a negative significant interaction occurred between PEG400% and hole diameter for the response RSQzero. This could be explained by the positive interaction of these variables after 2 h (Q%2h) and their negative interaction after 12 h (Q%12h) as previously mentioned. These opposite directions interactions may have led to a less linear release profile.
Interaction of coating level% and hole diameter
Response surface plots for the effects of coating level% and hole diameter on different responses is shown in Fig. 4. A positive significant interaction occurred between coating level% and hole diameter for the response Q%6h. That is the effect of coating level% on Q%6h increased in presence of the hole. This could be due to higher water influxes on higher hole diameter, higher volume of avicel in the core after water imbibition, and more cracks formation in thicker coats which led to higher TM release.
Fig. 4.
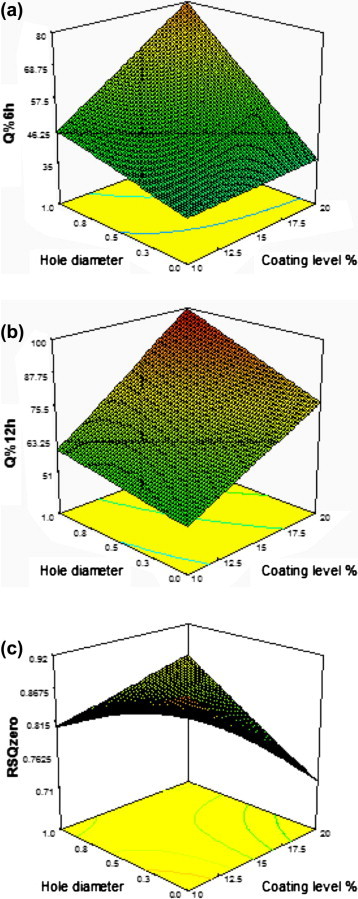
Response surface plot for the effect of coating level% and hole diameter on: (a) Q%6h, (b) Q%12h, and (c) RSQzero.
A positive significant interaction between coating level% and hole diameter for the response RSQzero was also noticed. This may be due to faster and more cracks formation in presence of the hole which resulted into reduced lag time.
Elucidation of an optimized formula for TM release
The constraints listed in Table 1 were used for numerical optimization of a formula with the desired responses by the aid of the statistical program. The tested trials resulted into a wide range of responses; Q%2h ranged from 0.504% to 31.447%, Q%6h from 1.562% to 87.934%, Q%12h from 2.7825% to 99.99%, and RSQzero from 0.5596 to 0.97495. Unfortunately, upon exploring the experimental space, no specific formula in the tested ranges of the independent variables could satisfy the required constraints. The release profiles of the tested formulae showed either a lag time of 0.5 h to 2 h and then very rapid release rates (formulae D2, D4, D6, D7, and D8), a very low release rate for the whole 12 h (formulae D1 and D5) or a very long lag time of about 7 h then high release rate (formula D3). This may be due to the presence of avicel as a component of tablet cores. At low levels of water entrance, the TM release was low and the coat remained intact for the whole 12 h. At high levels of water entrance, avicel imbibes high amounts of water, and its disintegrant nature led to cracks formation in the coat, which disturbed the zero release profile characteristic of osmotic release formulae.
The present study aimed at testing the feasibility of using the direct compression technique to prepare TM tablet cores for CPOP tablets. Direct compression is considered the easiest technique for tablet production. Direct compression of tablet cores for formation of osmotic release oral drug delivery systems have been used by many researchers [16,29,33]. This technique showed good results especially with the use of expandable polymers in the core formulation [3,20,33].
In preliminary experiments performed early in the present work, spray dried lactose and mannitol were used each alone as a diluent for formation of directly compressed tablet cores. Yet, the fluffy nature of TM powder hindered the direct compression of tablet cores with both diluents. The resultant cores were capped or laminated. Avicel is often regarded as one of the best excipients having high binding capacity in directly compressed cores [12]. So, in the present study, it was used with spray dried lactose in a ratio 1:1 to aid direct compression of TM tablet cores. The cores prepared with avicel and lactose acquired suitable hardness and friability and were satisfactory for coating, so the 23 design was initiated and the suggested CPOP tablets were prepared.
The prepared tablets failed to fulfill the required release profile of TM. This was due to disintegrant nature of avicel which led to cracks in the coat that is supposed to remain intact for the whole release period of 12 h. The cracks formed in coat also led to fluctuation of drug release in some trials. This is thought to be due to the ununiformity of cracks formed. However, microcrystalline cellulose had been used by some workers for formulation of osmotic tablets [24,32,34].
To recapitulate, the direct compression of CPOP tablets cores – under the studied conditions especially the use of avicel in the core – was found to be not suitable for optimizing TM release.
In an attempt to use lactose monohydrate as a sole soluble diluent for formation of tablet cores suitable for optimizing TM release from CPOP tablets, wet granulation technique was tried. This was to avoid cracks formation in the coat, and keeping the coat intact for the whole release period of 12 h. Preliminary trials of CPOP tablets of these wet granulated cores were subjected to release studies. Although the percentages released from these trial formulations did not meet the constraints listed in Table 1, yet these formulations appeared to be promising with an intact membrane for 12 h and high RSQzero. Further improvement of these formulations to optimize TM release will be done in further studies. The improvements on these formulations will include changing dibutylphthalate and PEG400 concentrations, coat thickness, and hole size. A new statistical design with new levels of factors will be used for optimizing trimetazidine release from CPOP tablets of wet granulated cores according to the same constraints of the present study.
Conclusions
The results of the statistical analysis revealed that PEG400% had a significant positive effect on Q%2h, Q%6h, and Q%12h. Coating level% had a significant positive effect on Q%6h and Q%12h and a significant negative effect on RSQzero. Hole diameter had a significant positive effect on Q%2h, Q%6h, Q%12h, and RSQzero.
Unfortunately, upon exploring the experimental space, no formula in the tested range of variables could satisfy the required constraints. (10% ⩽ Y1 ⩽ 20%, 40% ⩽ Y2 ⩽ 60%, 80% ⩽ Y3 ⩽ 100%, and Y4: Maximize > 0.9). This may be due to the presence of avicel as a component of tablet cores. The disintegrant nature of avicel led to cracks formation in the coat on high water entrance, which disturbed the zero order release profile characteristic of CPOP formulae. Thus, TM release could not be optimized from the prepared CPOP formulae of directly compressed cores under the studied conditions.
Preliminary trials of CPOP tablets of wet granulated cores using lactose monohydrate as a sole diluents were promising with an intact membrane for 12 h and high RSQzero. Further improvement of these formulations to optimize TM release will be done in further studies.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgments
The authors are thankful for Global Napi Pharmaceuticals Company for supplying some chemicals for this research and for Dr. Mohamed A.A.A. Eldegwy for his help during this work.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Malaterre V., Ogorka J., Loggia N., Gurny R. Oral osmotically driven systems: 30 years of development and clinical use. Eur J Pharm Biopharm. 2009;73:311–323. doi: 10.1016/j.ejpb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Conley R., Gupta S.K., Sathyan G. Clinical spectrum of the osmotic-controlled release oral delivery system (OROS), an advanced oral delivery form. Curr Med Res Opin. 2006;22:1879–1892. doi: 10.1185/030079906x132613. [DOI] [PubMed] [Google Scholar]
- 3.Shokri J., Ahmadi P., Rashidi P., Shahsavari M., Rajabi-Siahboomi A., Nokhodchi A. Swellable elementary osmotic pump (SEOP): an effective device for delivery of poorly water-soluble drugs. Eur J Pharm Biopharm. 2008;68:289–297. doi: 10.1016/j.ejpb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Zentner G.M., Rork G.S., Himmelstein K.J. Osmotic flow through controlled-porosity films: an approach to delivery of water-soluble compounds. J Control Release. 1985;2:217–229. [Google Scholar]
- 5.Zentner GM, Rork GS. Controlled-porosity osmotic pump. U.S. Patent 4,968,507; 1990.
- 6.Prabakaran D., Singh P., Kanaujia P., Jaganathan K.S., Rawat A., Vyas S.P. Modified push – pull osmotic system for simultaneous delivery of theophylline and salbutamol: development and in vitro characterization. Int J Pharm. 2004;284:95–108. doi: 10.1016/j.ijpharm.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Okimoto K., Tokunaga Y., Ibuki R., Irie T., Uekama K., Rajewski R.A. Applicability of (SBE) 7m -β-CD in controlled-porosity osmotic pump tablets (OPTs) Int J Pharm. 2004;286:81–88. doi: 10.1016/j.ijpharm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Zhao P., Zhang J., Yin X.-G., Maharaj P., Narraindoo S., Cui L.-Q. The effect of trimetazidine on cardiac function in diabetic patients with idiopathic dilated cardiomyopathy. Life Sci. 2013;92:633–638. doi: 10.1016/j.lfs.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Abdelbary A., El-Gazayerly O.N., El-Gendy N.A., Ali A.A. Floating tablet of trimetazidine dihydrochloride: an approach for extended release with zero-order kinetics. AAPS PharmSciTech. 2010;11:1058–1067. doi: 10.1208/s12249-010-9468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Péter K., János B., Zsolt Z., Klára P.-H. Study of the compaction behaviour and compressibility of binary mixtures of some pharmaceutical excipients during direct compression. Chem Eng Process. 2009;48:859–863. [Google Scholar]
- 11.Bolton S. 3rd ed. Marcel Dekker Inc.; New York, USA: 1997. Pharmaceutical statistics; practical and clinical application. [Google Scholar]
- 12.Huang Y.-B., Tsai Y.-H., Yang W.-C., Chang J.-S., Wu P.-C., Takayama K. Once-daily propranolol extended-release tablet dosage form: formulation design and in vitro/in vivo investigation. Eur J Pharm Biopharm. 2004;58:607–614. doi: 10.1016/j.ejpb.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Kim M.-S., Kim J.-S., You Y.-H., Park H.J., Lee S., Park J.-S. Development and optimization of a novel oral controlled delivery system for tamsulosin hydrochloride using response surface methodology. Int J Pharm. 2007;341:97–104. doi: 10.1016/j.ijpharm.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 14.Makhija S.N., Vavia P.R. Controlled porosity osmotic pump-based controlled release systems of pseudoephedrine I. Cellulose acetate as a semipermeable membrane. J Control Release. 2003;89:5–18. doi: 10.1016/s0168-3659(02)00482-0. [DOI] [PubMed] [Google Scholar]
- 15.Kim M.S., Kim J.S., Lee S., Jun S.W., Park J.S., Woo J.S. Optimization of tamsulosin hydrochloride controlled release pellets coated with Surelease and neutralized HPMCP. J Pharm Pharmacol. 2006;58:1611–1616. doi: 10.1211/jpp.58.12.0007. [DOI] [PubMed] [Google Scholar]
- 16.Liu L., Wang X. Solubility-modulated monolithic osmotic pump tablet for atenolol delivery. Eur J Pharm Biopharm. 2008;68:298–302. doi: 10.1016/j.ejpb.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Bhanushali R., Wakode R., Bajaj A. Monolithic osmotic tablets for controlled delivery of antihypertensive drug. J Pharm Innov. 2009;4:63–70. [Google Scholar]
- 18.Lu E., Jiang Z., Zhang Q., Jiang X. A water-insoluble drug monolithic osmotic tablet system utilizing gum arabic as an osmotic, suspending and expanding agent. J Control Release. 2003;92:375–382. doi: 10.1016/s0168-3659(03)00371-7. [DOI] [PubMed] [Google Scholar]
- 19.Waterman K.C., Macdonald B.C., Roy M.C. Extrudable core system: development of a single-layer osmotic controlled-release tablet. J Control Release. 2009;134:201–206. doi: 10.1016/j.jconrel.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Guan J., Zhou L., Nie S., Yan T., Tang X., Pan W. A novel gastric-resident osmotic pump tablet: in vitro and in vivo evaluation. Int J Pharm. 2010;383:30–36. doi: 10.1016/j.ijpharm.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 21.Liu L., Xu X. Preparation of bilayer-core osmotic pump tablet by coating the indented core tablet. Int J Pharm. 2008;352:225–230. doi: 10.1016/j.ijpharm.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 22.Xu W., Li N., Gao C. Preparation of controlled porosity osmotic pump tablets for salvianolic acid and optimization of the formulation using an artificial neural network method. Acta Pharm Sin B. 2011;1:64–70. [Google Scholar]
- 23.Muschert S., Siepmann F., Leclercq B., Siepmann J. Dynamic and static curing of ethylcellulose: PVA–PEG graft copolymer film coatings. Eur J Pharm Biopharm. 2011;78:455–461. doi: 10.1016/j.ejpb.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Kumaravelrajan R., Narayanan N., Suba V., Bhaskar K. Simultaneous delivery of Nifedipine and Metoprolol tartarate using sandwiched osmotic pump tablet system. Int J Pharm. 2010;399:60–70. doi: 10.1016/j.ijpharm.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 25.The United States Pharmacopoeial convention. The United States Pharmacopoeia USP 34; 2011.
- 26.Meidan V.M., Al-khalili M., Michniak B.B. Enhanced iontophoretic delivery of buspirone hydrochloride across human skin using chemical enhancers. Int J Pharm. 2003;264:73–83. doi: 10.1016/s0378-5173(03)00390-9. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery D.C. 5th ed. John Wiley & Sons, Inc.; New York, USA: 2001. Design and Analysis of Experiments. [Google Scholar]
- 28.Garg A., Gupta M., Bhargava H.N. Effect of formulation parameters on the release characteristics of propranolol from asymmetric membrane coated tablets. Eur J Pharm Biopharm. 2007;67:725–731. doi: 10.1016/j.ejpb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Yang Q.W., Flament M.P., Siepmann F., Busignies V., Leclerc B., Herry C. Curing of aqueous polymeric film coatings: importance of the coating level and type of plasticizer. Eur J Pharm Biopharm. 2010;74:362–370. doi: 10.1016/j.ejpb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 30.The Stationary Office. British Pharmacopoeia 2012;2011.
- 31.Gonnissen Y., Gonçalves S.I.V., De Geest B.G., Remon J.P., Vervaet C. Process design applied to optimise a directly compressible powder produced via a continuous manufacturing process. Eur J Pharm Biopharm. 2008;68:760–770. doi: 10.1016/j.ejpb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Herbig S.M., Cardinal J.R., Korsmeyer R.W., Smith K.L. Asymmetric-membrane tablet coatings for osmotic drug delivery. J Control Release. 1995;35:127–136. [Google Scholar]
- 33.Malaterre V., Ogorka J., Loggia N., Gurny R. Approach to design push–pull osmotic pumps. Int J Pharm. 2009;376:56–62. doi: 10.1016/j.ijpharm.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Liu L., Ku J., Khang G., Lee B., Rhee J.M., Lee H.B. Nifedipine controlled delivery by sandwiched osmotic tablet system. J Control Release. 2000;68:145–156. doi: 10.1016/s0168-3659(00)00243-1. [DOI] [PubMed] [Google Scholar]



